Single- and Multilayer Build-Up of an Antibacterial Temperature- and UV-Curing Sol–Gel System with Atmospheric Pressure Plasma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
FTIR Studies
2.2. Film Structure and Surface Properties
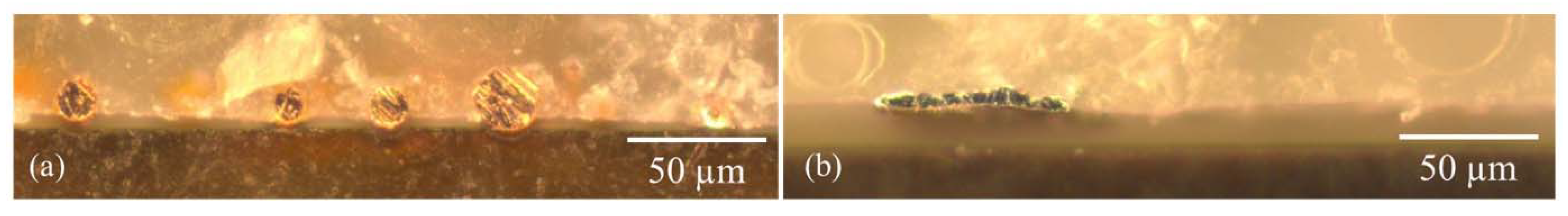
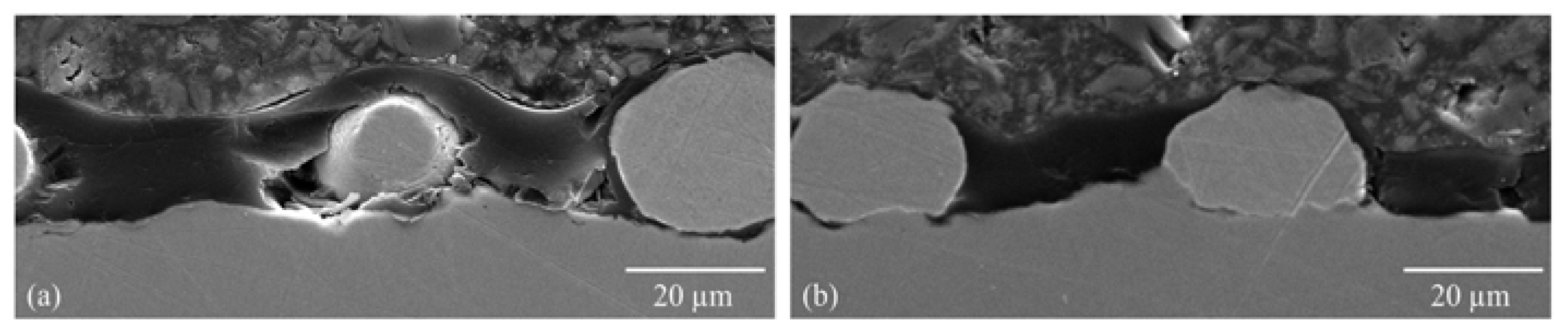
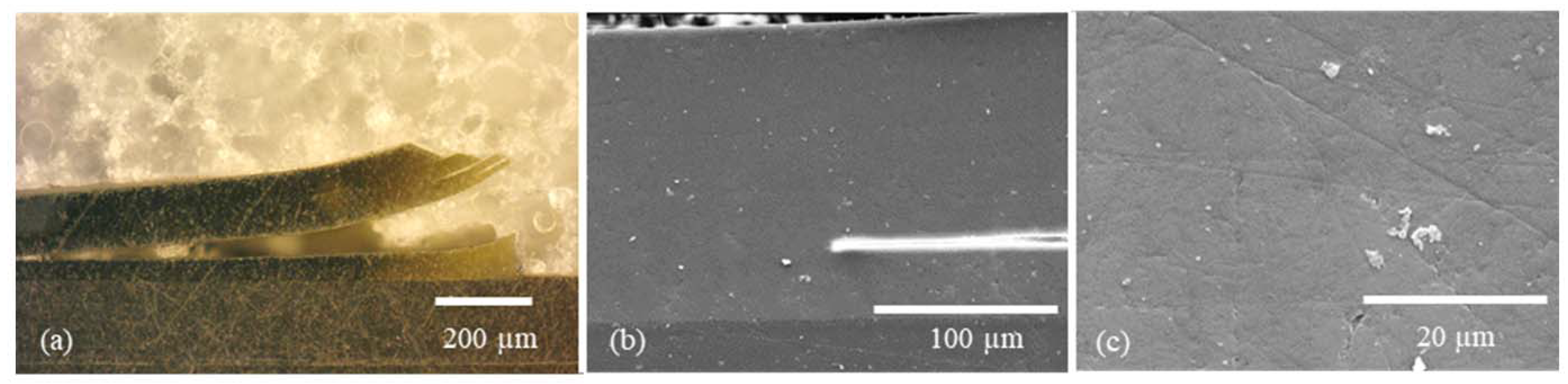
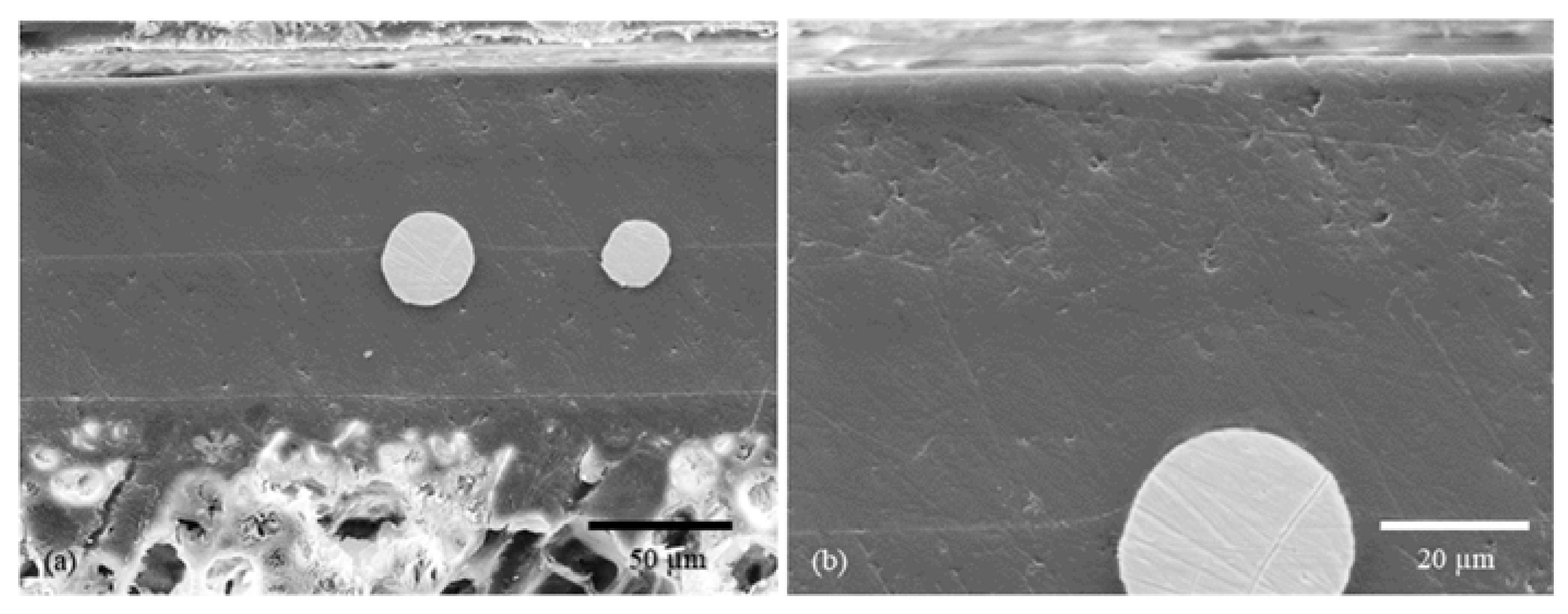
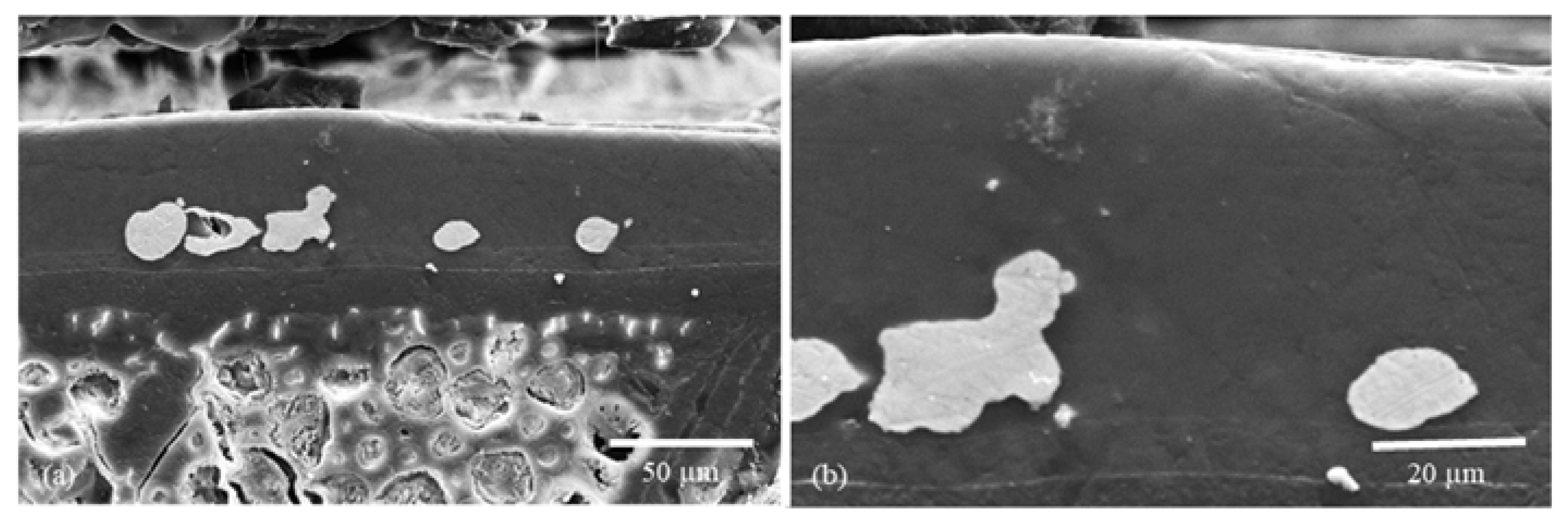
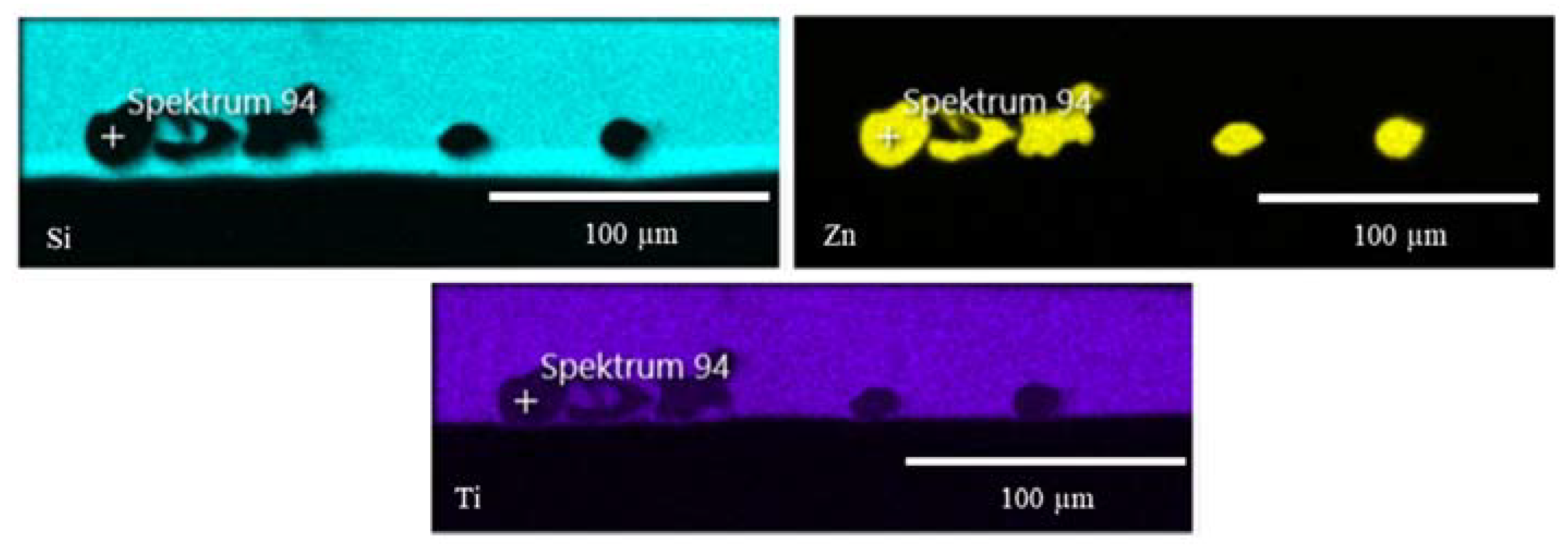
2.2.1. Morphology Studies
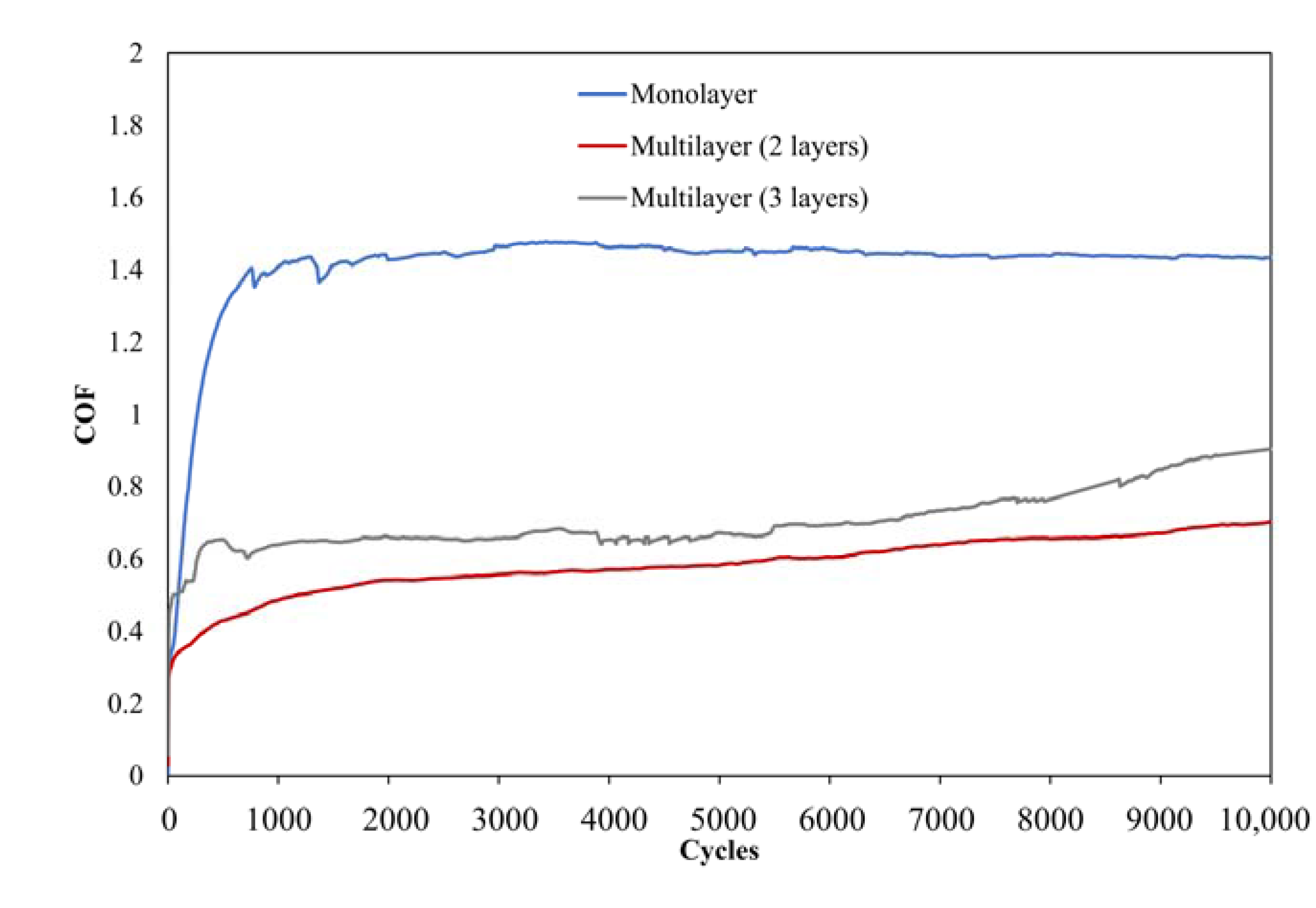
2.2.2. Critical Loads and Tribology
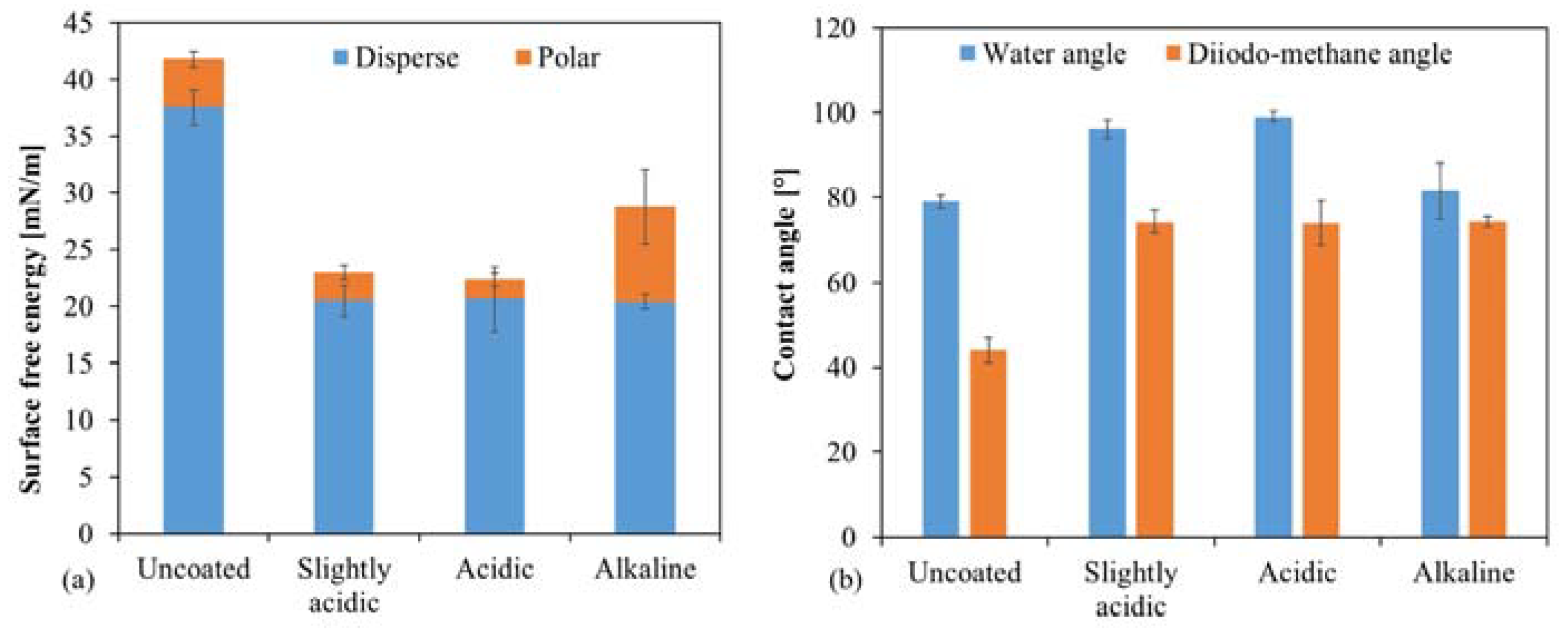
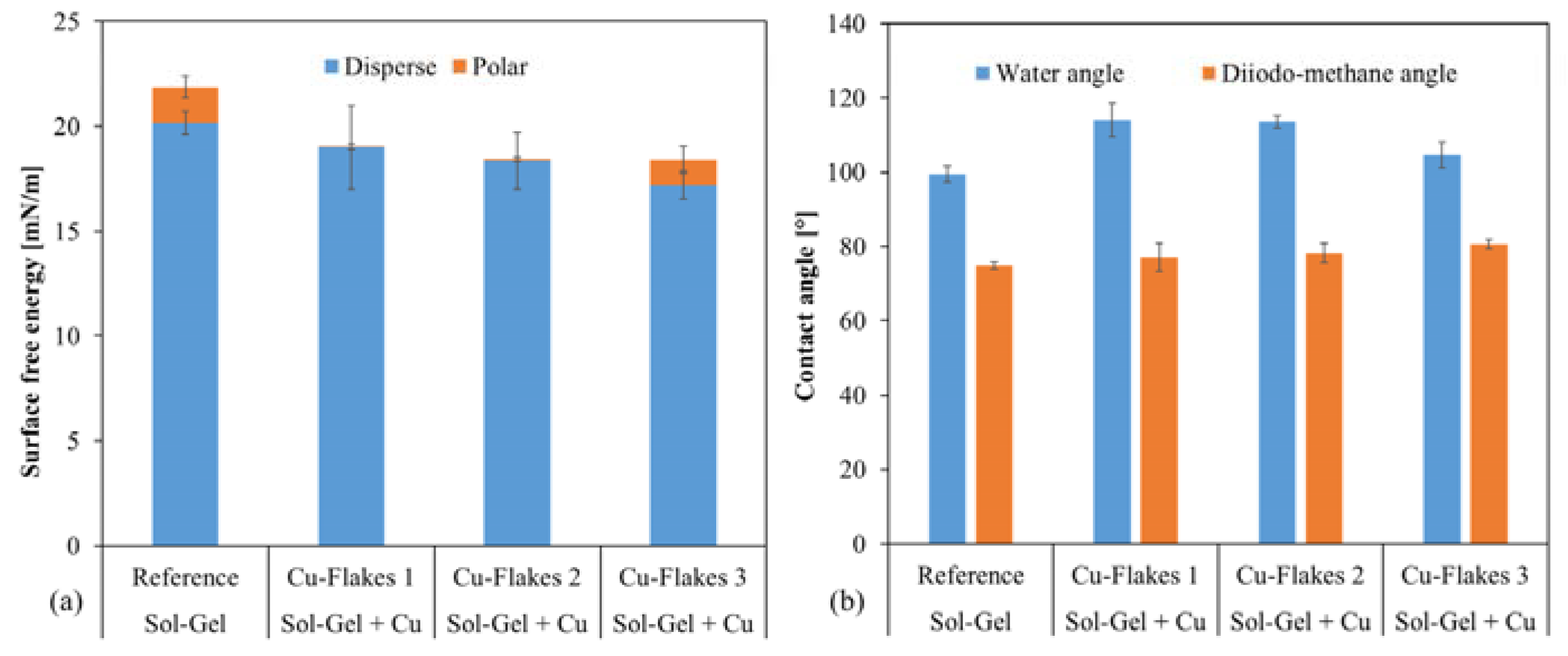
2.2.3. Wettability
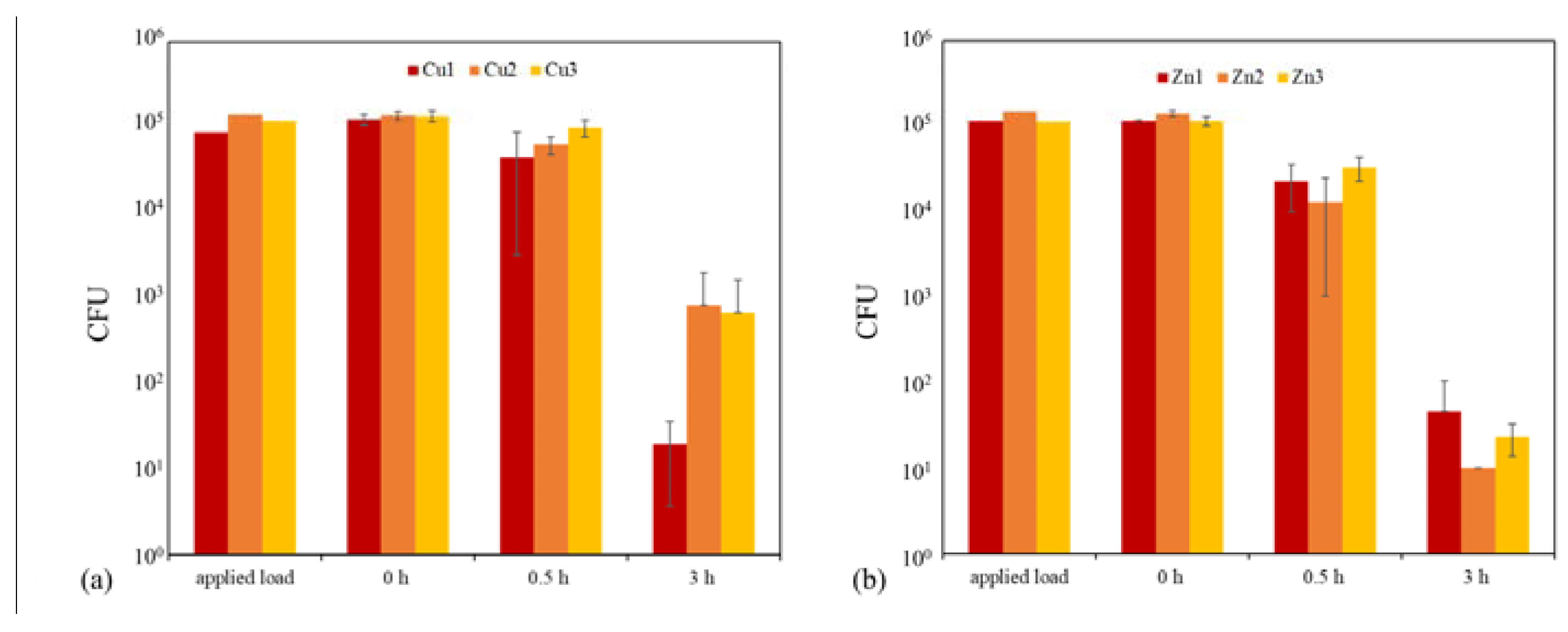
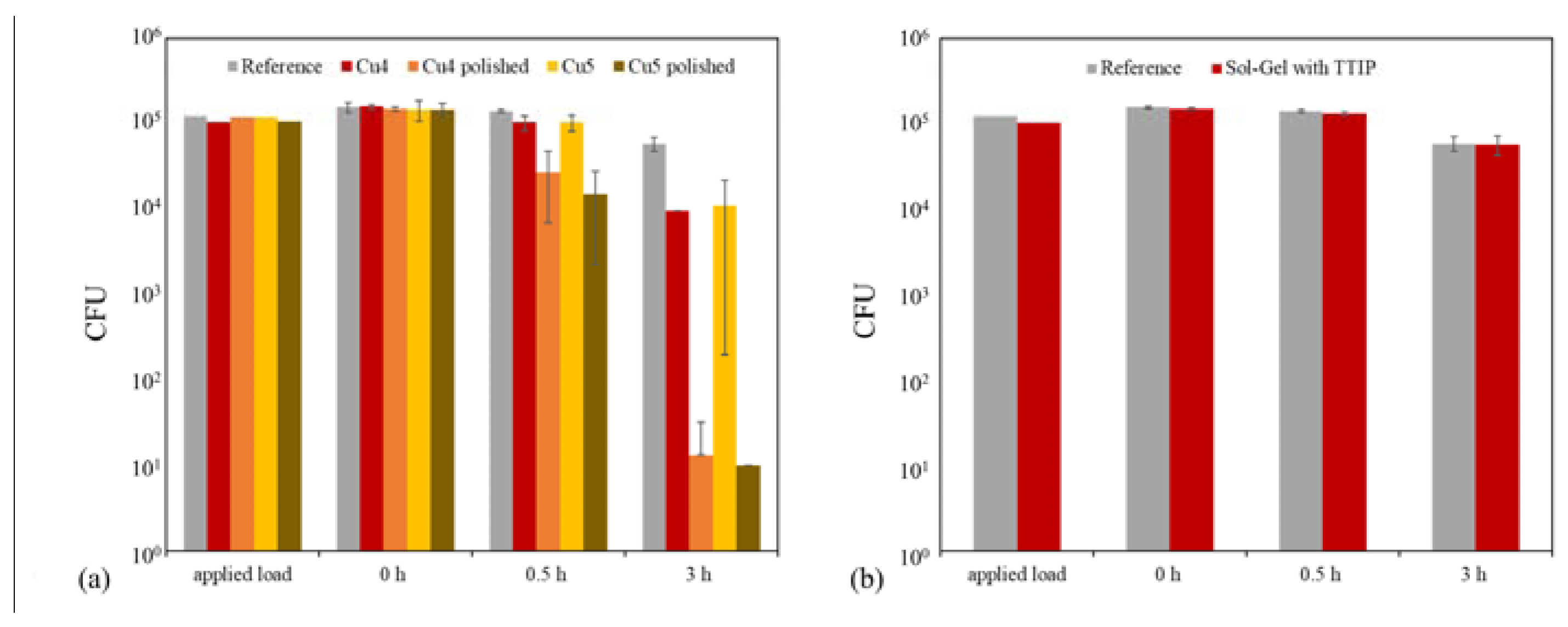
2.3. Antibacterial Tests
3. Conclusions
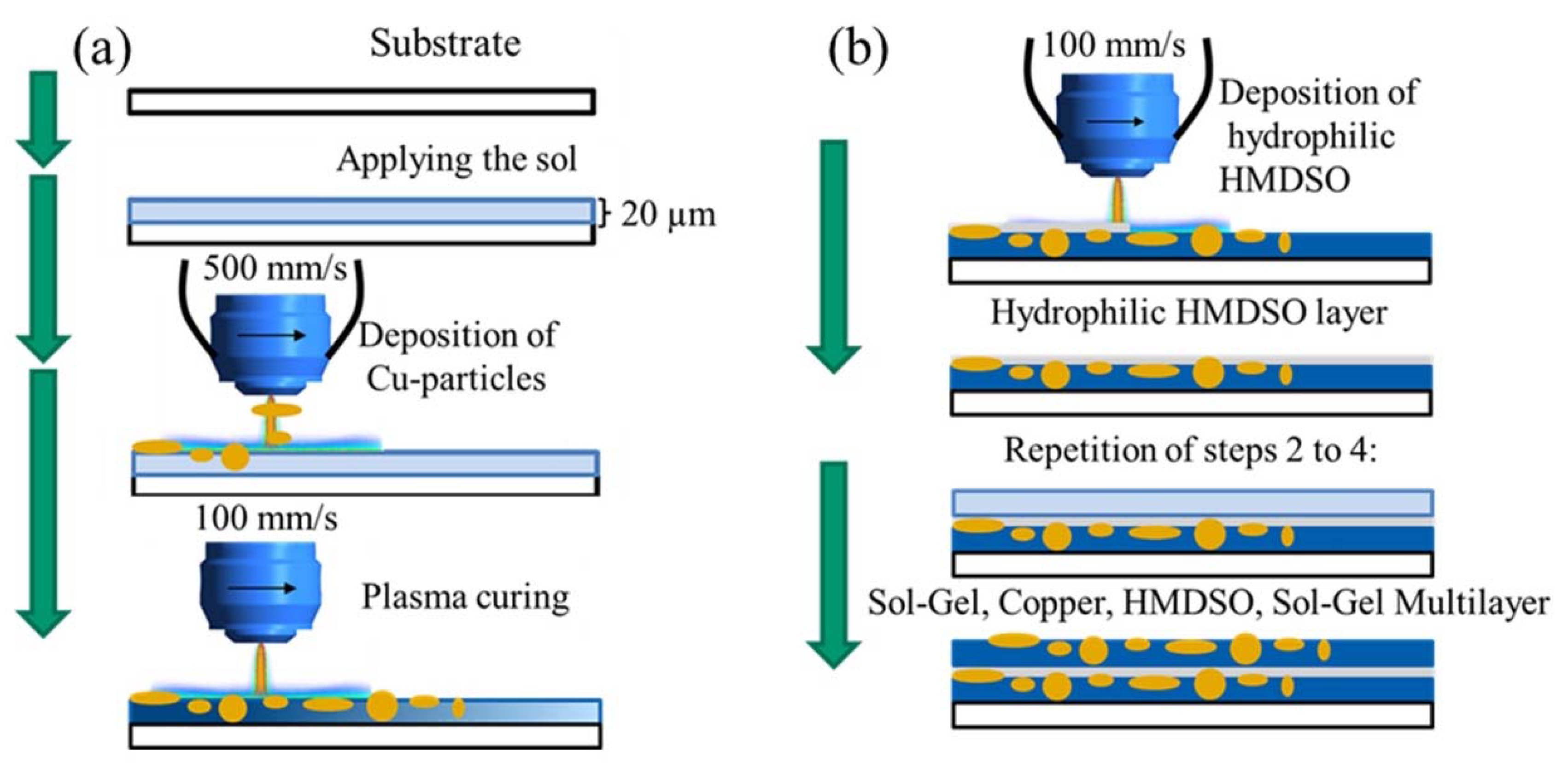
4. Experimental Setup and Characterization
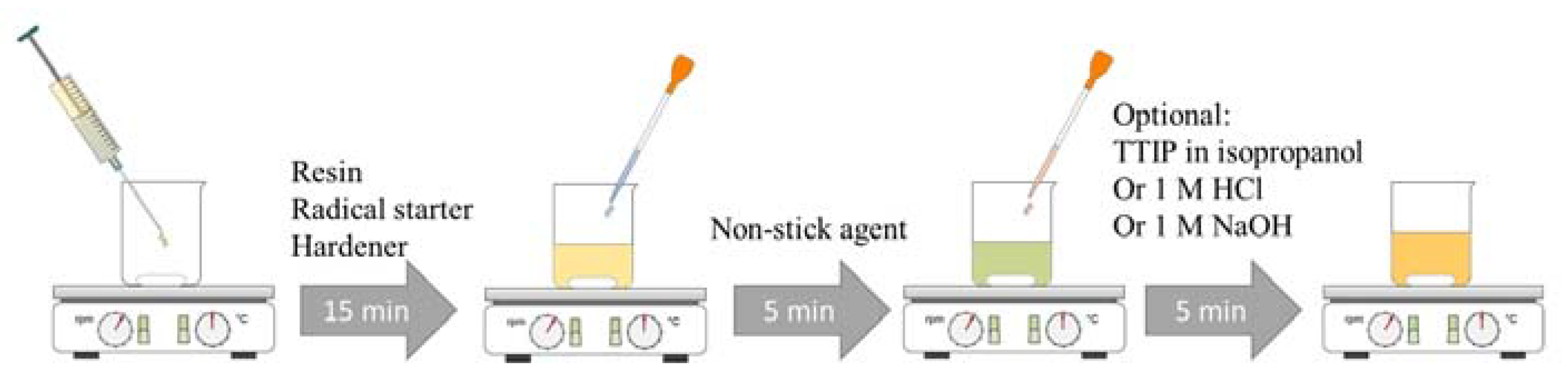
4.1. Sol–Gel Preparation
4.2. Sample Preparation
4.3. Curing Process with Atmospheric Pressure Plasma
4.4. Post-Growth Characterization
4.4.1. Optical Characterization
4.4.2. Structural Characterization
4.4.3. Tribological Characterization
4.4.4. Biological Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedel, M.; Poelman, M.; Zago, M.; Vandermiers, C.; Cossement, D.; Olivier, M.-G.; Deflorian, F. Influence of formulation and application parameters on the performances of a sol–gel/clay nanocomposite on the corrosion resistance of hot-dip galvanized steel. Part II. Effect of curing temperature and time. Surf. Coat. Technol. 2015, 274, 9–17. [Google Scholar] [CrossRef]
