Sustainable Tannin Gels for the Efficient Removal of Metal Ions and Organic Dyes
Abstract
:1. Introduction
2. Results and Discussion
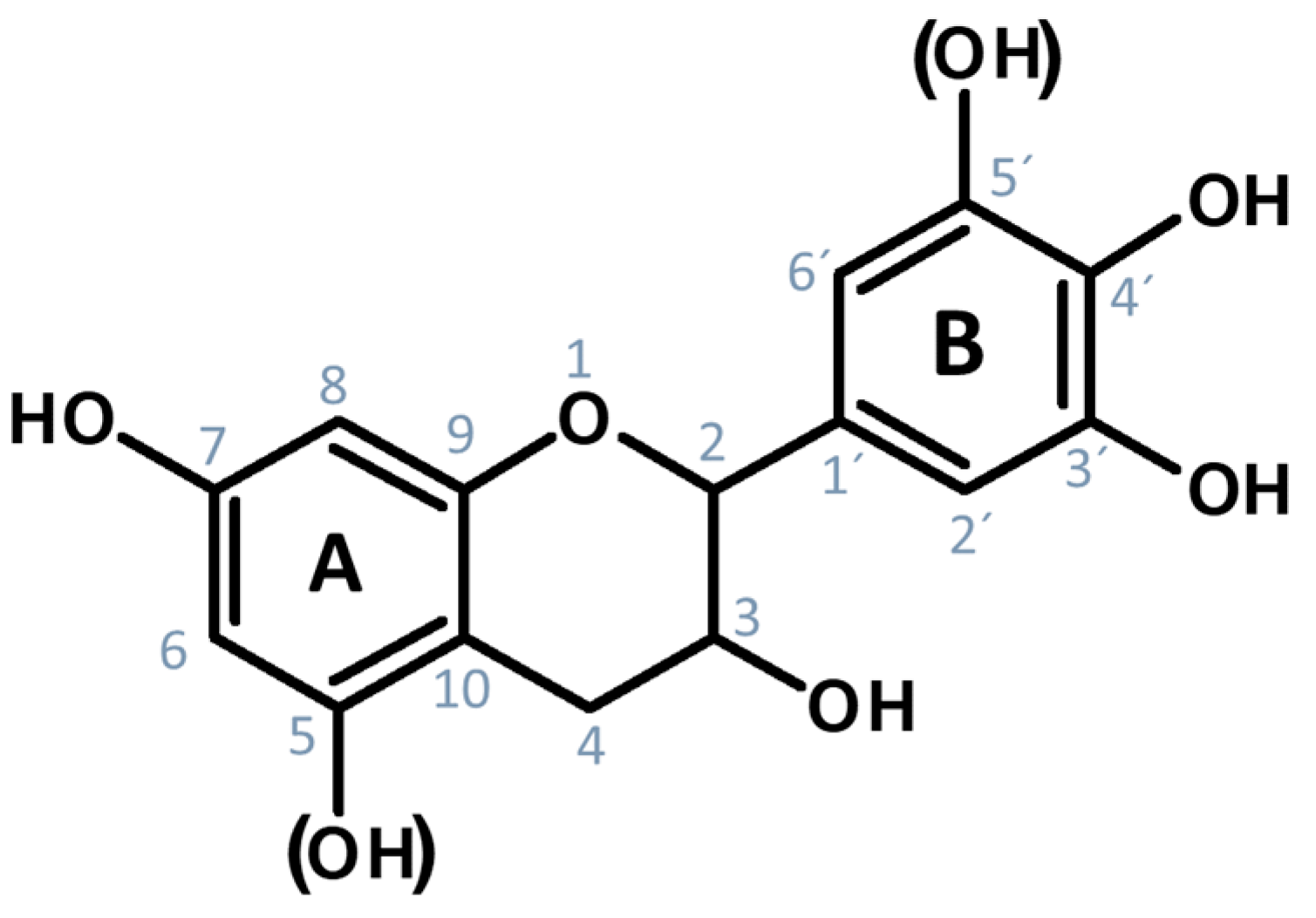
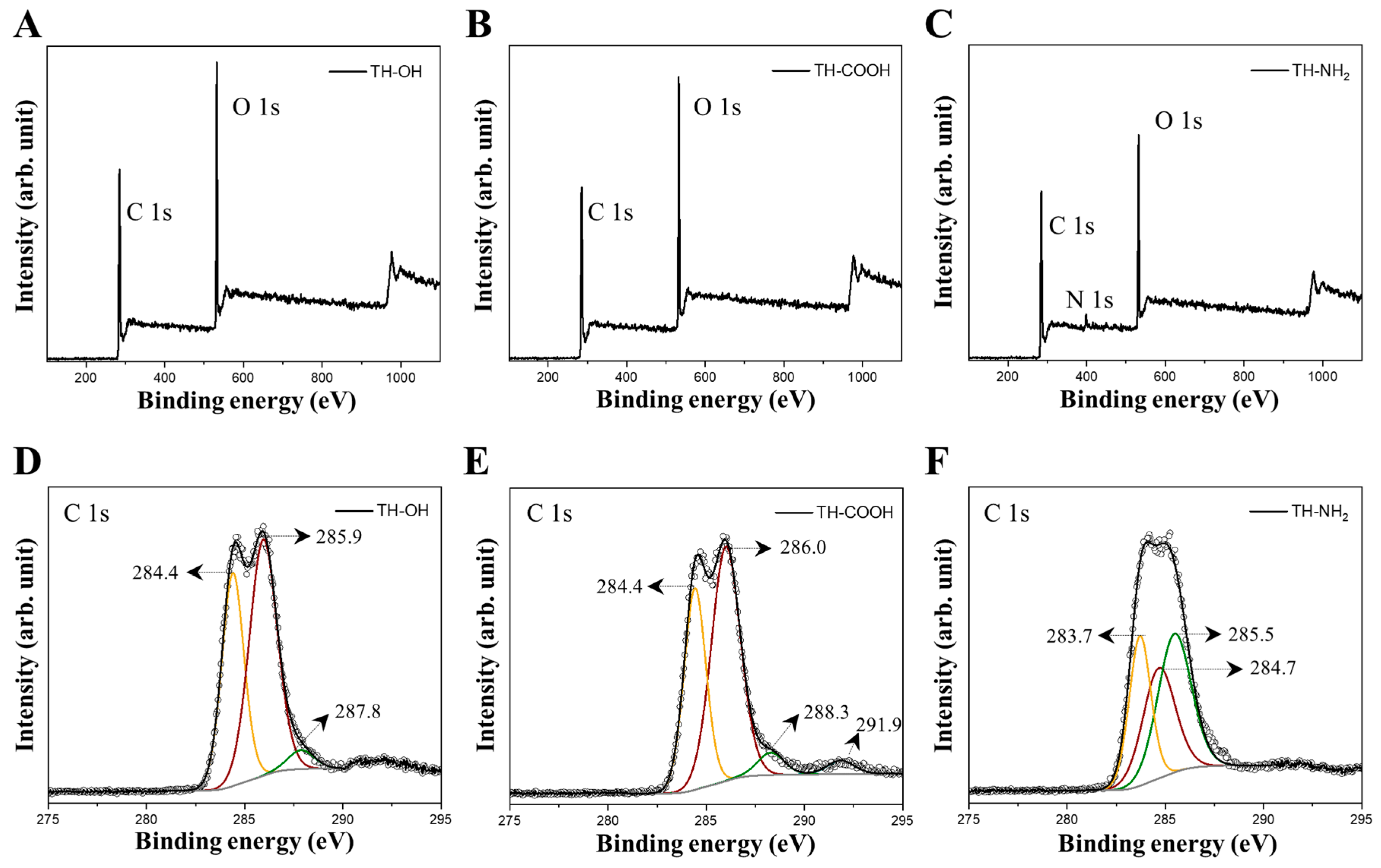
2.1. Characterization of Monolithic Tannin Gels and Their Functionalization
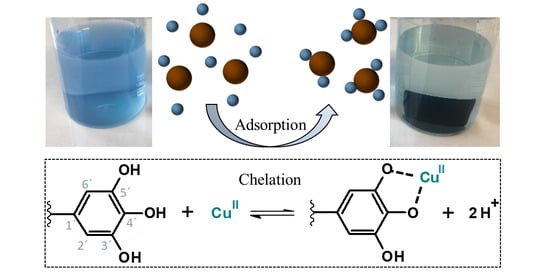
2.2. Adsorption Studies
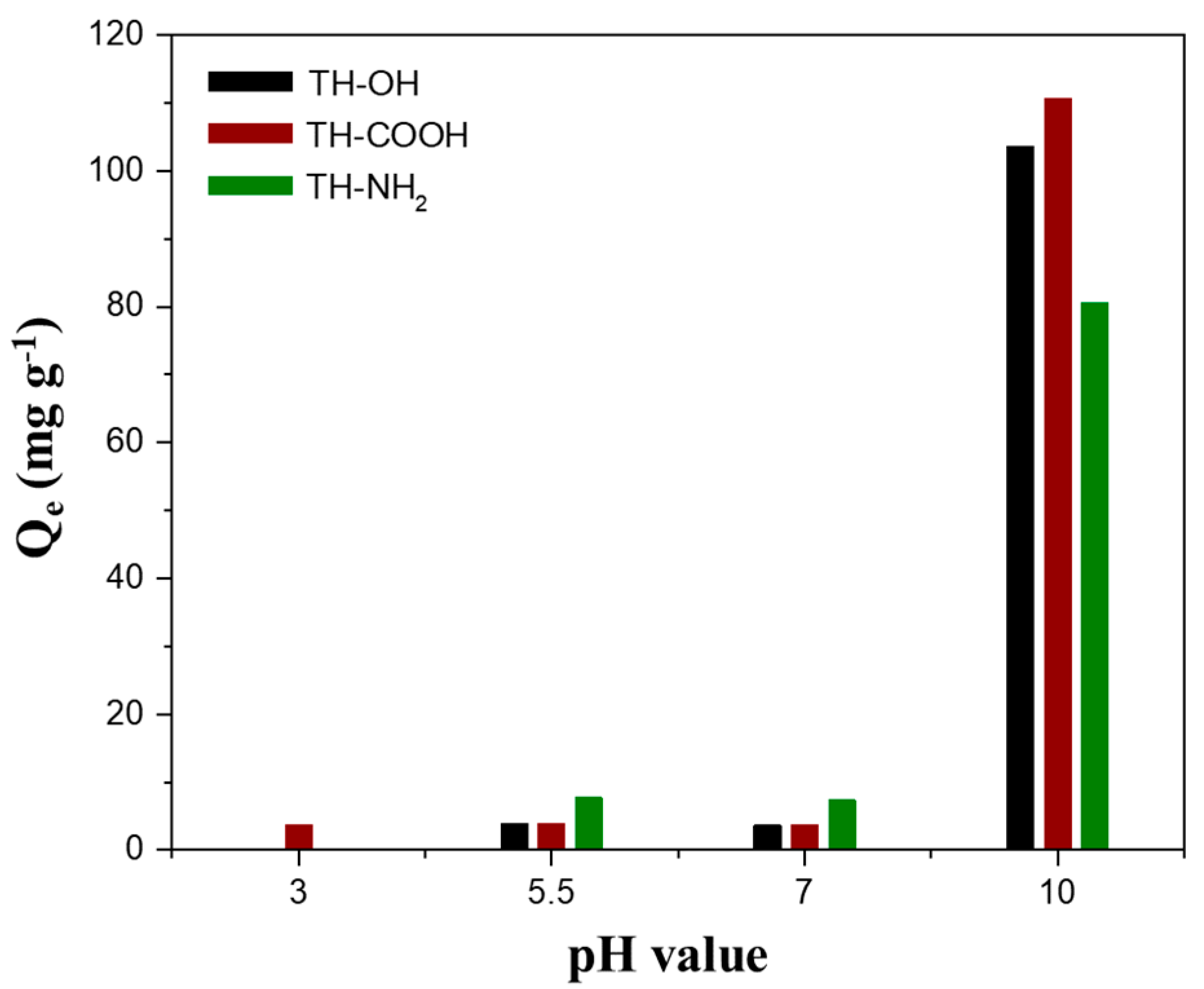
2.2.1. Effect of Initial pH
2.2.2. Effect of Surface Modification
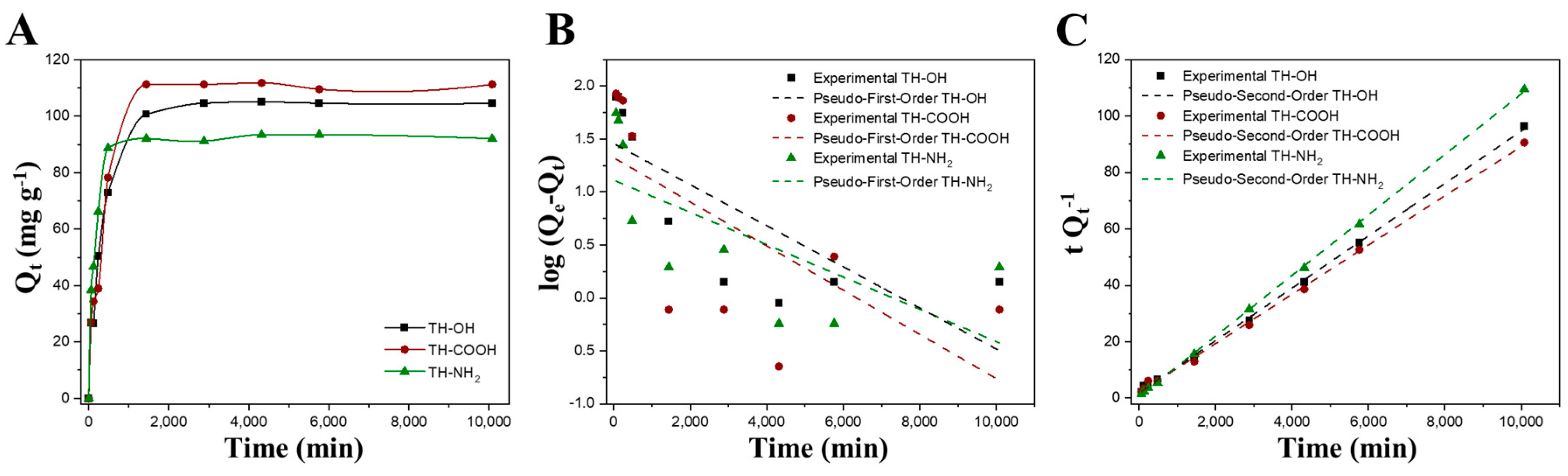
2.2.3. Adsorption Kinetics
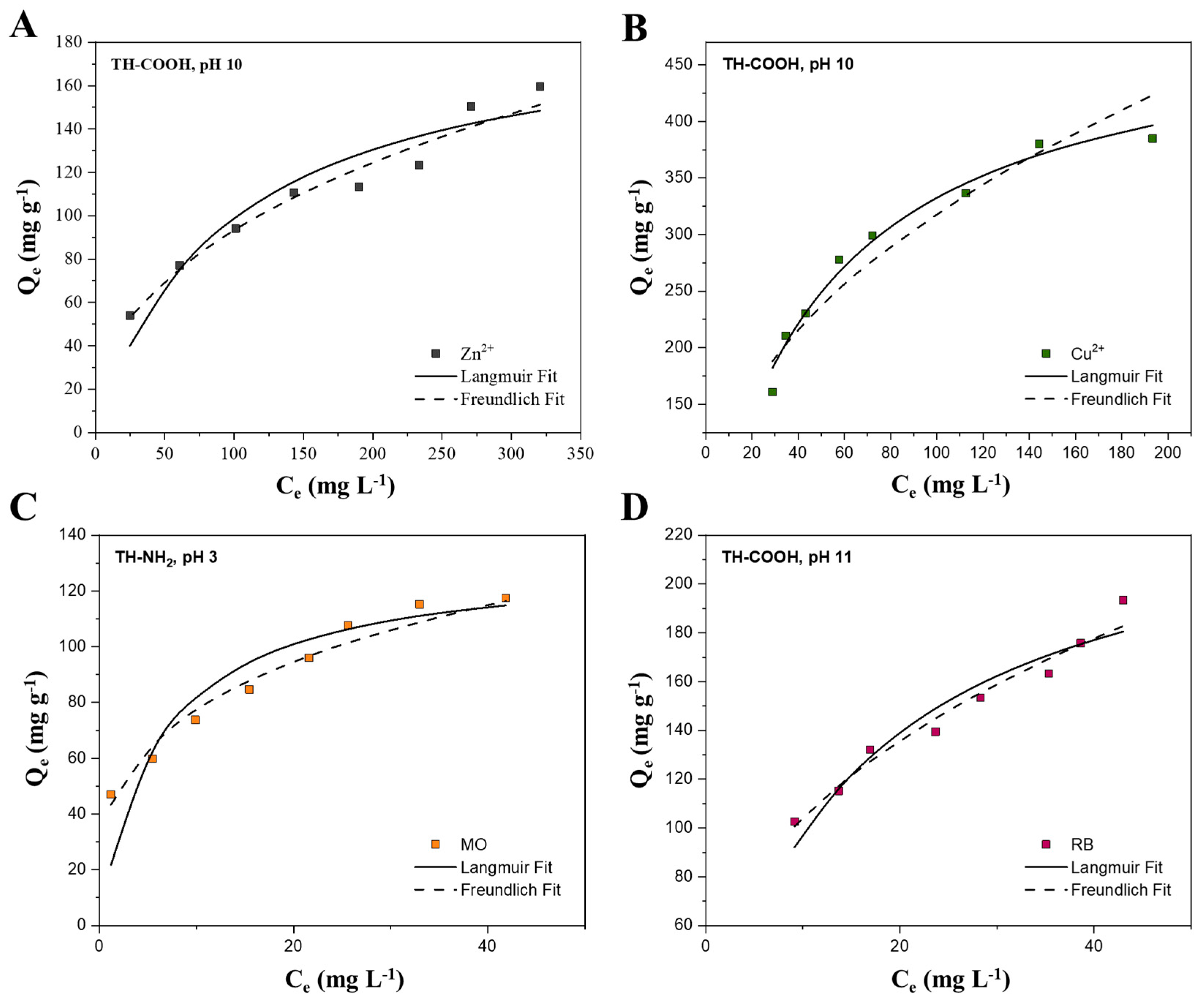
2.2.4. Adsorption Isotherms at Optimal Process Conditions
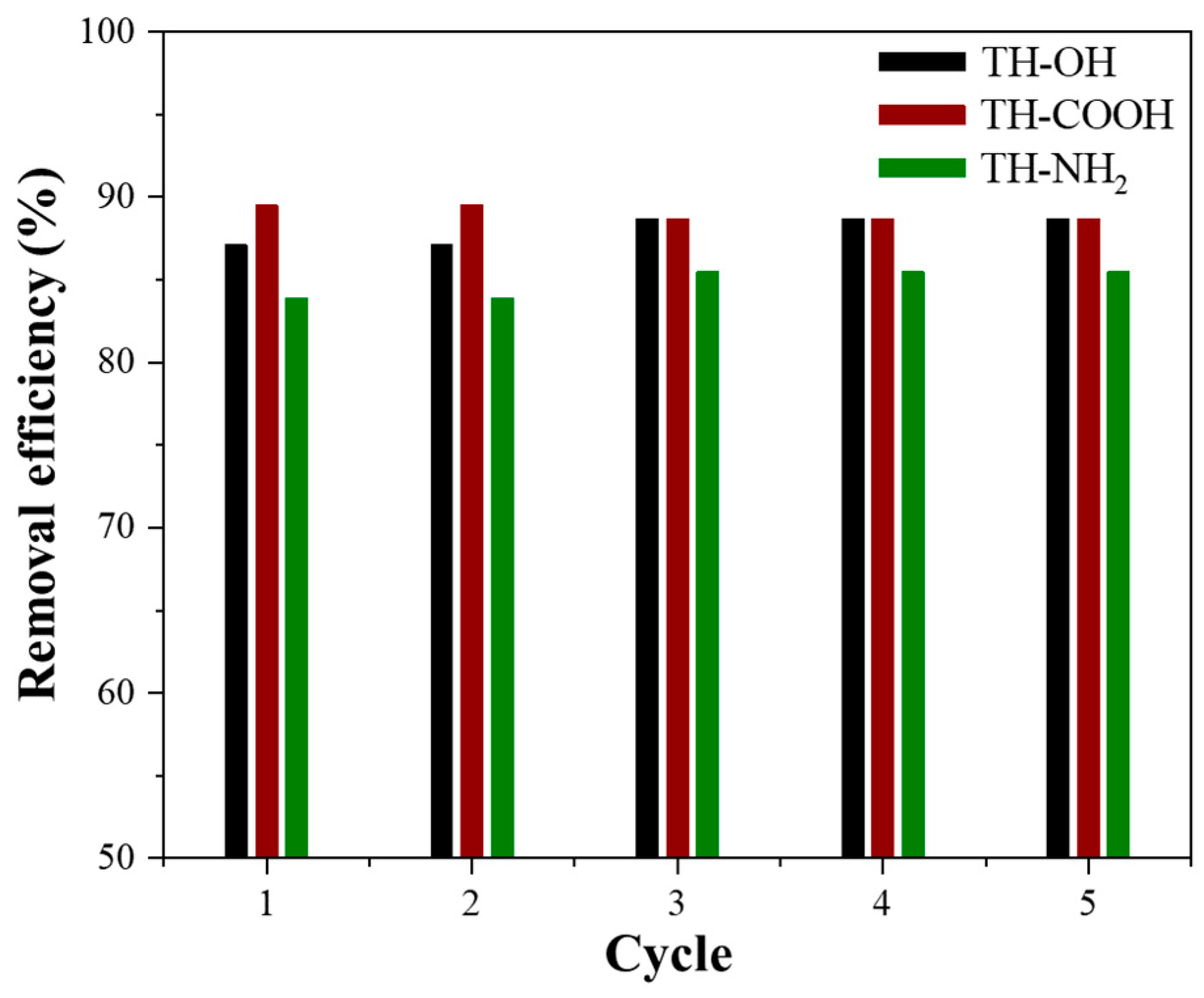
2.2.5. Regeneration Studies
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Monolithic Tannin 5-HMF (TH) Aerogels
4.3. Characterization of the Monolithic Gels
4.4. Adsorption Experiments
4.4.1. Equilibrium Adsorption Experiments
4.4.2. Kinetic Adsorption Experiments
4.4.3. Selective Adsorption Experiments
4.4.4. Reusability Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavitha, V.U.; Kandasubramanian, B. Tannins for wastewater treatment. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Pan, Y.; Rao, C.; Tan, X.; Ling, Y.; Singh, A.; Kumar, A.; Li, B.; Liu, J. Cobalt-seamed C-methylpyrogallol[4]arene nanocapsules-derived magnetic carbon cubes as advanced adsorbent toward drug contaminant removal. Chem. Eng. J. 2022, 433, 133857. [Google Scholar] [CrossRef]
- Visvanathan, C.; Ben Aim, R.; Parameshwaran, K. Membrane separation bioreactors for wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2000, 30, 1–48. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Sun, H.; Xia, N.; Liu, Z.; Kong, F.; Wang, S. Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere 2019, 236, 124370. [Google Scholar] [CrossRef] [PubMed]
