The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides
Abstract
1. Introduction
2. Results and Discussion
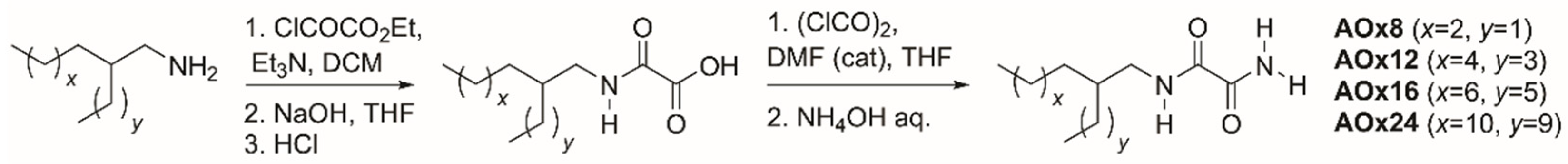
2.1. Synthesis
2.2. Gelation Behavior
2.3. Hansen Solubility Parameters
2.4. 1H Nuclear Magnetic Resonance (NMR) Studies
Effect of Solvent
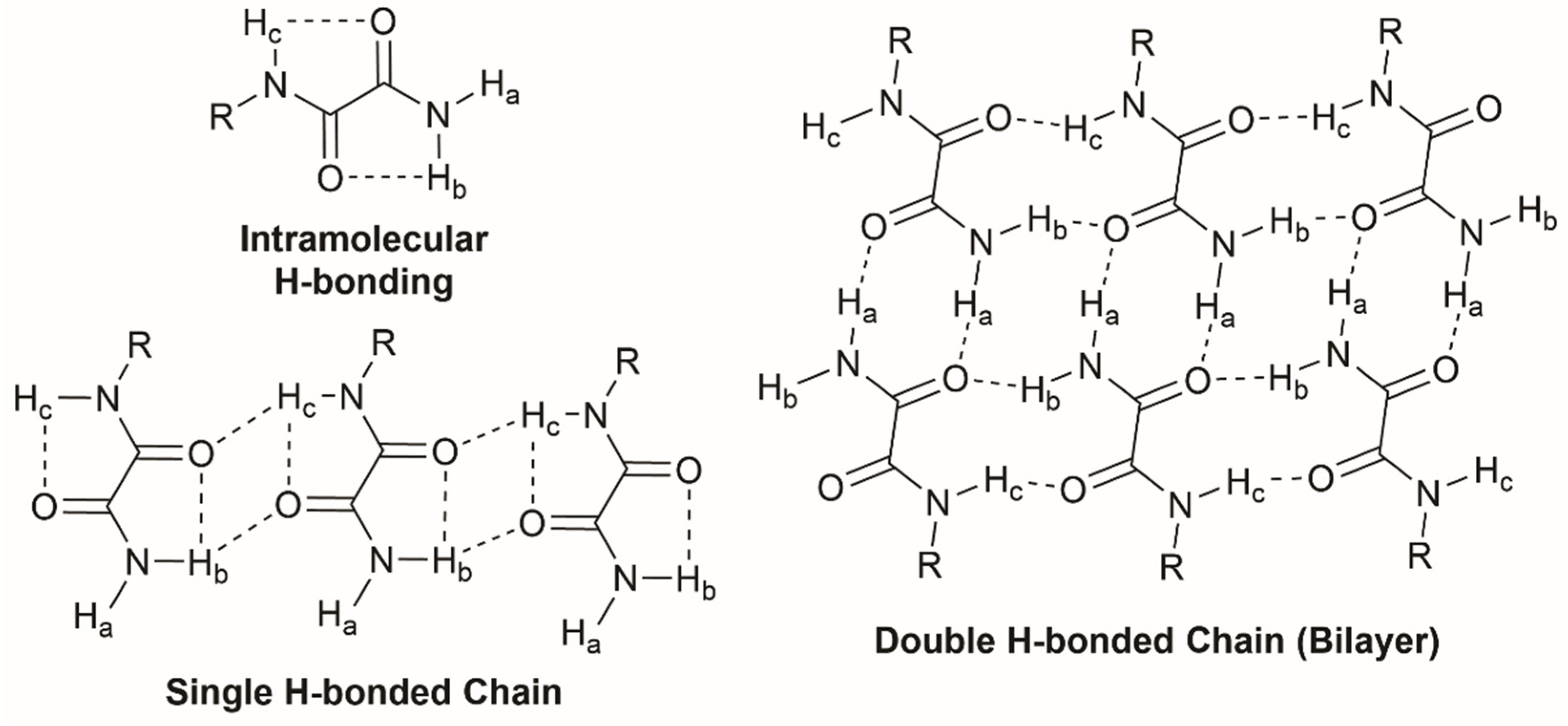
2.5. FT-IR Spectroscopy
2.6. Thermal Stability
2.7. Rheology Studies
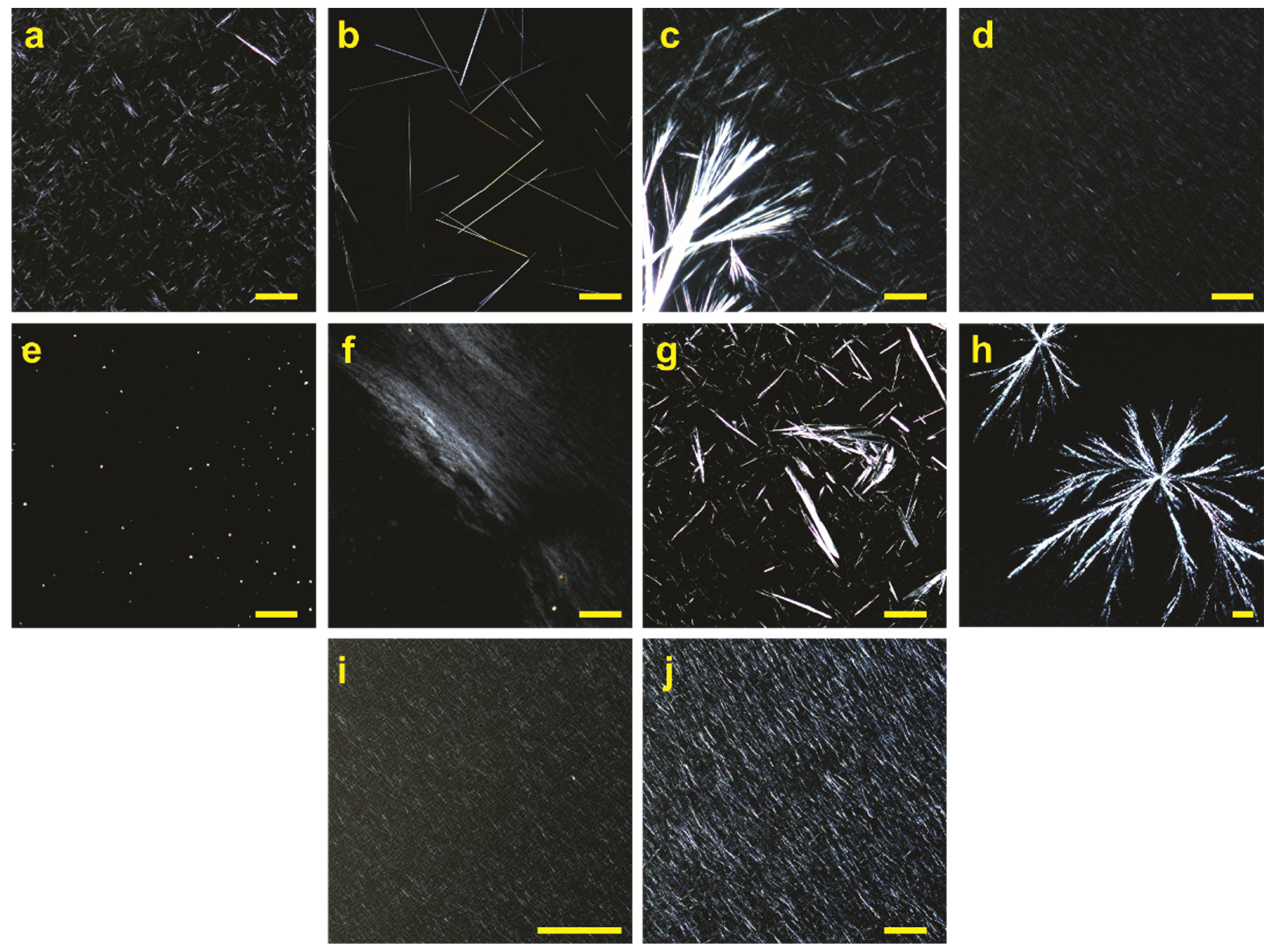
2.8. Polarized Optical Microscopy (POM)
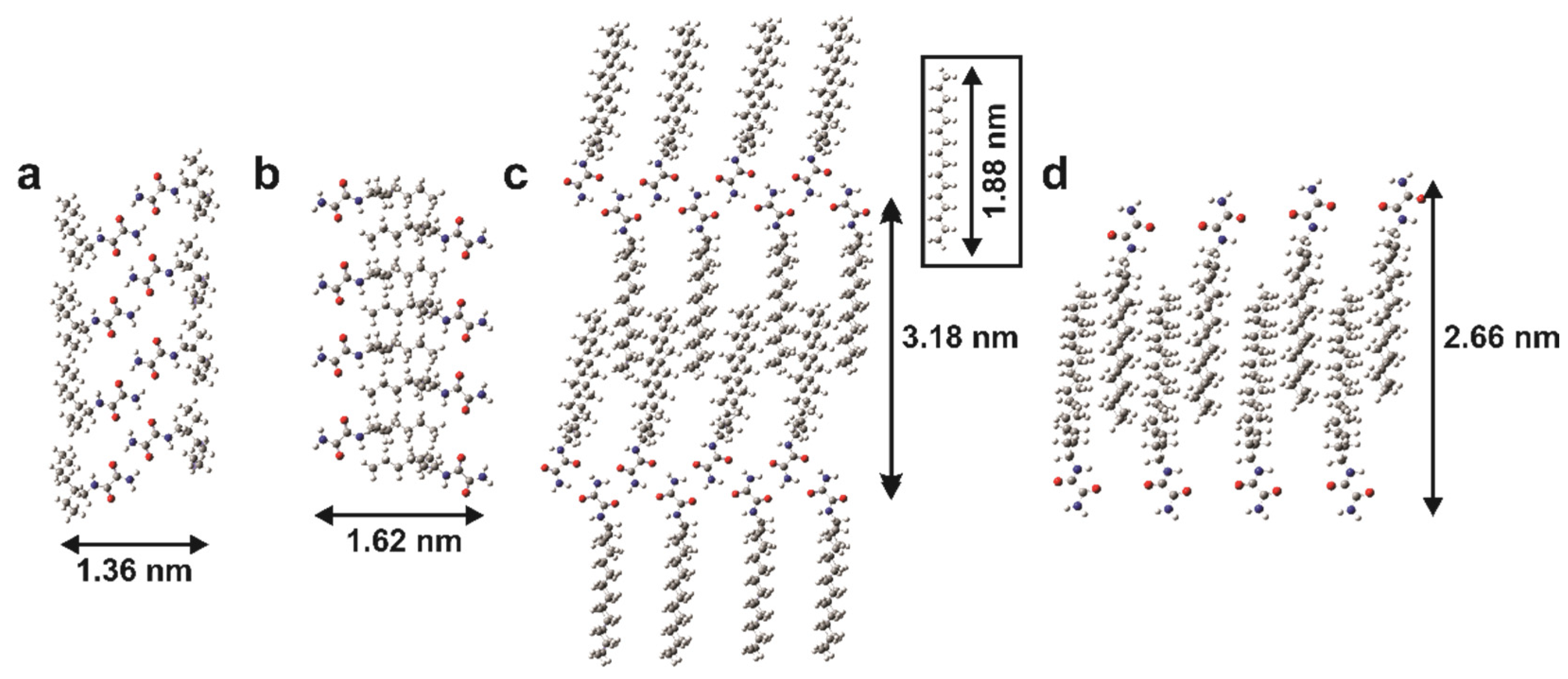
2.9. X-ray Diffraction Study
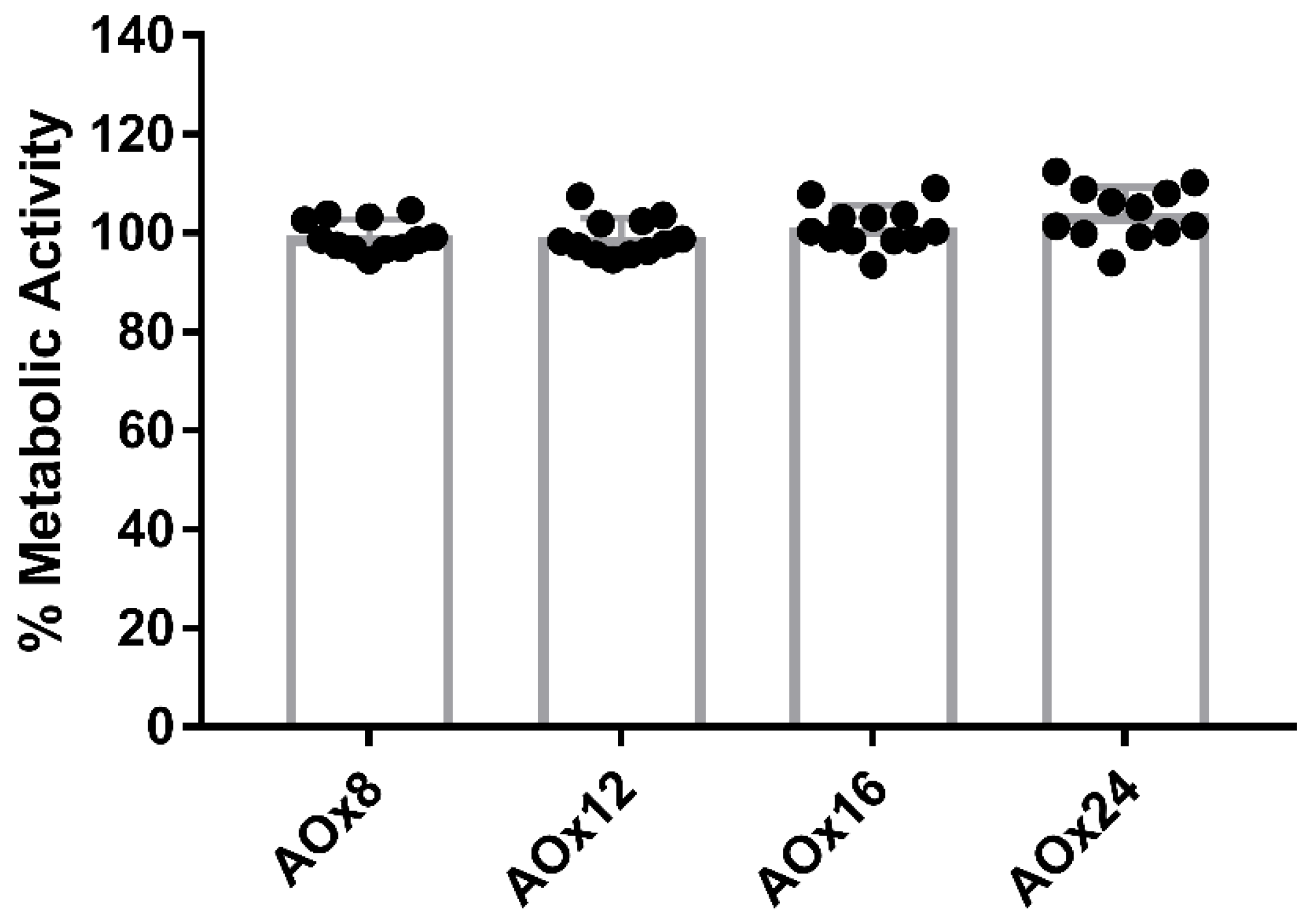
2.10. Cell Metabolism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.3. Mass Spectrometry (MS)
4.4. Gelation Tests
4.5. Hansen Solubility Parameter Analysis
4.6. Fourier Transform Infra-Red (FT-IR) Spectroscopy
4.7. Inverted Vial Gel Melting Experiments
4.8. Rheology Measurements
4.9. Polarized Optical Microscopy (POM)
4.10. X-ray Diffraction (XRD)
4.11. Computer Modelling
4.12. Cell Culture, Flow Cytometric Analysis and Cytotoxicity Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ouyang, G.; Niua, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Weiss, R.; Terech, P. Molecular Gels, 1st ed.; Weiss, R., Terech, P., Eds.; Springer: Dordrect, The Netherlands, 2006; Volume 256. [Google Scholar]
- Okesola, B.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Sureshan, K.M. How far are we in combating marine oil spills by using phase-selective organogelators? ChemSusChem 2020, 13, 5343–5360. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control Release 2018, 271, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Panja, S. Dosimetric gelator probes and their application as sensors. J. Ind. Chem. Soc. 2022, 99, 100359–100378. [Google Scholar] [CrossRef]
- Panja, S.; Panjab, A.; Ghosh, K. Supramolecular gels in cyanide sensing: A review. Mater. Chem. Front. 2021, 5, 584–602. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–468. [Google Scholar] [CrossRef]
- Saydé, T.; El Hamoui, O.; Alies, B.; Gaudin, K.; Lespes, G.; Battu, S. Biomaterials for three-dimensional cell culture: From applications in oncology to nanotechnology. Nanomaterials 2021, 11, 481. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.-I.; Shinkai, S. What kind of “soft materials” can we design from molecular gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef]