- Toide, T.; Rosero-Navarro, N.C.; Miura, A.; Kozuka, H.; Tadanaga, K. Preparation of transparent and mechanically hard inorganic-organic hybrid thick films from 3-glycidoxypropyltrimethoxysilane and zirconium propoxide. J. Sol-Gel Sci. Technol. 2022, 104, 478–483. [Google Scholar] [CrossRef]
- Han, Y.-H.; Taylor, A.; Mantle, M.D.; Knowles, K.M. Sol–gel-derived organic–inorganic hybrid materials. J. Non-Cryst. Solids 2007, 353, 313–320. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Orwat, K.; Bernard, P.; Wróblewski, S.; Mendez, J.D. Traditional vs. UV-Cured coatings—An inquiry-based experiment for introducing green chemistry. Maced. J. Chem. Chem. Eng. 2018, 37, 215–224. [Google Scholar] [CrossRef]
- Even, A.; Vignaud, G.; Guitter, N.; Le Bozec, N.; Tingaut, P.; Grohens, Y. Influence of radical photoinitiator content on UV curing process and UV-cured hybrid sol–gel films. J. Coat. Technol. Res. 2020, 17, 333–343. [Google Scholar] [CrossRef]
- Da Silva, F.S.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M.; Caires, C.; Lima, A.R.; Silva, C.M.; Oliveira, S.L.; Caires, A.; et al. Corrosion resistance and antibacterial properties of copper coating deposited by cold gas spray. Surf. Coat. Technol. 2019, 361, 292–301. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, J.; Cui, L.-Y.; Zeng, R.-C.; Wang, Q.-Z.; Han, Q.-X.; Qiu, J.; Chen, X.-B.; Chen, D.-C.; Guan, S.-K.; et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. [Google Scholar] [CrossRef]
- Yang, N.; Lu, K. Thermophysical property and electrical conductivity of titanium isopropoxide—Polysiloxane derived ceramics. J. Eur. Ceram. Soc. 2019, 39, 4029–4037. [Google Scholar] [CrossRef]