- Ehgartner, C.R.; Werner, V.; Selz, S.; Hüsing, N.; Feinle, A. Carboxylic acid-modified polysilsesquioxane aerogels for the selective and reversible complexation of heavy metals and organic molecules. Microporous Mesoporous Mater. 2021, 312, 110759. [Google Scholar] [CrossRef]
- Ji, Y.; Wen, Y.; Wang, Z.; Zhang, S.; Guo, M. Eco-friendly fabrication of a cost-effective cellulose nanofiber-based aerogel for multifunctional applications in Cu(II) and organic pollutants removal. J. Clean. Prod. 2020, 255, 120276. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean. Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Mack, C.; Wilhelmi, B.; Duncan, J.R.; Burgess, J.E. Biosorption of precious metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Bacelo, H.A.M.; Boaventura, R.A.R.; Botelho, C.M.S. Tannin-Adsorbents for Water Decontamination and for the Recovery of Critical Metals: Current State and Future Perspectives. Biotechnol. J. 2019, 14, e1900060. [Google Scholar] [CrossRef] [PubMed]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Jha, V.K.; Matsuda, M.; Miyake, M. Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni2+, Cu2+, Cd2+ and Pb2+. J. Hazard. Mater. 2008, 160, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Mousavi, H.Z.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Perdigoto, M.L.N.; Martins, R.C.; Rocha, N.; Quina, M.J.; Gando-Ferreira, L.; Patrício, R.; Durães, L. Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J. Colloid Interface Sci. 2012, 380, 134–140. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Motalebi Damuchali, A.; McPhedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Liu, S.; Lu, C.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalt. Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine bark as bio-adsorbent for Cd, Cu, Ni, Pb and Zn: Batch-type and stirred flow chamber experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef]
- Biswas, S.; Pal, A. Application of biopolymers as a new age sustainable material for surfactant adsorption: A brief review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100145. [Google Scholar] [CrossRef]
