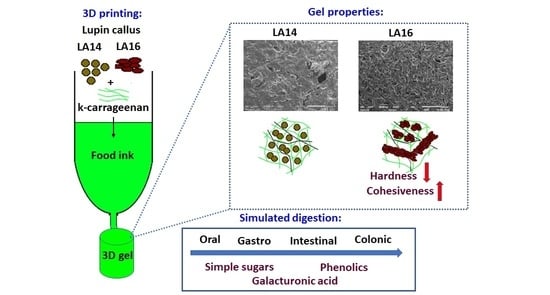
Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius
Abstract
1. Introduction
2. Results and Discussion
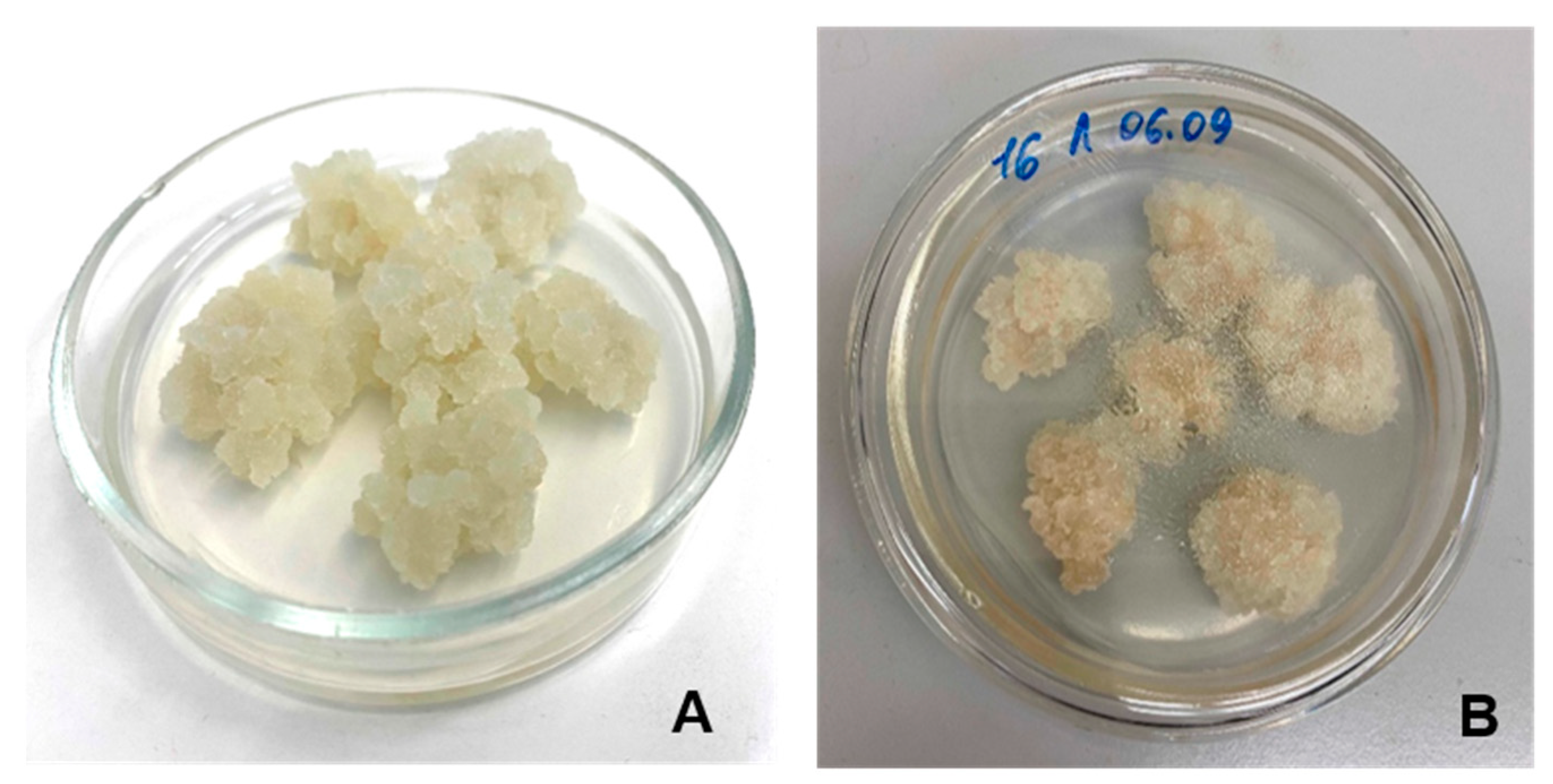
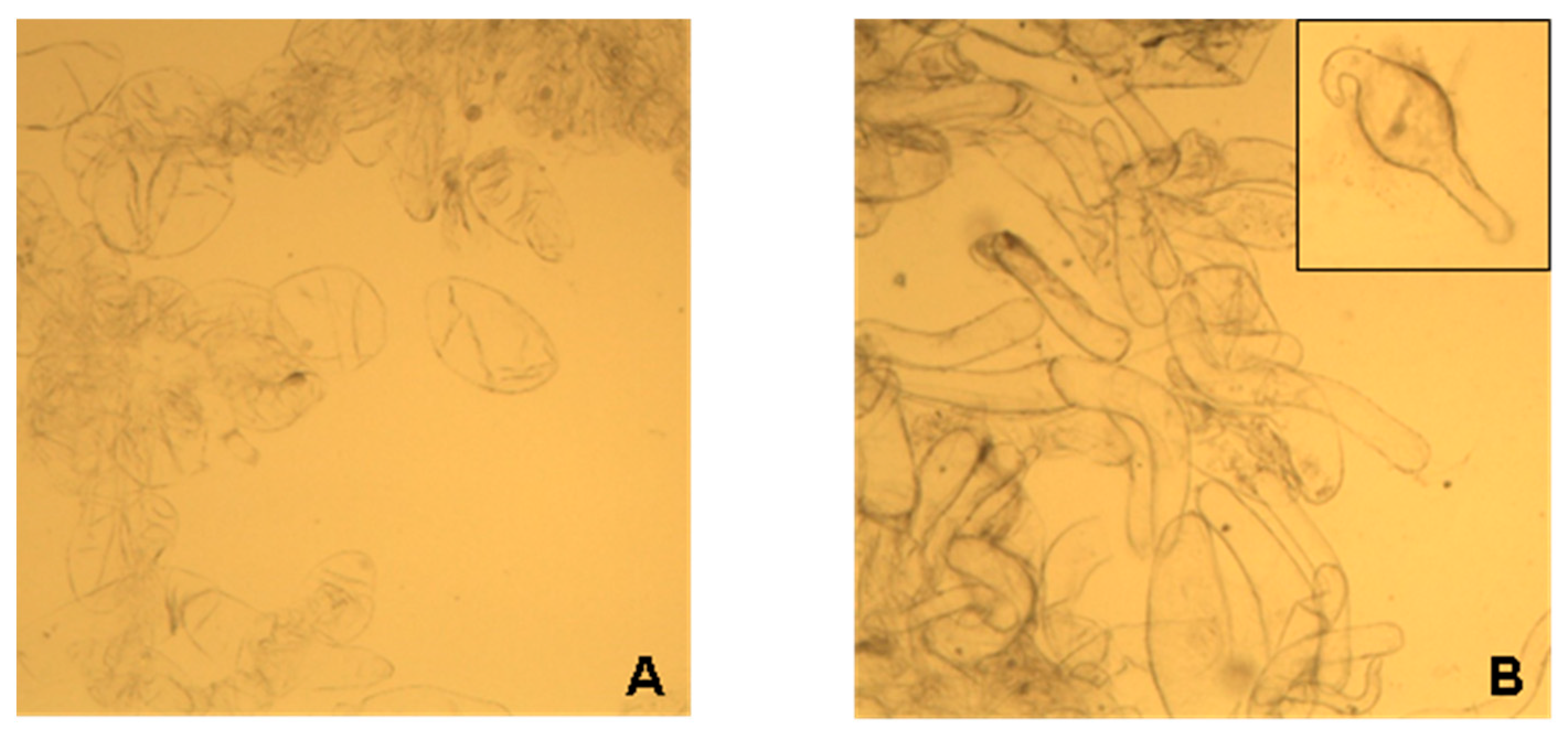
2.1. Characterization of Lupin Callus
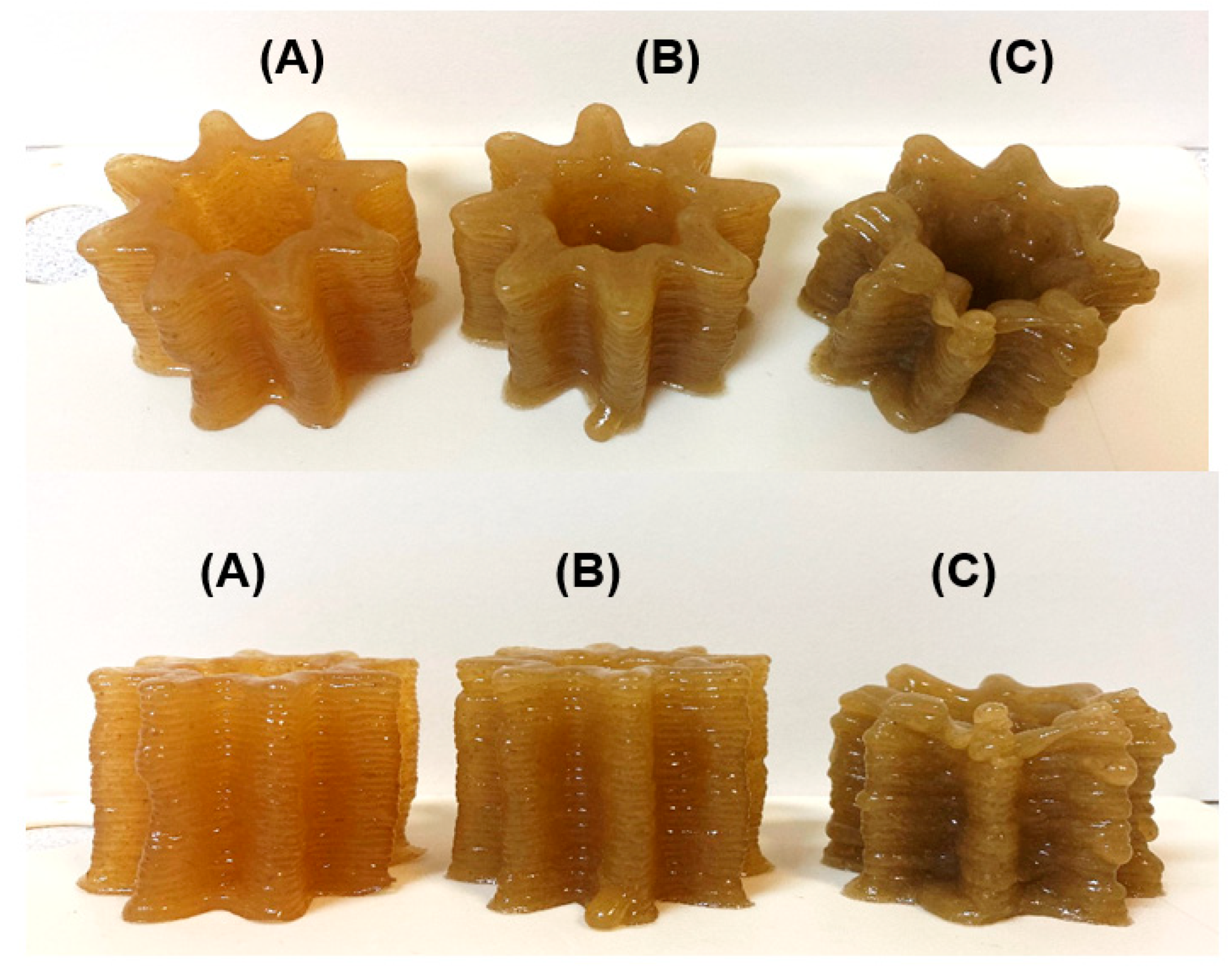
2.2. 3D Printing
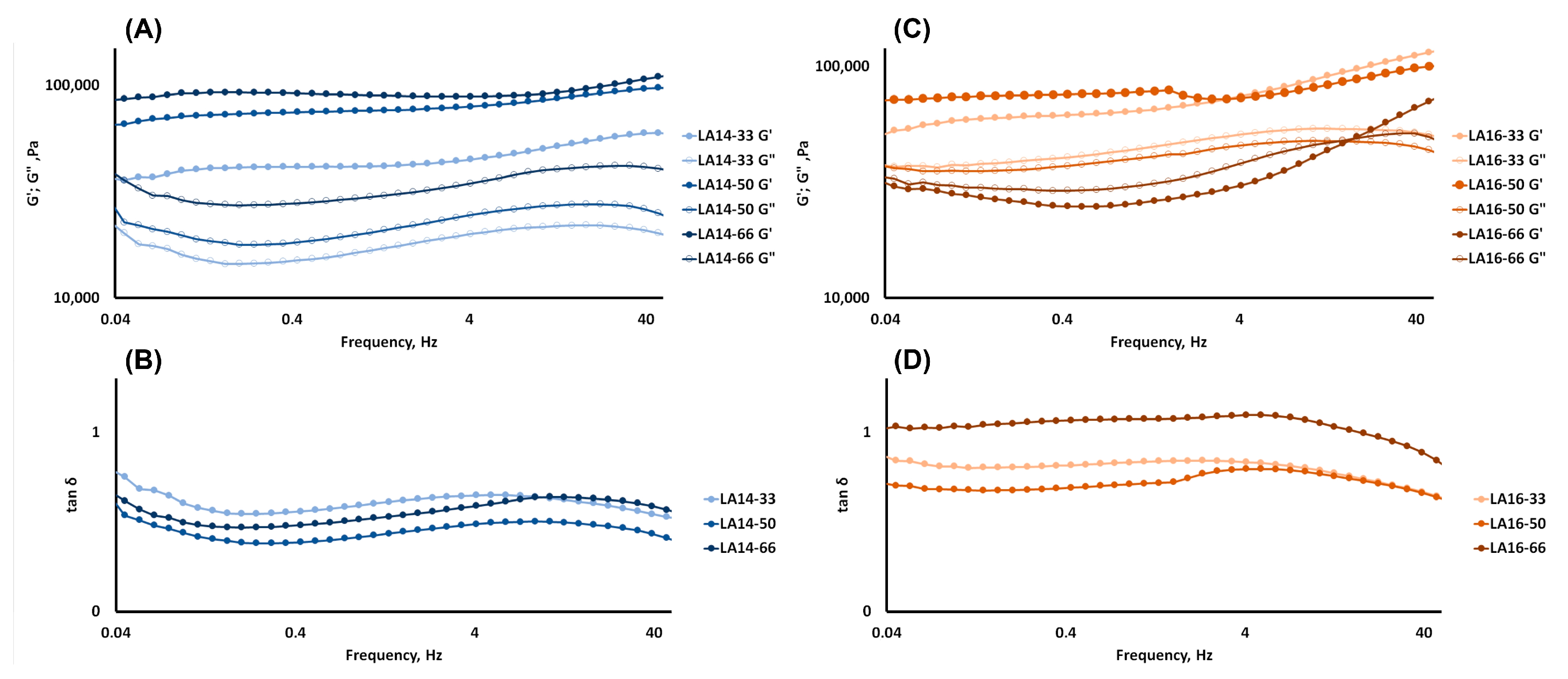
2.3. Rheological and Mechanical Properties of 3D-Printed Gels
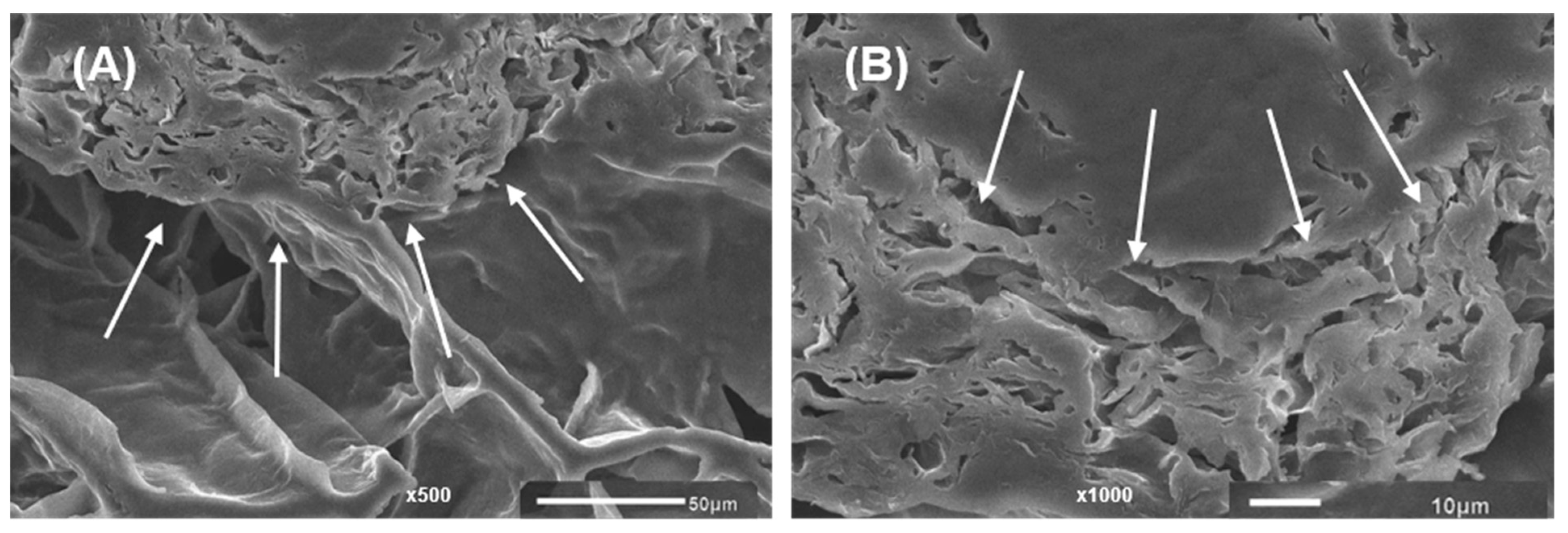
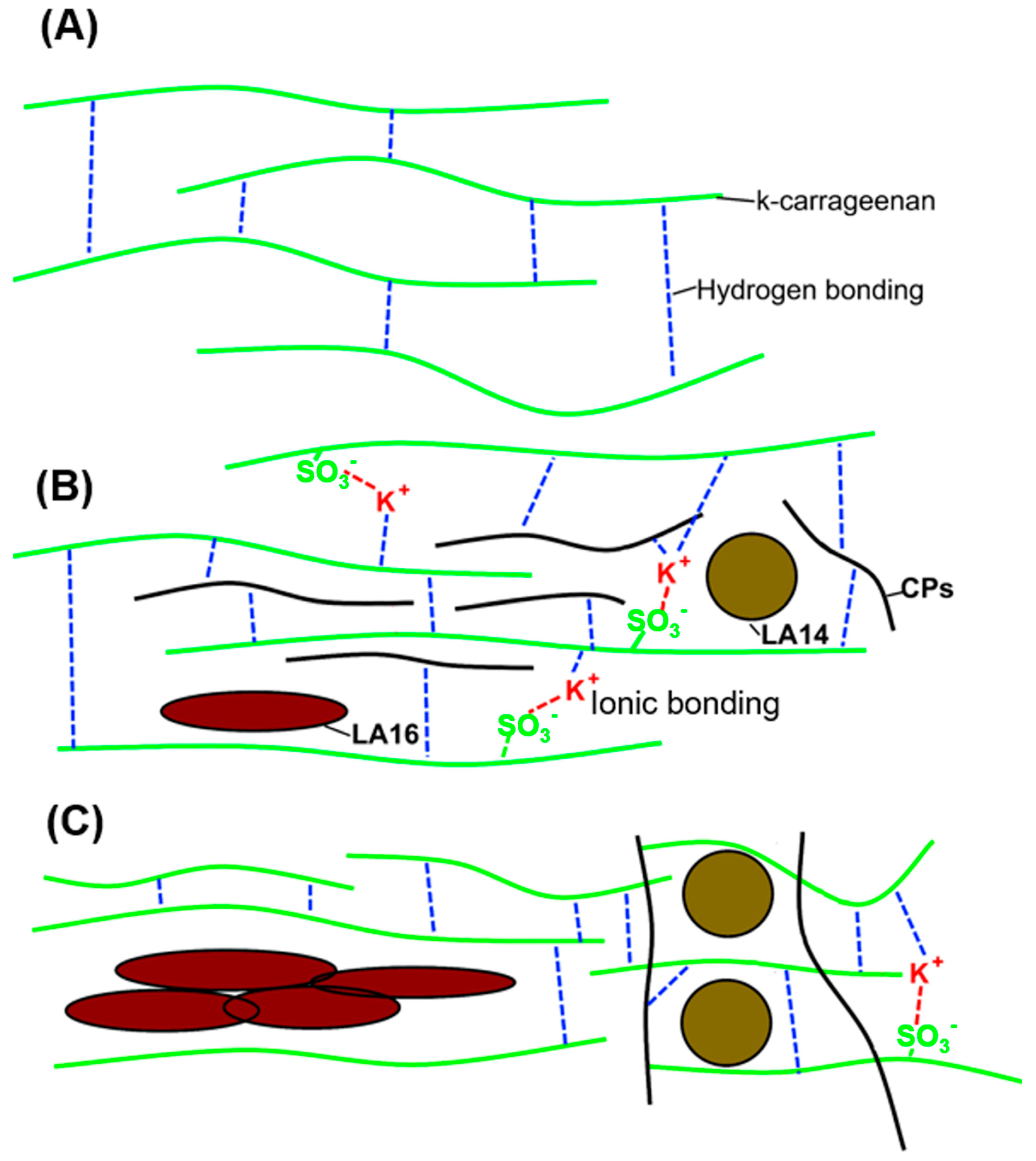
2.4. Microstructure of 3D-Printed Gels
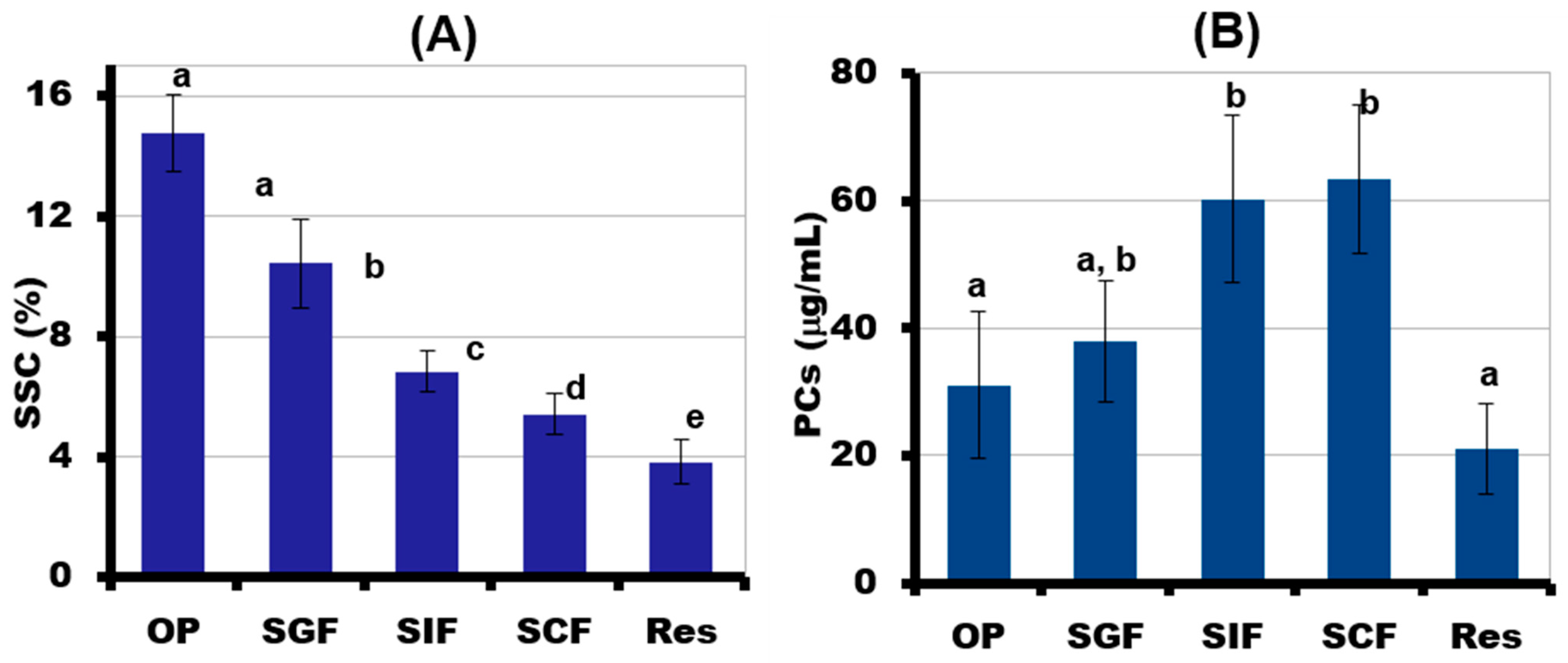
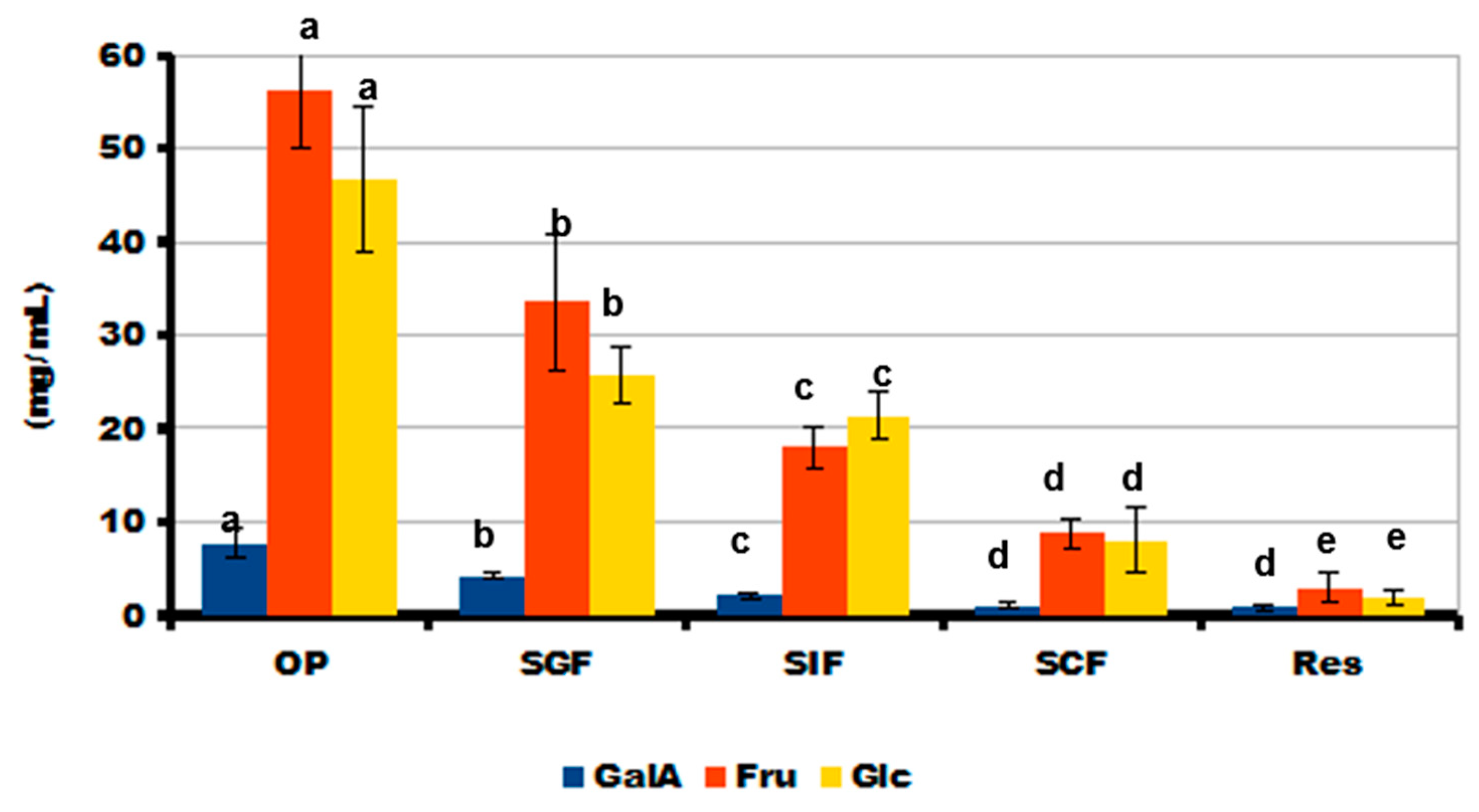
2.5. Simulated Digestibility of 3D-Printed Gel Containing LA16-33 Callus
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Obtaining and Characterizing Calluses
4.3. Inks Preparation and 3D Printing
4.4. Measurement of Mechanical Properties
4.5. Measurement of Rheological Properties
4.6. In Vivo Oral Phase (OP) and Static In Vitro Gastrointestinal Digestion
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key, T.; Papier, K.; Tong, T. Plant-based diets and long-term health: Findings from the EPIC-Oxford study. Proc. Nutr. Soc. 2022, 81, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.-M.; Heiniö, R.-L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—A concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food ingredients and food made with plant cell and tissue cultures: State-of-the art and future trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Trujillo-de Santiago, G.; Álvarez, M.M.; Chuck-Hernández, C. Advances and prospective applications of 3D food printing for health improvement and personalized nutrition. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5722–5741. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Paasi, J.; Pennanen, K.; Flores Ituarte, I.; Lille, M.; Partanen, J.; Sozer, N. Techno-Economic Prospects and Desirability of 3D Food Printing: Perspectives of Industrial Experts, Researchers and Consumers. Foods 2020, 9, 1725. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Cuan-Urquizo, E. Towards the Development of 3D-Printed Food: A Rheological and Mechanical Approach. Foods 2022, 11, 1191. [Google Scholar] [CrossRef]
- Outrequin, T.C.R.; Gamonpilas, C.; Siriwatwechakul, W.; Sreearunothai, P. Extrusion-based 3D printing of food biopolymers: A highlight on the important rheological parameters to reach printability. J. Food Eng. 2023, 342, 111371. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q. 3D food printing: Applications of f-based materials in extrusion-based food printing. Crit. Rev. Food Sci. Nutr. 2021, 62, 7184–7198. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, H.W.; Park, H.J. Callus-based 3D printing for food exemplified with carrot tissues and its potential for innovative food production. J. Food Eng. 2020, 271, 109781. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Seisun, D.; Zalesny, N. Strides in food texture and hydrocolloids. Food Hydrocoll. 2021, 117, 106575. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.L.; Norton, I.T.; Mills, T.B. Comparing the viscoelastic properties of gelatin and different concentrations of kappa-carrageenan mixtures for additive manufacturing applications. J. Food Eng. 2019, 246, 58–66. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking rheology and printability of a multicomponent gel system of carrageenan-xanthan-starch in extrusion based additive manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Tian, H.; Wang, K.; Lan, H.; Wang, Y.; Hu, Z.; Zhao, L. Effect of hybrid gelator systems of beeswax-carrageenan-xanthan on rheological properties and printability of litchi inks for 3D food printing. Food Hydrocoll. 2021, 113, 106482. [Google Scholar] [CrossRef]
- Pant, A.; Lee, A.Y.; Karyappa, R.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.-X.; Wong, G.; Chua, C.K.; Zhang, Y. 3D food printing of fresh vegetables using food hydrocolloids for dysphagic patients. Food Hydrocoll. 2021, 114, 106546. [Google Scholar] [CrossRef]
- Kim, S.M.; Wen, Y.; Kim, H.W.; Park, H.J. Textural and sensory qualities of low-calorie surimi with carrageenan inserted as a protein substitute using coaxial extrusion 3D food printing. J. Food Eng. 2022, 333, 111141. [Google Scholar] [CrossRef]
- Diañez, I.; Gallegos, C.; Fuente, E.B.; Martínez, I.; Valencia, C.; Sánchez, M.C.; Diaz, M.J.; Franco, J.M. 3D printing in situ gelification of κ-carrageenan solutions: Effect of printing variables on the rheological response. Food Hydrocoll. 2019, 87, 321–330. [Google Scholar] [CrossRef]