- Chwatal, S.; Stummer, M.; Steiner, H.; Brandner, A.; Pölzl, S.; Kittinger, C.; Lackner, J.M.; Hinterer, A.; Waldhauser, W.; Coclite, A.M. Cross-linking processes in antimicrobial UV-sol-gel systems initiated by atmospheric pressure plasma. Thin Solid Film. 2022, 763, 139598. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Multilayer Coatings for Tribology: A Mini Review. Nanomaterials 2022, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Khadem, M.; Penkov, O.V.; Yang, H.-K.; Kim, D.-E. Tribology of multilayer coatings for wear reduction: A review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Oh, T.; Choi, C.K. Comparison between SiOC Thin Film by plasma enhance chemical vapor deposition and SiO2 Thin Film by Fourier Transform Infrared Spectroscopy. J. Korean Phy. Soc. 2010, 56, 1150–1155. [Google Scholar] [CrossRef]
- Roesch, P. Siloxane und Silanole als Modellverbindungen für Oberflächendefekte: Hydrolyse- und Kondensationsreaktionen. Ph.D. Thesis, Umboldt University of Berlin, Berlin, Germany, 2018. [Google Scholar]
- Rubio, J.; Mazo, M.A.; Martín-Ilana, A.; Tamayo, A. FT-IR study of the hydrolysis and condensation of 3-(2-amino-ethylamino)propyl-trimethoxy silane. Boletín Soc. Española Cerámica Y Vidr. 2018, 57, 160–168. [Google Scholar] [CrossRef]
