Balsam Poplar Buds Extracts-Loaded Gels and Emulgels: Development, Biopharmaceutical Evaluation, and Biological Activity In Vitro
Abstract
:1. Introduction
2. Results
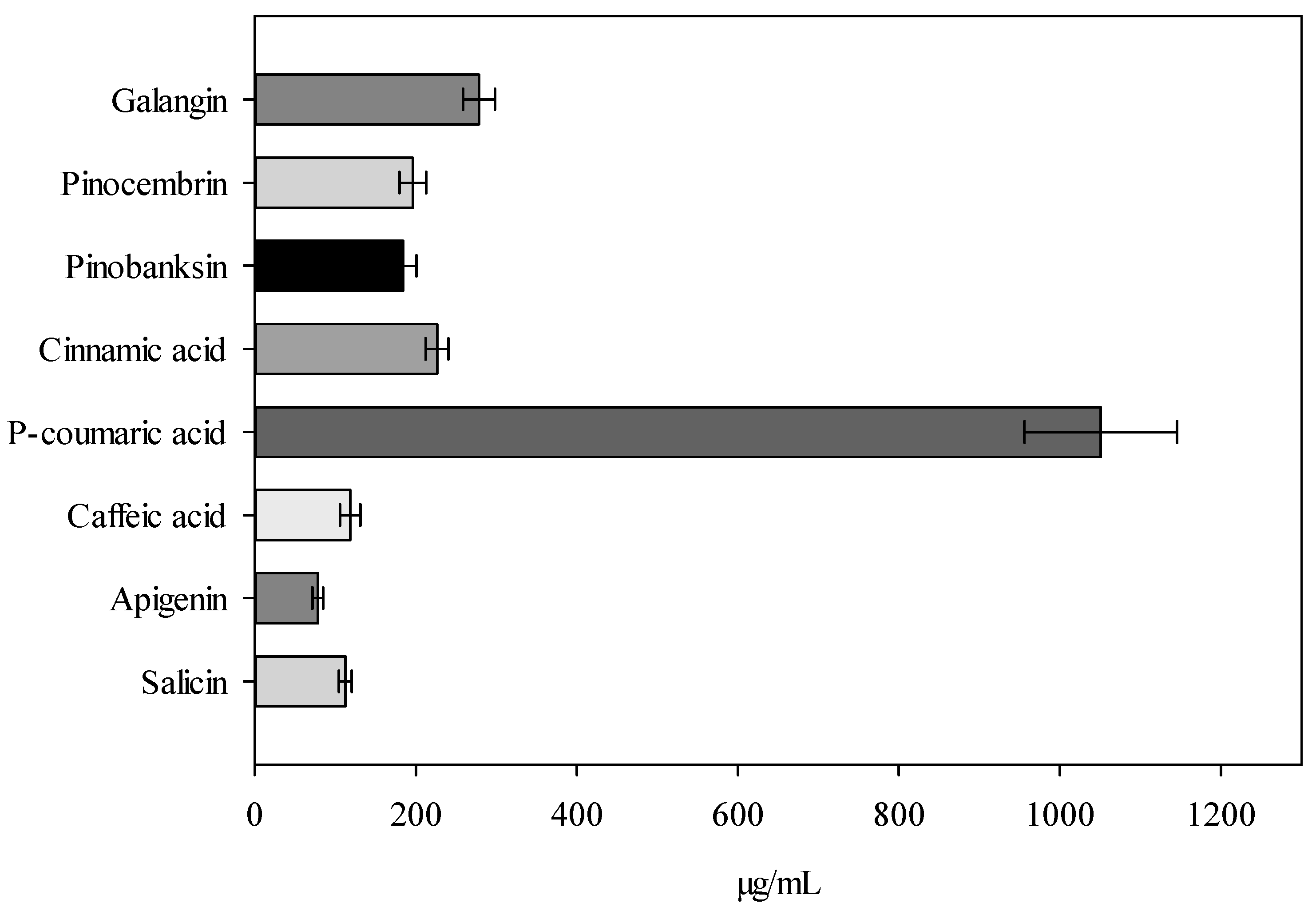
2.1. Analysis of Active Compounds in Balsam Poplar Buds Extracts
2.2. Production of Gels with a Liquid Extract of Balsam Poplar Buds
2.3. Investigation of the Physicochemical Properties of Semi-Solid Formulations with Balsam Poplar Buds Liquid Extract
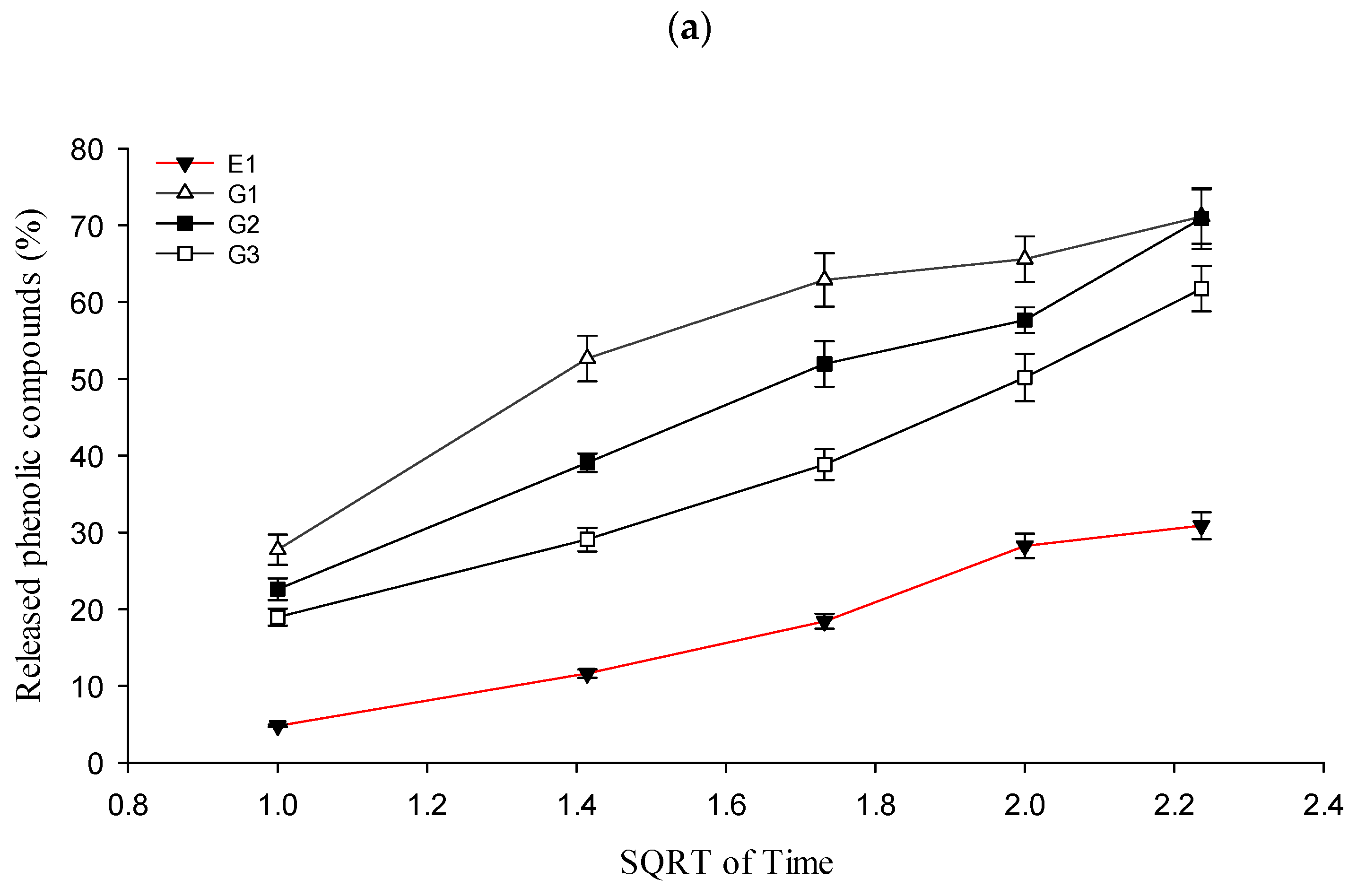
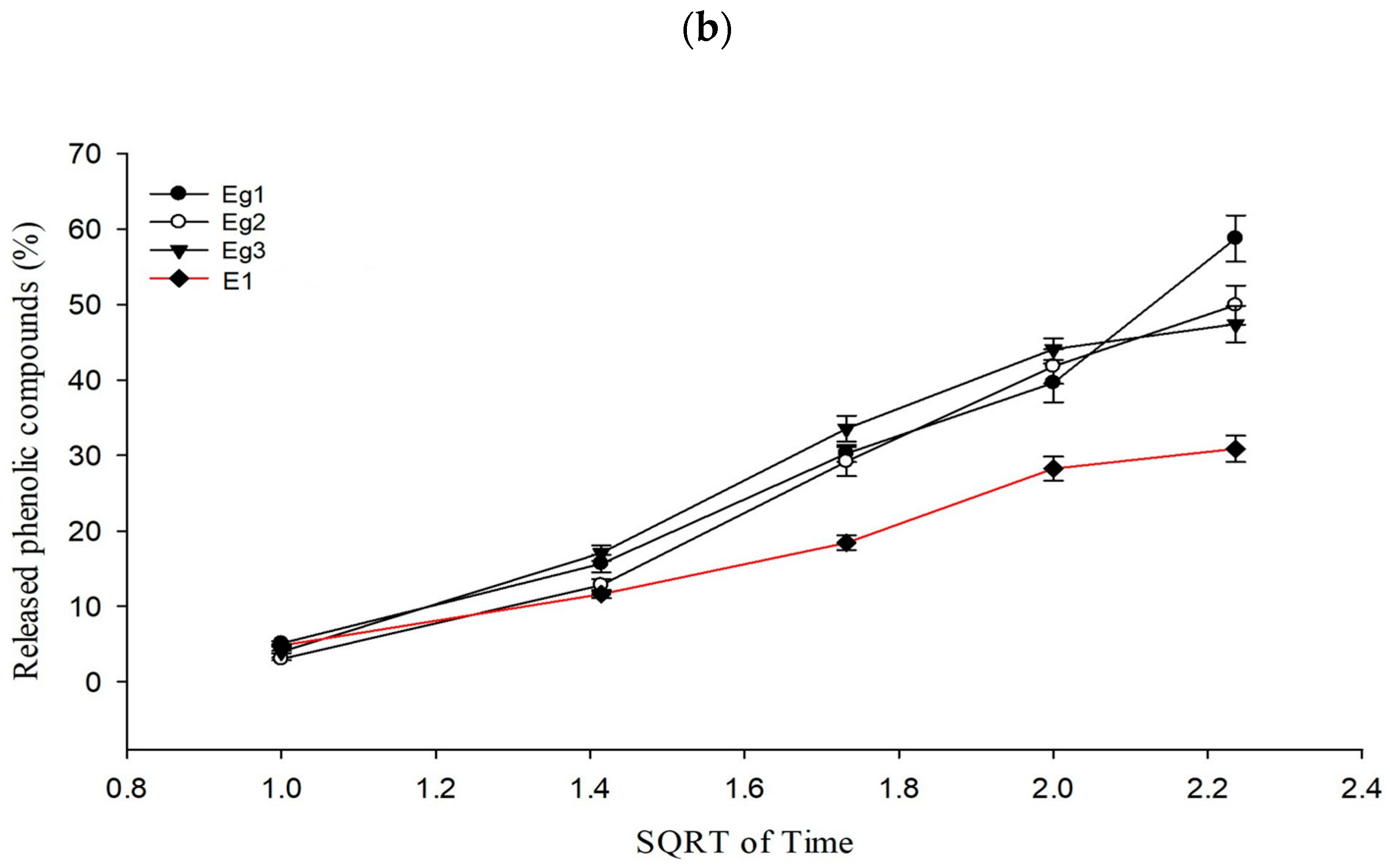
2.4. In Vitro Release Test from Formulations
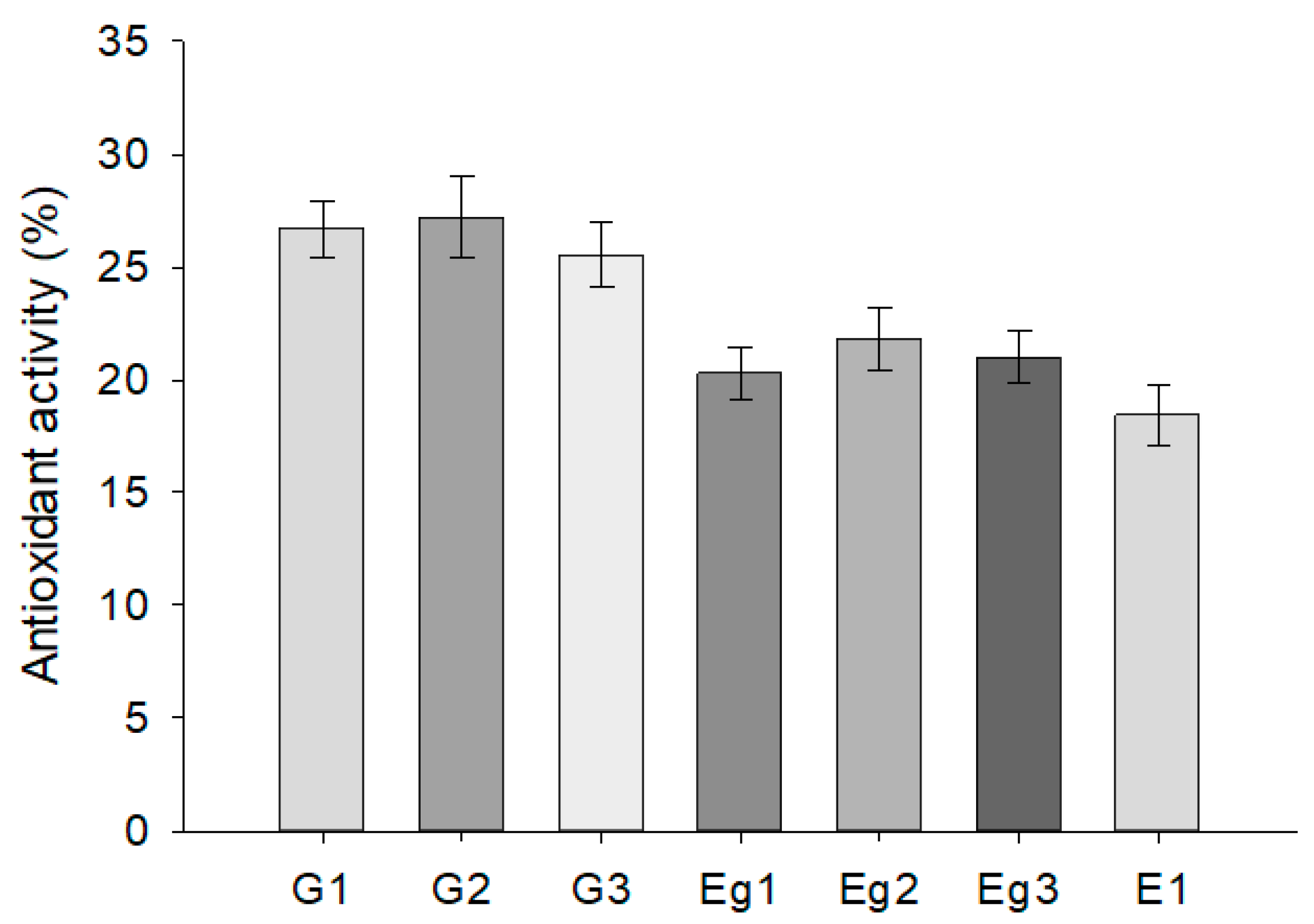
2.5. Evaluation of Semi-Solid Preparations’ Antioxidant Activity
2.6. Antimicrobal Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Liquid Extracts of Balsam Poplar Buds Preparation
5.3. Determination of the Total Phenolic Compounds and Flavonoid Content in Balsam Poplar Buds Extract
5.4. HPLC Analysis
5.5. Modeling of Semi-Solid Pharmaceutical Forms
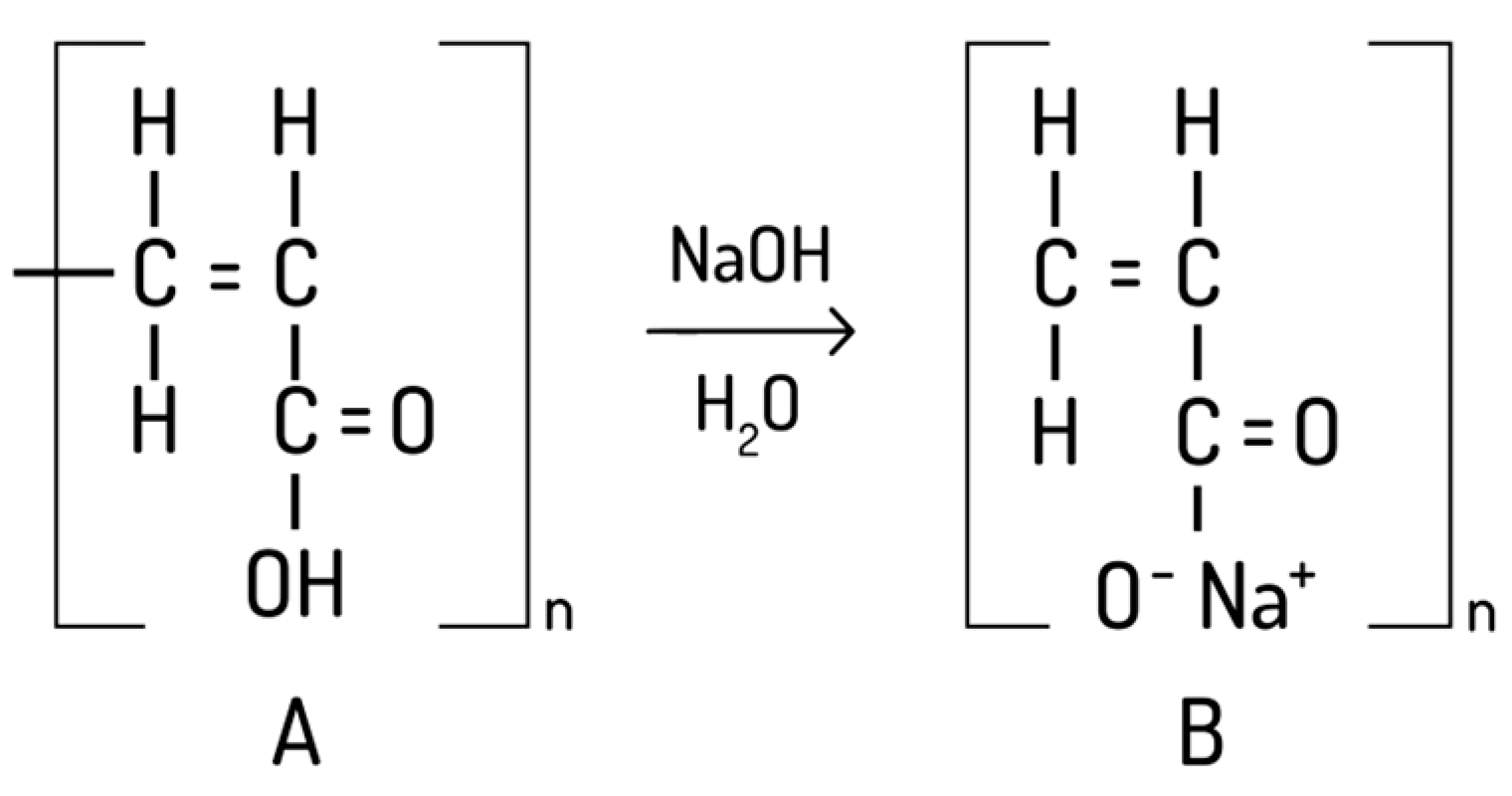
5.5.1. Gels with Balsam Poplar Buds Extract
5.5.2. Emulsions with Balsam Poplar Buds Extract
5.5.3. Emulgels with Balsam Poplar Buds Extract
5.6. Assessment of Physicochemical Parameters of Semi-Solid Pharmaceutical Formulations
5.7. In Vitro Release Test of Phenolic Compounds from Semi-Solid Pharmaceutical Formulations
5.8. Assessment of the Antioxidant Activity of Semi-Solid Pharmaceutical Formulations
5.9. Antimicrobial Activity