- Gholamipour-Shirazi, A.; Norton, I.T.; Mills, T. Designing hydrocolloid based food-ink formulations for extrusion 3D printing. Food Hydrocoll. 2019, 95, 161–167. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gao, X.; Cheng, C.; Liu, C.; Wang, Q.; Han, X. The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems. Mar. Drugs 2020, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Kharina, M.; Emelyanov, V.; Mokshina, N.; Ibragimova, N.; Gorshkova, T. Pretreatment of Sugar Beet Pulp with Dilute Sulfurous Acid is Effective for Multipurpose Usage of Carbohydrates. Appl. Biochem. Biotechnol. 2016, 179, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Ric-Varas, P.; Barceló, M.; Rivera, J.A.; Cerezo, S.; Matas, A.J.; Schückel, J.; Knox, J.P.; Posé, S.; Pliego-Alfaro, F.; Mercado, J.A. Exploring the Use of Fruit Callus Culture as a Model System to Study Color Development and Cell Wall Remodeling during Strawberry Fruit Ripening. Plants 2020, 9, 805. [Google Scholar] [CrossRef]
- Camacho-Fernández, C.; Seguí-Simarro, J.M.; Mir, R.; Boutilier, K.; Corral-Martínez, P. Cell Wall Composition and Structure Define the Developmental Fate of Embryogenic Microspores in Brassica napus. Front. Plant Sci. 2021, 2, 737139. [Google Scholar] [CrossRef]