- Tian, R.; Seitz, O.; Li, M.; Hu, W.W.; Chabal, Y.J.; Gao, J. Infrared characterization of interfacial Si-O bond formation on silanized flat SiO2/Si surfaces. Langmuir 2010, 26, 4563–4566. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.; Wolfs, D.P.; van der Linde, M.C.; Hovens, J.; Tinnemans, A. Transparent UV curable antistatic hybrid coatings on polycarbonate prepared by the sol–gel method. Prog. Org. Coat. 2004, 51, 312–319. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, R.; Li, X.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. A comparative study on 3D printed silicone-epoxy/acrylate hybrid polymers via pure photopolymerization and dual-curing mechanisms. J. Mater. Sci. 2019, 54, 5101–5111. [Google Scholar] [CrossRef]
- Aleksy, N.; Kermouche, G.; Vautrin, A.; Bergheau, J.-M. Mechanical study of polymers in scratch test. Int. J. Mater. Form. 2008, 1, 595–598. [Google Scholar] [CrossRef]
- Guediche, A.; Compoint, F.; Boscher, C.; Stelian, C.; Piombini, H. Indentation hardness and scratch tests for thin layers manufactured by sol-gel process. Thin Solid Film. 2021, 724, 138618. [Google Scholar] [CrossRef]
- Jiang, H.; Browning, R.; Whitcomb, J.D.; Ito, M.; Shimouse, M.; Chang, T.A.; Sue, H.-J. Mechanical Modeling of Scratch Behavior of Polymeric Coatings on Hard and Soft Substrates. Tribol. Lett. 2010, 37, 159–167. [Google Scholar] [CrossRef]
- Gabrielli, L.; Russo, L.; Poveda, A.; Jones, J.R.; Nicotra, F.; Jiménez-Barbero, J.; Cipolla, L. Epoxide opening versus silica condensation during sol-gel hybrid biomaterial synthesis. Chemistry 2013, 19, 7856–7864. [Google Scholar] [CrossRef] [PubMed]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, copper, and copper hydroxy salt decorated fumed silica hybrid composites as antibacterial agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Szymańska, K.; Szymański, K.; Moszyński, D.; Mozia, S. Effect of copper salts on the characteristics and antibacterial activity of Cu-modified titanate nanotubes. J. Environ. Chem. Eng. 2020, 8, 104550. [Google Scholar] [CrossRef]
- Kim, S.W. Characterization of UV curable hybrid hard coating materials prepared by sol-gel method. Korean J. Chem. Eng. 2011, 28, 298–303. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S. Sol–Gel Process and Applications. In Handbook of Advanced Ceramics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 883–910. ISBN 9780123854698. [Google Scholar]
- Fedel, M. Effect of sol–gel layers obtained from GLYMO/MTES mixtures on the delamination of a cataphoretic paint on AA1050. J. Coat. Technol. Res. 2017, 14, 425–435. [Google Scholar] [CrossRef]
- Guo, L.; Feng, W.; Liu, X.; Lin, C.; Li, B.; Qiang, Y. Sol–gel synthesis of antibacterial hybrid coatings on titanium. Mater. Lett. 2015, 160, 448–451. [Google Scholar] [CrossRef]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef]
- Versace, D.L.; Oubaha, M.; Copperwhite, R.; Croutxé-Barghorn, C.; MacCraith, B.D. Waveguide fabrication in UV-photocurable sol–gel materials: Influence of the photoinitiating system. Thin Solid Film. 2008, 516, 6448–6457. [Google Scholar] [CrossRef]









| Assignments | Peak (cm−1) | Relative Intensity * Slightly Acidic|Alkaline 0 h, 24 h|0 h, 24 h | References |
|---|---|---|---|
| Si-OH | 950 | 0.35, 0.29|0.34, 0.24 | [3,4,13,14,15,16] |
| Si-OCH2CH3 | 1163 | 0.33, 0.26|0.34, 0.22 | [3,4,17,18] |
| 1100 | 0.68, 0.62|0.74, 0.65 | ||