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Keskin, M. Chapter 7—Natural Bee Products and Their Apitherapeutic Applications. Bioact. Nat. Prod. 2020, 66, 175–196. [Google Scholar] [CrossRef]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.P.; Solomon, P.R.; Thangaraj, B. (Eds.) Salicaceae BT—Biodiesel from Flowering Plants; Springer: Singapore, 2022; pp. 491–494. [Google Scholar]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus × canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, E.; D’Eliseo, D.; Ieri, F.; Romani, A.; Santi, L.; Bernini, R.; Sabatti, M.; Velotti, F. Perspectives on Populus spp. (Salicaceae) bud extracts as antioxidant and anti-inflammatory agents. Nat. Prod. Res. 2022, 36, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of Ethanolic and Aqueous Populus balsamifera L. Bud Extracts by Different Extraction Methods: Chemical Composition, Antioxidant and Antibacterial Activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Ivanauskas, L.; Perminaite, K.; Ramanauskiene, K. Ophthalmic In Situ Gels with Balsam Poplar Buds Extract: Formulation, Rheological Characterization, and Quality Evaluation. Pharmaceutics 2021, 13, 953. [Google Scholar] [CrossRef]
- Smithson, J.; Kellick, K.A.; Mergenhagen, K. Nutritional Modulators of Pain in the Aging Population; Academic Press: Cambridge, MA, USA, 2017; Volume 16, pp. 191–198. [Google Scholar]
- Król, W.; Bankova, V.; Sforcin, J.M.; Szliszka, E.; Czuba, Z.; Kuropatnicki, A.K. Propolis: Properties, Application, and Its Potential. Evid.-Based Complement. Altern. Med. 2013, 2013, 807578. [Google Scholar] [CrossRef]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Evans, W.C.; Evans, D. Trease and Evans’ Pharmacognosy. Chapter 5—A Taxonomic Approach to the Study of Medicinal Plants and Animal-Derived Drugs; W.B. Saunders-Elsevier: Amsterdam, The Netherlands, 2009; pp. 18–44. [Google Scholar] [CrossRef]
