Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of SFNL and Hydrogel Scaffold
2.1.1. Selection of Optimum Formula
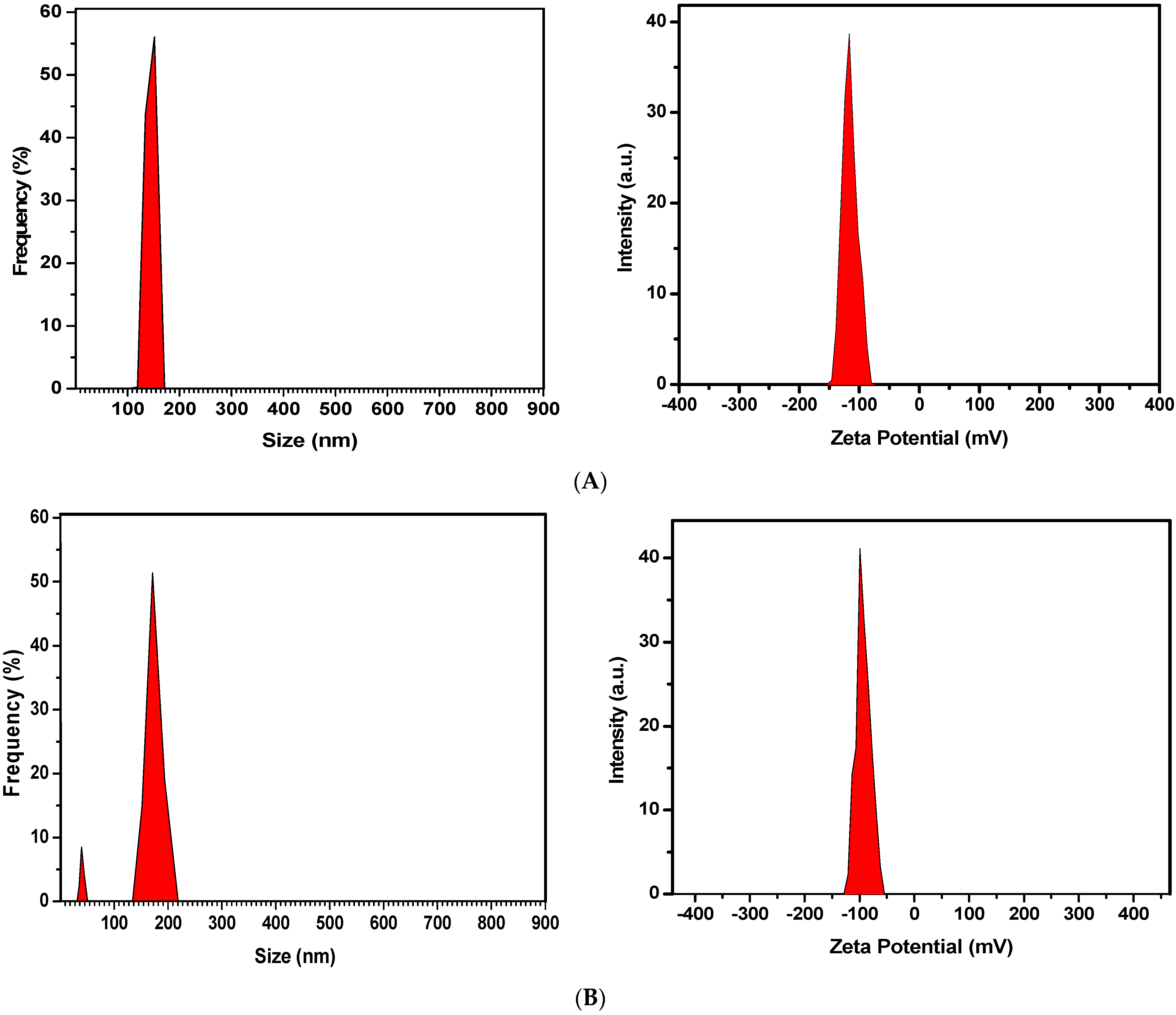
2.1.2. Particle Size, Biodistribution, and Zeta Potential of Optimized Formulation
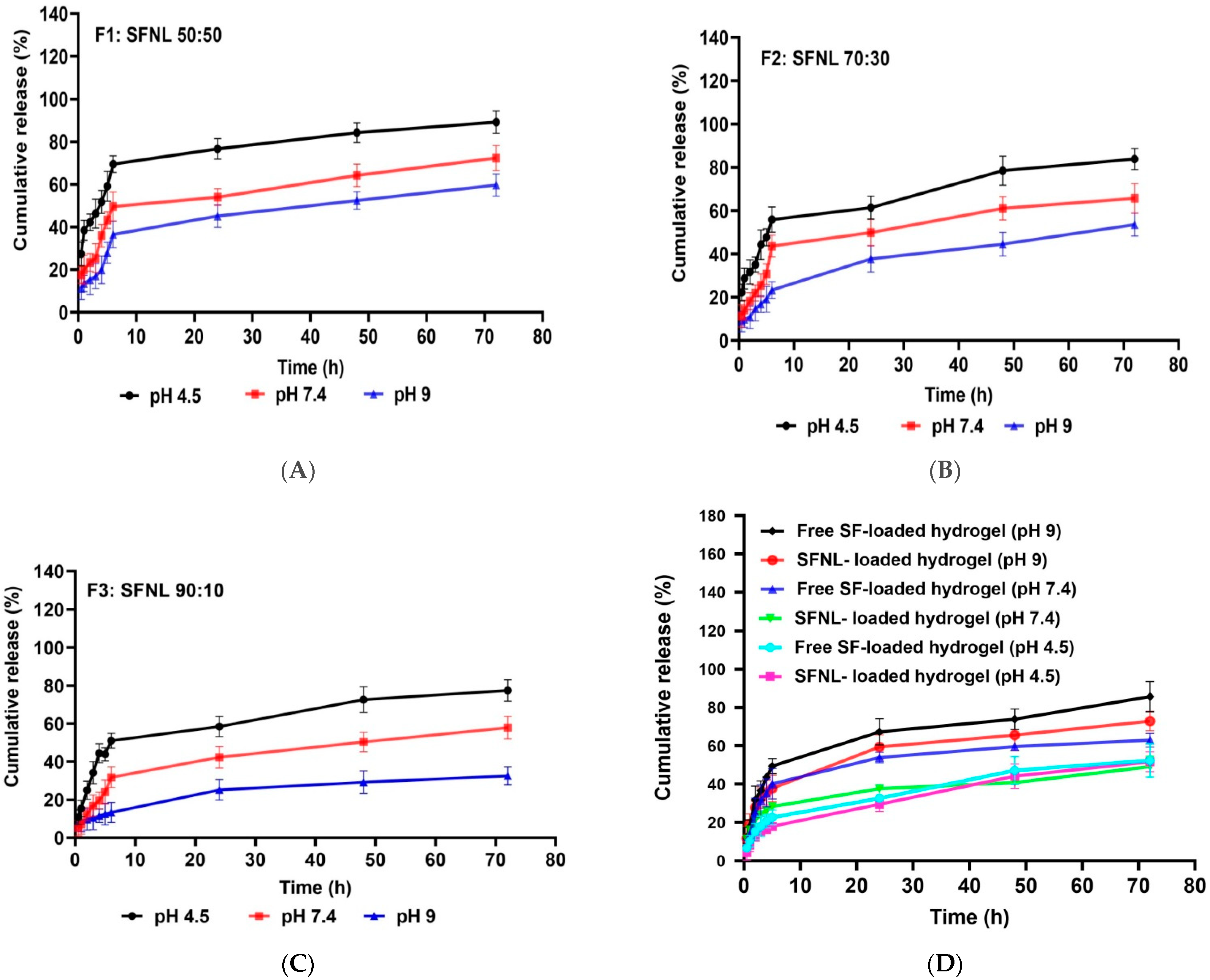
2.1.3. The Release Profile of SFNL and Hydrogel Containing SFNL
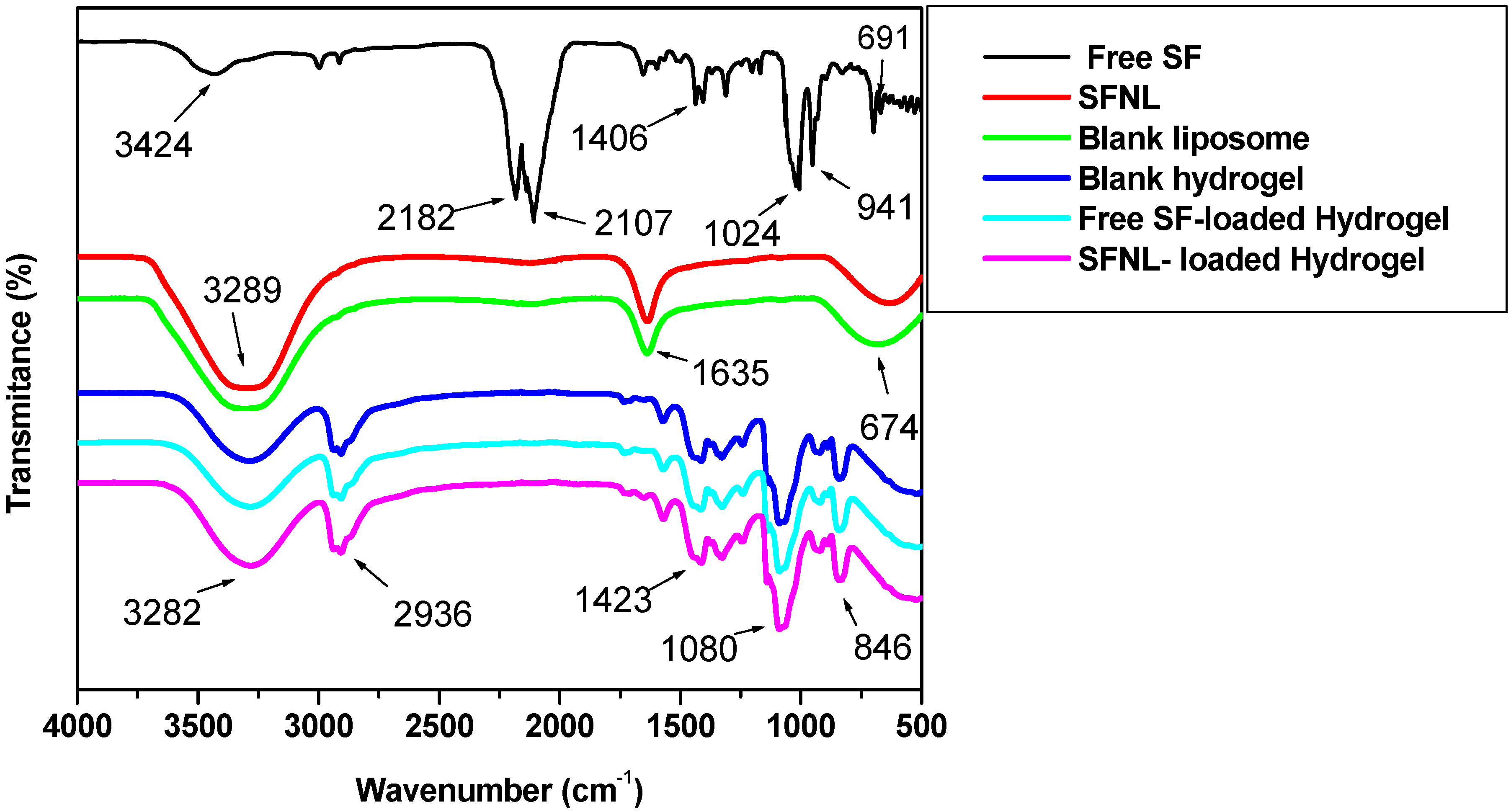
2.1.4. Fourier-Transform Infrared Spectroscopy (FTIR)
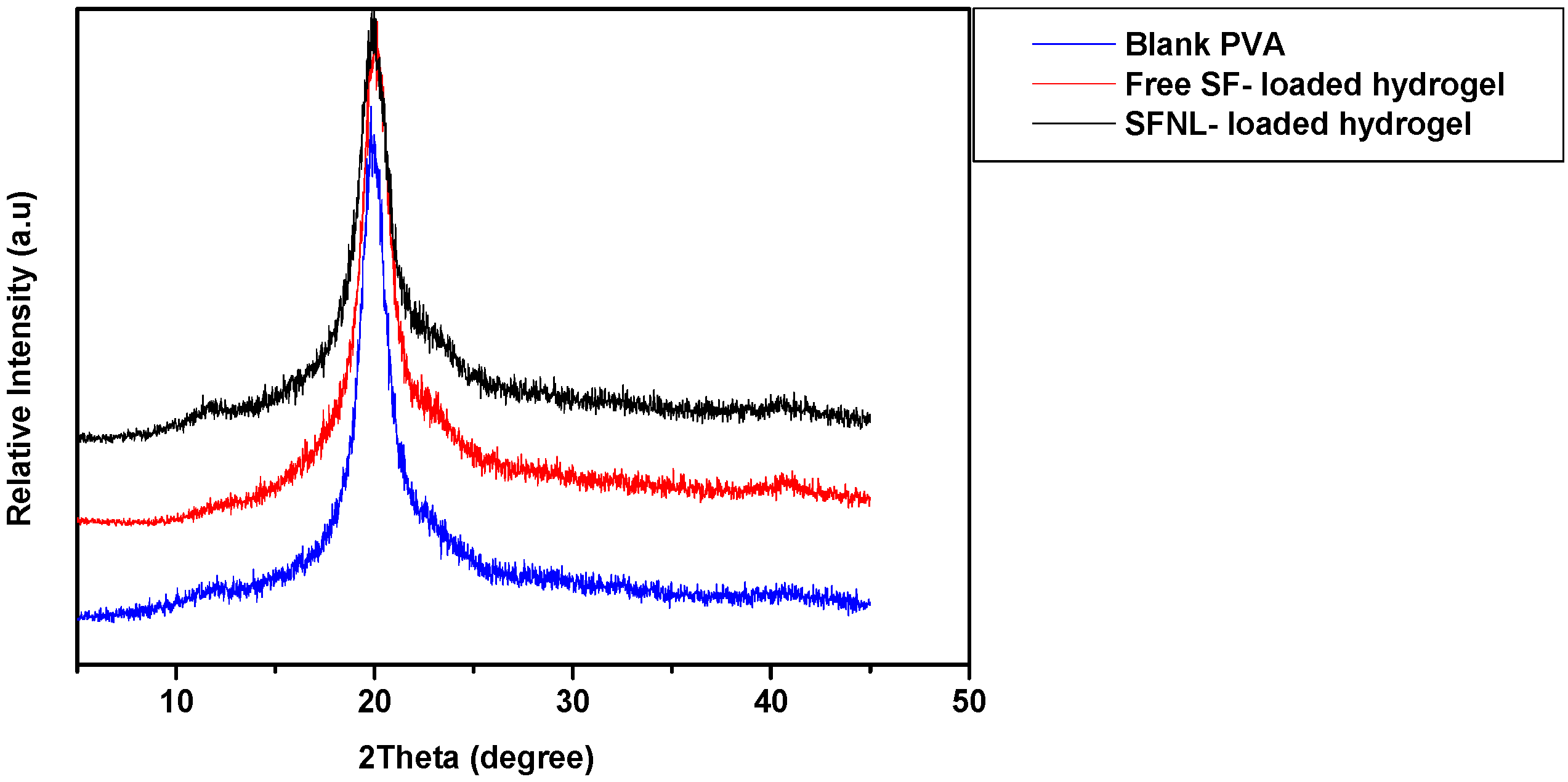
2.1.5. X-ray Diffraction (XRD)
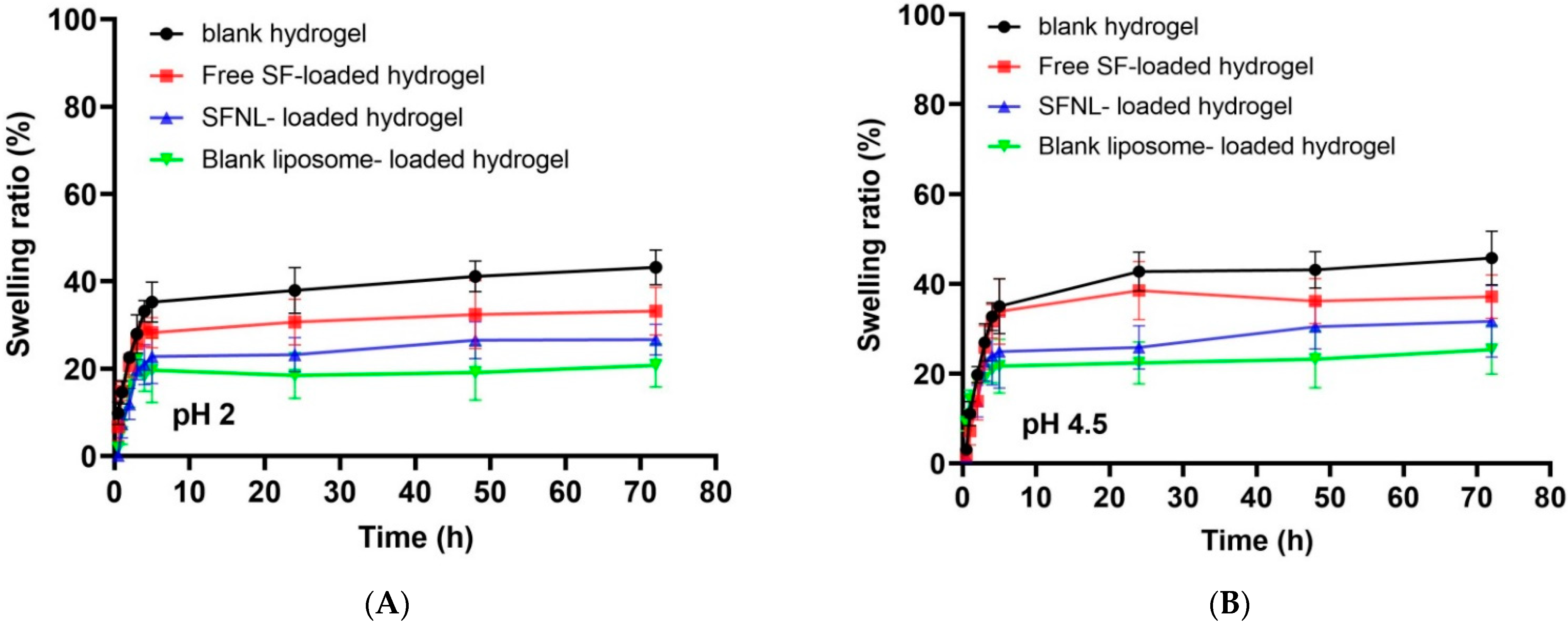
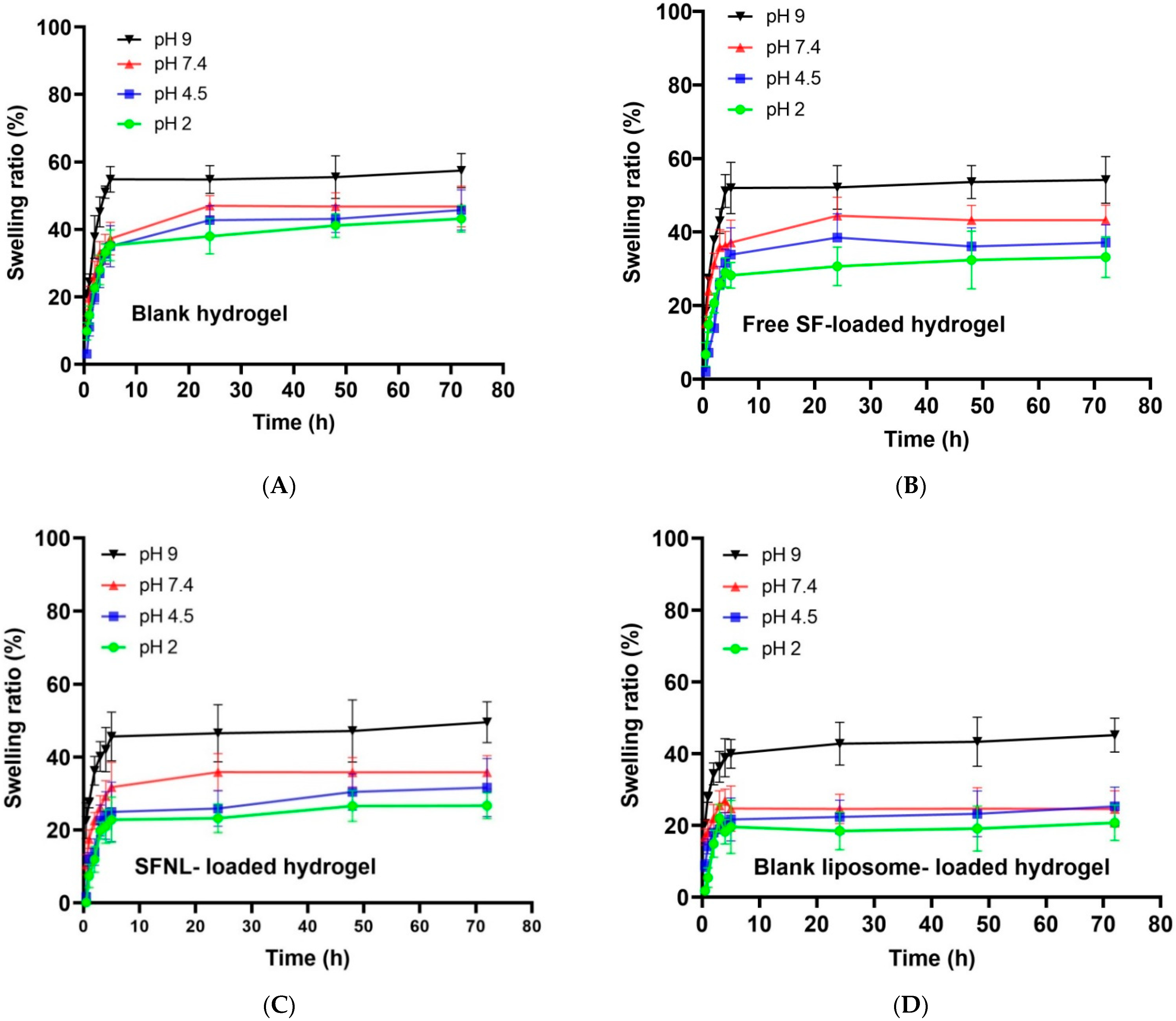
2.1.6. Determination of Swelling Ratio (%)
Swelling Ratio of Hydrogel Scaffolds
pH-Dependent Swelling Behavior of Hydrogel Scaffolds
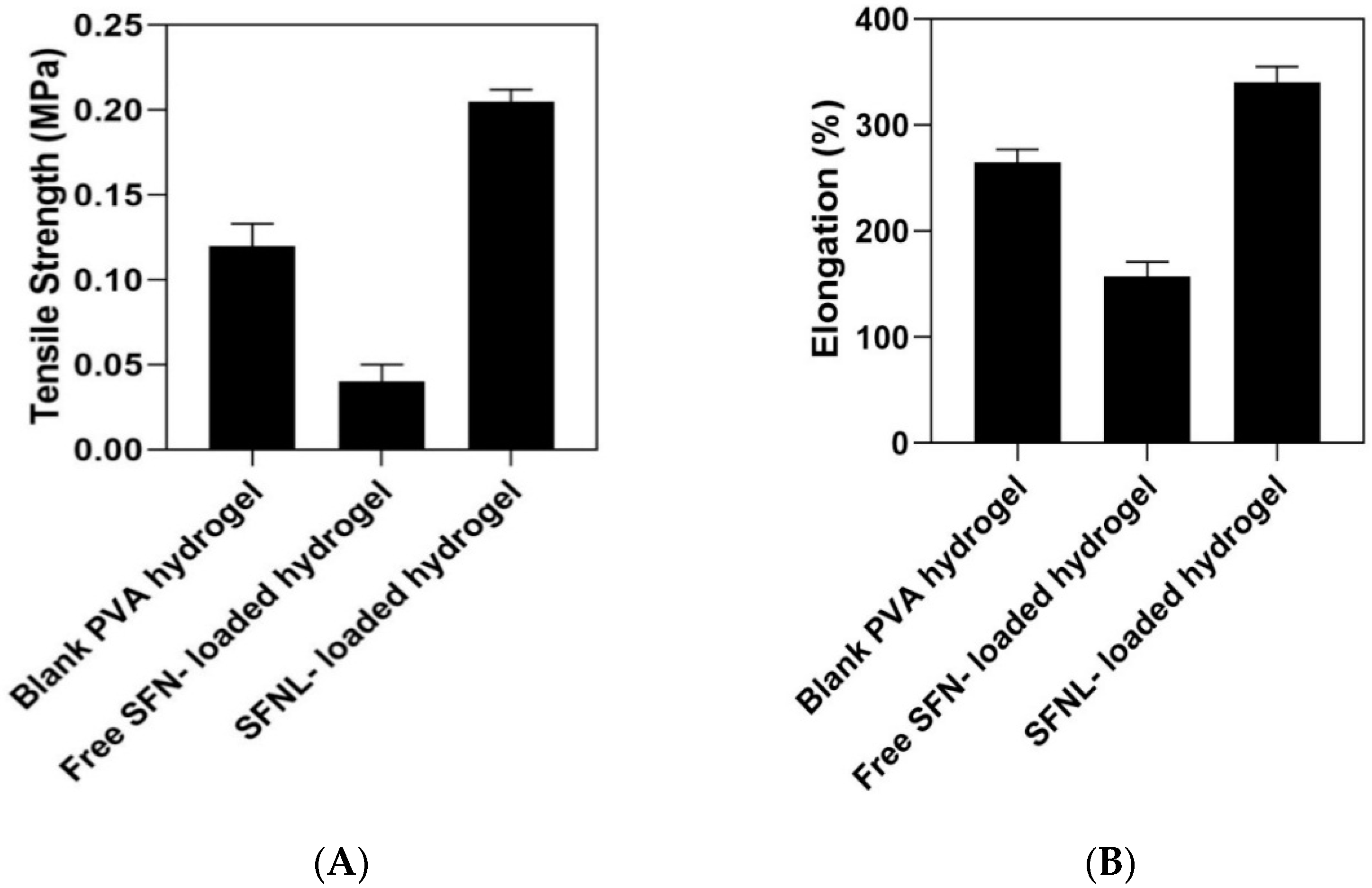
2.1.7. Mechanical Properties of Hydrogel Scaffolds
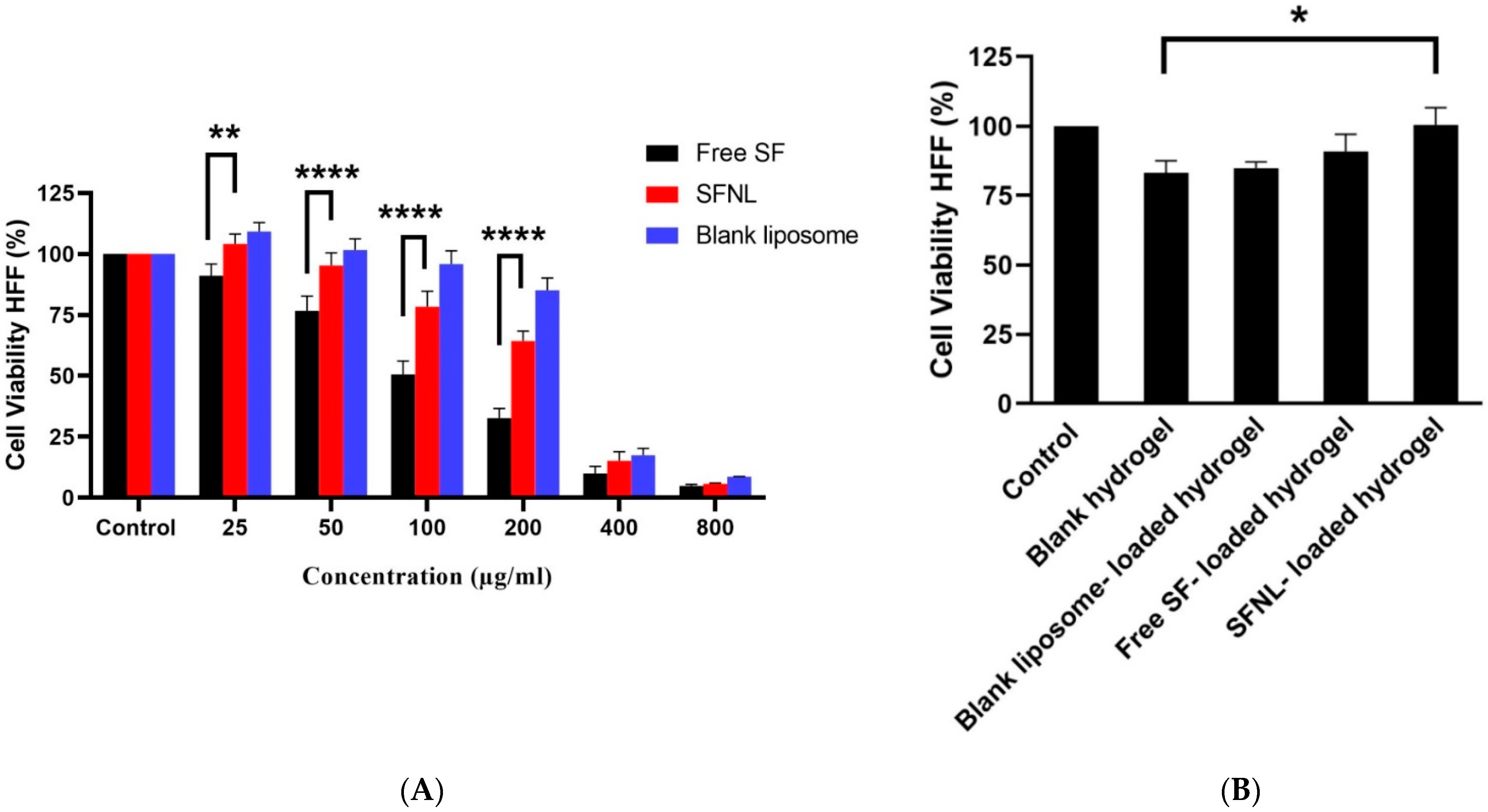
2.2. In Vitro Cell Proliferation
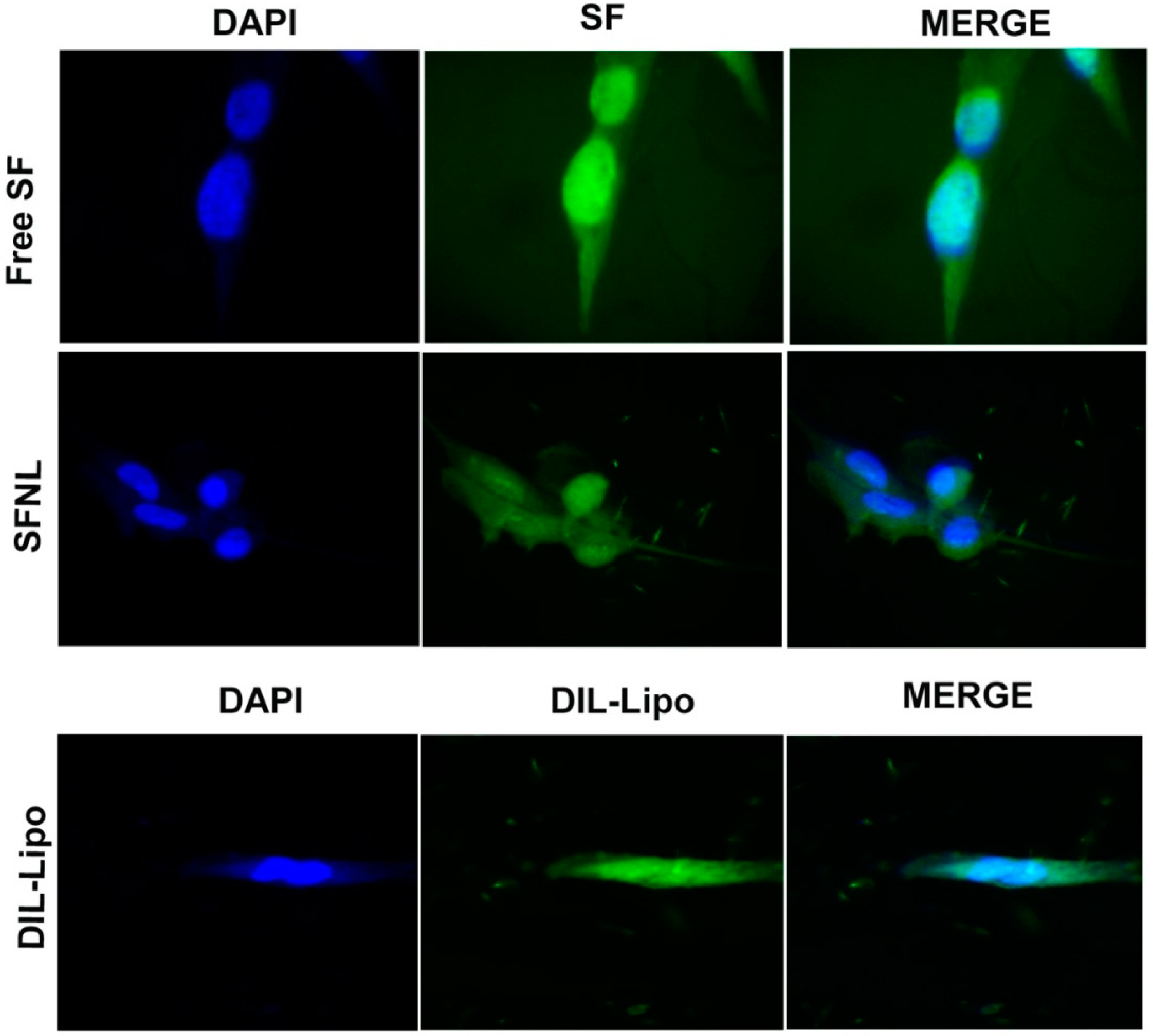
2.3. Cellular Uptake
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Preparation of Sulforaphane-Loaded Nanoliposome (SFNL)
4.4. Preparation of PVA/PEG400 Hydrogel Scaffold Containing SFNL
4.5. Physicochemical Characterization of Synthesized Nanoparticles and PVA/PEG400 Hydrogels
4.5.1. Size, Distribution, and Zeta Potential of Liposomes
4.5.2. FT-IR Analysis
4.5.3. XRD
4.5.4. Entrapment Efficiency% (EE%)
4.5.5. Release Profile
4.5.6. Swelling Rate Assay
4.5.7. Mechanical Analysis
4.6. Cellular Analysis
4.6.1. Cytocompatibility Assay
4.6.2. Qualitative Evaluation of Cellular Uptake
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urciuolo, F.; Passariello, R.; Imparato, G.; Casale, C.; Netti, P.A. Bioengineered wound healing skin models: The role of immune response and endogenous ECM to fully replicate the dynamic of scar tissue formation in vitro. Bioengineering 2022, 9, 233. [Google Scholar] [CrossRef]