| 1067 | 0.94, 0.63|0.97, 0.60 | ||
| 950 | 0.35, 0.29|0.34, 0.24 | ||
| Si-O-Si | 1039 | 1.00, 0.83|1.00, 0.80 | [3,4,13,14,15,16,17,18] |
| 1013 | 0.85, 1.00|0.84, 1.00 | ||
| 700 | 0.23, 0.25|0.21, 0.25 | ||
| Si-CH3 | 1260 | 0.14, 0.10|0.39, 0.10 | [3,4] |
| 800–760 | 0.48, 0.31|0.48, 0.30 | ||
| C=C | 1640 | 0.11, 0.14|0.11, 0.16 | |
| C=O | 1716 | 0.29, 0.30|0.29, 0.29 | [3,4,5] |
| Plasma Powder Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Powder | C [A] | MS [mm/s] | TT [s] | PG | GF [L/min] | S-P D [mm] | PF [g/min] |
| Powder 1 | Copper | 180 | 500 | 10 | Argon | 10 | 150 | 2 |
| Powder 2 | Copper | 120 | 500 | 10 | Argon | 10 | 150 | 2 |
| Powder 3 | Zinc | 180 | 500 | 10 | Argon | 10 | 150 | 2 |
| Powder 4 | Zinc | 120 | 500 | 10 | Argon | 10 | 150 | 2 |
| Powder 5 | Copper Flakes | - | 50 | 100 | - | - | 250 | 2 |
| Powder 6 | Copper Flakes | - | 75 | 75 | - | - | 250 | 2 |
| Powder 7 | Copper Flakes | - | 100 | 50 | - | - | 250 | 2 |
| Plasma Treatment | ||||||
|---|---|---|---|---|---|---|
| Parameter | C [A] | MS [mm/s] | TT [s] | PG | GF [L/min] | S-P D [mm] |
| Plasma 1 | 250 | 100 | 160 | Argon | 10 | 250 |
| Plasma 2 | 180 250 | 100 | 40 120 | Argon | 10 | 250 |
| Plasma 3 | 180 250 | 100 | 80 80 | Argon | 10 | 250 |
| Plasma 4 | 180 250 | 100 | 40 80 | Argon | 10 | 250 |
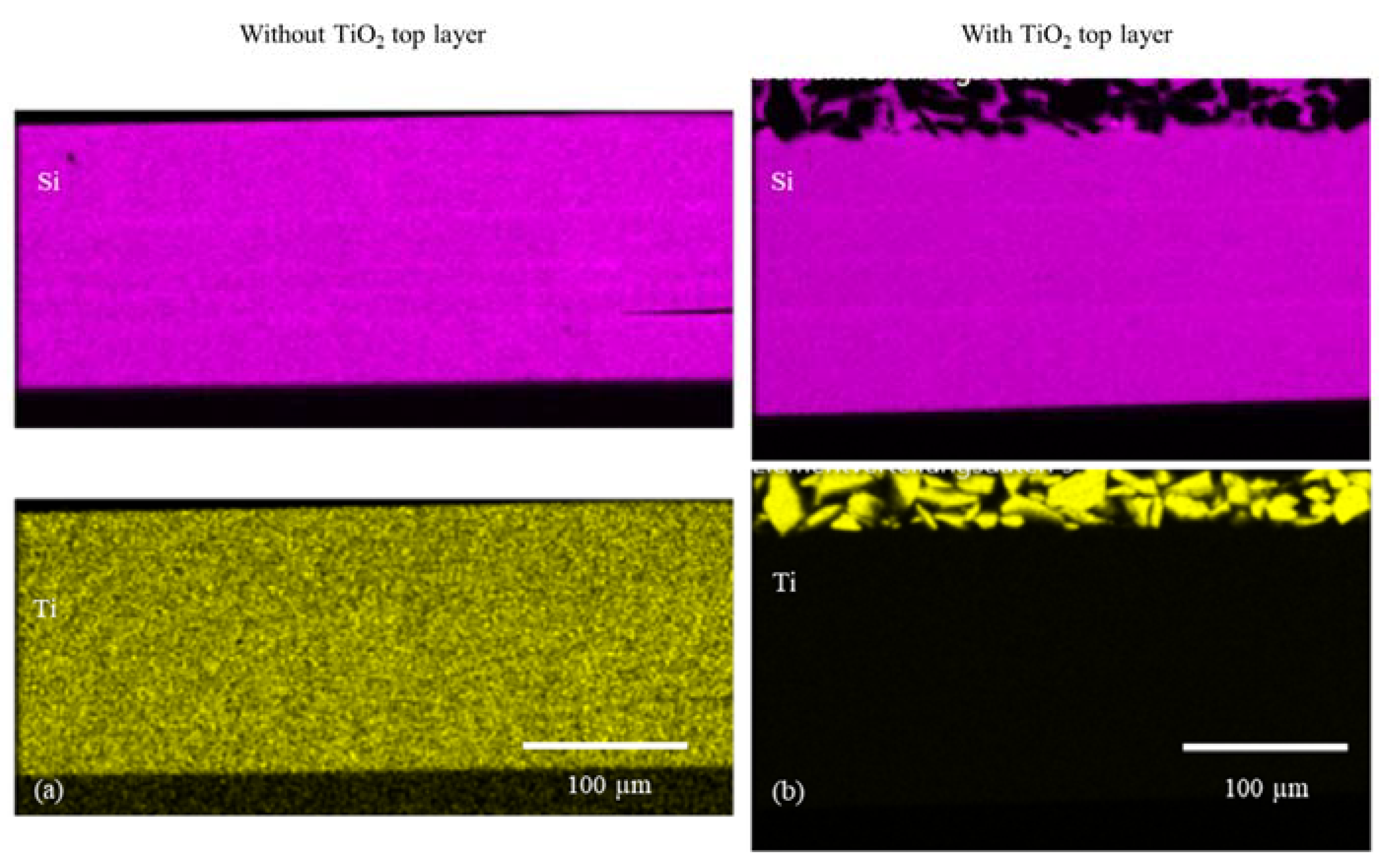
| Without TiO2 − Top Layer | With TiO2 − Top Layer | |||
|---|---|---|---|---|
| Element | In Layer | Top Layer | In Layer | Top Layer |
| O | 65.1 | 65.8 | 62.7 | 75.1 |
| Si | 32.7 | 32.0 | 34.7 | 5.9 |
| Ti | 2.2 | 2.3 | 2.6 | 19.0 |
| Coating | Layer Thickness [µm] |