- Kis, B.; Avram, S.; Pavel, I.Z.; Lombrea, A.; Buda, V.; Dehelean, C.A.; Soica, C.; Yerer, M.B.; Bojin, F.; Folescu, R.; et al. Recent Advances Regarding the Phytochemical and Therapeutic Uses of Populus nigra L. Buds. Plants 2020, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Poupard, P.; Coutière, P.; Woillez, M.; Richard, T.; Mérillon, J.-M.; Vitrac, X. Phenolic Composition and Antioxidant Properties of Poplar Bud (Populus nigra) Extract: Individual Antioxidant Contribution of Phenolics and Transcriptional Effect on Skin Aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and Treatments of Photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Stanciauskaite, M.; Marksa, M.; Rimkiene, L.; Ramanauskiene, K. Evaluation of Chemical Composition, Sun Protection Factor and Antioxidant Activity of Lithuanian Propolis and Its Plant Precursors. Plants 2022, 11, 3558. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Grenier, A.; Simard, F.; Gendreau, I.; Pichette, A.; Legault, J.; Pouliot, R. Dihydrochalcone Derivatives from Populus balsamifera L. Buds for the Treatment of Psoriasis. Int. J. Mol. Sci. 2020, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Rath, G.; Goyal, A.K. Comprehensive Review on Additives of Topical Dosage Forms for Drug Delivery. Drug Deliv. 2015, 22, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef]
- Olejnik, A.; Goscianska, J.; Nowak, I. Active Compounds Release from Semisolid Dosage Forms. J. Pharm. Sci. 2012, 101, 4032–4045. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Souto, E.B.; Müller, R.H.; Junyaprasert, V.B. Physicochemical Characterization and in Vitro Release Studies of Ascorbyl Palmitate-Loaded Semi-Solid Nanostructured Lipid Carriers (NLC Gels). J. Microencapsul. 