- Escuder, B.; Miravet, J. Functional Molecular Gels, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Dastidar, P. Designing supramolecular gelators: Challenges, frustrations, and hopes. Gels 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Kumari, H. Low molecular weight supramolecular gels under shear: Rheology as the tool for elucidating structure–function correlation. Chem. Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef] [PubMed]
- De Loos, M.; Feringa, B.L.; van Esch, J.H. Design and application of self-assembled low molecular weight hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- Chu, C.-W.; Schalley, C.A. Recent advances on supramolecular gels: From stimuli-responsive gels to co-assembled and self-sorted systems. Org. Mater. 2021, 3, 25–40. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, applicability, and advanceability. Adv. Mater. 2019, 31, 1806204–1806227. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef]
- Van Esch, J.H. We can design molecular gelators, but do we understand them? Langmuir 2009, 25, 8392–8394. [Google Scholar] [CrossRef]
- Yang, H.-K.; Zhang, C.; He, X.-N.; Wang, P.-Y. Effects of alkyl chain lengths on 12-hydroxystearic acid derivatives based supramolecular organogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126319–126328. [Google Scholar] [CrossRef]
- Bietsch, J.; Olson, M.; Wang, G. Fine-tuning of molecular structures to generate carbohydrate based super gelators and their applications for drug delivery and dye absorption. Gels 2021, 7, 134. [Google Scholar] [CrossRef]
- Golodnizky, D.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of the polar head group and aliphatic tail in the self-assembly of low molecular weight molecules in oil. Food Struct. 2021, 30, 100240–100251. [Google Scholar] [CrossRef]
- Meyer, A.R.; Bender, C.R.; Dos Santos, D.M.; Ziembowicz, F.I.; Frizzo, C.P.; Villetti, M.A.; Reichert, J.M.; Zanatta, N.; Bonacorso, H.G.; Martins, M.A.P. Effect of slight structural changes on the gelation properties of N-phenylstearamide supramolecular gels. Soft Matter 2018, 14, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, K.; Liu, J.; Zhang, Q.; Feng, X.; Fang, Y. New dicholesteryl-based gelators: Chirality and spacer length effect. Langmuir 2008, 24, 2992–3000. [Google Scholar] [CrossRef]
