A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy
Abstract
1. Introduction
2. Results and Discussions
2.1. Screening of Excipients
2.2. Formulation Development
2.2.1. Preparation of a Placebo Nanoemulsion and Excipients Optimization
2.2.2. Preparation of a Drug-Loaded FTQ-NE
2.3. BBD: Mathematical Model Fitting and Optimization of FTQ-NE
2.3.1. Effects of Independent Variables on the Particle Size
2.3.2. Effects of Independent Variables on the PDI
2.3.3. Effects of Independent Variables on the %Transmittance
2.4. Thermodynamic Stability
2.5. Characterization of the Optimized FTQ-NE
2.5.1. Dilution Test
2.5.2. Particle Size, Polydispersity Index (PDI), and Zeta Potential
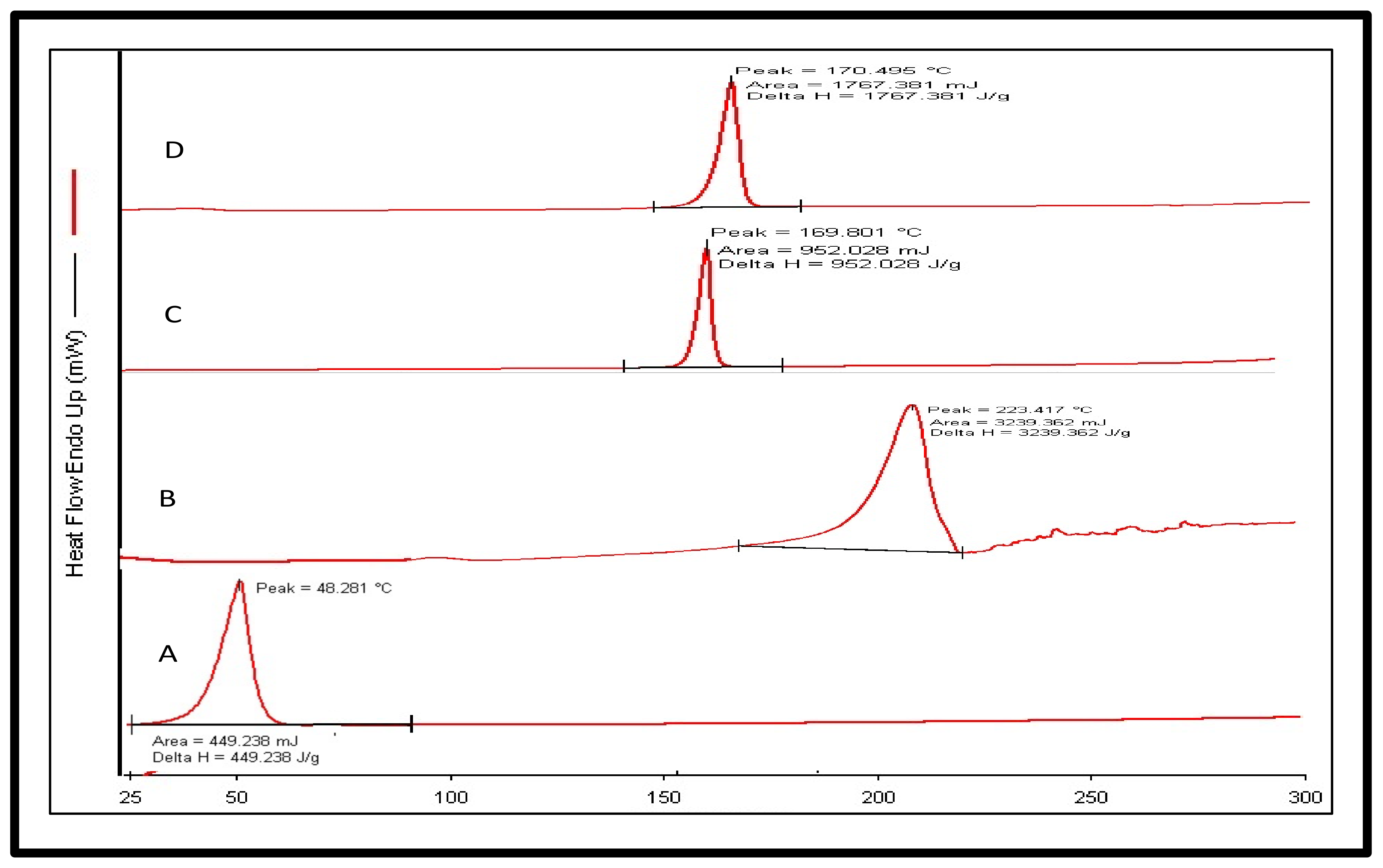
2.5.3. Differential Scanning Calorimetry (DSC)
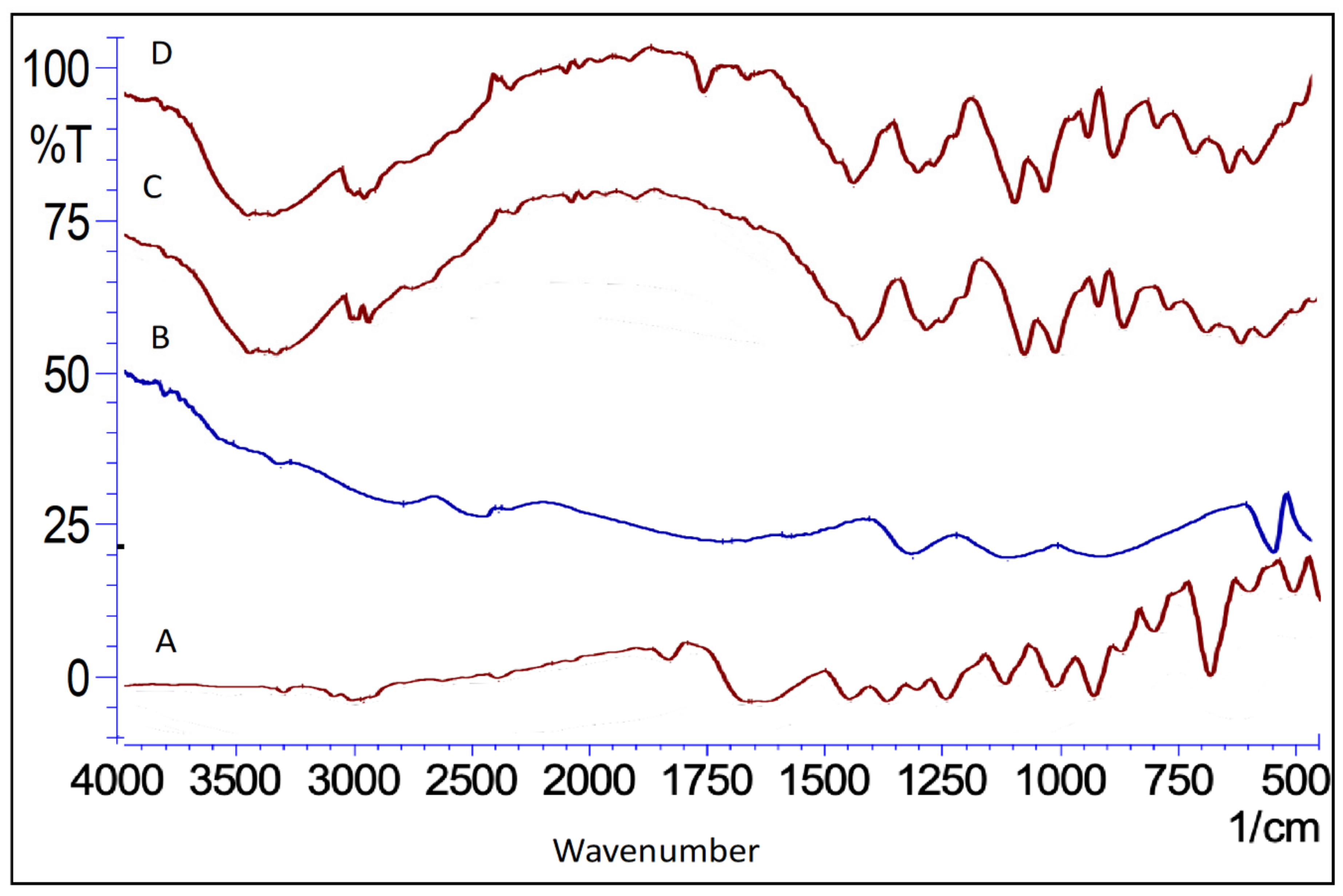
2.5.4. Fourier Transforms Infrared Spectroscopy (FTIR)
2.5.5. X-ray Diffraction
2.6. Surface Morphology
2.7. Characterization of the FTQ-NEG
2.7.1. Spreadability and Extrudability
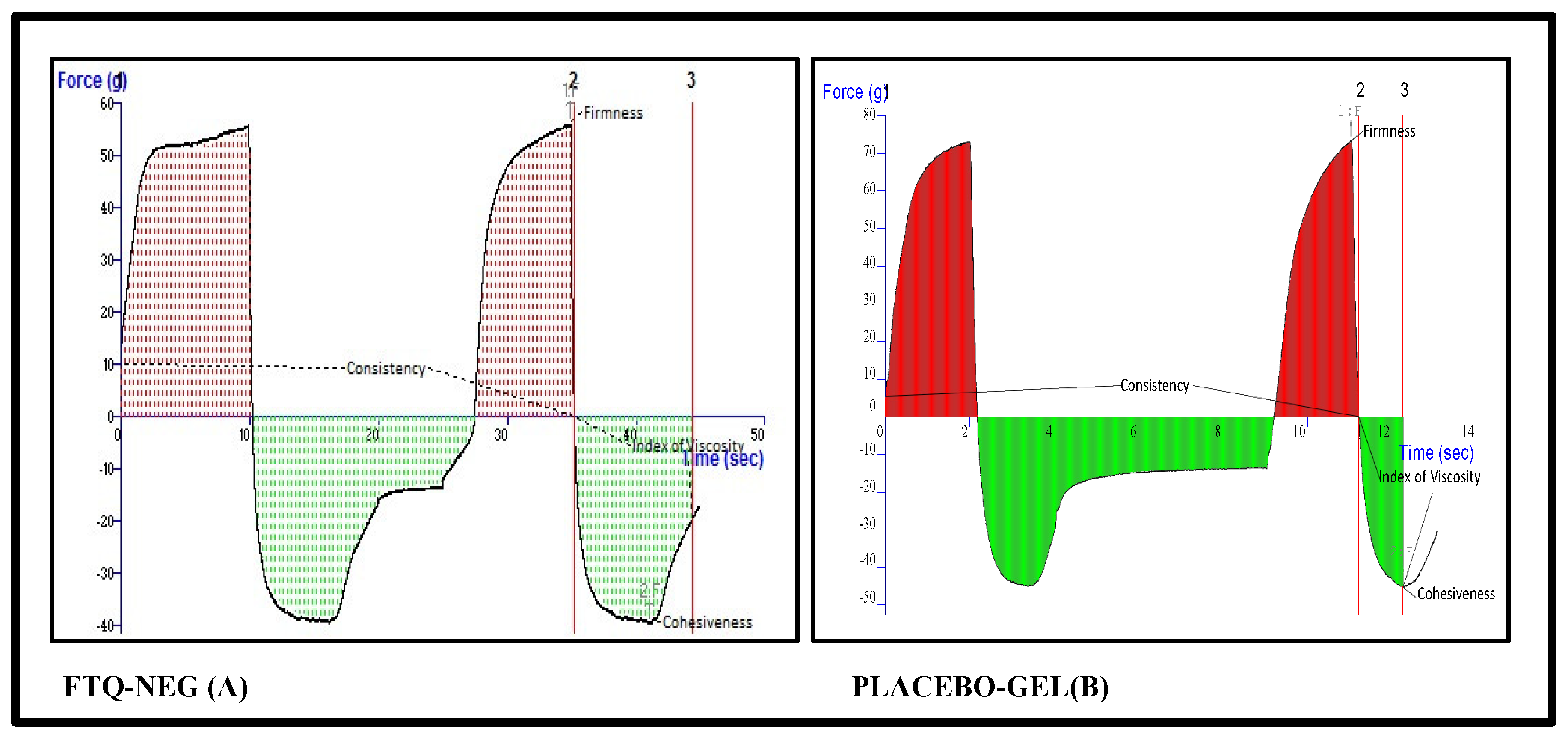
2.7.2. Texture Analysis of the Placebo and Formulation
2.8. Drug Release/Permeation Profile
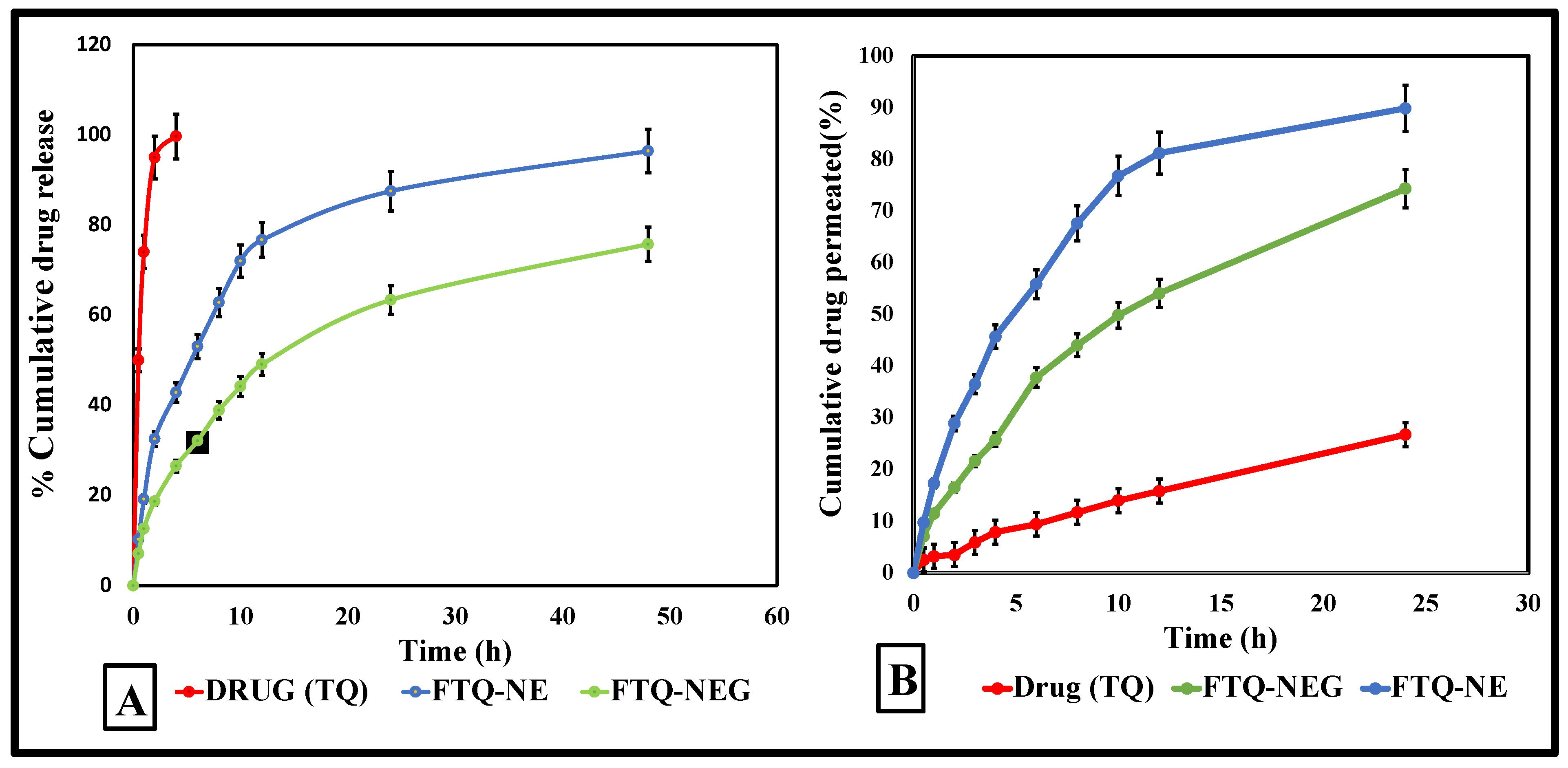
2.8.1. In Vitro Drug Release Study
2.8.2. Ex Vivo Skin Permeation Study
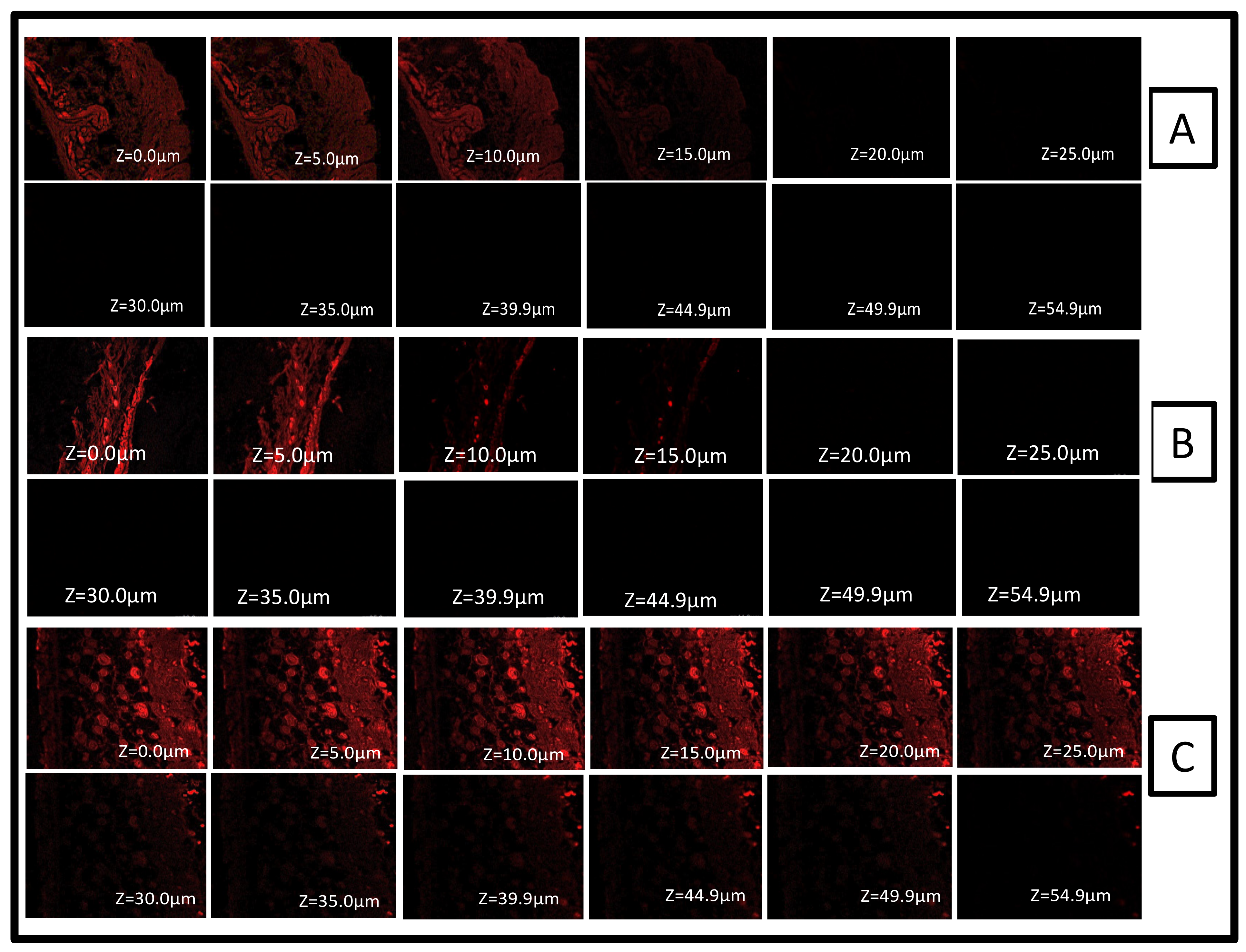
2.9. Confocal Laser Scanning Microscopy
2.10. Stability Study
3. Conclusions
4. Materials and Methods
4.1. HPLC Analytical Method
4.2. Screening of Excipients
4.3. Formulation Development
4.3.1. Preparation of a Placebo Formulation
4.3.2. Preparation of FTQ-NE
4.3.3. Optimization of Prepared FTQ-NE: DoE
4.4. Thermal Stability Studies
4.4.1. Heating Cooling Cycle
4.4.2. Centrifugation
4.4.3. Freeze–Thaw Cycle
4.5. Characterization of the Optimized FTQ-NE
4.5.1. Dilution Test
4.5.2. Filter Paper Test
4.5.3. Cobalt Chloride Test
4.5.4. Transmittance
4.5.5. Refractive Index (RI) and Viscosity
4.5.6. Particle Size, Polydispersity Index (PDI), and Zeta Potential
4.5.7. Differential Scanning Calorimetry (DSC)
4.5.8. Fourier Transform Infrared Spectroscopy (FTIR)
4.5.9. X-ray Diffraction (XRD)
4.6. Surface Morphology Studies of an Optimized FTQ-NE
4.6.1. Transmission Electron Microscopy (TEM)
4.6.2. Scanning Electron Microscopy (SEM)
4.6.3. Fluorescent Microscopy
4.7. Preparation of a Drug-Loaded Nanoemulgel Using an Optimized FTQ-NE
4.8. Physicochemical Characterization of NEG
4.8.1. Spreadability
4.8.2. Extrudability
4.8.3. Texture Analyzer
4.8.4. Homogeneity and pH
4.9. Drug Release/Permeation Profile
4.9.1. In Vitro Drug Release Study
4.9.2. Ex vivo Skin Permeation Study
4.10. Confocal Laser Scanning Microscopy
4.11. Storage Stability of the Optimized Nanoemulsion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solmaz Mohammed, N.; Hilal, Ö.; Nurşen, B. Pharmacological and Toxicological Properties of Eugenol. Turkish J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Imam, S.S.; Afroz Ahmad, M.; Najmi, A.K.; Mujeeb, M.; Aqil, M. Neuroprotective Study of Nigella Sativa-Loaded Oral Provesicular Lipid Formulation: In Vitro and Ex Vivo Study. Drug Deliv. 2014, 21, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Barkat, M.A.; Harshita; Ahmad, J.; Khan, M.A.; Beg, S.; Ahmad, F.J. Insights into the Targeting Potential of Thymoquinone for Therapeutic Intervention against Triple-Negative Breast Cancer. Curr. Drug Targets 2018, 19, 70–80. [Google Scholar] [CrossRef]