2008, 25, 111–120. [Google Scholar] [CrossRef]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Lopez-Galindo, A. Uses of Clay Minerals in Semisolid Health Care and Therapeutic Products. Appl. Clay Sci. 2007, 36, 37–50. [Google Scholar] [CrossRef]
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced Skin Performance of Emulgel vs. Cream as Systems for Topical Delivery of Herbal Actives (Immortelle Extract and Hemp Oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Supriya, U.; Seema, C.B.; Preeti, K. Emulgel: A Boon for Dermatological Diseases. Int. J. Pharm. Res. Allied Sci. 2014, 3, 1–9. [Google Scholar]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An Effective Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Damour, A.; Garcia, M.; Seneschal, J.; Lévêque, N.; Bodet, C. Eczema Herpeticum: Clinical and Pathophysiological Aspects. Clin. Rev. Allergy Immunol. 2020, 59, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Houlleberghs, M.; Verheyden, L.; Voorspoels, F.; Chandran, C.V.; Duerinckx, K.; Radhakrishnan, S.; Martens, J.A.; Breynaert, E. Magneto-hydrodynamic mixing: A new technique for preparing carbomer hydrogels. AIChE J. 2023, 69, 17911. [Google Scholar] [CrossRef]
- Kolman, M.; Smith, C.; Chakrabarty, D.; Amin, S. Rheological stability of carbomer in hydroalcoholic gels: Influence of alcohol type. Int. J. Cosmet. Sci. 2021, 43, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in Vitro Antimicrobial Activity of Populus Buds and Poplar-Type Propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Kis, B.; Pavel, I.Z.; Avram, S.; Moaca, E.A.; Herrero San