- Dodson, J.R.; Parker, H.L.; García, A.M.; Hicken, A.; Asemave, K.; Farmer, T.J.; He, H.; Clark, J.H.; Hunt, A.J. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Şengil, I.A.; Özacar, M. Competitive biosorption of Pb2+, Cu2+ and Zn2+ ions from aqueous solutions onto valonia tannin resin. J. Hazard. Mater. 2009, 166, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, N. Plant Polyphenols. Basics of Life Sciences; Hemingway, R.F., Lakes, P., Eds.; Springer: Boston, MA, USA, 1992; ISBN 978-1-4613-6540-2. [Google Scholar]
- Oo, C.W.; Kassim, M.J.; Pizzi, A. Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II). Ind. Crops Prod. 2009, 30, 152–161. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermayer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Özacar, M.; Soykan, C.; Şengil, I.A. Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins. J. Appl. Polym. Sci. 2006, 102, 786–797. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI-TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar] [CrossRef]
- Xie, F.; Fan, R.; Yi, Q.; Zhang, Q.; Luo, Z. NaOH Modification of Persimmon Powder-formaldehyde Resin to Enhance Cu2+ and Pb2+ Removal from Aqueous Solution. Procedia Environ. Sci. 2016, 31, 817–826. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Alvares Rodrigues, L.; Koibuchi Sakane, K.; Alves Nunes Simonetti, E.; Patrocínio Thim, G. Cr total removal in aqueous solution by PHENOTAN AP based tannin gel (TFC). J. Environ. Chem. Eng. 2015, 3, 725–733. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr(VI). J. Hazard. Mater. 2018, 352, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liao, X.; Shi, B. Tannin-immobilized mesoporous silica bead (BT-SiO2) as an effective adsorbent of Cr(III) in aqueous solutions. J. Hazard. Mater. 2010, 173, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Ogata, T.; Nakano, Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Water Res. 2005, 39, 4281–4286. [Google Scholar] [CrossRef]