- Alzobaidi, N.; Quasimi, H.; Emad, N.A.; Alhalmi, A.; Naqvi, M. Bioactive Compounds and Traditional Herbal Medicine: A Promising Approaches for the Treatment of Dementia. Degener. Neurol. Neuromuscul. Dis. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aquib, M.; Najmi, A.K.; Akhtar, M. Antidepressant Effect of Thymoquinone in Animal Models of Depression. Drug Res. 2014, 65, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and Evaluation of Thymoquinone Niosomes: Application of Developed and Validated RP-HPLC Method in Delivery System. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced Efficacy and Bioavailability of Thymoquinone Using Nanoliposomal Dosage Form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A Novel Strategy to Combat Cancer: A Review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef]
- Khan, R.; Jain, P.; Aqil, M.; Agarwal, S.P.; Mirza, M.A.; Iqbal, Z. Pharmacokinetic Evaluation of Fulvic Acid-Ketoconazole Complexes: A Validation and Line Extension Study. J. Drug Deliv. Sci. Technol. 2020, 55, 101469. [Google Scholar] [CrossRef]
- Mirza, M.A.; Talegaonkar, S.; Ahmad, F.J.; Iqbal, Z. A Novel and Multifunctional Excipient for Vaginal Drug Delivery. J. Excipients Food Chem. 2011, 2, 98–112. [Google Scholar]
- Khan, R.; Jain, P.; Zakir, F.; Aqil, M.; Alshehri, S.; Mirza, M.A.; Iqbal, Z. Quality and In Vivo Assessment of a Fulvic Acid Complex: A Validation Study. Sci. Pharm. 2022, 90, 33. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative Importance of Humic and Fulvic Acid on ROS Generation, Dissolution, and Toxicity of Sulfide Nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Jose, A.; Murray, C.; Williams, C.; Jones, B.; Millington, O.; Bagg, J.; Ramage, G. Carbohydrate Derived Fulvic Acid: An in Vitro Investigation of a Novel Membrane Active Antiseptic Agent against Candida Albicans Biofilms. Front. Microbiol. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, M.; Dai, W.; Xiang, C.; Li, B.; Jia, Q. Antidiarrhoeal Mechanism Study of Fulvic Acids Based on Molecular Weight Fractionation. Fitoterapia 2019, 137, 104270. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative Effects of Humic and Fulvic Acids as Biostimulants on Growth, Antioxidant Activity and Nutrient Content of Yarrow (Achillea Millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, Characteristics and Oil Industry Applications of Nanoemulsions: A Review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured Lipid Carrier Co-Delivering Tacrolimus and TNF-α SiRNA as an Innovate Approach to Psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Deshmukh, R.; Rahman, M.A. Nanoemulsion: Promising Nanocarrier System for Delivery of Herbal Bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Emad, N.A.; Ahmed, B.; Alhalmi, A.; Alzobaidi, N.; Al-Kubati, S.S. Recent Progress in Nanocarriers for Direct Nose to Brain Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102642. [Google Scholar] [CrossRef]
