Preparation of MOF-Based Core-Shell Gel Particles with Catalytic Activity and Their Plugging Performance
Abstract
1. Introduction
2. Results and Discussion
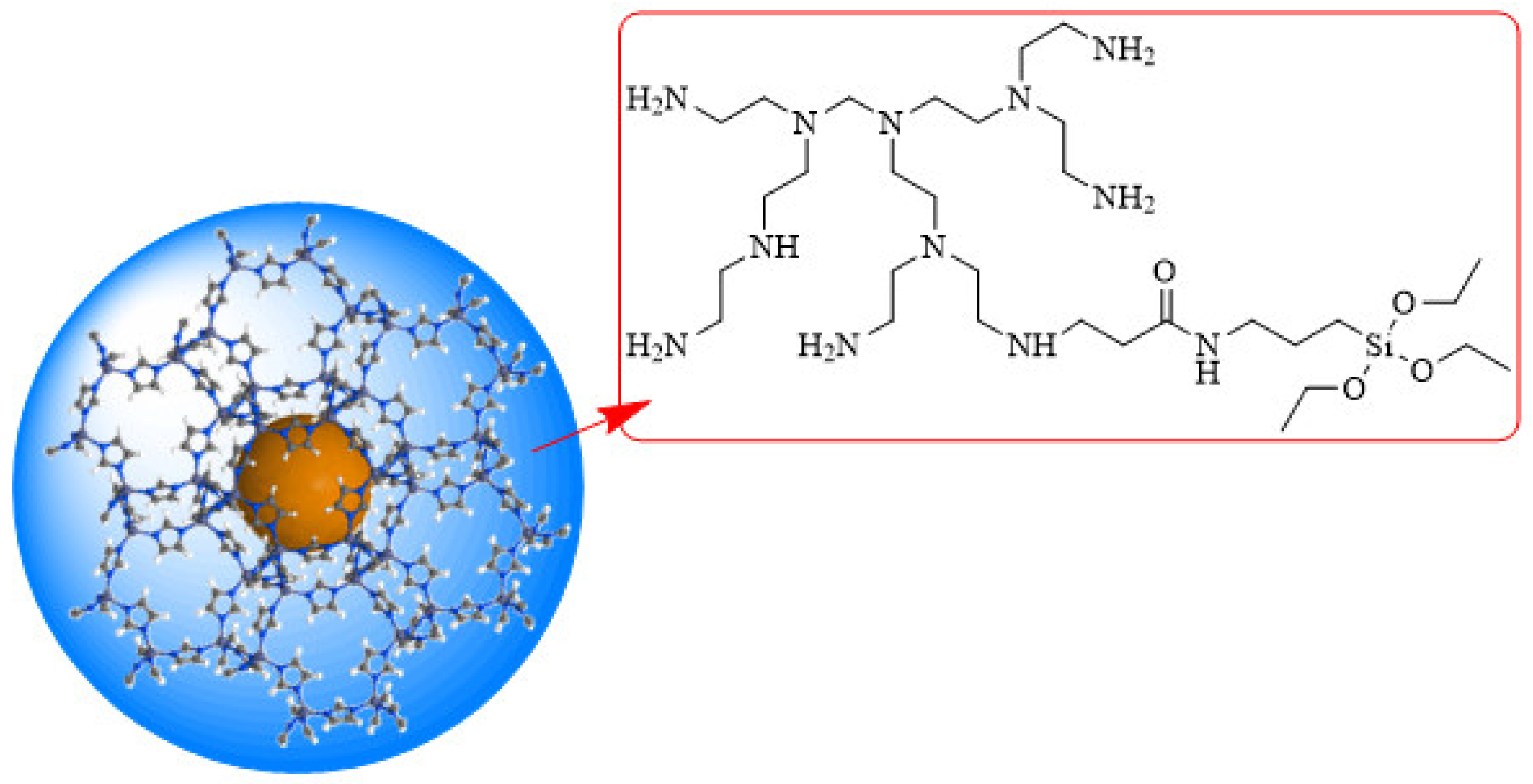
2.1. Physicochemical Characterization of Modified ZIF
2.1.1. SEM Analysis of the Modified ZIF
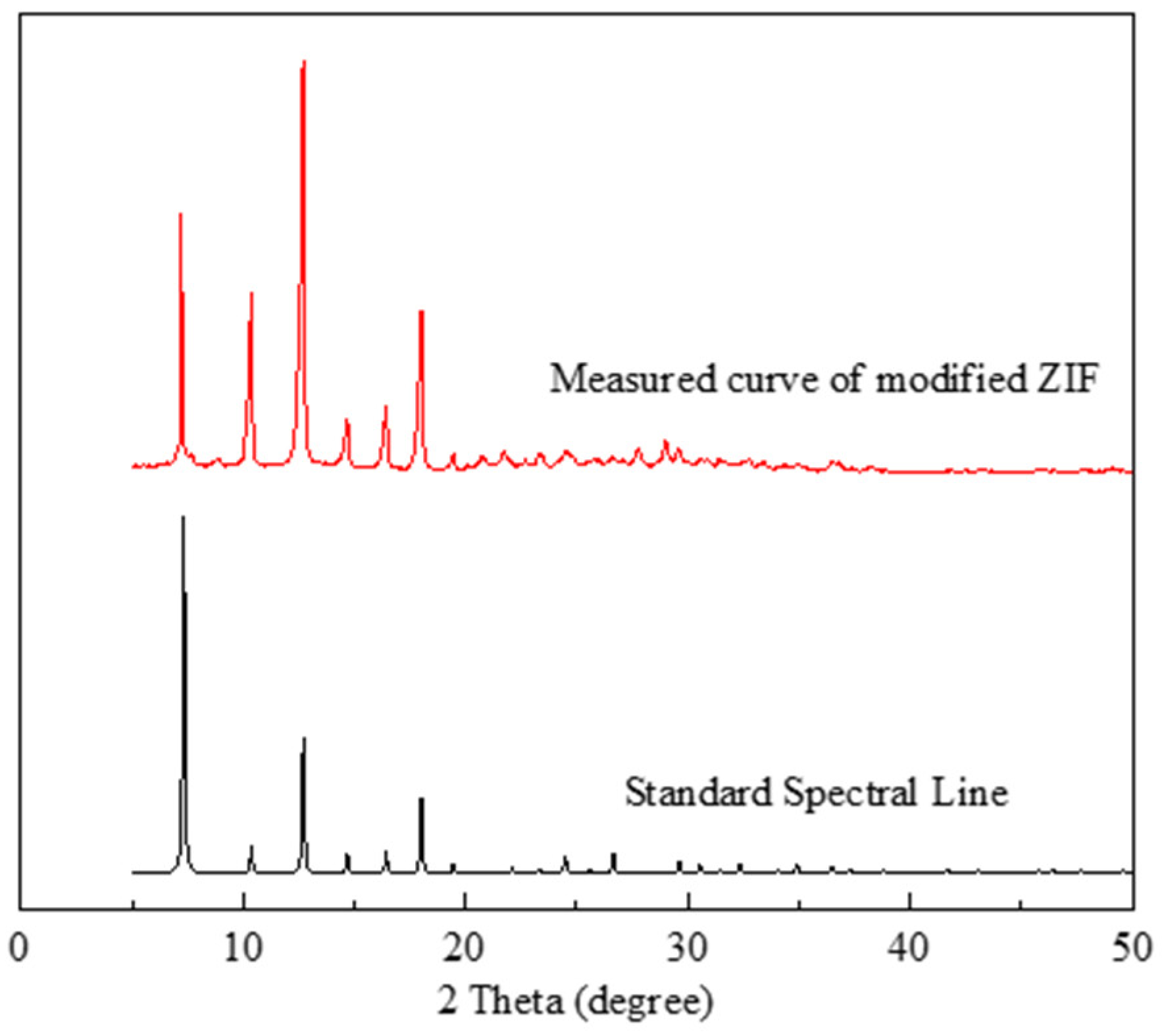
2.1.2. XRD Analysis of the Modified ZIF
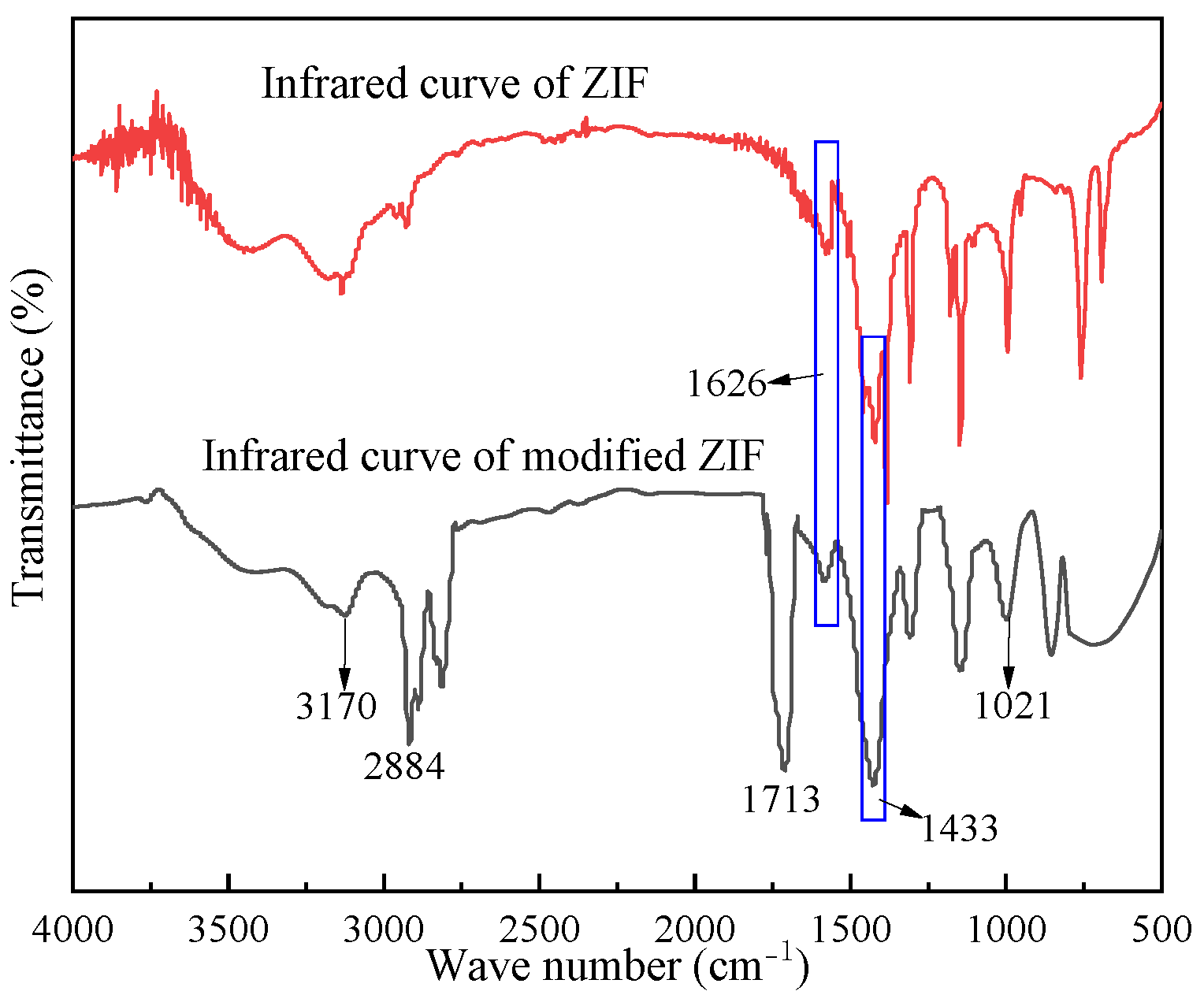
2.1.3. FTIR Analysis of the Modified ZIF