- Kuosmanen, R.T.; Truong, K.N.; Rissanen, K.T.; Sievänen, E.I. The effect of the side chain on gelation properties of bile acid alkyl amides. ChemistryOpen 2021, 10, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Gutiérrez, J.R.; Mojica-Sánchez, J.P.; Macias-López, E.G.; Martínez-Martínez, F.J.; Magaña-Vergara, N.E.; Mendoza-Muñoz, N. Structure–property relationship of novel supramolecular gels based on coumarins. N. J. Chem. 2021, 45, 13369–13379. [Google Scholar] [CrossRef]
- Komiyama, T.; Harada, Y.; Hase, T.; Mori, S.; Kimura, S.; Yokoya, M.; Yamanaka, M. Effect of alkyl chain length of N-alkyl-N′-(2-benzylphenyl)ureas on gelation. Chem. Asian J. 2021, 16, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Sundararajan, P.R. Low molecular weight organogels based on long-chain carbamates. Langmuir 2005, 21, 3802–3807. [Google Scholar] [CrossRef]
- Chen, S.; An, Z.; Tong, X.; Chen, Y.; Ma, M.; Shi, Y.; Wang, X. Stronger intermolecular forces or closer molecular spacing? Key impact factor research of gelator self-assembly mechanism. Langmuir 2017, 33, 14389–14395. [Google Scholar] [CrossRef]
- Yang, H.-K.; Zhao, H.; Yang, P.-R.; Huang, C.-H. How do molecular structures affect gelation properties of supramolecular gels? Insights from low-molecular-weight gelators with different aromatic cores and alkyl chain lengths. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 242–250. [Google Scholar] [CrossRef]
- Chen, S.; He, H.; Tang, G.; Wu, B.; Ma, M.; Shi, Y.; Wang, X. Topological structure influences on the gel formation process and mechanical properties of l-lysine based supramolecular gels. RSC Adv. 2015, 5, 101437–101443. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Tan, M.; Wang, L.; Kong, D.; Yang, Z. A structure–gelation ability study in a short peptide-based ‘super hydrogelator’ system. Soft Matter 2011, 7, 3897–3905. [Google Scholar] [CrossRef]
- Martínez-Martínez, F.J.; Padilla-Martínez, I.I.; Brito, M.A.; Geniz, E.D.; Rojas, R.C.; Saavedra, J.B.R.; Höpfl, H.; Tlahuextl, M.; Contreras, R. Three-center intramolecular hydrogen bonding in oxamide derivatives. NMR and X-ray diffraction study. J. Chem. Soc. Perkin Trans. 2 1998, 2, 401–406. [Google Scholar] [CrossRef]
