Heat-Treated Aramid Pulp/Silica Aerogel Composites with Improved Thermal Stability and Thermal Insulation
Abstract
:1. Introduction
2. Results and Discussion
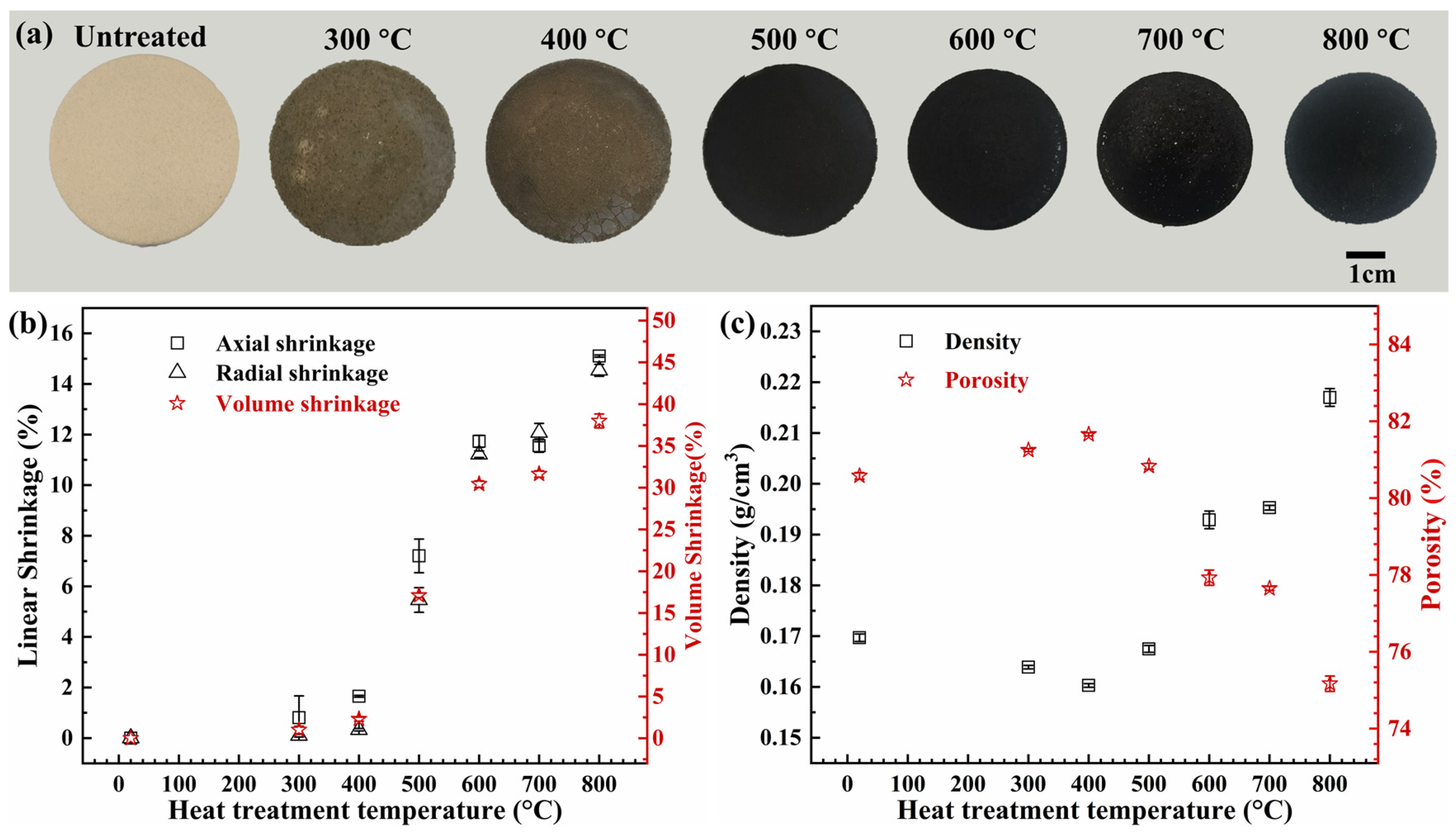
2.1. Shrinkage, Density and Porosity
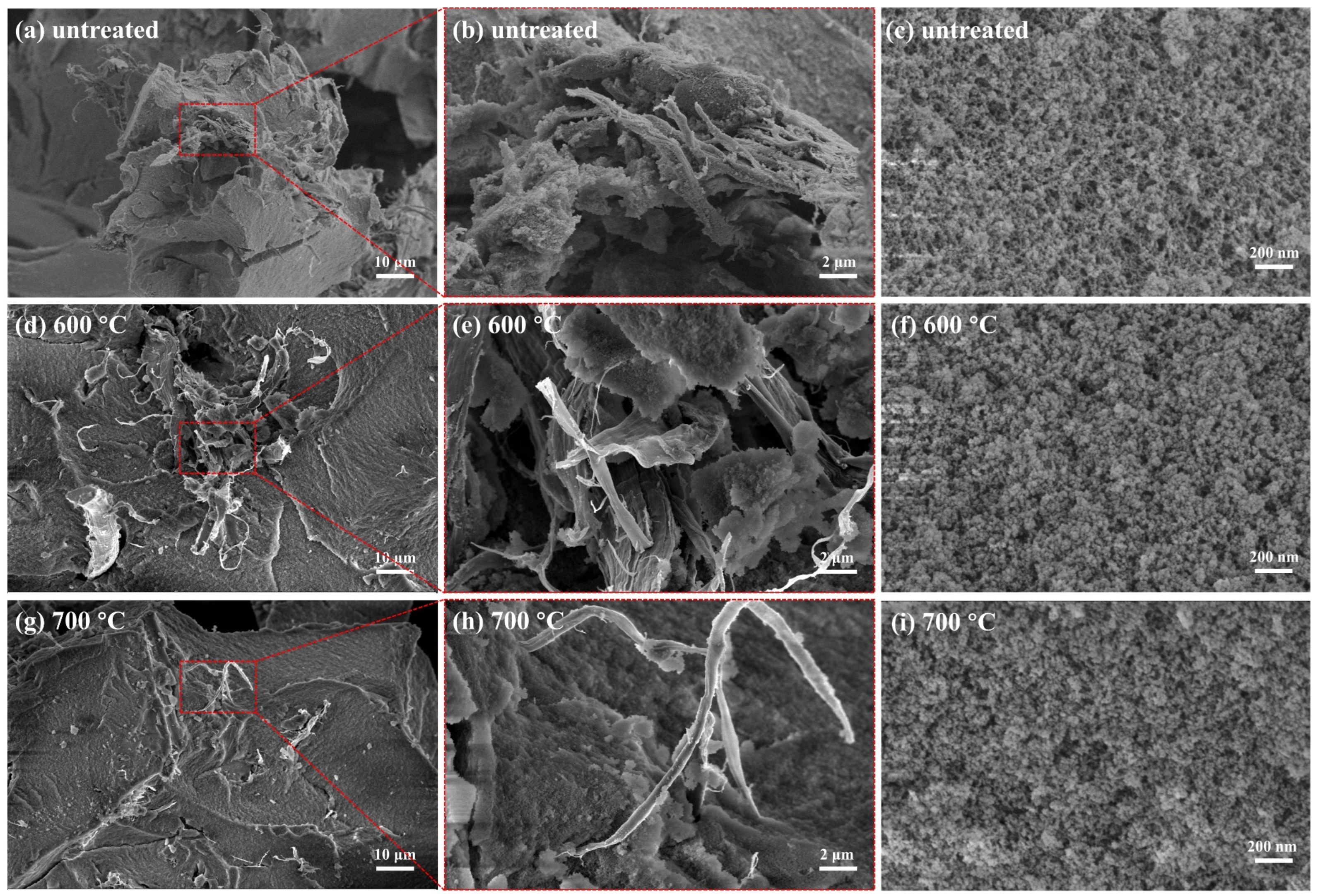
2.2. Microstructure
2.3. Pore Structure
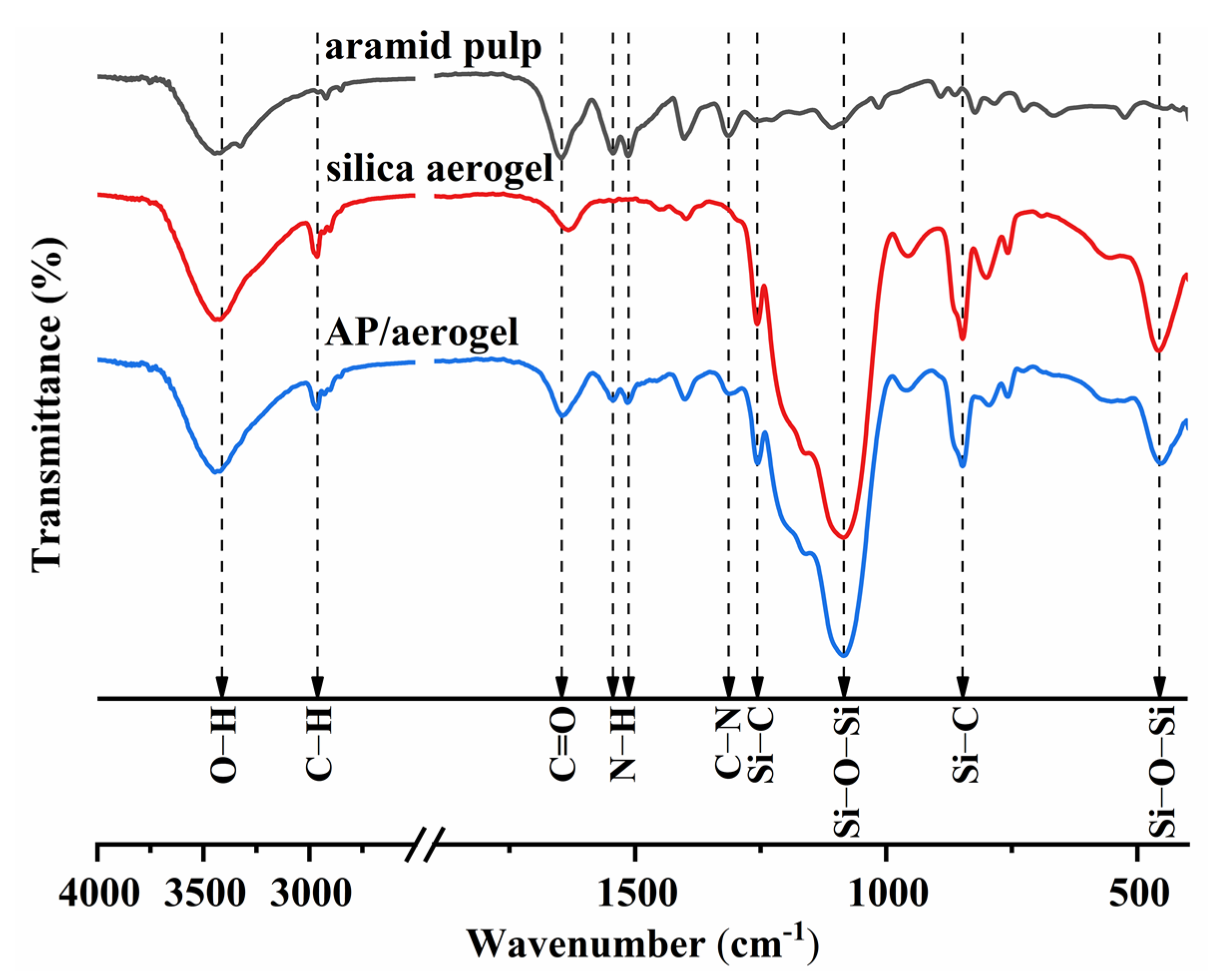
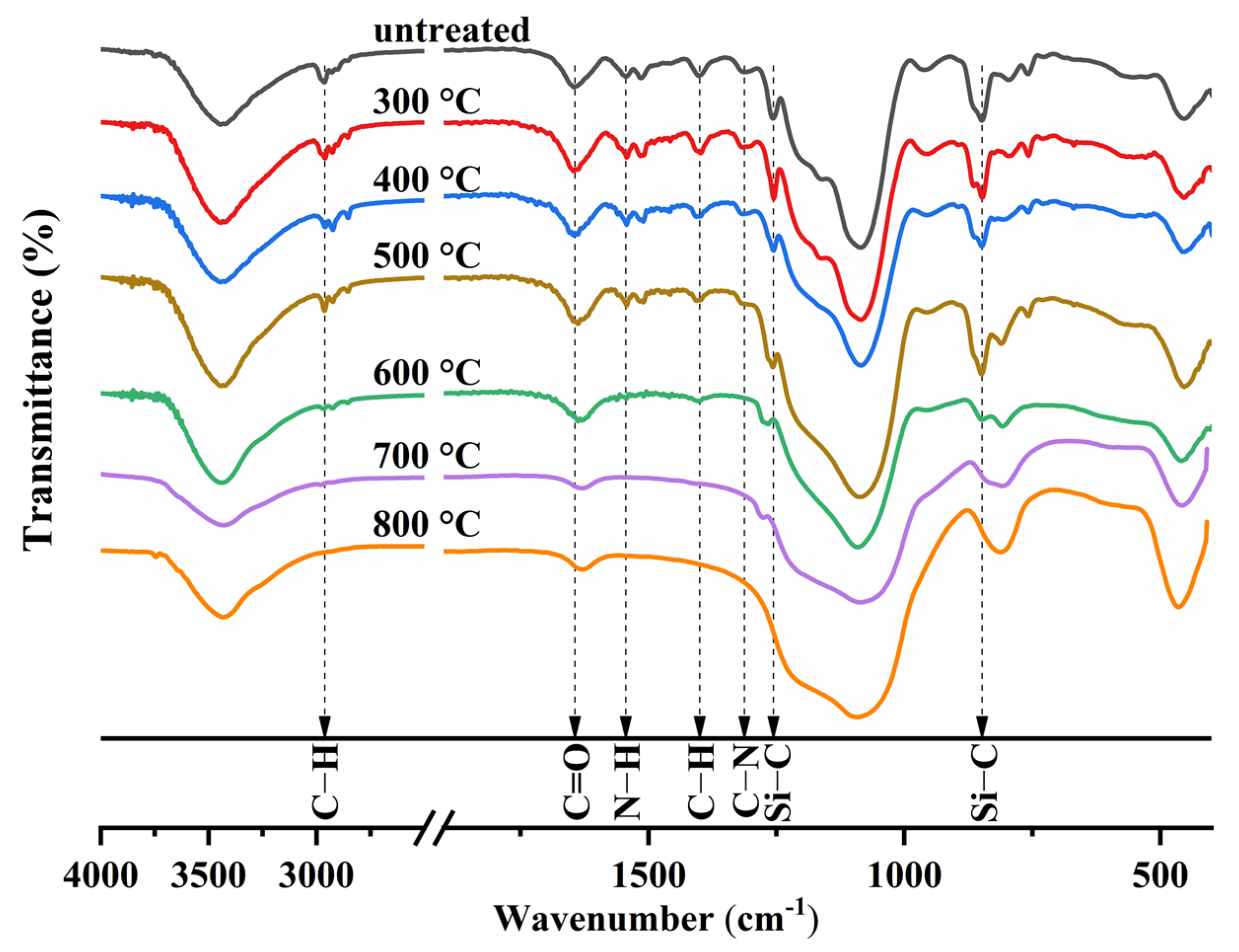
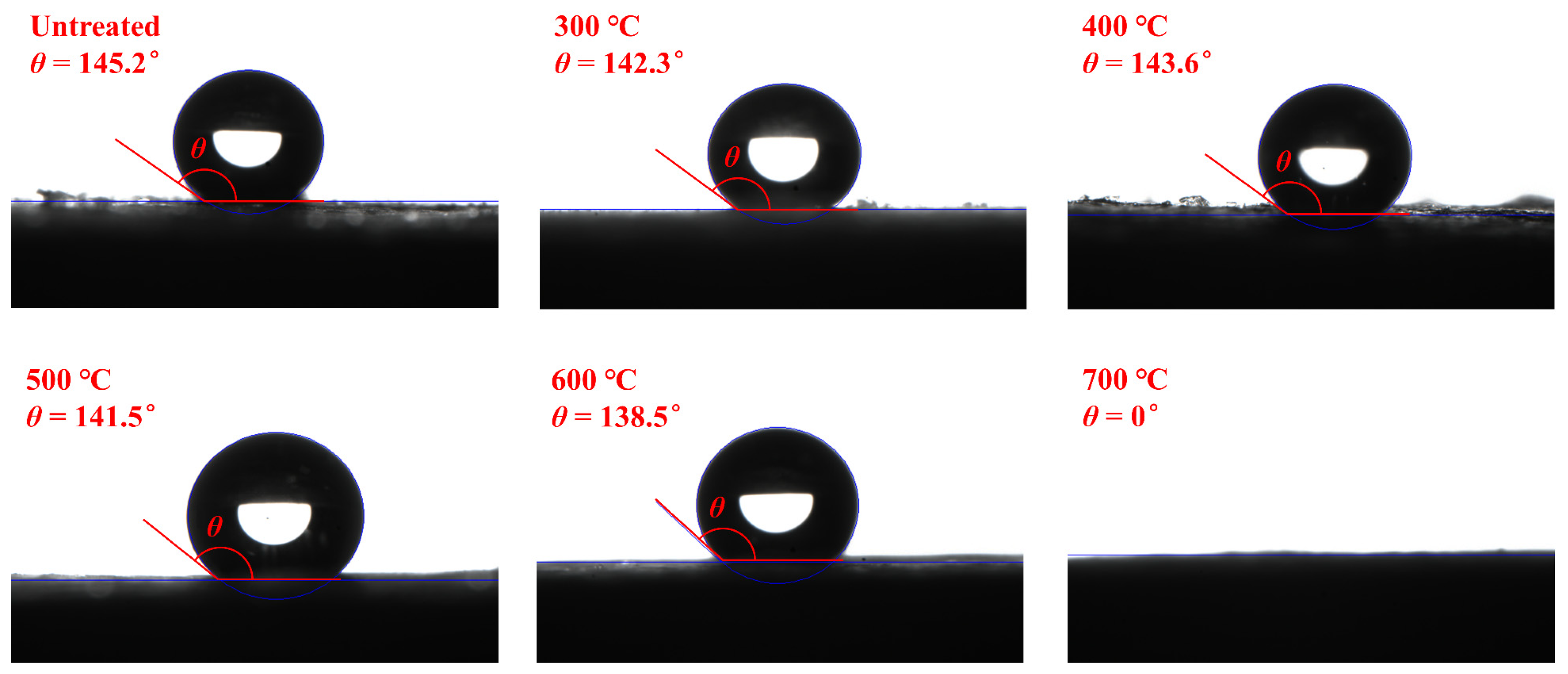
2.4. FTIR and Hydrophobicity
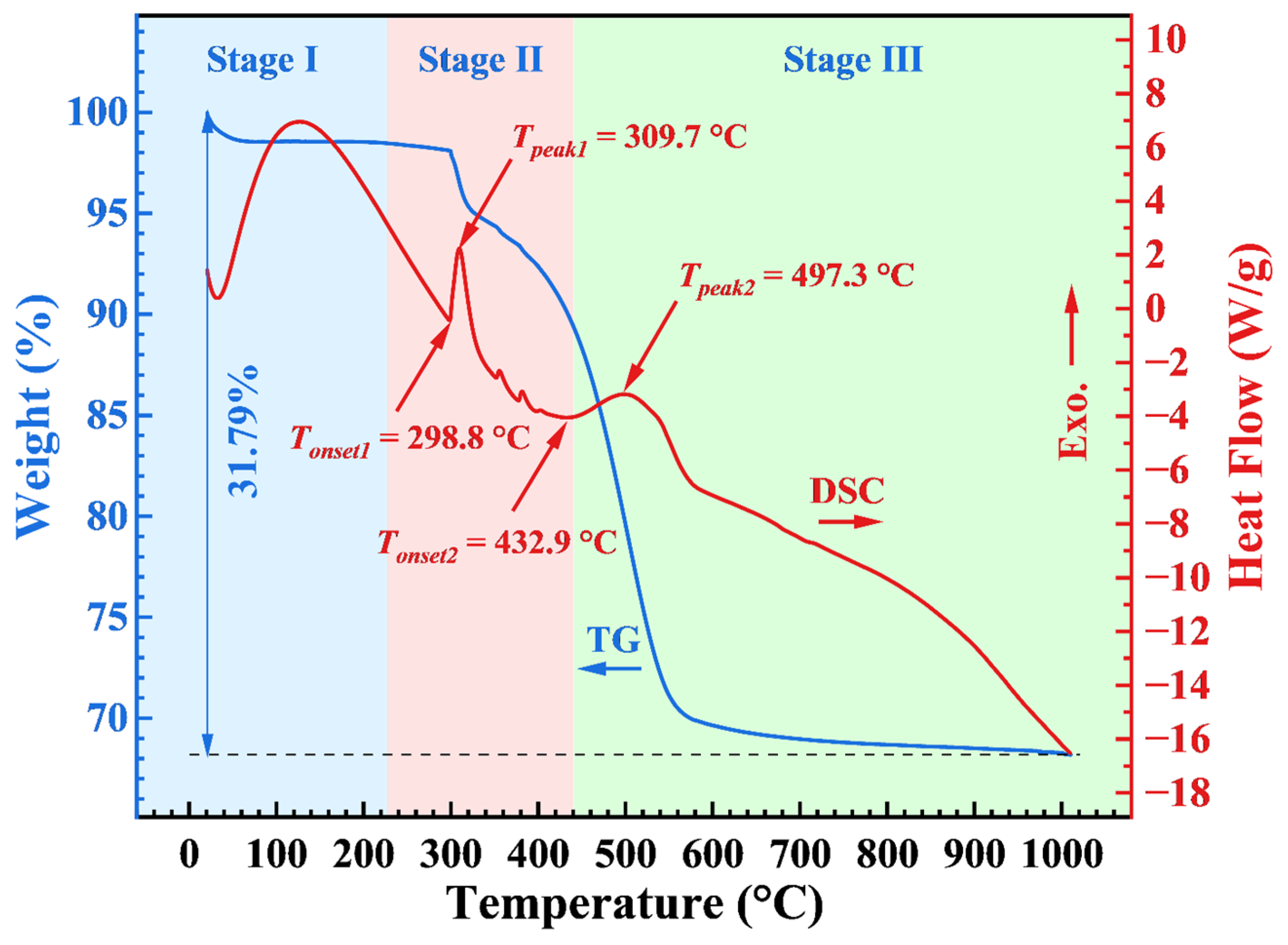
2.5. Thermal Stability
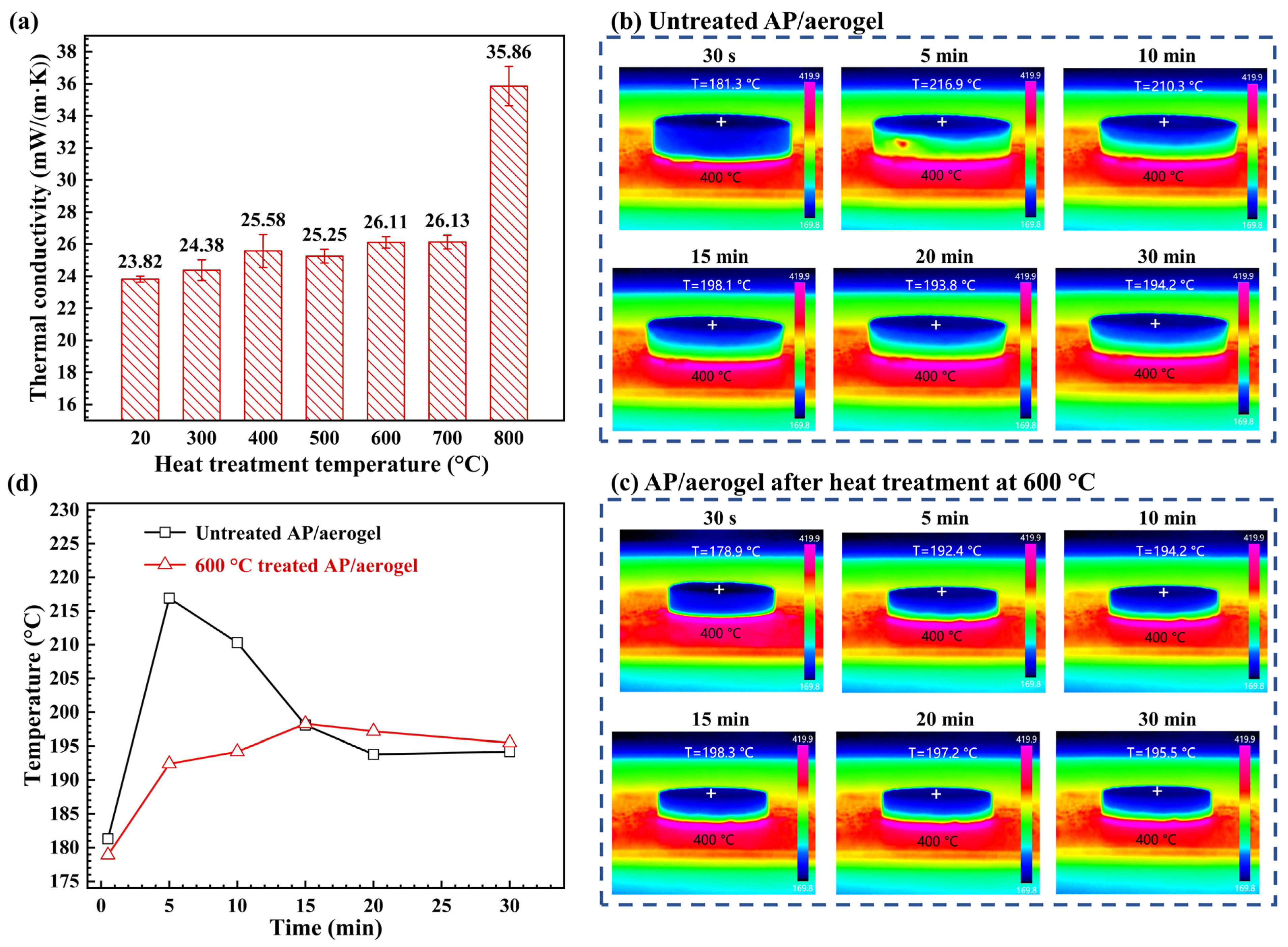
2.6. Thermal Insulation
3. Conclusions
4. Materials and Methods
4.1. Materials
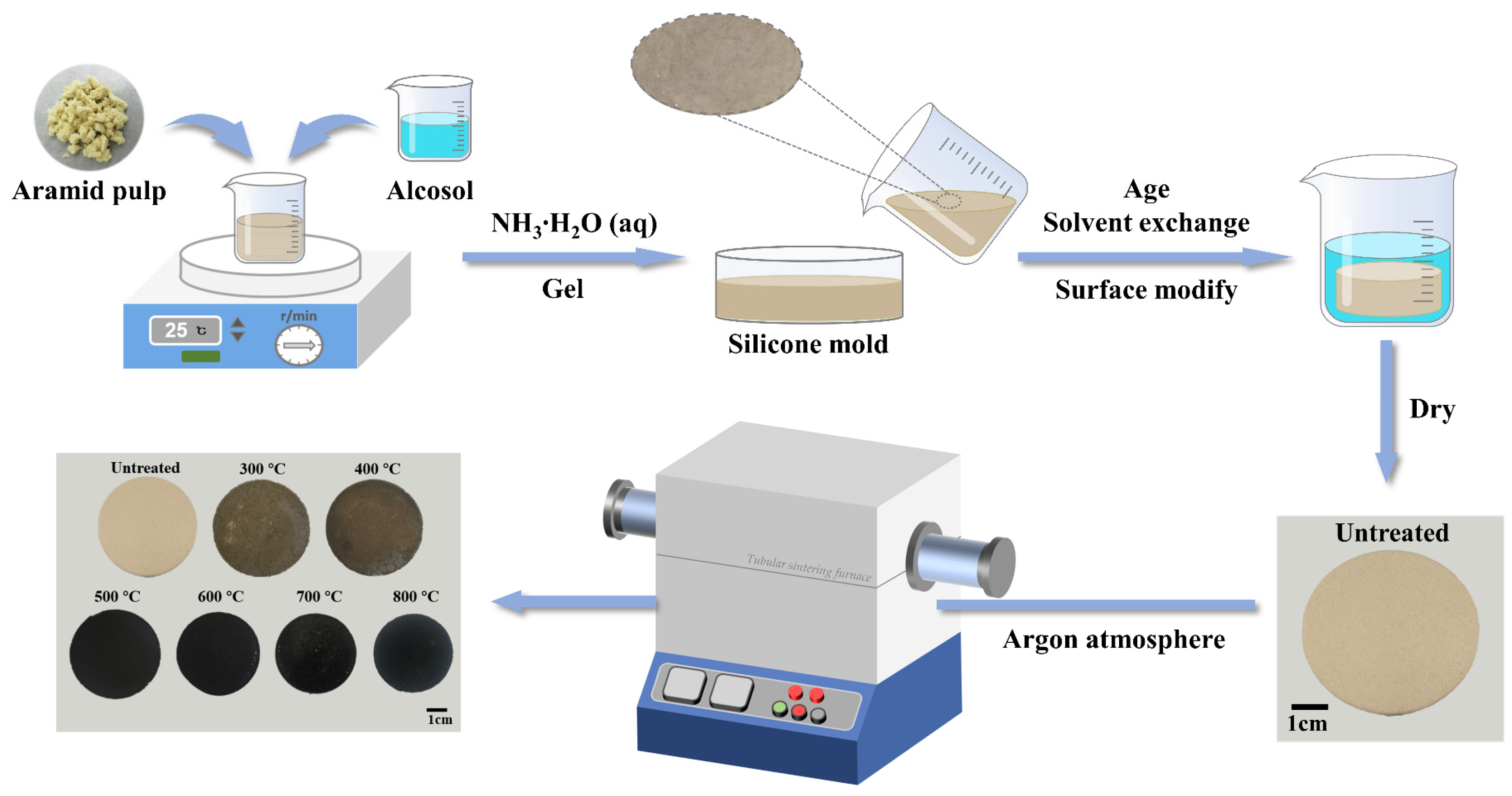
4.2. Preparation of Heat-Treated AP/Aerogels
4.3. Methods of Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive Manufacturing of Silica Aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward Aerogel Based Thermal Superinsulation in Buildings: A Comprehensive Review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A Review and Evaluation of Thermal Insulation Materials and Methods for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Tang, G.H.; Zhao, Y.; Guo, J.F. Multi-Layer Graded Doping in Silica Aerogel Insulation with Temperature Gradient. Int. J. Heat Mass Transf. 2016, 99, 192–200. [Google Scholar] [CrossRef]