|---|---|
| Sol–Gel with TTIP | 145.8 ± 9.7 |
| Sol–Gel with TTIP + TiO2 | 232.5 ± 25.3 |
| Plasma Adhesion Layer Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Precursor | C [A] | MS [mm/s] | TT [s] | PG | GF [L/min] | S-P D [mm] | PrF [mL/min] |
| Adhesion 1 | HMDSO | 180 | 100 | 40 | Argon | 10 | 250 | 80 |
| Coating | Parameters | Scratch Hardness [N] |
|---|---|---|
| Alkaline One Layer | Plasma 1 | 12.7 ± 0.5 |
| Slightly acidic One Layer | Plasma 1 | 14.3 ± 0.5 |
| Acidic One Layer | Plasma 1 | 14.7 ± 0.5 |
| Alkaline Three Layers | Plasma 1 | 8.3 ± 0.5 |
| Slightly acidic Three Layers | Plasma 1 | 8.7 ± 0.5 |
| Acidic Three Layers | Plasma 1 | 6.0 ± 0.8 |
| Copper Sol–Gel One Layer | Plasma 1 | 12.7 ± 0.5 |
| Zinc Sol–Gel One Layer | Plasma 1 | 14.7 ± 0.5 |
| Sol–Gel with TTIP Five Layers | Plasma 2 | 18.0 ± 0.8 |
| Sol–Gel with TTIP + TiO2 Five Layers | Plasma 2 | 17.7 ± 0.5 |
| Sample | Parameter | Reduction in CFU after 0.5 h | Reduction in CFU after 3 h |
|---|---|---|---|
| Cu1 | Powder 1 Plasma 1 | 63.53% | 99.98% |
| Cu2 | Powder 2 Plasma 1 | 54.30% | 99.39% |
| Cu3 | Powder 1 Plasma 2 | 26.72% | 99.50% |
| Zn1 | Powder 3 Plasma 1 | 80.26% | 99.96% |
| Zn2 | Powder 4 Plasma 1 | 90.87% | 99.99% |
| Zn3 | Powder 3 Plasma 2 | 71.20% | 99.98% |
| Cu4 | Powder 1 Plasma 4 | 34.82% | 93.89% |
| Cu4 polished | Powder 1 Plasma 4 | 81.70% | 99.99% |
| Cu5 | Powder 2 Plasma 4 | 29.22% | 92.49% |
| Cu5 polished | Powder 2 Plasma 4 | 89.70% | 99.99% |
| Cu-Flakes 1 | Powder 5 Plasma 2 | 98.23% | 99.99% |
| Cu-Flakes 2 | Powder 6 Plasma 2 | 99.68% | 99.99% |