- Betekhtin, A.; Rojek, M.; Milewska-Hendel, A.; Gawecki, R.; Karcz, J.; Kurczynska, E.; Hasterok, R. Spatial Distribution of Selected Chemical Cell Wall Components in the Embryogenic Callus of Brachypodium distachyon. PLoS ONE 2016, 11, e0167426. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Salam, H.K.; Hamada, A.M.; Radi, A.A. Alleviating excess boron stress in tomato calli by applying benzoic acid to various biochemical strategies. Plant Physiol. Biochem. 2022, 182, 216–226. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Braz da Silva, D., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Vancauwenberghe, V.; Mfortaw, M.W.B.M.; Vanstreels, E.; Verboven, P.; Lammertyn, J.; Nicolai, B. 3D printing of plant tissue for innovative food manufacturing: Encapsulation of alive plant cells into pectin based bio-ink. J. Food Eng. 2019, 263, 454–464. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A.; Majewska, B. Effect of Spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fucinos, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893. [Google Scholar] [CrossRef]
- Keerthana, K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J. Food Eng. 2020, 287, 110116. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; Van de Velde, F.; Stieger, M. Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food Funct. 2017, 8, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Pascua, Y.; Koc, H.; Foegeding, E.A. Food structure: Roles of mechanical properties and oral processing in determining sensory texture of soft materials. Curr. Opin. Colloid Interface Sci. 2013, 18, 324–333. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Ramos, F.; Saraiva, A.; Raposo, A. Texture-Modified Food for Dysphagic Patients: A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 5125. [Google Scholar] [CrossRef]
- Sungsinchai, S.; Niamnuy, C.; Wattanapan, P.; Charoenchaitrakool, M.; Devahastin, S. Texture modification technologies and their opportunities for the production of dysphagia foods: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1898–1912. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interaction with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana Cell Culture Establishment, and Assessment of Its Cytotoxic, Antibacterial, α-Amylase Inhibitor, and Antioxidant In Vitro Bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Khan, T.; Ahmad, N.; Zaman, G.; Khan, T.; Ahmad, W.; Batool, S.; Hussain, Z.; Drouet, S.; Hano, C.; et al. Chemical Elicitors-Induced Variation in Cellular Biomass, Biosynthesis of Secondary Cell Products, and Antioxidant System in Callus Cultures of Fagonia indica. Molecules 2021, 26, 6340. [Google Scholar] [CrossRef] [PubMed]
- Maliński, M.P.; Kikowska, M.A.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Thiem, B. Phytochemical Screening, Phenolic Compounds and Antioxidant Activity of Biomass from Lychnis flos-cuculi L. In Vitro Cultures and Intact Plants. Plants 2021, 10, 206. [Google Scholar] [CrossRef]