- Li, X.-W.; Tao, L.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Bimetallic complexes constructed from asymmetrical N,N′-bis(substituted)-oxamide: Cytotoxicities, and reactivities towards DNA and protein. Eur. J. Med. Chem. 2012, 54, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-T.; Li, X.-W.; Wu, Z.-Y. (3-{[N-(5-Chloro-2-hydroxyphenyl)oxamoyl]amino}propyl)dimethylazanium perchlorate. Acta Crystallogr. E 2011, 68, o8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-J.; Zheng, K.; Wu, Z.-Y.; Li, Y. N-[3-(Dimethylamino)propyl]-N′-(2-hydroxy-5-methylphenyl)oxamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, 895. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.-C.; Jian, X.-E.; Chen, P.; Huang, C.; Yin, J.; Huang, J.C.; Li, J.-S.; Zhao, P.-L. Design, synthesis and biological evaluation of novel indole-based oxalamide and aminoacetamide derivatives as tubulin polymerization inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126816–126822. [Google Scholar] [CrossRef] [PubMed]
- Sunduru, N.; Sharma, M.; Srivastava, K.; Rajakumar, S.; Puri, S.K.; Saxena, J.K.; Chauhan, P.M.S. Synthesis of oxalamide and triazine derivatives as a novel class of hybrid 4-aminoquinoline with potent antiplasmodial activity. Bioorg. Med. Chem. 2009, 17, 6451–6462. [Google Scholar] [CrossRef]
- Curreli, F.; Choudhury, S.; Pyatkin, I.; Zagorodnikov, V.P.; Bulay, A.K.; Altieri, A.; Kwon, Y.D.; Kwong, P.D.; Debnath, A.K. Design, synthesis, and antiviral activity of entry inhibitors that target the CD4-binding site of HIV-1. J. Med. Chem. 2012, 55, 4764–4775. [Google Scholar] [CrossRef]
- Yerdelen, K.O.; Koca, M.; Kasap, Z.; Anil, B. Preparation, anticholinesterase activity, and docking study of new 2-butenediamide and oxalamide derivatives. J. Enzym. Inhib. Med. Chem. 2014, 30, 671–678. [Google Scholar] [CrossRef]
- Malik, N.P.; Naz, M.; Ashiq, U.; Jamal, R.A.; Gul, S.; Saleem, F.; Khan, K.M.; Yousuf, S. Oxamide derivatives as potent α-glucosidase inhibitors: Design, synthesis, in vitro inhibitory screening and in silico docking studies. ChemistrySelect 2021, 6, 7188–7201. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Julfakyan, K.; Lu, J.; Emwas, A.-H.; Anjum, D.H.; Omar, H.; Tamanoi, F.; Zink, J.I.; Khashab, N.M. Biodegradable oxamide-phenylene-based mesoporous organosilica nanoparticles with unprecedented drug payloads for delivery in cells. Chem. Eur. J. 2016, 22, 14806–14811. [Google Scholar] [CrossRef] [PubMed]