- Alhalmi, A.; Beg, S.; Kohli, K.; Waris, M.; Singh, T. Nanotechnology Based Approach for Hepatocellular Carcinoma Targeting. Curr. Drug Targets 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-Thickened Nanoemulsion System for Topical Delivery of Lipophilic Drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef]
- Bashir, M.; Ahmad, J.; Asif, M.; Khan, S.U.D.; Irfan, M.; Ibrahim, A.Y.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Haseeb, A.; et al. Nanoemulgel, an Innovative Carrier for Diflunisal Topical Delivery with Profound Anti-Inflammatory Effect: In Vitro and in Vivo Evaluation. Int. J. Nanomedicine 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of Nanoemulsions Stabilized by Model Food-Grade Emulsifiers Using High-Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B.; Karl, Y.; Bujanover, S.; Becker, Y. Oleic Acid, a Skin Penetration Enhancer, Affects Langerhans Cells and Corneocytes. J. Control. Release 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Oral Naringenin Nanocarriers: Fabrication, Optimization, Pharmacokinetic and Chemotherapeutic Efficacy Assessments. Nanomedicine 2017, 12, 1243–1260. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, M.; Wang, X.; Zhong, J. Droplet and Creaming Stability of Fish Oil-Loaded Gelatin/Surfactant-Stabilized Emulsions Depends on Both the Adsorption Ways of Emulsifiers and the Adjusted PH. Food Sci. Hum. Wellness 2020, 9, 280–288. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential Biological Fate of Ingested Nanoemulsions: Influence of Particle Characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of Sub-Micron Emulsions by Ultrasound and Microfluidization Techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Dhiman, A.; Prabhakar, P.K. Micronization in Food Processing: A Comprehensive Review of Mechanistic Approach, Physicochemical, Functional Properties and Self-Stability of Micronized Food Materials. J. Food Eng. 2021, 292, 110248. [Google Scholar] [CrossRef]
- Ahmed, S.; Gull, A.; Alam, M.; Aqil, M.; Sultana, Y. Ultrasonically Tailored, Chemically Engineered and “QbD” Enabled Fabrication of Agomelatine Nanoemulsion; Optimization, Characterization, Ex-Vivo Permeation and Stability Study. Ultrason. Sonochem. 2018, 41, 213–226. [Google Scholar] [CrossRef]
- Gull, A.; Ahmed, S.; Ahmad, F.J.; Nagaich, U.; Chandra, A. Effect of Polyherbal Microemulsion on Staphylococcus Epidermidis: Formulation Development, CCD Based Optimization, Characterization, and Antibacterial Activity by Scanning Electron Microscopy. J. Drug Deliv. Sci. Technol. 2020, 57, 101641. [Google Scholar] [CrossRef]
- Jyotshna; Gupta, A.C.; Bawankule, D.U.; Verma, A.K.; Shanker, K. Nanoemulsion Preconcentrate of a Pentacyclic Triterpene for Improved Oral Efficacy: Formulation Design and in-Vivo Antimalarial Activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101734. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. [Google Scholar] [CrossRef] [PubMed]