- Malviya, N.; Saxena, R.; Sharma, C. Understanding Hydrogels and Insight on the Latest Hydrogel Applications in Pharmaceutical and Allied Sciences. In Advancements in Controlled Drug Delivery Systems; IGI Global: Hershey, PA, USA, 2022; pp. 281–308. [Google Scholar]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef]
- Patel, M.; Fisher, J.P. Biomaterial scaffolds in pediatric tissue engineering. Pediatr. Res. 2008, 63, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Maarof, M.; Mohd, N.F.; Hiraoka, Y.; Tabata, Y.; Fauzi, M.B. Injectable Crosslinked Genipin Hybrid Gelatin–PVA Hydrogels for Future Use as Bioinks in Expediting Cutaneous Healing Capacity: Physicochemical Characterisation and Cytotoxicity Evaluation. Biomedicines 2022, 10, 2651. [Google Scholar] [CrossRef]
- Weller, C.; Team, V. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–134. [Google Scholar]
- Thi-Hiep, N.; Van Hoa, D.; Van Toi, V. Injectable in situ crosslinkable hyaluronan-polyvinyl phosphonic acid hydrogels for bone engineering. J. Biomed. Sci. Eng. 2013, 6, 35657. [Google Scholar] [CrossRef]
- Sarmadi, M.; Shamloo, A.; Mohseni, M. Utilization of molecular dynamics simulation coupled with experimental assays to optimize biocompatibility of an electrospun PCL/PVA scaffold. PLoS ONE 2017, 12, e0169451. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem.-Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Sarabu, S.; Butreddy, A.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Kogan, M.; Bi, V.; Durig, T.; Repka, M.A. Preliminary investigation of peroxide levels of Plasdone™ copovidones on the purity of atorvastatin calcium amorphous solid dispersions: Impact of plasticizers on hot melt extrusion processability. J. Drug Deliv. Sci. Technol. 2022, 70, 103190. [Google Scholar] [CrossRef]