- Neugebauer, A.; Chen, K.; Tang, A.; Allgeier, A.; Glicksman, L.R.; Gibson, L.J. Thermal Conductivity and Characterization of Compacted, Granular Silica Aerogel. Energy Build. 2014, 79, 47–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Zhao, X.; Jia, Q.; Li, H. Preparation and Microstructure of CuO-CoO-MnO/SiO2 Nanocomposite Aerogels and Xerogels as Catalyst Carriers. Prog. Nat. Sci. Mater. Int. 2011, 21, 330–335. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Wang, M.; Cai, J.; Xu, J.; Xia, C. Facile Preparation of Highly-Dispersed Cobalt-Silicon Mixed Oxide Nanosphere and Its Catalytic Application in Cyclohexane Selective Oxidation. Nanoscale Res. Lett. 2011, 6, 586. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Ramalho, A.L.; Durães, L. Silica-Based Aerogel Composites Reinforced with Different Aramid Fibers for Thermal Insulation in Space Environments. J. Mater. Sci. 2021, 56, 13604–13619. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Yin, L.; Xu, J.; Zhang, B.; Wang, L.; Tao, W.; Teng, X.; Ning, W.; Zhang, Z. A Facile Fabrication of Highly Dispersed CeO2/SiO2 Aerogel Composites with High Adsorption Desulfurization Performance. Chem. Eng. J. 2022, 428, 132581. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Hegde, N.D.; Hirashima, H. Absorption and Desorption of Organic Liquids in Elastic Superhydrophobic Silica Aerogels. J. Colloid Interface Sci. 2007, 305, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, Y.; Dong, Z.; Wang, J.; Hou, X. Hydrophobic and Nanoporous Chitosan-Silica Composite Aerogels for Oil Absorption. J. Appl. Polym. Sci. 2015, 132, 41770. [Google Scholar] [CrossRef]