- Issaoui, H.; Sallem, F.; Lafaille, J.; Grassl, B.; Charrier–El Bouhtoury, F. Biosorption of Heavy Metals from Water onto Phenolic Foams Based on Tannins and Lignin Alkaline Liquor. Int. J. Environ. Res. 2021, 15, 369–381. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everet, D.H.; Haul, R.A.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kaufman, T.S. The multiple faces of Eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-Functionalized Covalent Organic Framework for the Removal of Heavy-Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef]
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2009, 63, 319–326. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Bello, A.; Virtanen, V.; Salminen, J.P.; Leiviskä, T. Aminomethylation of spruce tannins and their application as coagulants for water clarification. Sep. Purif. Technol. 2020, 242, 116765. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Alsohaimi, I.H.; Alqadami, A.A.; Khan, M.A.; Kamel, M.M.; Aldosari, O.; Siddiqui, M.R.; Hamedelniel, A.E. Recyclable glutaraldehyde cross-linked polymeric tannin to sequester hexavalent uranium from aqueous solution. J. Mol. Liq. 2019, 281, 29–38. [Google Scholar] [CrossRef]
- Facchi, S.P.; de Oliveira, A.C.; Bezerra, E.O.T.; Vlcek, J.; Hedayati, M.; Reynolds, M.M.; Kipper, M.J.; Martins, A.F. Polycationic condensed tannin/polysaccharide-based polyelectrolyte multilayers prevent microbial adhesion and proliferation. Eur. Polym. J. 2020, 130, 109677. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic Acid Induced Self-Assembly of Three-Dimensional Graphene with Good Adsorption and Antibacterial Properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Yue, R.; Wen, X.; Mao, Y.; Su, Y.; Shen, Q.; Song, H.; Zhang, H.; Ba, X. Eco-friendly fabrication of Au nanoparticles immobilized on tannin-aminopropyltriethoxysilane-coated halloysite nanotubes for thermally tunable catalysis. J. Mater. Sci. 2020, 55, 17094–17107. [Google Scholar] [CrossRef]