- Mazarakis, N.; Higgins, R.A.; Anderson, J.; Toh, Z.Q.; Luwor, R.B.; Snibson, K.J.; Karagiannis, T.C.; Do, L.A.H.; Licciardi, P.V. The effects of the dietary compound L-sulforaphane against respiratory pathogens. Int. J. Antimicrob. Agents. 2021, 58, 106460. [Google Scholar] [CrossRef]
- Ishida, K.; Kaji, K.; Sato, S.; Ogawa, H.; Takagi, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J. Nutr. Biochem. 2021, 89, 108573. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Leal, E.C.; Alves, I.; Bose, C.; Palade, P.T.; Singh, P.; Awasthi, S.; Børsheim, E.; Dalgaard, L.T.; Singh, S.P. Dietary supplementation with sulforaphane ameliorates skin aging through activation of the Keap1-Nrf2 pathway. J. Nutr. Biochem. 2021, 98, 108817. [Google Scholar] [CrossRef]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane-role in aging and neurodegeneration. Geroscience 2019, 41, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Fattahi Bafghi, A.; Haghirosadat, B.F.; Yazdian, F.; Mirzaei, F.; Pourmadadi, M.; Pournasir, F.; Hemati, M.; Pournasir, S. A novel delivery of curcumin by the efficient nanoliposomal approach against Leishmania major. Prep. Biochem. Biotechnol. 2021, 51, 990–997. [Google Scholar] [CrossRef]
- Afereydoon, S.; Haghiralsadat, F.; Hamzian, N.; Shams, A.; Hemati, M.; Naghib, S.M.; Shabani, M.; Zandieh-Doulabi, B.; Tofighi, D. Multifunctional PEGylated Niosomal Nanoparticle-Loaded Herbal Drugs as a Novel Nano-Radiosensitizer and Stimuli-Sensitive Nanocarrier for Synergistic Cancer Therapy. Front. Bioeng. Biotechnol. 2022, 10, 917368. [Google Scholar] [CrossRef]