Juan, M.; Schwiebs, A.; Radeke, H.H.; Muntean, D.; Diaconeasa, Z.; Minda, D.; et al. Antimicrobial Activity, in Vitro Anticancer Effect (MCF-7 Breast Cancer Cell Line), Antiangiogenic and Immunomodulatory Potentials of Populus nigra L. Buds Extract. BMC Complement. Med. Ther. 2022, 22, 74. [Google Scholar] [CrossRef]
- De Marco, S.; Piccioni, M.; Pagiotti, R.; Pietrella, D. Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas Aeruginosa. Evid.-Based Complement. Altern. Med. 2017, 2017, 5163575. [Google Scholar] [CrossRef]
- Dennie, L. Safety and Efficacy of 0.5% Carbomer 980 Gel for Treatment of Symptoms of Common Cold: Results of 2 Randomized Trials. Drugs RD 2019, 19, 191–200. [Google Scholar] [CrossRef]
- Acosta, N.; Sánchez, E.; Calderón, L.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Dom, S.; Heras, Á. Physical Stability Studies of Semi-Solid Formulations from Natural Compounds Loaded with Chitosan Microspheres. Mar. Drugs 2015, 13, 5901–5919. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nada, A.A.; Eid, B.M. Polysaccharide-Based Polymer Gels and Their Potential Applications. In Polymer Gels; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Verma, A.; Singh, S.; Kaur, R.P.; Jain, U.K. Topical Gels as Drug Delivery Systems: A Review. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 374–382. [Google Scholar]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation Effects of Topical Emulsions on Transdermal and Dermal Delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Fisk, I.D.; Mitchell, J.R.; Wolf, B.; Hill, S.E.; Gray, D.A. Sunflower-Seed Oil Body Emulsions: Rheology and Stability Assessment of a Natural Emulsion. Food Hydrocoll. 2008, 22, 1224–1232. [Google Scholar] [CrossRef]