- Xu, S.; Cui, M.; Chen, R.; Qiu, Q.; Xie, J.; Fan, Y.; Dai, X.; Dong, B. Design of facile technology for the efficient removal of hydroxypropyl guar gum from fracturing fluid. PLoS ONE 2021, 16, e0247948. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Wang, F.; Bano, Z.; Xia, M. Exploration of adsorption mechanism of 2-phosphonobutane-1,2,4-tricarboxylic acid onto kaolinite and montmorillonite via batch experiment and theoretical studies. J. Hazard. Mater. 2021, 403, 123810. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Ethylenediamintetraessigsäure (EDTA) und ihre Alkalisalze [MAK Value Documentation in German language, 2009]. In MAK-Collection for Occupational Health and Safety; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009.
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439880494. [Google Scholar]
- Bel’Kov, M.V.; Ksendzova, G.A.; Kuzovkov, P.V.; Polozov, G.I.; Skornyakov, I.V.; Sorokin, V.L.; Tolstorozhev, G.B.; Shadyro, O.I. Intramolecular hydrogen bonds and antioxidant activity of aminophenols. J. Appl. Spectrosc. 2007, 74, 635–641. [Google Scholar] [CrossRef]
- Gdaniec, M.; Gilski, M.; Denisov, G.S. γ-Resorcylic acid, its monohydrate and its pyridinium complex. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 1622–1626. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, S.S.; Salahi, E.; Khanlarkhani, A. Kinetics, isotherms and thermodynamic modeling of Mn2+ and Zn2+ single and binary removal using mercapto functionalized silica aerogel. J. Dispers. Sci. Technol. 2019, 40, 657–667. [Google Scholar] [CrossRef]
- McKay, G.; Porter, J.F. Equilibrium parameters for the sorption of copper, cadmium and zinc ions onto peat. J. Chem. Technol. Biotechnol. 1997, 69, 309–320. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, K.L.; Liu, S.Q.; Wang, A.T.; Yan, C. Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 55–61. [Google Scholar] [CrossRef]