- Dua, J.; Rana, A.; Bhandari, A. Liposome: Methods of preparation and applications. Int. J. Pharm. Stud. Res 2012, 3, 14–20. [Google Scholar]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Clares, B.; Gallardo, V.; Medina, M.; Ruiz, M. Multilamellar liposomes of triamcinolone acetonide: Preparation, stability, and characterization. J. Liposome Res. 2009, 19, 197–206. [Google Scholar] [CrossRef]
- Tabandeh, H.; Mortazavi, S.A. An investigation into some effective factors on encapsulation efficiency of alpha-tocopherol in MLVs and the release profile from the corresponding liposomal gel. Iran. J. Pharm. Res. IJPR 2013, 12, 21. [Google Scholar] [PubMed]
- Mohammed, A.; Weston, N.; Coombes, A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Begum, M.Y.; Abbulu, K.; Sudhakar, M. Design and evaluation of flurbiprofen liposomes. J. Pharm. Res. 2011, 4, 653–655. [Google Scholar]
- Ghaffari, M.; Kalantar, S.M.; Hemati, M.; Dehghani Firoozabadi, A.; Asri, A.; Shams, A.; Jafari Ghalekohneh, S.; Haghiralsadat, F. Co-delivery of miRNA-15a and miRNA-16–1 using cationic PEGylated niosomes downregulates Bcl-2 and induces apoptosis in prostate cancer cells. Biotechnol. Lett. 2021, 43, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, S.; Cao, F.; Ding, Z.; Liu, J.; Chen, Q.; Gao, J.; Huang, X. Investigations on the influence of the structural flexibility of nanoliposomes on their properties. J. Liposome Res. 2022, 32, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, W.; Zhang, X.; Su, P.; Yue, F.; Zeng, S.; Du, S. Improved surface adhesion and wound healing effect of madecassoside liposomes modified by temperature-responsive PEG-PCL-PEG copolymers. Eur. J. Pharm. Sci. 2020, 151, 105373. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Jafary, F.; Moosavizadeh, S.; Moradi, A. Targeting cell cycle protein in gastric cancer with CDC20siRNA and anticancer drugs (doxorubicin and quercetin) co-loaded cationic PEGylated nanoniosomes. Int. J. Nanomed. 2019, 14, 6575–6585. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Oogi, C.; Takashima, Y.; Seta, Y. Effects of surface charge and flexibility of liposomes on dermal drug delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 155–162. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, M.; Rabasco, A. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin. Drug Deliv. 2011, 8, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Ternullo, S.; Basnet, P.; Holsæter, A.M.; Flaten, G.E.; de Weerd, L.; Škalko-Basnet, N. Deformable liposomes for skin therapy with human epidermal growth factor: The effect of liposomal surface charge. Eur. J. Pharm. Sci. 2018, 125, 163–171. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.; Avilés-Castrillo, J.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Ferriol, A.; del Carmen Morán, M. Enhanced performance of gelatin 5-fluorouracil-containing nanoparticles against squamous cell carcinoma in simulated chronic wounds conditions. Mater. Sci. Eng. C 2021, 124, 112073. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Yazdian, F.; Jafari, F.; Moradi, A.; Malekpour-Dehkordi, Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Luan, Y.; Liu, W.; Chen, T.; Liu, P.; Liu, Z. Preparation of pH-responsive cellulose nanofibril/sodium alginate based hydrogels for drug release. J. Appl. Polym. Sci. 2022, 139, 51647. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-hydrogel delivery system enhances the potential of resveratrol in combating vaginal chlamydia infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA blending on structural and ion transport properties of CS: AgNt-based polymer electrolyte membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef]
- Abdel Bary, E.; Soliman, Y.A.; Fekri, A.; Harmal, A.N. Aging of novel membranes made of PVA and cellulose nanocrystals extracted from Egyptian rice husk manufactured by compression moulding process. Int. J. Environ. Stud. 2018, 75, 750–762. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, C.; Azer, A.; Liu, H. Dispersibility and characterization of polyvinyl alcohol–coated magnetic nanoparticles in poly (glycerol sebacate) for biomedical applications. J. Nanoparticle Res. 2019, 21, 275. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; Zhang, L.; He, C.; Mo, X.; Wang, H. Polyvinyl alcohol/hydroxyethylcellulose containing ethosomes as a scaffold for transdermal drug delivery applications. Appl. Biochem. Biotechnol. 2020, 191, 1624–1637. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, N.; Kouchak, M.; Mohamadipour, P.; Sadeghi-Nejad, B. Preparation and physicochemical characterization of topical chitosan-based film containing griseofulvin-loaded liposomes. J. Adv. Pharm. Technol. Res. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Ghanaatian, E.; Entezam, M. Mechanical properties and drug release rate of poly (vinyl alcohol)/poly (ethylene glycol)/clay nanocomposite hydrogels: Correlation with structure and physical properties. J. Appl. Polym. Sci. 2019, 136, 47843. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Lee, S.T.; Wong, T.B. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1551–1564. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly (vinyl alcohol)(PVA)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Lopes, L.C.; Rubira, A.F.; Muniz, E.C. Development and application of chitosan/poly (vinyl alcohol) films for removal and recovery of Pb (II). Chem. Eng. J. 2012, 183, 253–260. [Google Scholar] [CrossRef]
- Razzak, M.T.; Darwis, D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Y.; Liu, H.; Cheng, R.; Ni, Q.; Ye, T.; Cui, W. Local release of gemcitabine via in situ UV-crosslinked lipid-strengthened hydrogel for inhibiting osteosarcoma. Drug Deliv. 2018, 25, 1642–1651. [Google Scholar] [CrossRef]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef]
- Tan, X.-L.; Shi, M.; Tang, H.; Han, W.; Spivack, S.D. Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. J. Nutr. 2010, 140, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Yang, H.; Shen, L.; Bu, H.; Li, G. Stable and biocompatible hydrogel composites based on collagen and dialdehyde carboxymethyl cellulose in a biphasic solvent system. Carbohydr. Polym. 2019, 222, 114974. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dan, W.; Xiong, S.; Kang, Y.; Dhinakar, A.; Wu, J.; Gu, Z. Development of collagen/polydopamine complexed matrix as mechanically enhanced and highly biocompatible semi-natural tissue engineering scaffold. Acta Biomater. 2017, 47, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Tran, T.Q.; Van Chinh, L.; Nguyen, L.P.; An, T.N.T.; Anh, N.H.T.; Nguyen, D.T. Anti-tumor effect of liposomes containing extracted Murrayafoline A against liver cancer cells in 2D and 3D cultured models. Open Chem. 2022, 20, 463–473. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Alcalá-Alcalá, S.; Tapia-Guerrero, Y.; Magaña, J.J.; Del Prado-Audelo, M.L.; Leyva-Gómez, G. Cross-linked polyvinyl alcohol-xanthan gum hydrogel fabricated by freeze/thaw technique for potential application in soft tissue engineering. RSC Adv. 2022, 12, 21713–21724. [Google Scholar] [CrossRef]

| Formula | Cholesterol: SPC: DPPC: DSPE-PEG2000 (Molar Ratio) | EE% | %Release (6 h) | %Release (24 h) | %Release (48 h) | %Release (72 h) |
|---|---|---|---|---|---|---|
| F1 | 50:25:25:5 | 34 ± 7.3 | 49.6 ± 6.8 | 53.9 ± 3.9 | 64.1 ± 5.3 | 72.3 ± 5.9 |
| F2 | 30:35:35:5 | 60 ± 8.4 | 43.6 ± 5.1 | 49.9 ± 6.1 | 61.0 ± 5.4 | 65.7 ± 6.7 |
| F3 | 10:45:45:5 | 88 ± 6.9 | 31.8 ± 5.4 | 42.3 ± 5.6 | 50.3 ± 5.1 | 57.8 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemati, H.; Haghiralsadat, F.; Hemati, M.; Sargazi, G.; Razi, N. Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing. Gels 2023, 9, 748. https://doi.org/10.3390/gels9090748
Hemati H, Haghiralsadat F, Hemati M, Sargazi G, Razi N. Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing. Gels. 2023; 9(9):748. https://doi.org/10.3390/gels9090748
Chicago/Turabian StyleHemati, Hamide, Fateme Haghiralsadat, Mahdie Hemati, Ghasem Sargazi, and Nastaran Razi. 2023. "Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing" Gels 9, no. 9: 748. https://doi.org/10.3390/gels9090748
APA StyleHemati, H., Haghiralsadat, F., Hemati, M., Sargazi, G., & Razi, N. (2023). Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing. Gels, 9(9), 748. https://doi.org/10.3390/gels9090748