- Thapa, C.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and Optimization of Nanostructured Lipid Carriers to Enhance Oral Bioavailability of Telmisartan Using Box–Behnken Design. J. Drug Deliv. Sci. Technol. 2018, 44, 431–439. [Google Scholar] [CrossRef]
- Patel, D.; Patel, M.; Soni, T.; Suhagia, B. Topical Arginine Solid Lipid Nanoparticles: Development and Characterization by QbD Approach. J. Drug Deliv. Sci. Technol. 2021, 61, 102329. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-Responsive Lipid Microbubbles for Drug Delivery: A Review of Preparation Techniques to Optimise Formulation Size, Stability and Drug Loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and Bioavailability Assessment of Ramipril Nanoemulsion Formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes Nanocarriers for Improved Oral Bioavailability of Acyclovir: A Complete Characterization through in Vitro, Ex-Vivo and in Vivo Assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A Vaginal Drug Delivery Model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef]
- Mirza, M.A.; Rahman, M.A.; Talegaonkar, S.; Iqbal, Z. In Vitro/in Vivo Performance of Different Complexes of Itraconazole Used in the Treatment of Vaginal Candidiasis. Brazilian J. Pharm. Sci. 2012, 48, 759–772. [Google Scholar] [CrossRef][Green Version]
- Alhalmi, A.; Amin, S.; Beg, S.; Al-Salahi, R.; Mir, S.R.; Kohli, K. Formulation and Optimization of Naringin Loaded Nanostructured Lipid Carriers Using Box-Behnken Based Design: In Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 103590. [Google Scholar] [CrossRef]
- Chin, L.Y.; Tan, J.Y.P.; Choudhury, H.; Pandey, M.; Sisinthy, S.P.; Gorain, B. Development and Optimization of Chitosan Coated Nanoemulgel of Telmisartan for Intranasal Delivery: A Comparative Study. J. Drug Deliv. Sci. Technol. 2021, 62, 102341. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Husain, M.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Hansen, S.; Windbergs, M.; Lehr, C.M. Development of Artemether-Loaded Nanostructured Lipid Carrier (NLC) Formulation for Topical Application. Int. J. Pharm. 2014, 477, 208–217. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Adlhart, C.; Spano, F.; Rotaru, G.-M.; Derler, S.; Zhai, L.; Spencer, N.D.; Rossi, R.M. In Vivo Confirmation of Hydration-Induced Changes in Human-Skin Thickness, Roughness and Interaction with the Environment. Biointerphases 2016, 11, 031015. [Google Scholar] [CrossRef]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation Technology—A Greener Processing Technique for the Generation of Pharmaceutical Nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, Characterization and Evaluation of Anti-Inflammatory and Anti-Nociceptive Effects of Brucine-Loaded Nanoemulgel. Colloids Surfaces B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Mendoza-Muñoz, N.; Leyva-Gómez, G. Nanoemulsions and Nanosized Ingredients for Food Formulations. Handb. Food Nanotechnol. 