| Cu-Flakes 3 | Powder 7 Plasma 2 | 97.60% | 99.99% |
| Sol–Gel with TTIP | Plasma 2 | 11.86% | 62.06% |
| Reference | Plasma 2 | 9.94% | 62.42% |
| Sol–Gel Coating | ||||
|---|---|---|---|---|
| Sample | Layer Thickness Applied [µm] | Layer Thickness after Treatment [µm] | Room Temperature [°C] | Humidity % |
| Sol–Gel Coating (any pH value) | 20 | 5 | 25 | 60 |
| TTIP Sol–Gel Coating | 20 | 5 | 25 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwatal, S.; Pölzl, S.; Kittinger, C.; Lackner, J.M.; Coclite, A.M.; Waldhauser, W. Single- and Multilayer Build-Up of an Antibacterial Temperature- and UV-Curing Sol–Gel System with Atmospheric Pressure Plasma. Gels 2023, 9, 675. https://doi.org/10.3390/gels9090675
Chwatal S, Pölzl S, Kittinger C, Lackner JM, Coclite AM, Waldhauser W. Single- and Multilayer Build-Up of an Antibacterial Temperature- and UV-Curing Sol–Gel System with Atmospheric Pressure Plasma. Gels. 2023; 9(9):675. https://doi.org/10.3390/gels9090675
Chicago/Turabian StyleChwatal, Simon, Sabine Pölzl, Clemens Kittinger, Jürgen Markus Lackner, Anna Maria Coclite, and Wolfgang Waldhauser. 2023. "Single- and Multilayer Build-Up of an Antibacterial Temperature- and UV-Curing Sol–Gel System with Atmospheric Pressure Plasma" Gels 9, no. 9: 675. https://doi.org/10.3390/gels9090675
APA StyleChwatal, S., Pölzl, S., Kittinger, C., Lackner, J. M., Coclite, A. M., & Waldhauser, W. (2023). Single- and Multilayer Build-Up of an Antibacterial Temperature- and UV-Curing Sol–Gel System with Atmospheric Pressure Plasma. Gels, 9(9), 675. https://doi.org/10.3390/gels9090675