- Park, J.-S.; Seong, Z.-K.; Kim, M.-S.; Ha, J.-H.; Moon, K.-B.; Lee, H.-J.; Lee, H.-K.; Jeon, J.-H.; Park, S.U.; Kim, H.-S. Production of Flavonoids in Callus Cultures of Sophora flavescens Aiton. Plants 2020, 9, 688. [Google Scholar] [CrossRef]
- Erst, A.A.; Petruk, A.A.; Erst, A.S.; Krivenko, D.A.; Filinova, N.V.; Maltseva, S.Y.; Kulikovskiy, M.S.; Banaev, E.V. Optimization of Biomass Accumulation and Production of Phenolic Compounds in Callus Cultures of Rhodiola rosea L. Using Design of Experiments. Plants 2022, 11, 124. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Ayoubi, Y.; Sahiner, N. Therapeutic and Nutraceutical Effects of Polyphenolics from Natural Sources. Molecules 2022, 27, 6225. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Khin, M.N.; Goff, H.D.; Nsor-Atindana, J.; Ahammed, S.; Liu, F.; Zhong, F. Effect of texture and structure of polysaccharide hydrogels containing maltose on release and hydrolysis of maltose during digestion: In vitro study. Food Hydrocoll. 2021, 112, 106326. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several Calcareous red algae: Isolation of alginate from Corallina Pilulifera, P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–52. [Google Scholar] [CrossRef]



| Callus | SSC (%) | Protein a (%) | EHP b (%) | HHP b (%) | PCs b (%) |
|---|---|---|---|---|---|
| LA14 | 2.68 ± 0.01 | 0.28 ± 0.01 | 9.2 ± 0.1 | 25.0 ± 2.6 | 1.25 ± 0.02 |
| LA16 | 4.33 ± 0.11 # | 0.30 ± 0.00 # | 14.9 ± 0.4 # | 30.2 ± 0.4 # | 0.53 ± 0.01 # |
| Gels | Storage Modulus | Viscosity | |||