- Fatieiev, Y.; Croissant, J.G.; Julfakyan, K.; Deng, L.; Anjum, D.H.; Gurinov, A.; Khashab, N.M. Enzymatically degradable hybrid organic–inorganic bridged silsesquioxane nanoparticles for in vitro imaging. Nanoscale 2015, 7, 15046–15050. [Google Scholar] [CrossRef] [PubMed]
- Uzan, S.; Barış, D.; Çolak, M.; Aydın, H.; Hoşgören, H. Organogels as novel carriers for dermal and topical drug delivery vehicles. Tetrahedron 2016, 72, 7517–7525. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic diamides and tert-butoxide: Two types of ligands enabling practical access to alkyl aryl ethers via cu-catalyzed coupling reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef]
- Morarji, D.V.; Gurjar, K.K. Theoretical and experimental studies: Cu(I)/Cu(II) catalytic cycle in CuI/oxalamide-promoted C–N bond formation. Organometallics 2019, 38, 2502–2511. [Google Scholar] [CrossRef]
- Chan, V.S.; Krabbe, S.W.; Li, C.; Sun, L.; Liu, Y.; Nett, A.J. Identification of an oxalamide ligand for copper-catalyzed C−O couplings from a pharmaceutical compound library. ChemCatChem 2019, 11, 5748–5753. [Google Scholar] [CrossRef]
- Kaous, A.; Winkel, C. Oxalamide Derivative as Umami Flavoring Agent. U.S. Patent 008470384B2, 5 June 2013. [Google Scholar]
- Çolak, M.; Bariş, D.; Pirinççioǧlu, N.; Hoşgören, H. Novel bis(aminoalcohol)oxalamide organogelators and their diglycolylamide analogs: Evaluation of gelation efficiency in various organic fluids. Turk. J. Chem. 2017, 41, 658–671. [Google Scholar] [CrossRef]
- Džolić, Z.; Cametti, M.; Dalla Cort, A.; Mandolini, L.; Žinić, M. Fluoride-responsive organogelator based on oxalamide-derived anthraquinone. Chem. Commun. 2007, 3535–3537. [Google Scholar] [CrossRef]
- Makarević, J.; Jokić, M.; Raza, Z.; Caplar, V.; Katalenić, D.; Štefanić, Z.; Kojić-Prodić, B.; Žinić, M. Chiral bis(tyrosinol) and bis(p-hydroxyphenylglycinol) oxalamide gelators. Influence of aromatic groups and hydrogen bonding on gelation properties. Croat. Chem. Acta 2004, 77, 403–414. [Google Scholar]
- Makarević, J.; Štefanić, Z.; Horvat, L.; Žinić, M. Intermolecular central to axial chirality transfer in the self-assembled biphenyl containing amino acid-oxalamide gelators. Chem. Commun. 2012, 48, 7407–7409. [Google Scholar] [CrossRef]