2020, 207–256. [Google Scholar] [CrossRef]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere Mediated Topical Delivery of Thymoquinone in the Treatment of Psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, F.A.; Asif, M.; Asghar, S.; Iqbal, M.S.; Khan, I.U.; Khan, S.; Irfan, M.; Syed, H.K.; Khames, A.; Mahmood, H.; et al. Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation. Cells, 2021; 10, 2404. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent Advances in Carrier Mediated Nose-to-Brain Delivery of Pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Alam, M.A.; Ahmad, F.J.; Sarafroz, M.; Ansari, K.; Sharma, S.; Amir, M. Ultrasonication Techniques Used for the Preparation of Novel Eugenol-Nanoemulsion in the Treatment of Wounds Healings and Anti-Inflammatory. J. Drug Deliv. Sci. Technol. 2018, 46, 461–473. [Google Scholar] [CrossRef]
- Dadhania, P.; Vuddanda, P.R.; Jain, A.; Velaga, S.; Singh, S. Intranasal Delivery of Asenapine Loaded Nanostructured Lipid Carriers: Formulation, Characterization, Pharmacokinetic and Behavioural Assessment. RSC Adv. 2016, 6, 2032–2045. [Google Scholar] [CrossRef]
- Manickam, S.; Sivakumar, K.; Pang, C.H. Investigations on the Generation of Oil-in-Water (O/W) Nanoemulsions through the Combination of Ultrasound and Microchannel. Ultrason. Sonochem. 2020, 69, 105258. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Rahman, M.; Hasnain, M.S.; Imam, S.S. Application of Design of Experiments (DoE) in Pharmaceutical Product and Process Optimization; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128157992. [Google Scholar]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Introduction to Quality by Design (QbD): Fundamentals, Principles, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128157992. [Google Scholar]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion Loaded Polymeric Hydrogel for Topical Delivery of Curcumin in Psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Jena, J.; Jena, G.K.; Patra, C.N.; Rao, M.E.B. QBD-Based Systematic Development of Bosentan SNEDDS: Formulation, Characterization and Pharmacokinetic Assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 31–42. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarim Imam, S.; Zafar, A.; Ali, A.; Aqil, M.; Gull, A. In Vitro and Preclinical Assessment of Factorial Design Based Nanoethosomes Transgel Formulation of an Opioid Analgesic. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1793–1802. [Google Scholar] [CrossRef]
- Rahman, M.; Al-Ghamdi, S.A.; Alharbi, K.S.; Beg, S.; Sharma, K.; Anwar, F.; Al-Abbasi, F.A.; Kumar, V. Ganoderic Acid Loaded Nano-Lipidic Carriers Improvise Treatment of Hepatocellular Carcinoma. Drug Deliv. 2019, 26, 782–793. [Google Scholar] [CrossRef]