- Melo, D.Q.; Neto, V.O.S.; Oliveira, J.T.; Barros, A.L.; Gomes, E.C.C.; Raulino, G.S.C.; Longuinotti, E.; Nascimento, R.F. Adsorption equilibria of Cu2+, Zn2+, and Cd2+ on EDTA-functionalized silica spheres. J. Chem. Eng. Data 2013, 58, 798–806. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1919, 40, 1361–1403. [Google Scholar] [CrossRef]
- Walker, E.A.; Morton, P. The application of the Freundlich isotherm to the adsorption of sugars from solution by a column of charcoal. Analyst 1964, 89, 512–519. [Google Scholar] [CrossRef]
- Tahari, N.; de Hoyos-Martinez, P.L.; Izaguirre, N.; Houwaida, N.; Abderrabba, M.; Ayadi, S.; Labidi, J. Preparation of chitosan/tannin and montmorillonite films as adsorbents for Methyl Orange dye removal. Int. J. Biol. Macromol. 2022, 210, 94–106. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Wang, Y.; Gou, X.; Duan, Y. Adsorption and removal of rhodamine B from aqueous solution by tannic acid functionalized graphene. Colloids Surf. A Physicochem. Eng. Asp. 2015, 477, 35–41. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yenisoy-Karakaş, S.; Aygün, A.; Güneş, M.; Tahtasakal, E. Physical and chemical characteristics of polymer-based spherical activated carbon and its ability to adsorb organics. Carbon 2004, 42, 477–484. [Google Scholar] [CrossRef]
- Hema, M.; Arivoli, S. Rhodamine B adsorption by activated carbon: Kinetic and equilibrium studies. Indian J. Chem. Technol. 2009, 16, 38–45. [Google Scholar]
- Tao, X.; Wu, Y.; Cha, L. Shaddock peels-based activated carbon as cost-saving adsorbents for efficient removal of Cr (VI) and methyl orange. Environ. Sci. Pollut. Res. 2019, 26, 19828–19842. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Selmer, I.; Behnecke, A.S.; Quiño, J.; Braeuer, A.S.; Gurikov, P.; Smirnova, I. Model development for sc-drying kinetics of aerogels: Part 1. Monoliths and single particles. J. Supercrit. Fluids 2018, 140, 415–430. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