- Štandeker, S.; Novak, Z.; Knez, Ž. Removal of BTEX Vapours from Waste Gas Streams Using Silica Aerogels of Different Hydrophobicity. J. Hazard. Mater. 2009, 165, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS/TEOS Co-Precursor Silica Aerogels Dried at Ambient Pressure. J. Non-Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Odling, G.; Logan, H.; Chan, A.; Bissel, A.J.; Pulham, C.R.; Oliver, D.E. Large Scale Recyclable Monolithic Methyltrimethoxysilane Aerogels Formed by Self-Reinforcement. Mater. Adv. 2023, 4, 3724–3732. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Parvathy Rao, A.; Kulkarni, M.M. Influence of Gel Aging and Na2SiO3/H2O Molar Ratio on Monolithicity and Physical Properties of Water–Glass-Based Aerogels Dried at Atmospheric Pressure. J. Non-Cryst. Solids 2004, 350, 224–229. [Google Scholar] [CrossRef]
- Choi, H.; Parale, V.G.; Lee, K.-Y.; Nah, H.-Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H.-H. Polypropylene/Silica Aerogel Composite Incorporating a Conformal Coating of Methyltrimethoxysilane-Based Aerogel. J. Nanosci. Nanotechnol. 2019, 19, 1376–1381. [Google Scholar] [CrossRef]
- Kim, H.M.; Noh, Y.J.; Yu, J.; Kim, S.Y.; Youn, J.R. Silica Aerogel/Polyvinyl Alcohol (PVA) Insulation Composites with Preserved Aerogel Pores Using Interfaces between the Superhydrophobic Aerogel and Hydrophilic PVA Solution. Compos. Part A Appl. Sci. Manuf. 2015, 75, 39–45. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L.; Li, C.; Pan, Y.; Huang, Y.; Cheng, X. Silica Aerogel/Aramid Pulp Composites with Improved Mechanical and Thermal Properties. J. Non-Cryst. Solids 2016, 454, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L.; Cheng, X.; He, S.; Li, C.; Zhang, H. Flexible Silica Aerogel Composites Strengthened with Aramid Fibers and Their Thermal Behavior. Mater. Des. 2016, 99, 349–355. [Google Scholar] [CrossRef]