|---|---|---|---|---|---|
| A (Pa) | B (Slope) | R2 | ηapp 0.045, Hz | ηapp 10.500, Hz | |
| LA14-33 | 69,196 | 0.1047 | 0.926 | 145,473.3 ± 29,131.1 a | 834.9 ± 134.9 a |
| LA14-50 | 77,682 | 0.0355 | 0.623 | 300,836.7 ± 18,894.7 b | 1702.3 ± 2.9 b |
| LA14-66 | 31,914 | 0.1159 | 0.603 | 359,313.3 ± 59,577.9 b | 1601.8 ± 324.1 c |
| LA16-33 | 43,765 | 0.0635 | 0.893 | 278,873.3 ± 36,029.6 a | 1887.0 ± 328.1 a |
| LA16-50 | 77,554 | 0.0474 | 0.935 | 162,349.0 ± 30,465.1 b | 962.0 ± 189.0 b |
| LA16-66 | 92,050 | 0.0181 | 0.406 | 154,626.0 ± 63,583.1 b | 855.9 ± 372.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belova, K.; Dushina, E.; Popov, S.; Zlobin, A.; Martinson, E.; Vityazev, F.; Litvinets, S. Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius. Gels 2023, 9, 45. https://doi.org/10.3390/gels9010045
Belova K, Dushina E, Popov S, Zlobin A, Martinson E, Vityazev F, Litvinets S. Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius. Gels. 2023; 9(1):45. https://doi.org/10.3390/gels9010045
Chicago/Turabian StyleBelova, Kseniya, Elena Dushina, Sergey Popov, Andrey Zlobin, Ekaterina Martinson, Fedor Vityazev, and Sergey Litvinets. 2023. "Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius" Gels 9, no. 1: 45. https://doi.org/10.3390/gels9010045
APA StyleBelova, K., Dushina, E., Popov, S., Zlobin, A., Martinson, E., Vityazev, F., & Litvinets, S. (2023). Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius. Gels, 9(1), 45. https://doi.org/10.3390/gels9010045