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design Expert Assisted Nanoformulation Design for Co-Delivery of Topotecan and Thymoquinone: Optimization, in Vitro Characterization and Stability Assessment. J. Mol. Liq. 2017, 242, 382–394. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Li, J.; Zhi, D.; Zhang, X.; Liu, H.; Wang, H.; Li, H. Development, Optimization and Evaluation of Emodin Loaded Nanoemulsion Prepared by Ultrasonic Emulsification. J. Drug Deliv. Sci. Technol. 2015, 27, 46–55. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A.; Kazmi, I.; Al-Abbasi, F.A.; Choudhry, H.; Alenezi, S.K.; Barkat, M.A.; Beg, S.; et al. Cationic Solid Lipid Nanoparticles of Resveratrol for Hepatocellular Carcinoma Treatment: Systematic Optimization, in Vitro Characterization and Preclinical Investigation. Int. J. Nanomed. 2020, 15, 9283–9299. [Google Scholar] [CrossRef]
- Zafar, S.; Negi, L.M.; Verma, A.K.; Kumar, V.; Tyagi, A.; Singh, P.; Iqbal, Z.; Talegaonkar, S. Sterically Stabilized Polymeric Nanoparticles with a Combinatorial Approach for Multi Drug Resistant Cancer: In Vitro and in Vivo Investigations. Int. J. Pharm. 2014, 477, 454–468. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and Evaluation of Thymoquinone-Encapsulated Chitosan Nanoparticles for Nose-to-Brain Targeting: A Pharmacoscintigraphic Study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Ali, A.; Ali, S.; Aqil, M.; Imam, S.S.; Ahad, A.; Qadir, A. Thymoquinone Loaded Dermal Lipid Nano Particles: Box Behnken Design Optimization to Preclinical Psoriasis Assessment. J. Drug Deliv. Sci. Technol. 2019, 52, 713–721. [Google Scholar] [CrossRef]
- Ali, H.H.; Hussein, A.A. Oral Nanoemulsions of Candesartan Cilexetil: Formulation, Characterization and in Vitro Drug Release Studies. AAPS Open 2017, 3, 4. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective Effect of Ropinirole Lipid Nanoparticles Enriched Hydrogel for Parkinson’s Disease: In Vitro, Ex Vivo, Pharmacokinetic and Pharmacodynamic Evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef]
- Zakir, F.; Ahmad, A.; Mirza, M.A.; Kohli, K.; Ahmed, F.J. Exploration of a Transdermal Nanoemulgel as an Alternative Therapy for Postmenopausal Osteoporosis. J. Drug Deliv. Sci. Technol. 2021, 65, 102745. [Google Scholar] [CrossRef]
- Gardouh, A.R.; Nasef, A.M.; Mostafa, Y.; Gad, S. Design and Evaluation of Combined Atorvastatin and Ezetimibe Optimized Self- Nano Emulsifying Drug Delivery System. J. Drug Deliv. Sci. Technol. 2020, 60, 102093. [Google Scholar] [CrossRef]
- Fasolo, D.; Pippi, B.; Meirelles, G.; Zorzi, G.; Fuentefria, A.M.; von Poser, G.; Teixeira, H.F. Topical Delivery of Antifungal Brazilian Red Propolis Benzophenones-Rich Extract by Means of Cationic Lipid Nanoemulsions Optimized by Means of Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 56, 101573. [Google Scholar] [CrossRef]
- Singh, H.P.; Utreja, P.; Tiwary, A.K.; Jain, S. Elastic Liposomal Formulation for Sustained Delivery of Colchicine: In Vitro Characterization and in Vivo Evaluation of Anti-Gout Activity. AAPS J. 2009, 11, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Nazish, I.; Ahmed, F.J. Nanocarrier-Based Topical Drug Delivery for an Antifungal Drug. Drug Dev. Ind. Pharm. 2014, 40, 527–541. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Abdel, I.; Ibrahim, A.; Singh, T.; Baothman, A.A.; Choudhry, H.; et al. Paclitaxel and Naringenin-Loaded Solid Lipid Nanoparticles Surface Modified with Cyclic Peptides with Improved Tumor Targeting Ability in Glioblastoma Multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef]