- Ghica, M.E.; Almeida, C.M.R.; Rebelo, L.S.D.; Cathoud-Pinheiro, G.C.; Costa, B.F.O.; Durães, L. Novel Kevlar® Pulp-Reinforced Alumina-Silica Aerogel Composites for Thermal Insulation at High Temperature. J. Sol-Gel Sci. Technol. 2022, 101, 87–102. [Google Scholar] [CrossRef]
- Choi, I.; Lee, D.G. Surface Modification of Carbon Fiber/Epoxy Composites with Randomly Oriented Aramid Fiber Felt for Adhesion Strength Enhancement. Compos. Part A Appl. Sci. Manuf. 2013, 48, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Hu, Y.; Yang, G.; Shi, L.; Cheng, B.; Zhuang, X. Aramid Fibril Aerogel from Steam-Exploded PPTA Pulp for Thermal Insulation. J. Polym. Res. 2022, 29, 144. [Google Scholar] [CrossRef]
- Zhang, S.; He, G.; Liang, G.; Cui, H.; Zhang, W.; Wang, B. Comparison of F-12 Aramid Fiber with Domestic Armid Fiber III on Surface Feature. Appl. Surf. Sci. 2010, 256, 2104–2109. [Google Scholar] [CrossRef]
- Kim, C.; Yun, M.-G.; Kim, S.; Jeon, G.-W. Mathematical Model to Predict the Moduli of Wet-Laid Pulp/Fiber/Resin Composite Materials. Int. J. Precis. Eng. Manuf. 2022, 23, 1315–1324. [Google Scholar] [CrossRef]
- Teng, C.; Wang, T.; Qin, M.; Kong, H.; Zhang, J.; Yu, M. Facile Dispersion of Aramid Pulp by Matrix Sizing Agent and Reinforced Rubber Composites. Polym. Compos. 2020, 41, 4583–4592. [Google Scholar] [CrossRef]