2.2. Performance Study of the Modified ZIF in Drilling Fluid
2.2.1. Simulated Liquid Phase Environment
2.2.2. SEM Analysis of the Modified ZIF after Aging
2.2.3. XRD Analysis of the Modified ZIF after Aging
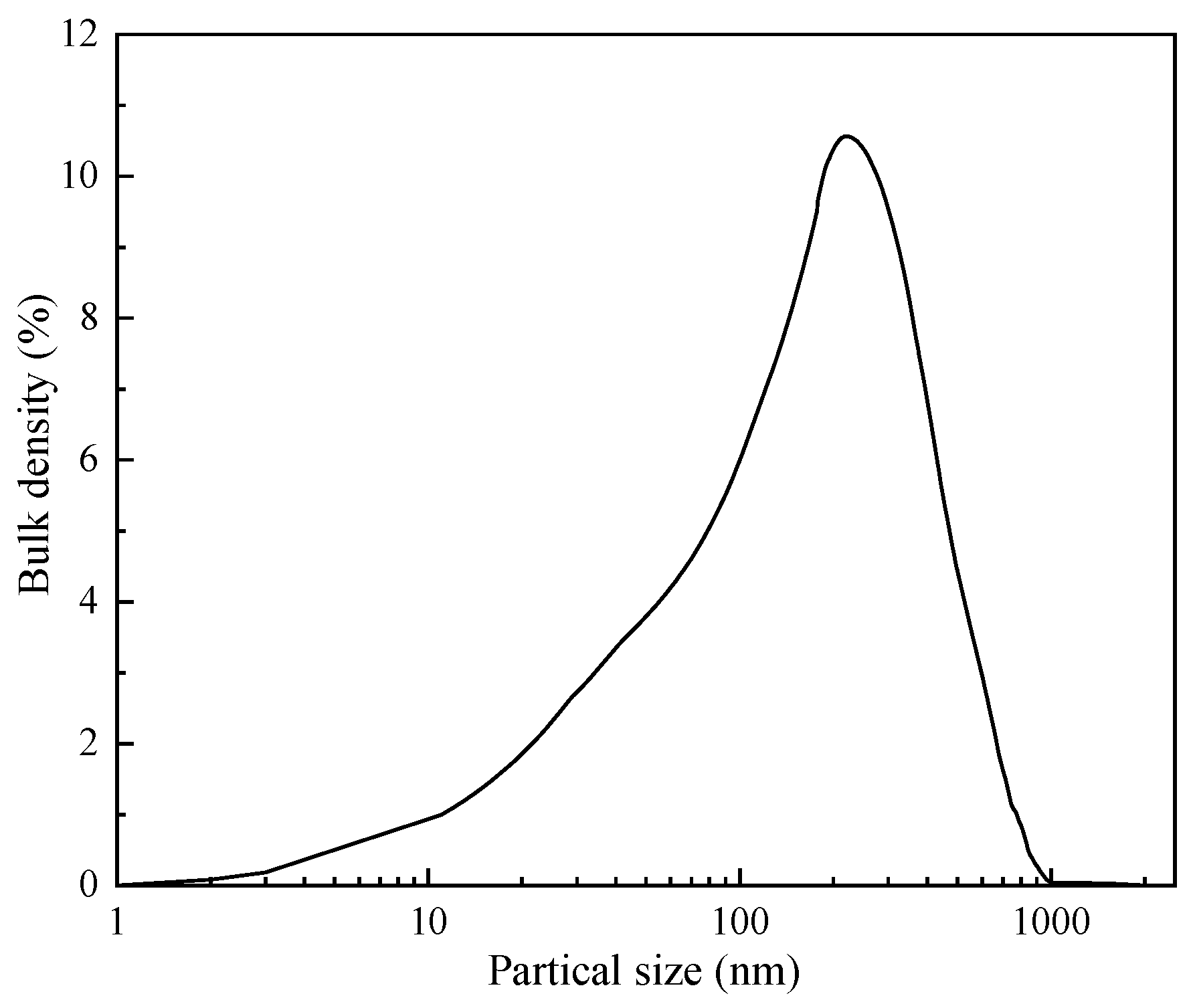
2.2.4. Particle Size Distribution of Modified ZIF after Aging
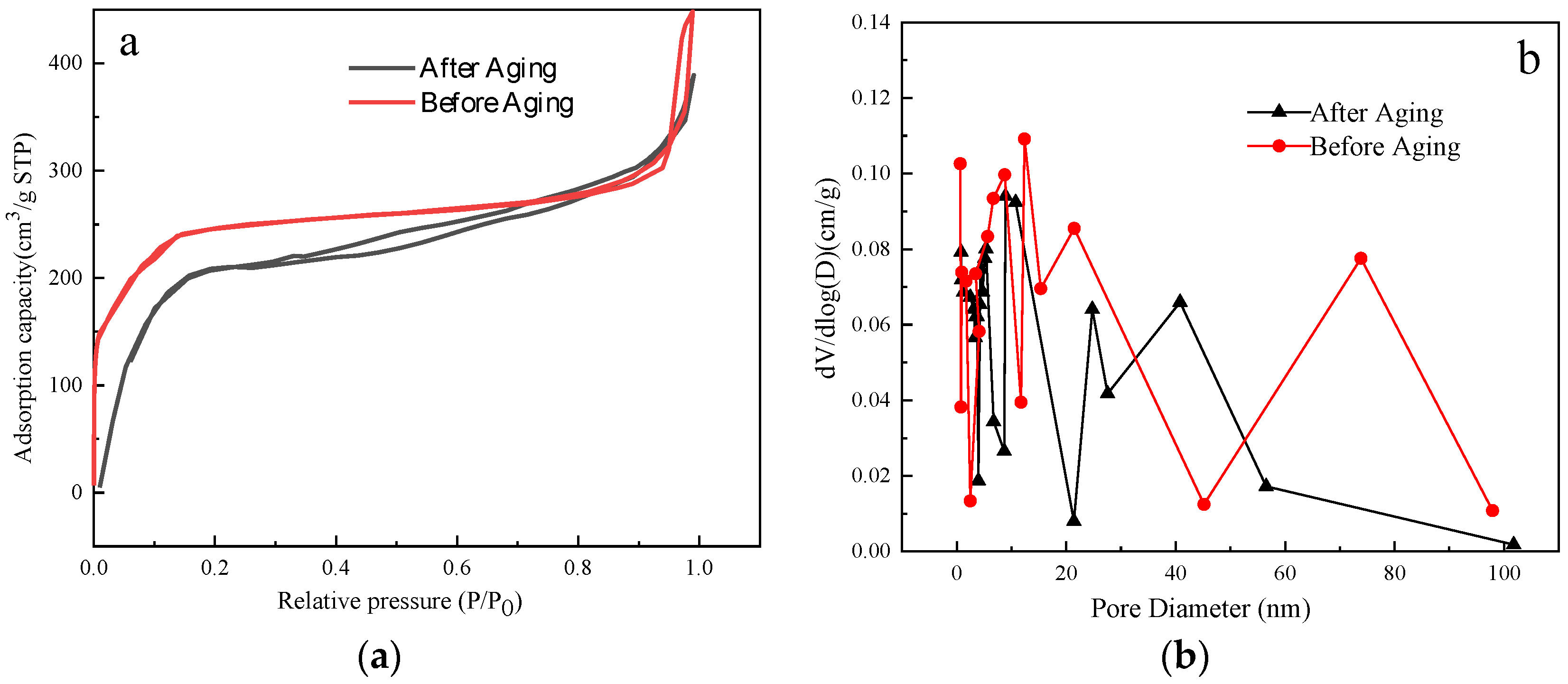
2.2.5. BET Analysis of Modified ZIF
2.2.6. Catalytic Performance of the Modified ZIF on SMP-3 and SPNH
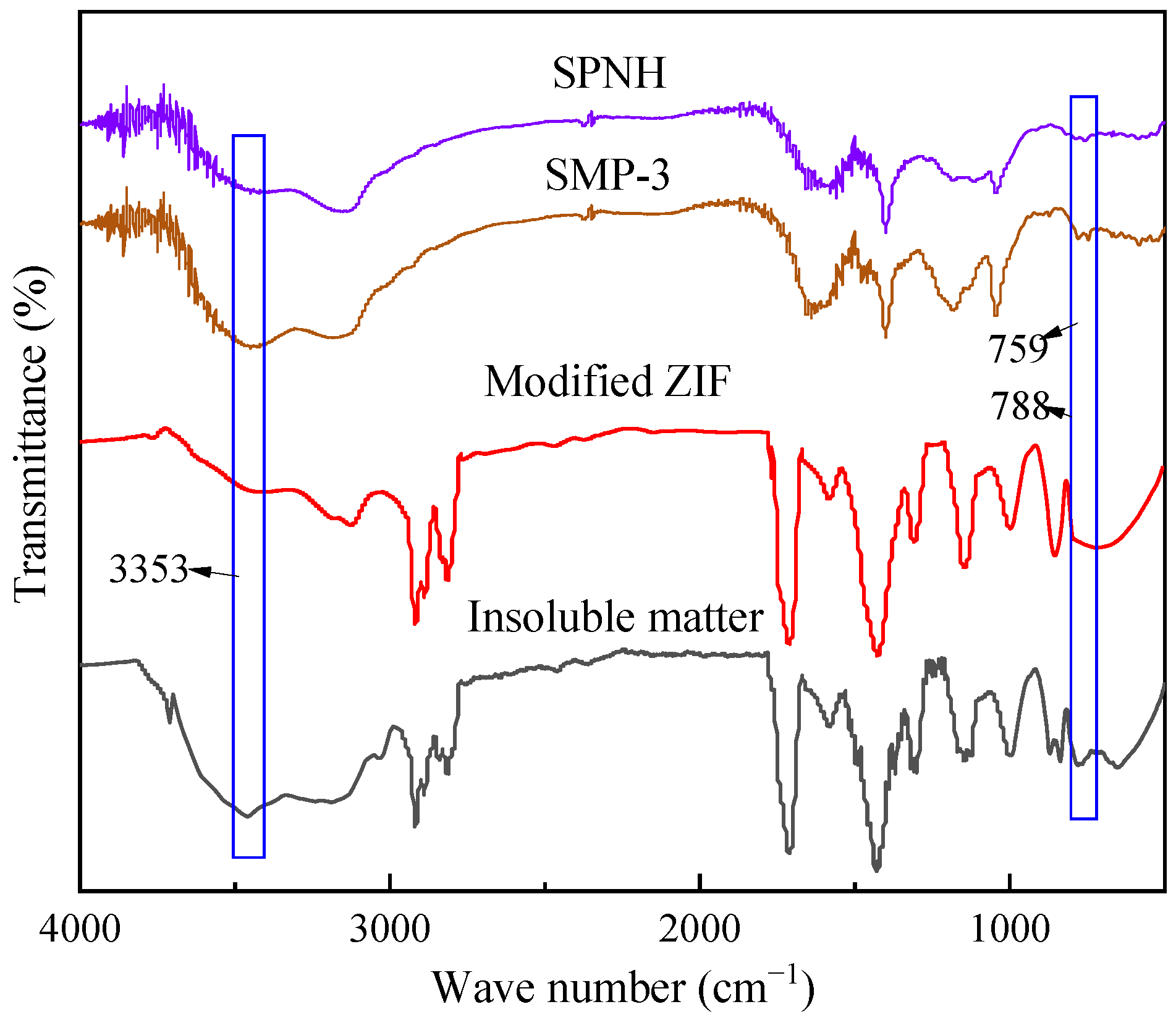