- Lamas, D.L.; Constenla, D.T.; Raab, D. Effect of Degumming Process on Physicochemical Properties of Sunflower Oil. Biocatal. Agric. Biotechnol. 2016, 6, 138–143. [Google Scholar] [CrossRef]
- Campanholi, K.d.S.S.; Junior, R.C.d.S.; Jaski, J.M.; Silva, J.B.d.; Oliveira, M.C.d.; Santos, R.S.d.; Pozza, M.S.d.S.; Castro-Hoshino, L.V.d.; Baesso, M.L.; Cardozo-Filho, L.; et al. Thermo and Photoresponsive Emulgel Loaded with Copaifera reticulata Ducke and Chlorophylls: Rheological, Mechanical, Photodynamic and Drug Delivery Properties in Human Skin. Pharmaceutics 2022, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Stolić Jovanović, A.; Martinović, M.; Žugić, A.; Nešić, I.; Tosti, T.; Blagojević, S.; Tadić, V.M. Derivatives of L-Ascorbic Acid in Emulgel: Development and Comprehensive Evaluation of the Topical Delivery System. Pharmaceutics 2023, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Naga Sravan Kumar Varma, V.; Maheshwari, P.V.; Navya, M.; Reddy, S.C.; Shivakumar, H.G.; Gowda, D.V. Calcipotriol Delivery into the Skin as Emulgel for Effective Permeation. Saudi Pharm. J. 2014, 22, 591–599. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Sun-Hee, Y.; Seung-Hee, N. Physiochemical, Nutritional and Functional Characterization of 10 Different Pear Cultivars (Pyrus Spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- More, G.K.; Makola, R.T. In-Vitro Analysis of Free Radical Scavenging Activities and Suppression of LPS-Induced ROS Production in Macrophage Cells by Solanum Sisymbriifolium Extracts. Sci. Rep. 2020, 10, 6493. [Google Scholar] [CrossRef] [PubMed]
| Quantity in Gel g/100 g | |||