- Lertwassana, W.; Parnklang, T.; Mora, P.; Jubsilp, C.; Rimdusit, S. High Performance Aramid Pulp/Carbon Fiber-Reinforced Polybenzoxazine Composites as Friction Materials. Compos. Part B Eng. 2019, 177, 107280. [Google Scholar] [CrossRef]
- Soria, F.A.; Zhang, W.; Van Duin, A.C.T.; Patrito, E.M. Thermal Stability of Organic Monolayers Grafted to Si(111): Insights from ReaxFF Reactive Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2017, 9, 30969–30981. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Huang, S.; Wu, X.; Shi, L.; Liu, Q. Thermal Stability and Pyrolysis Characteristics of MTMS Aerogels Prepared in Pure Water. J. Nanopart. Res. 2020, 22, 334. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Parale, V.G.; Park, H.-H. Organically Modified Silica Aerogel with Different Functional Silylating Agents and Effect on Their Physico-Chemical Properties. J. Non-Cryst. Solids 2016, 453, 164–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Deng, X.; Deng, Y.; Wu, X.; Shi, L.; Li, M.; Liu, Q.; Cheng, X.; Li, Z. Improving the Flame Retardance of Hydrophobic Silica Aerogels through a Facile Post-Doping of Magnesium Hydroxide. Adv. Powder Technol. 2021, 32, 1891–1901. [Google Scholar] [CrossRef]
- Yang, S.; Strobach, E.; Bierman, D.; Zhao, L.; Bhatia, B.; Wang, E.N. Effect of Al2O3 ALD Coating on Thermal Stability of Silica Aerogel. J. Porous Mater. 2022, 29, 193–200. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Chen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of Heat Treatment on Hydrophobic Silica Aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Joao, G.; Zhang, Y.; Huang, S.; Liu, Q. Reducing the Flammability of Hydrophobic Silica Aerogels by Tailored Heat Treatment. J. Nanopart. Res. 2020, 22, 83. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, X.; Song, H.; Hu, Z.; Cao, B. The Influence of Thermal Treatment on the Microstructure and Thermal Insulation Performance of Silica Aerogels. J. Non-Cryst. Solids 2017, 470, 178–183. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wu, X.; Liu, Q.; Li, M.; Shi, L.; Cheng, X. Surface Chemistry, Skeleton Structure and Thermal Safety of Methylsilyl Modified Silica Aerogels by Heat Treatment in an Argon Atmosphere. J. Non-Cryst. Solids 2023, 611, 122335. [Google Scholar] [CrossRef]
- Lun, Z.; Gong, L.; Zhang, Z.; Deng, Y.; Zhou, Y.; Pan, Y.; Cheng, X. Improvement of the Thermal Insulation Performance of Silica Aerogel by Proper Heat Treatment: Microporous Structures Changes and Pyrolysis Mechanism. Gels 2022, 8, 141. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7477-8. [Google Scholar]
- Cai, P.; Li, Z.; Wang, T.; Wang, Q. Effect of Aspect Ratios of Aramid Fiber on Mechanical and Tribological Behaviors of Friction Materials. Tribol. Int. 2015, 92, 109–116. [Google Scholar] [CrossRef]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Dominguez, A.; Riccardo, J.L. Capillary Condensation in Heterogeneous Mesoporous Networks Consisting of Variable Connectivity and Pore-Size Correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346–2355. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Z.; Kong, Y.; Ren, J.; Jiang, X.; Shen, X. Auto-Continuous Synthesis of Robust and Hydrophobic Silica Aerogel Microspheres from Low-Cost Aqueous Sodium Silicate for Fast Dynamic Organics Removal. Gels 2022, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Villar-Rodil, S.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M.D. Atomic Force Microscopy and Infrared Spectroscopy Studies of the Thermal Degradation of Nomex Aramid Fibers. Chem. Mater. 2001, 13, 4297–4304. [Google Scholar] [CrossRef]
- Demétrio da Silva, V.; Jacobi, M.M.; Schrekker, H.S.; Amico, S.C. Aramid Pulp with Physisorbed Imidazolium Ionic Liquids for Solvent-Casted Enhanced Styrene-Butadiene Rubber Composites. J. Appl. Polym. Sci. 2018, 135, 46693. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Huang, S.; Liu, Q. Effects of Various Methylchlorosilanes on Physicochemical Properties of Ambient Pressure Dried Silica Aerogels. J. Nanopart. Res. 2019, 21, 234. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Gurav, J.L.; Rao, A.V.; Rao, A.P.; Nadargi, D.Y.; Bhagat, S.D. Physical Properties of Sodium Silicate Based Silica Aerogels Prepared by Single Step Sol–Gel Process Dried at Ambient Pressure. J. Alloys Compd. 2009, 476, 397–402. [Google Scholar] [CrossRef]
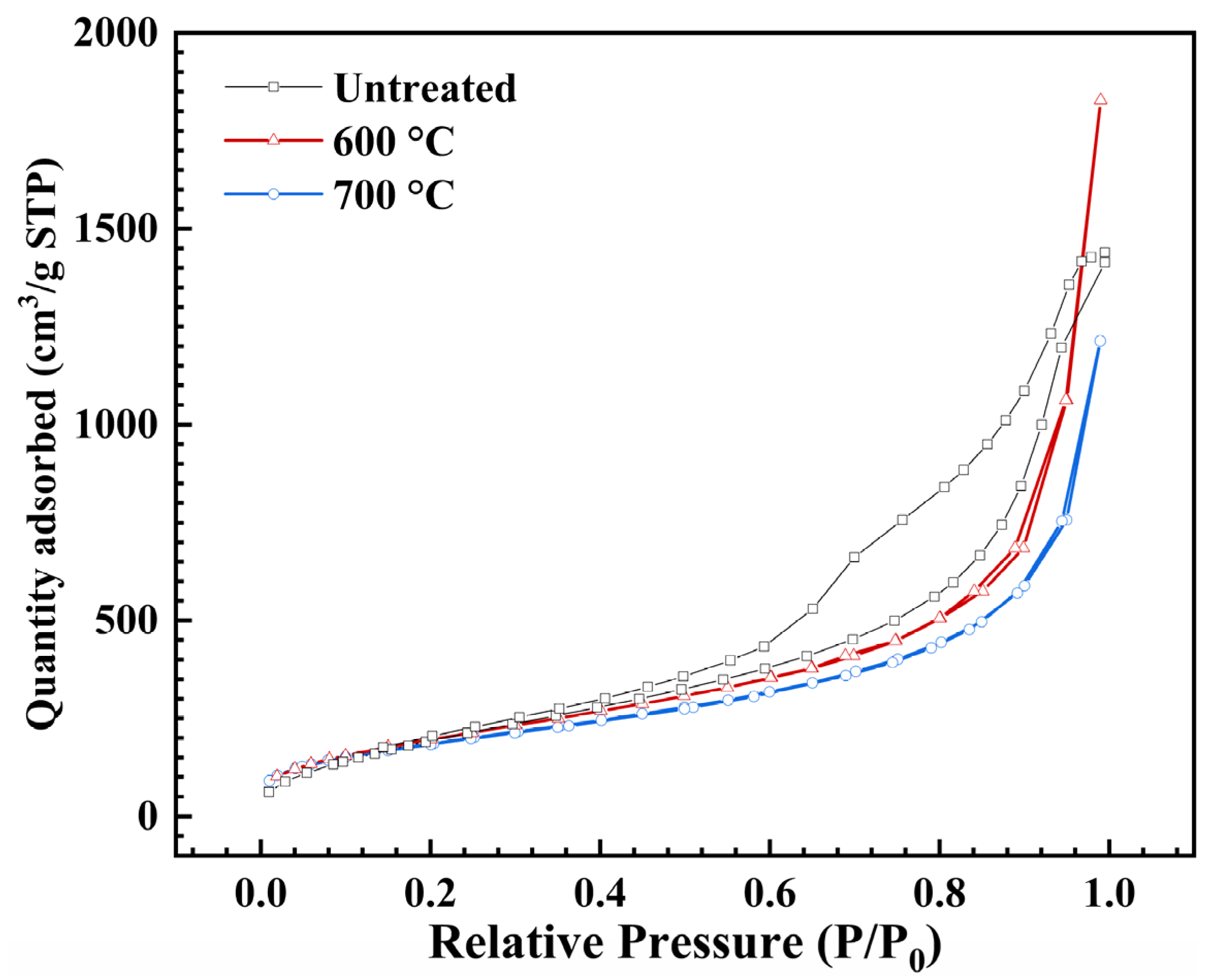
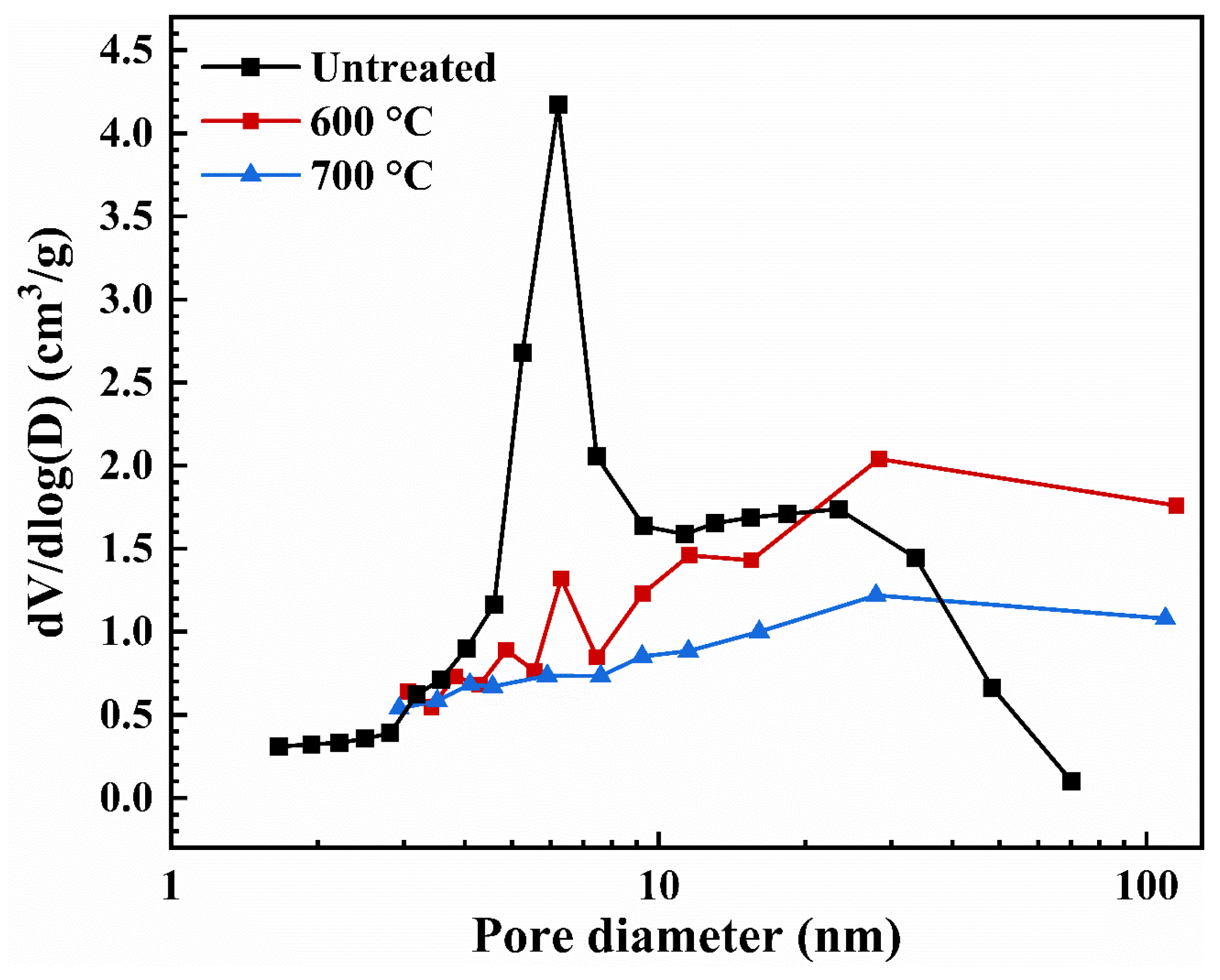

| Heat Treatment | BET Surface Area (m2/g) | Pore Volume a (cm3/g) | Average Pore Diameter b (nm) | Vpore c (cm3/g) | Dpore d (nm) |
|---|---|---|---|---|---|
| Untreated | 764.8 ± 15.5 | 2.23 | 8.11 | 5.39 | 28.19 |