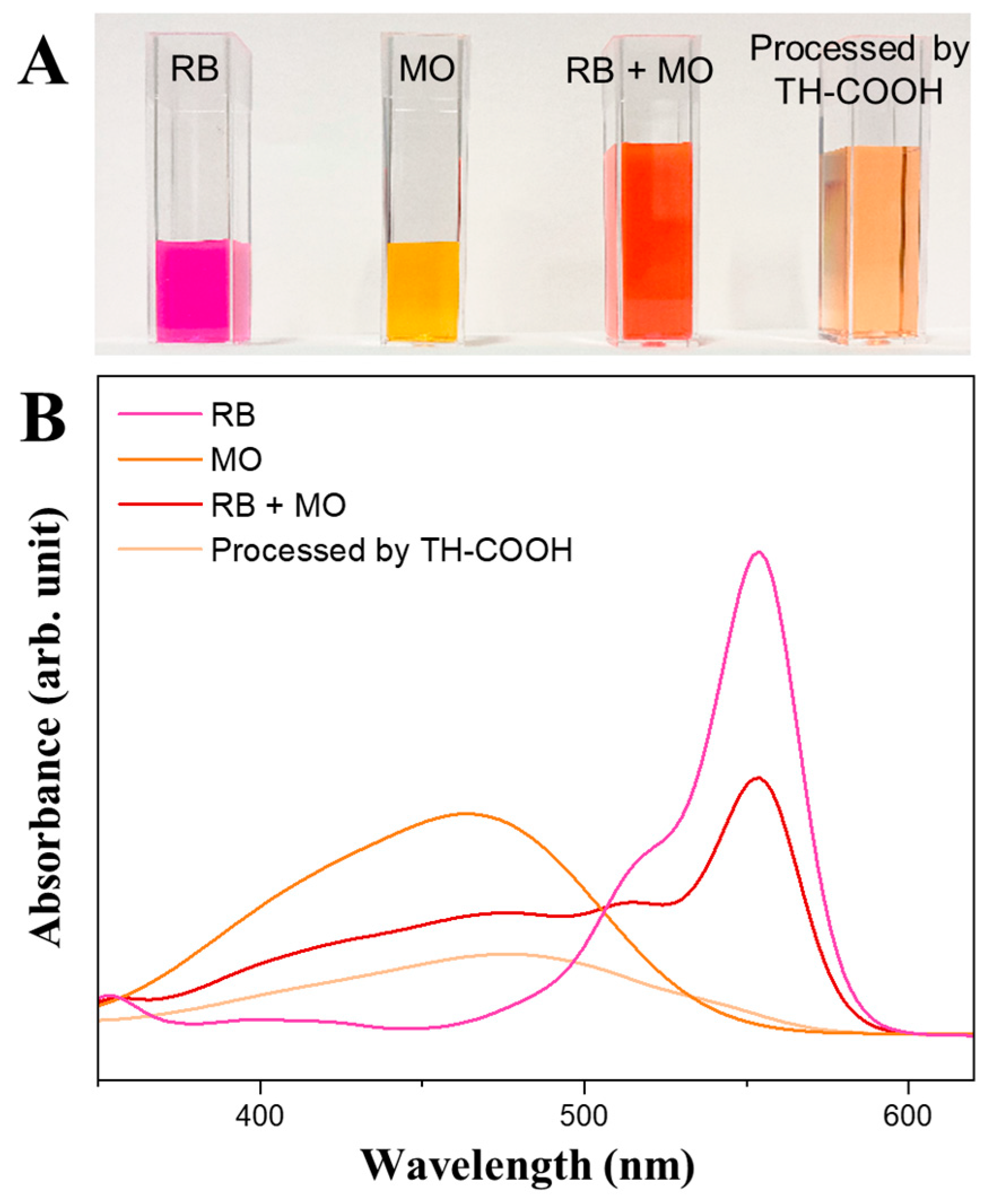

| Sample | Shrinkage (%) | Bulk Density (g cm−3) | Skeletal Density (g cm−3) | Porosity ϴ (%) | SBET (m2 g−1) | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| TH-OH | 23.5 ± 0.5 | 0.20 ± 0.01 | 1.48 ± 0.01 | 86.6 ± 0.6 | 441 ± 12 | 11.3 ± 1.6 |
| TH-COOH | 20.2 ± 1.2 | 0.19 ± 0.01 | 1.47 ± 0.02 | 87.3 ± 0.6 | 509 ± 6 | 12.7 ± 0.6 |
| TH-NH2 | 17.1 ± 1.5 | 0.17 ± 0.02 | 1.41 ± 0.01 | 88.0 ± 1.0 | 497 ± 4 | 11.8 ± 0.1 |
| Sample | Element | Binding Energy (eV) | Assignment |
|---|---|---|---|
| TH-OH | C 1s | 284.4 | C=C |
| - | - | 285.9 | C–OH, C–C, C–H |
| - | - | 287.8 | C=O, C–O |
| - | - | - | - |
| TH-COOH | C 1s | 284.4 | C=C, C–C, C–H |
| - | - | 286.0 | C-OH |
| - | - | 288.3 | O–C=O, –COOH |
| - | - | 291.9 | π-π* satellite |
| - | - | - | - |
| TH-NH2 | C 1s | 283.7 | C=C |
| - | - | 284.7 | C–C, C–H |
| - | - | 285.5 | C–OH |
| Linear Pseudo-First-Order | Linear Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| Adsorbate | K1 (min−1) | Qe (mg g−1) | R2 | K2 (g mg−1 min−1) | Qe (mg g−1) | R2 | Conditions |
| Zn2+ | −2.0 × 10−4 | 21.1 | 0.675 | 4.0 × 10−5 | 114.9 | 0.999 | TH-COOH, pH 10 |
| Cu2+ | −3.0 × 10−4 | 155.1 | 0.968 | 1.7 × 10−5 | 322.6 | 0.999 | TH-COOH, pH 10 |
| MO | −2.0 × 10−4 | 69.1 | 0.997 | 1.7 × 10−5 | 94.3 | 0.995 | TH-NH2, pH 3 |
| RB | −2.0 × 10−4 | 99.6 | 0.982 | 1.1 × 10−6 | 147.1 | 0.885 | TH-COOH, pH 11 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| Adsorbate | KL (L mg−1) | Qm (mg g−1) | R2 | KF (mg1−n Ln g−1) | 1/n | R2 | Conditions |
| Zn2+ | 0.011 | 192.3 | 0.957 | 459.7 | 0.408 | 0.982 | TH-COOH, pH 10 |
| Cu2+ | 0.019 | 500.0 | 0.990 | 6308.1 | 0.427 | 0.965 | TH-COOH, pH 10 |
| MO | 0.164 | 131.6 | 0.943 | 5206.0 | 0.279 | 0.985 | TH-NH2, pH 3 |