|---|---|---|---|
| Exipients | G1 | G2 | G3 |
| Balsam poplar buds extract | 20 | 20 | 20 |
| Carbomer 980 | 0.5 | 1 | 2 |
| 10% NaOH solution | 3–4 drops | 3–4 drops | 3–4 drops |
| Purified water | ad 100 | ad 100 | ad 100 |
| Composition of emulgels, quantity in emulgel g/100 g | |||
| Eg1 | Eg2 | Eg3 | |
| Balsam poplar buds extract | 20 | 20 | 20 |
| Span 60 | 1.75 | 1.75 | 1.75 |
| Sunflower oil | 18.18 | 18.18 | 18.18 |
| Tween 60 | 1.89 | 1.89 | 1.89 |
| Purified water | ad 60 | ad 60 | ad 60 |
| Carbomer 980 | 0.5 | 1 | 2 |
| 10% NaOH solution | 3–4 drops | 3–4 drops | 3–4 drops |
| Purified water | ad 40 | ad 40 | ad 40 |
| Composition of emulsions, quantity in emulsions g/100 g | |||
| E1 | |||
| Balsam poplar buds extract | 20 | ||
| Span 60 | 4 | ||
| Sunflower oil | 45 | ||
| Tween 60 | 5 | ||
| Purified water | ad 100 | ||
| 0 G1 | 0 G2 | 0 G3 | 0 Eg1 | 0 Eg2 | 0 Eg3 | 0 E1 | |
|---|---|---|---|---|---|---|---|
| pH | 5.76 | 5.98 | 5.91 | 5.17 | 5.54 | 5.28 | 4.61 |
| SD | 0.06 | 0.04 | 0.05 | 0.04 | 0.06 | 0.05 | 0.04 |
| Viscosity (mPa·s) | 248.5 | 255.2 | 458.1 | 194.7 | 1264.3 | 2481 | 2666.1 |
| SD | 13.3 | 14 | 38.3 | 9.4 | 85 | 196.6 | 188 |
| G1 | G2 | G3 | Eg1 | Eg2 | Eg3 | E1 | |
| pH | 5.93 | 5.93 | 5.95 | 5.13 | 5.66 | 5.3 | 4.65 |
| SD | 0.06 | 0.06 | 0.07 | 0.05 | 0.15 | 0.1 | 0.06 |
| Viscosity (mPa·s) | 237.5 | 237.5 | 408.4 | 202.8 | 1271.6 | 2322.4 | 2641 |
| SD | 9.7 | 9.7 | 14.3 | 5.2 | 41.3 | 92.7 | 96.3 |
| Appearance |  |  |  |  |  |  |  |
| Escherichia coli ATCC 29522 ⊘ mm | Pseudomonas aeruginosa ATCC 27859 ⊘ mm | Staphylococcus aureus ATCC 25923 ⊘ mm | Enterococcus faecalis ATCC 29212 ⊘ mm | Candida albicans ATCC 6193 ⊘ mm | |