- Portada, T.; Molčanov, K.; Šijakovic Vujičic, N.; Žinic, M. Biphenyl bis(amino alcohol) oxalamide gelators: Complex gelation involving coupled equilibria, central-to-axial chirality transfer, diastereoisomer interconversion, and self-sorting. Eur. J. Org. Chem. 2016, 2016, 1205–1214. [Google Scholar] [CrossRef]
- Valls, A.; Castillo, A.; Porcar, R.; Hietala, S.; Altava, B.; Garcĺa-Verdugo, E.; Luis, S.V. Urea-based low-molecular-weight pseudopeptidic organogelators for the encapsulation and slow release of (r)-limonene. J. Agric. Food Chem. 2020, 68, 7051–7061. [Google Scholar] [CrossRef]
- Santic, A.; Brinkkotter, M.; Portada, T.; Frkanec, L.; Cremer, C.; Schonhoff, M.; Mogus-Milankovic, A. Supramolecular ionogels prepared with bis(amino alcohol)oxamides as gelators: Ionic transport and mechanical properties. RSC Adv. 2020, 10, 17070–17078. [Google Scholar] [CrossRef] [PubMed]
- Miljanić, S.; Frkanec, L.; Meić, Z.; Žinić, M. Gelation ability of novel oxamide-based derivatives bearing a stilbene as a photo-responsive unit. Eur. J. Org. Chem. 2006, 2006, 1323–1334. [Google Scholar] [CrossRef]
- Luo, X.; Li, C.; Liang, Y. Self-assembled organogels formed by monoalkyl derivatives of oxamide. Chem. Commun. 2000, 2091–2092. [Google Scholar] [CrossRef]
- McFarland, C.; Vicic, D.A.; Debnath, A.K. Rapid microwave-assisted syntheses of derivatives of HIV-1 entry inhibitors. Synthesis 2006, 2006, 807–812. [Google Scholar]
- Makeiff, D.A.; Cho, J.Y.; Smith, B.; Carlini, R.; Godbert, N. Self-assembly of alkylamido isophthalic acids toward the design of a supergelator: Phase-selective gelation and dye adsorption. Gels 2022, 8, 285. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Cho, J.Y.; Godbert, N.; Smith, B.; Azyat, K.; Wagner, A.; Kulka, M.; Carlini, R. Supramolecular gels from alkylated benzimidazolone derivatives. J. Mol. Liq. 2021, 339, 116723–116737. [Google Scholar] [CrossRef]
- Luboradzki, R.; Gronwald, O.; Ikeda, A.; Shinkai, S. Sugar-integrated “supergelators” which can form organogels with 0.03–0.05% [g mL−1]. Chem. Lett. 2000, 29, 1148–1149. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and light control of the sol-gel phase transition in cholesterol-based organic gels. Novel helical aggregation modes as detected by circular dichroism and electron microscopic observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavanc, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Raynal, M.; Bouteiller, L. Organogel formation rationalized by hansen solubility parameters. Chem. Commun. 