| 600 | 748.7 ± 17.6 | 2.73 | 18.47 | 4.68 | 25.00 |
| 700 | 685.5 ± 12.5 | 1.74 | 15.93 | 4.62 | 26.96 |
| Sample | Heat Treatment | Aramid Pulp Component | Silica Aerogel Component | ||
|---|---|---|---|---|---|
| Tonset (°C) | Tpeak (°C) | Tonset (°C) | Tpeak (°C) | ||
| Pure TSA [37] | Untreated | - | - | 248 | 275 |
| 700 °C | - | - | 523 | 584 | |
| AP/aerogel | Untreated | 432.9 | 497.3 | 298.8 | 309.7 |
| 600 °C | 414.7 | 501.4 | 573.2 | 613.4 | |
| 700 °C | 415.4 | 493.4 | 553.1 | 628.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shen, K.; Hu, M.; Shulga, Y.M.; Chen, Z.; Liu, Q.; Li, M.; Wu, X. Heat-Treated Aramid Pulp/Silica Aerogel Composites with Improved Thermal Stability and Thermal Insulation. Gels 2023, 9, 749. https://doi.org/10.3390/gels9090749
Li Z, Shen K, Hu M, Shulga YM, Chen Z, Liu Q, Li M, Wu X. Heat-Treated Aramid Pulp/Silica Aerogel Composites with Improved Thermal Stability and Thermal Insulation. Gels. 2023; 9(9):749. https://doi.org/10.3390/gels9090749
Chicago/Turabian StyleLi, Zhi, Kai Shen, Min Hu, Yury M. Shulga, Zhenkui Chen, Qiong Liu, Ming Li, and Xiaoxu Wu. 2023. "Heat-Treated Aramid Pulp/Silica Aerogel Composites with Improved Thermal Stability and Thermal Insulation" Gels 9, no. 9: 749. https://doi.org/10.3390/gels9090749
APA StyleLi, Z., Shen, K., Hu, M., Shulga, Y. M., Chen, Z., Liu, Q., Li, M., & Wu, X. (2023). Heat-Treated Aramid Pulp/Silica Aerogel Composites with Improved Thermal Stability and Thermal Insulation. Gels, 9(9), 749. https://doi.org/10.3390/gels9090749