|---|---|---|---|---|---|
| p-coumaric acid (500 µg/mL) | 6.5 ± 0.5 | NI | 11.8 ± 0.8 | 10.7 ± 0.7 | 11.2 ± 0.3 |
| Balsam poplar buds extract | 7.8 ± 0.3 | NI | 20.8 ± 1.0 | 16.2 ± 1.0 | 14.8 ± 0.3 |
| G1 | NI | NI | 5.8 ± 0.3 | 5.0 ± 0.5 | <5 |
| G2 | NI | NI | 5.7 ± 0.3 | <5 | <5 |
| G3 | NI | NI | 5.5 ± 0.5 | <5 | <5 |
| Eg1 | NI | NI | 5.8 ± 0.3 | <5 | <5 |
| Eg2 | NI | NI | 5.6 ± 0.7 | <5 | <5 |
| Eg3 | NI | NI | 5.3 ± 0.6 | <5 | <5 |
| E1 | NI | NI | <5 | <5 | NI |
| 0.5% chlorhexidine gel | 19.3 ± 0.6 | 16.0 ± 0.9 | 22.3 ± 0.6 | 20.2 ± 1.3 | 12.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokubaite, M.; Pukenaite, G.; Marksa, M.; Ramanauskiene, K. Balsam Poplar Buds Extracts-Loaded Gels and Emulgels: Development, Biopharmaceutical Evaluation, and Biological Activity In Vitro. Gels 2023, 9, 821. https://doi.org/10.3390/gels9100821
Jokubaite M, Pukenaite G, Marksa M, Ramanauskiene K. Balsam Poplar Buds Extracts-Loaded Gels and Emulgels: Development, Biopharmaceutical Evaluation, and Biological Activity In Vitro. Gels. 2023; 9(10):821. https://doi.org/10.3390/gels9100821
Chicago/Turabian StyleJokubaite, Monika, Greta Pukenaite, Mindaugas Marksa, and Kristina Ramanauskiene. 2023. "Balsam Poplar Buds Extracts-Loaded Gels and Emulgels: Development, Biopharmaceutical Evaluation, and Biological Activity In Vitro" Gels 9, no. 10: 821. https://doi.org/10.3390/gels9100821
APA StyleJokubaite, M., Pukenaite, G., Marksa, M., & Ramanauskiene, K. (2023). Balsam Poplar Buds Extracts-Loaded Gels and Emulgels: Development, Biopharmaceutical Evaluation, and Biological Activity In Vitro. Gels, 9(10), 821. https://doi.org/10.3390/gels9100821