2011, 47, 8271–8273. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Suissa, G.; Raynal, M.; Bouteiller, L. Organogel formation rationalized by Hansen solubility parameters: Influence of gelator structure. Soft Matter 2015, 11, 2308–2312. [Google Scholar] [CrossRef]
- Martínez-Martínez, F.J.; Ariza-Castolo, A.; Tlahuext, H.; Tlahuextl, M.; Contreras, R. 1H,13C,15N, 2D and variable temperature NMR study of the role of hydrogen bonding in the structure and conformation of oxamide derivatives. J. Chem. Soc. Perkin Trans. 2 1993, 1481–1485. [Google Scholar] [CrossRef]
- Dhanishta, P.; Sai Siva Kumar, P.; Mishra, S.K.; Suryaprakash, N. Intramolecular hydrogen bond directed stable conformations of benzoyl phenyl oxalamides: Unambiguous evidence from extensive NMR studies and DFT-based computations. RSC Adv. 2018, 8, 11230–11240. [Google Scholar] [CrossRef] [PubMed]
- Coe, S.; Kane, J.J.; Nguyen, T.L.; Toledo, L.M.; Wininger, E.; Fowler, F.W.; Lauher, J.W. Molecular symmetry and the design of molecular solids: The oxalamide functionality as a persistent hydrogen bonding unit. J. Am. Chem. Soc. 1997, 119, 86–93. [Google Scholar] [CrossRef]
- Desseyn, H.O.; Perlepes, S.P.; Clou, K.; Blaton, N.; Van der Veken, B.J.; Dommisse, R.; Hansen, P.E. Theoretical, structural, vibrational, nmr, and thermal evidence of the inter- versus intramolecular hydrogen bonding in oxamides and thiooxamides. J. Phys. Chem. A 2004, 108, 5175–5182. [Google Scholar] [CrossRef]
- Wallace, M.; Iggo, J.A.; Adams, D.J. Using solution state NMR spectroscopy to probe NMR invisible gelators. Soft Matter 2015, 11, 7739–7747. [Google Scholar] [CrossRef]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Sijbrandi, N.J.; Kimenai, A.J.; Mes, E.P.C.; Broos, R.; Bar, G.; Rosenthal, M.; Odarchenko, Y.; Ivanov, D.A.; Dijkstra, P.J.; Feijen, J. Synthesis, morphology, and properties of segmented poly(ether amide)s with uniform oxalamide-based hard segments. Macromolecules 2012, 45, 3948–3961. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 8 December 2022).