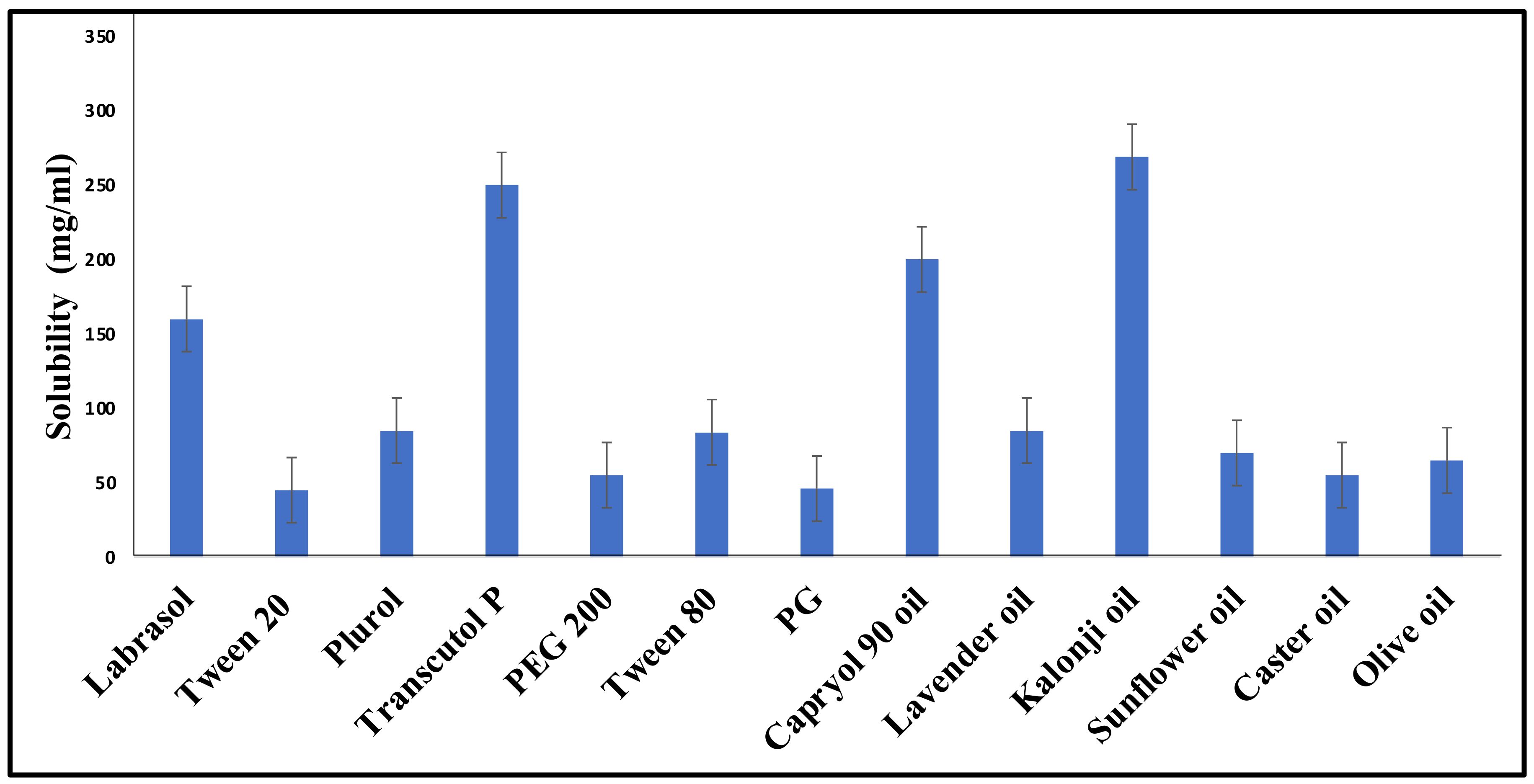
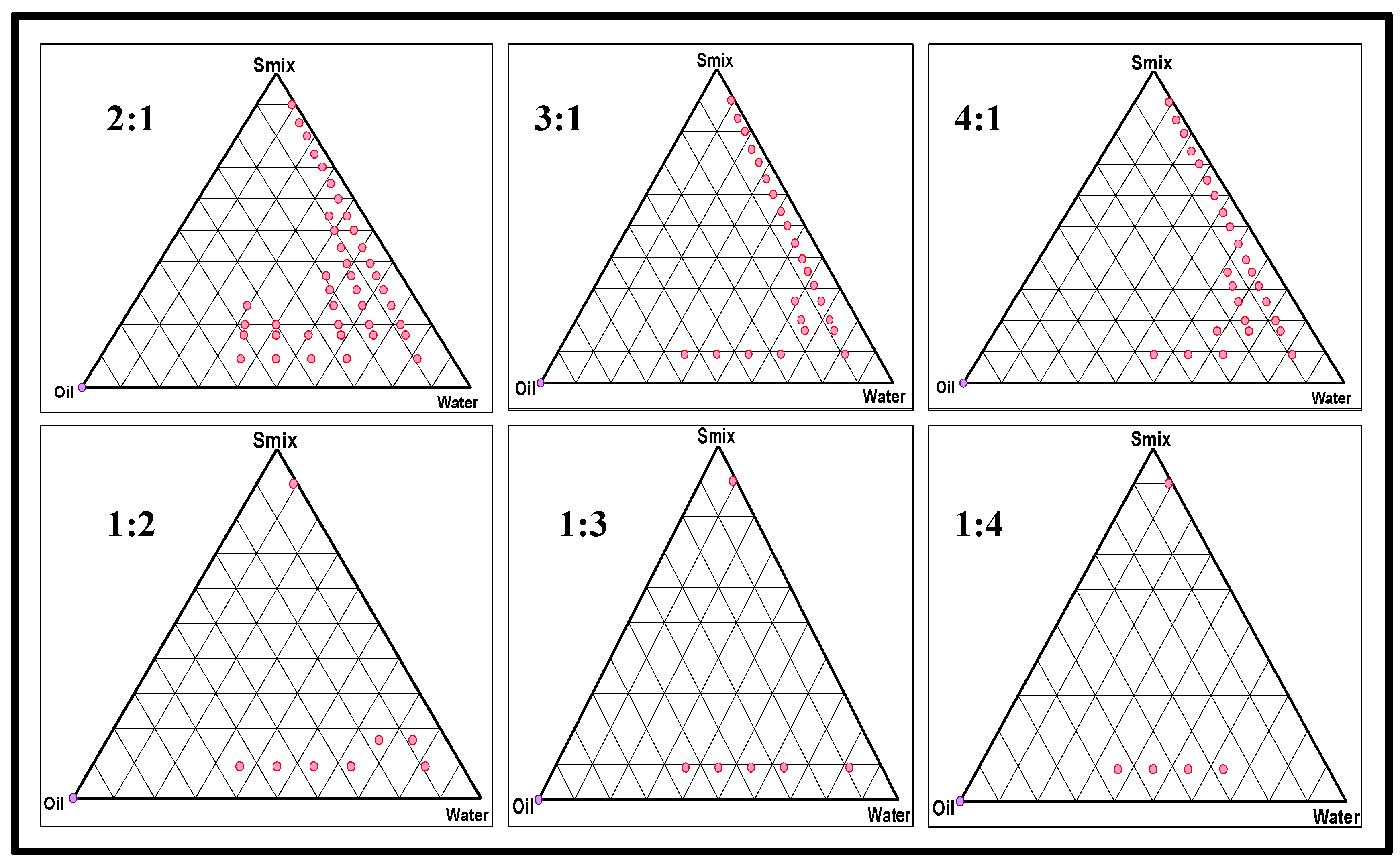
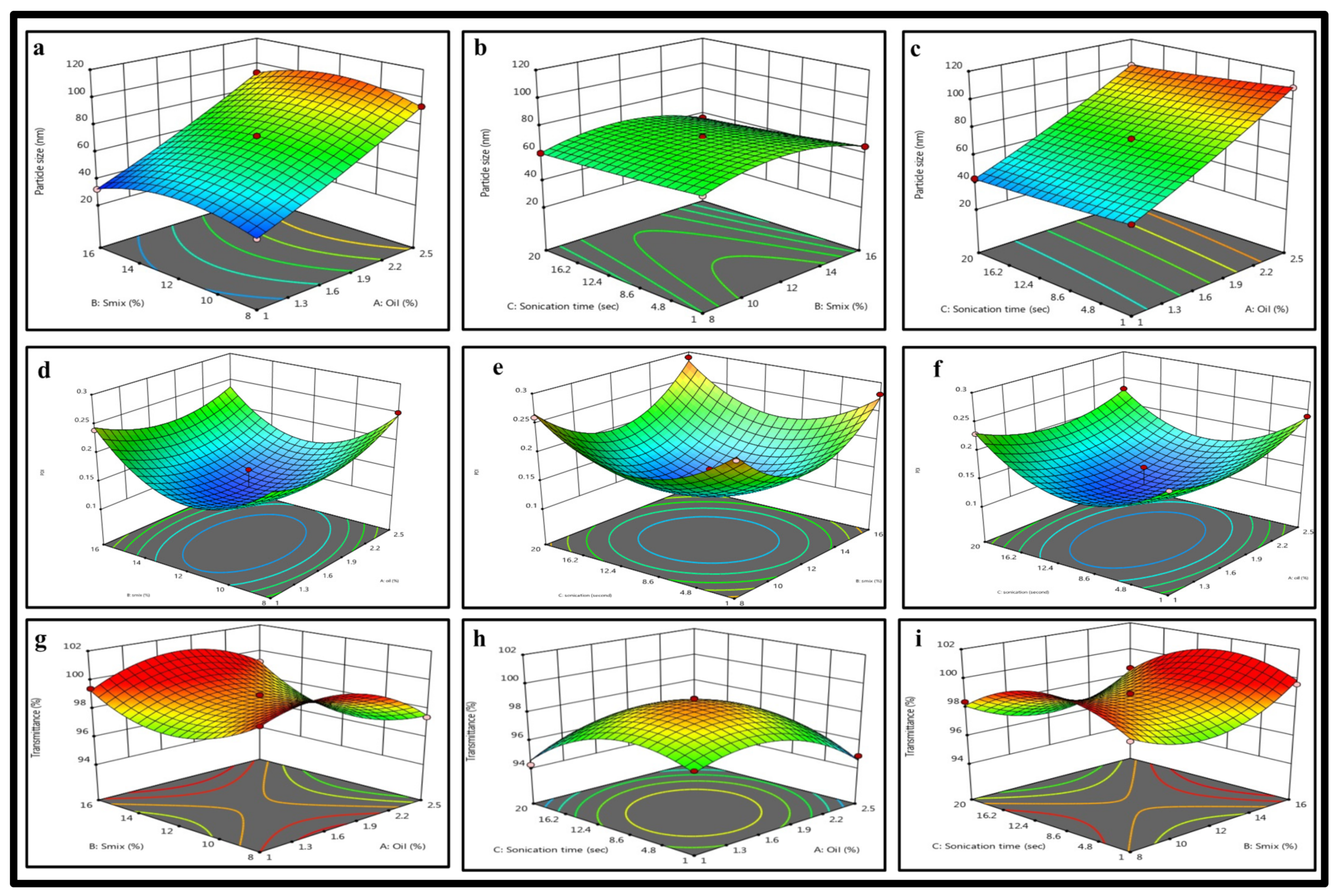

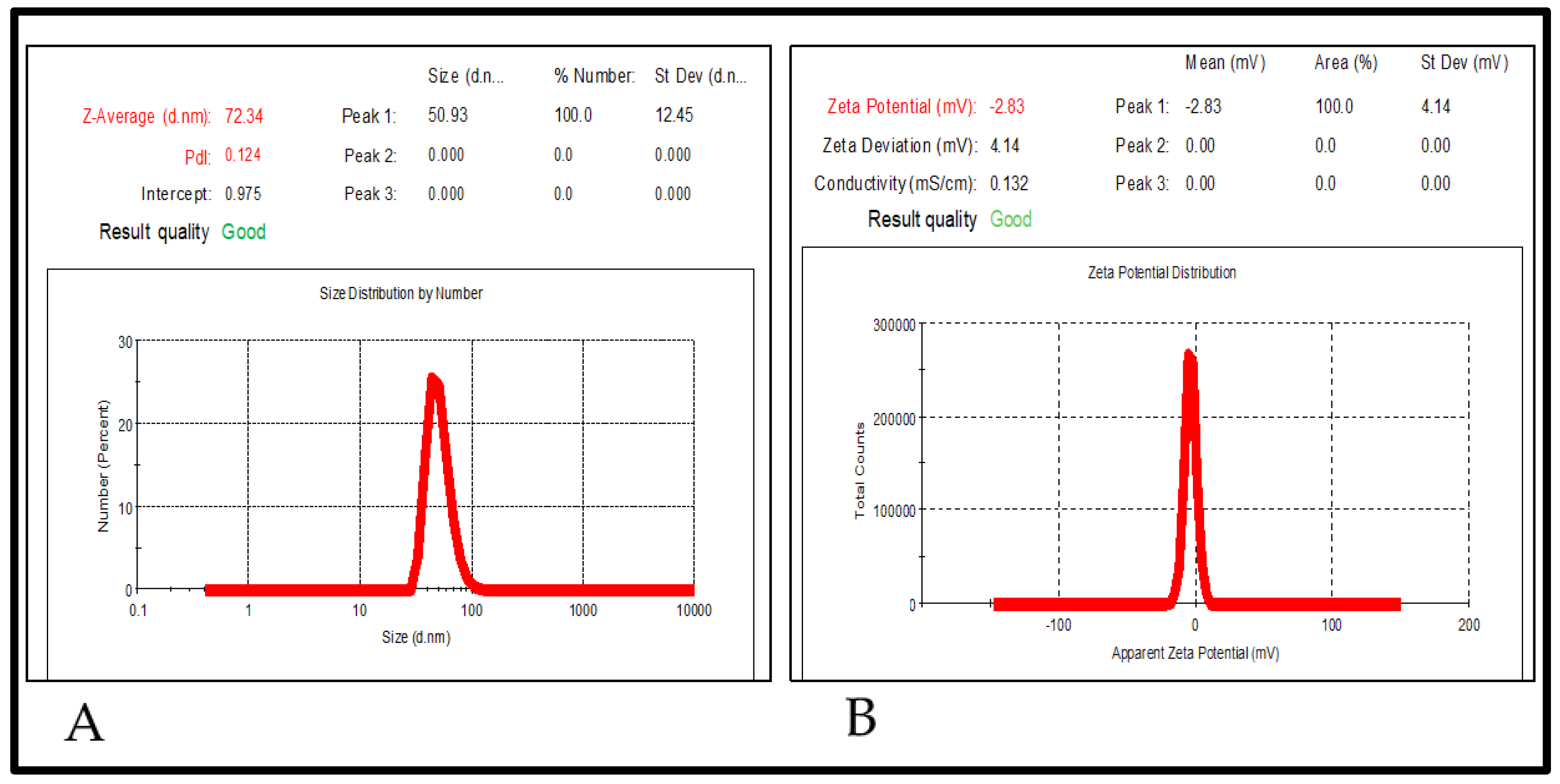


| Scheme | Oils | Surfactants | Co-Surfactants | Inference |
|---|---|---|---|---|
| 1 | Lavender | Tween 80 | Transcutol-P | No Phase separation |
| 2 | Sunflower | Tween 80 | Transcutol-P | Phase separation |
| 3 | Kalonji | Tween 80 | Transcutol-P | No phase separation |
| 4 | Castor | Tween 80 | Transcutol-P | Phase separation |
| 5 | Olive | Tween 80 | Transcutol-P | Phase separation |
| 6 | Sunflower | Labrasol | Transcutol-P | No phase separation |
| 7 | Kalonji | Labrasol | Transcutol-P | No phase separation |
| 8 | Lavender | Labrasol | Transcutol-P | No phase separation |
| 9 | Olive | Labrasol | Transcutol-P | No phase separation |
| 10 | Castor | Labrasol | Transcutol-P | No phase separation |
| Run | Oil (%) | Smix (%) | Sonication Time (s) | Particle Size (nm) | PDI | Transmittance (%) |
|---|---|---|---|---|---|---|
| 1 | 2.5 | 12 | 20 | 99.76 | 0.23 | 94.65 |
| 2 | 1.75 | 12 | 10.5 | 72.34 | 0.12 | 99.01 |
| 3 | 1 | 16 | 10.5 | 32.26 | 0.24 | 99.42 |
| 4 | 1 | 12 | 20 | 43.12 | 0.23 | 94.24 |
| 5 | 1 | 8 | 10.5 | 36.26 | 0.22 | 99.51 |
| 6 | 1.75 | 12 | 10.5 | 72.34 | 0.12 | 98.19 |
| 7 | 1.75 | 8 | 20 | 60.52 | 0.26 | 98.41 |
| 8 | 1 | 12 | 1 | 49.22 | 0.21 | 96.94 |
| 9 | 1.75 | 16 | 20 | 56.61 | 0.29 | 98.58 |
| 10 | 1.75 | 12 | 10.5 | 72.34 | 0.12 | 98.98 |
| 11 | 1.75 | 12 | 10.5 | 72.34 | 0.12 | 98.99 |
| 12 | 2.5 | 16 | 10.5 | 92.63 | 0.23 | 99.19 |
| 13 | 2.5 | 12 | 1 | 108.53 | 0.24 | 94.9 |
| 14 | 1.75 | 16 | 1 | 65.43 | 0.28 | 99.62 |
| 15 | 1.75 | 12 | 10.5 | 72.34 | 0.16 | 98.79 |
| 16 | 1.75 | 8 | 1 | 66.42 | 0.26 | 98.51 |
| 17 | 2.5 | 8 | 10.5 | 93.68 | 0.25 | 97.42 |
| Independent Variables | Levels | ||
|---|---|---|---|
| Low | Medium | High | |
| X1 = Oil (%) | 1 | 1.75 | 2.5 |
| X2 = Smix (%) | 8 | 12 | 16 |
| X3 = Sonication time (s) | 1 | 10.5 | 20 |
| Quadratic Model | %CV | R2 | Adjusted R2 | Predicted R2 | SD |
|---|---|---|---|---|---|
| Particle size (Y1) | 0.36 | 0.9999 | 0.9999 | 0.9991 | 0.24 |
| PDI (Y2) | 7.19 | 0.9713 | 0.9344 | 0.8712 | 0.01 |
| % Transmittance (Y3) | 0.38 | 0.9802 | 0.9547 | 0.8253 | 0.37 |
| Y1 = Particle size + 72.34 + 29.22 × A − 1.24 × B − 3.7 × C + 0.7375 × AB − 0.6675 × AC − 0.73 × BC + 2.14 × A2 − 10.77 × B2 + 0.6775 × C2 | (1) | ||||
| Y2 = PDI + 0.1280 + 0.0063 × A + 0.0063 × B + 0.0025 × C − 0.0100 × AB 0.0075 × AC + 0.0025 × BC + 0.031 × A2 + 0.0760 × B2 + 0.0685 × C2 | (2) | ||||
| Y3 = Transmittance + 98.79 − 0.4937 × A + 0.37 × B − 0.5113 × C + 0.465 × AB + 0.6125 × AC − 0.235 × BC − 1.75 × A2 + 1.85 × B2 − 1.86 × C2 | (3) | ||||
| Smix Ratios | Formulation Coding | Percentage of Component % (v/v) | Observation | Inference | ||||
|---|---|---|---|---|---|---|---|---|
| Oil | Smix | Water | Heating–Cooling | Freeze–Thaw | Centrifugation | |||