| RB | 0.066 | 243.9 | 0.966 | 5735.9 | 0.385 | 0.983 | TH-COOH, pH 11 |
| Adsorbent | Zn2+ Qm (mg g−1) | Cu2+ Qm (mg g−1) | MO Qm (mg g−1) | RB Qm (mg g−1) | Conditions | SSA (m² g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| TH aerogel | 192.3 * | 500.0 * | 131.6 + | 243.9 * | * TH-COOH, pH 10 + TH-NH2, pH 3 | 441–509 | This work |
| Pine tannin gel | 65.0 | - | - | - | pH 7 | 2–6 | [30] |
| Valonia tannin resin | 35.5 | 45.4 | - | - | pH 5 | 11–12 | [22] |
| Phenolic foams based on tannins | 29.1 | 46.5 | - | - | pH 5–7 | - | [36] |
| Chitosan/tannin/ montmorillonite film | - | - | 57.4 | - | pH 7 | - | [63] |
| Chitosan–tannin paper | - | 684.9 | - | - | pH 9 | - | [6] |
| Tannic acid functionalized graphene | - | - | - | 201.0 | pH 11 | - | [64] |
| Activated carbon | 3.9 | 6.8 | 94.6 | 61.0 | pH 5.5 | 79–1170 | [65,66,67,68] |
| Carbon aerogel | 1.8 | 561.7 | - | / | pH 6 | 544 | [69] |
| Carboxyl-functionalized silica aerogel | 111.0 | 78.0 | - | 154.0 | pH 8 | 145 | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koopmann, A.-K.; Ehgartner, C.R.; Euchler, D.; Claros, M.; Huesing, N. Sustainable Tannin Gels for the Efficient Removal of Metal Ions and Organic Dyes. Gels 2023, 9, 822. https://doi.org/10.3390/gels9100822
Koopmann A-K, Ehgartner CR, Euchler D, Claros M, Huesing N. Sustainable Tannin Gels for the Efficient Removal of Metal Ions and Organic Dyes. Gels. 2023; 9(10):822. https://doi.org/10.3390/gels9100822
Chicago/Turabian StyleKoopmann, Ann-Kathrin, Caroline Ramona Ehgartner, Daniel Euchler, Martha Claros, and Nicola Huesing. 2023. "Sustainable Tannin Gels for the Efficient Removal of Metal Ions and Organic Dyes" Gels 9, no. 10: 822. https://doi.org/10.3390/gels9100822
APA StyleKoopmann, A.-K., Ehgartner, C. R., Euchler, D., Claros, M., & Huesing, N. (2023). Sustainable Tannin Gels for the Efficient Removal of Metal Ions and Organic Dyes. Gels, 9(10), 822. https://doi.org/10.3390/gels9100822