- Xin, H.; Zhou, X.; Zhao, C.; Wang, H.; Lib, M. Low molecular weight organogel from the cubic mesogens containing dihydrazide group. J. Mol. Liq. 2011, 160, 17–21. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Rossat, C.; Volino, F. On the measurement of phase transition temperatures in physical molecular organogels. J. Colloid Interface Sci. 2000, 227, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Makarević, J.; Jokić, M.; Frkanec, L.; Katalenić, D.; Žinić, M. Gels with exceptional thermal stability formed by bis(amino acid) oxalamide gelators and solvents of low polarity. Chem. Commun. 2002, 2, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Willows, S.; Kulka, M. Harnessing the power of mast cells in unconventional immunotherapy strategies and vaccine adjuvants. Cells 2020, 9, 2713. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2015, 6, 620–632. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef]
- Almpanis, G.C.; Tsigkas, G.G.; Koutsojannis, C.; Mazarakis, A.; Kounis, G.N.; Kounis, N.G. Nickel allergy, Kounis syndrome and intracardiac metal devices. Int. J. Cardiol. 2010, 145, 364–365. [Google Scholar] [CrossRef]
- Moura, C.C.G.; Cristina, C.T.; Oliveira, C.V.; Dechichi, P.; Gabrielli, B.J.C. A study on biocompatibility of three endodontic sealers: Intensity and duration of tissue irritation. Iran Endod. J. 2014, 9, 137–143. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azyat, K.; Makeiff, D.; Smith, B.; Wiebe, M.; Launspach, S.; Wagner, A.; Kulka, M.; Godbert, N. The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides. Gels 2023, 9, 5. https://doi.org/10.3390/gels9010005
Azyat K, Makeiff D, Smith B, Wiebe M, Launspach S, Wagner A, Kulka M, Godbert N. The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides. Gels. 2023; 9(1):5. https://doi.org/10.3390/gels9010005
Chicago/Turabian StyleAzyat, Khalid, Darren Makeiff, Bradley Smith, Mickie Wiebe, Steve Launspach, Ashley Wagner, Marianna Kulka, and Nicolas Godbert. 2023. "The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides" Gels 9, no. 1: 5. https://doi.org/10.3390/gels9010005
APA StyleAzyat, K., Makeiff, D., Smith, B., Wiebe, M., Launspach, S., Wagner, A., Kulka, M., & Godbert, N. (2023). The Effect of Branched Alkyl Chain Length on the Properties of Supramolecular Organogels from Mono-N-Alkylated Primary Oxalamides. Gels, 9(1), 5. https://doi.org/10.3390/gels9010005