| Formulation P Smix = ratio 2:1 | P-1 P-2 P-3 P-4 | 2.22 2 2.5 7.84 | 17.78 14 17.5 47.06 | 80 85 80 45.1 | ✓ ✓ × ✓ | ✓ ✓ × × | ✓ ✓ × × | Passed Passed Failed Failed |
| Formulation Q Smix = ratio 3:1 | Q-1 Q-2 Q-3 Q-4 | 1.67 1.87 2 2.86 | 13.33 13.12 14 17.14 | 85 85 85 80 | × × ✓ ✓ | ✓ × ✓ × | ✓ × ✓ × | Failed Failed Passed Failed |
| Formulation R Smix = ratio 4:1 | R-1 R-2 R-3 R-4 | 2.78 3.13 2.5 2 | 22.22 21.88 12.5 18 | 75 75 85 80 | ✓ ✓ ✓ × | ✓ ✓ × × | ✓ ✓ × × | Passed Passed Failed Failed |
| Formulation Coding | Particle Size (nm) (Mean ± SD) (N = 3) | PDI (Mean ± SD) (N = 3) | pH (Mean ± SD) (N = 3) | RI (Mean ± SD) (N = 3) | Viscosity (mp) (Mean ± SD) (N = 3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | FTQ-NE | Blank | FTQ-NE | Blank | FTQ-NE | Blank | FTQ-NE | Blank | FTQ-NE | |
| P-1 | 150.6 ± 1.34 | 224.7 ± 1.30 | 0.24 ± 0.05 | 0.28 ± 0.03 | 5.9 ± 0.34 | 6.1 ± 0.44 | 1.401 ± 0.006 | 1.402 ± 0.008 | 43.31 ± 3.24 | 43.78 ± 2.42 |
| P-2 | 41.13 ± 1.45 | 72.34 ± 1.43 | 0.16 ± 0.02 | 0.13 ± 0.03 | 5.8 ± 0.25 | 5.8 ± 0.32 | 1.405 ± 0.003 | 1.405 ± 0.007 | 134.0 ± 6.42 | 138.5 ± 3.08 |
| Q-3 | 100.13 ± 1.74 | 187.6 ± 1.23 | 0.18 ± 0.04 | 0.23 ± 0.02 | 5.6 ± 0.13 | 5.7 ± 0.25 | 1.402 ± 0.002 | 1.368 ± 0.004 | 104.0 ± 3.40 | 106.0 ± 3.49 |
| R-1 | 106.3 ± 2.33 | 159.3 ± 1.57 | 0.38 ± 0.03 | 0.33 ± 0.04 | 6.4 ± 0.65 | 6.6 ± 0.27 | 1.405 ± 0.005 | 1.402 ± 0.004 | 52.60 ± 2.70 | 53.51 ± 2.02 |
| R-2 | 112.14 ± 2.74 | 171.2 ± 2.13 | 0.15 ± 0.06 | 0.18 ± 0.02 | 6.3 ± 0.43 | 6.7 ± 0.14 | 1.403 ± 0.006 | 1.407 ± 0.004 | 157.2 ± 6.72 | 159.6 ± 5.52 |
| Duration (Week) | FTQ-NE (Before Sonication) | FTQ-NE+US (After Sonication) | ||
|---|---|---|---|---|
| Particle Size (nm) (n = 3 ± SD) | PDI (n = 3) | Particle Size (nm) (n = 3 ± SD) | PDI (n = 3 ± SD) | |
| 0 | 97.12 ± 4.21 | 0.319 ± 0.072 | 72.34 ± 1.42 | 0.135 ± 0.014 |
| 1 | 131.3 ± 7.02 | 0.123 ± 0.02 | 95.03 ± 3.02 | 0.202 ± 0.035 |
| 3 | 145.2 ± 13.71 | 0.214 ± 0.032 | 105.8 ± 5.12 | 0.142 ± 0.046 |
| 7 | 241.4 ± 19.82 | 0.321 ± 0.057 | 108.2 ± 6.03 | 0.227 ± 0.013 |
| 10 | Phase separation | Phase separation | 112.3 ± 7.13 | 0.237 ± 0.016 |
| 13 | Phase separation | Phase separation | 125.3 ± 9.12 | 0.238 ± 0.024 |
| 15 | Phase separation | Phase separation | 135.8 ± 7.34 | 0.289 ± 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Mirza, M.A.; Aqil, M.; Hassan, N.; Zakir, F.; Ansari, M.J.; Iqbal, Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels 2022, 8, 733. https://doi.org/10.3390/gels8110733
Khan R, Mirza MA, Aqil M, Hassan N, Zakir F, Ansari MJ, Iqbal Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels. 2022; 8(11):733. https://doi.org/10.3390/gels8110733
Chicago/Turabian StyleKhan, Rahmuddin, Mohd Aamir Mirza, Mohd Aqil, Nazia Hassan, Foziyah Zakir, Mohammad Javed Ansari, and Zeenat Iqbal. 2022. "A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy" Gels 8, no. 11: 733. https://doi.org/10.3390/gels8110733
APA StyleKhan, R., Mirza, M. A., Aqil, M., Hassan, N., Zakir, F., Ansari, M. J., & Iqbal, Z. (2022). A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels, 8(11), 733. https://doi.org/10.3390/gels8110733