2.2.7. FITR Spectra of Insoluble Matter
2.2.8. Gel Permeation Chromatography (GPC) Determination of the Filtrate
2.2.9. Performance Evaluation of the Modified ZIF-Containing Drilling Fluid
3. Conclusions
4. Materials and Methods
4.1. Preparation of Modified ZIF
4.2. Structural Characteristics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, C.; Lei, G.; Hou, J.; Xu, X.; Wang, D.; Steenhuis, T.S. Enhanced Oil Recovery Using Micron-Size Polyacrylamide Elastic Microspheres: Underlying Mechanisms and Displacement Experiments. Ind. Eng. Chem. Res. 2015, 54, 10925–10934. [Google Scholar] [CrossRef]
- Rodrigues, R.K.; Martins, S.d.F.C.; Naccache, M.F.; de Souza Mendes, P.R. Rheological Modifiers in Drilling Fluids. J. Nonnewton. Fluid Mech. 2020, 286, 104397. [Google Scholar] [CrossRef]
- Dong, W.; Pu, X.; Ren, Y.; Zhai, Y.; Gao, F.; Xie, W. Thermoresponsive Bentonite for Water-Based Drilling Fluids. Materials 2019, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, X.; Li, L.; Yan, F.; Wang, J.; Zhang, Y. Preparation and Performance of an Oil-Soluble Polyethylene Wax Particles Temporary Plugging Agent. J. Chem. 2018, 2018, 7086059. [Google Scholar] [CrossRef]
- Hou, Y.; Li, H.; Xu, Y.; Niu, Q.; Wu, W. Influence of Polyethylene Glycol on the Deep Desulfurization of Catalytic Cracking Gasoline by Polyurethane Membranes via Pervaporation. Energy Fuels 2018, 32, 2089–2094. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Liu, Y.; Tongwa, P. Thermo-Dissoluble Polymer for in-Depth Mobility Control. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. [Google Scholar] [CrossRef]
- Du, G.; Peng, Y.; Pei, Y.; Zhao, L.; Wen, Z.; Hu, Z. Thermo-Responsive Temporary Plugging Agent Based on Multiple Phase Transition Supramolecular Gel. Energy Fuels 2017, 31, 9283–9289. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, S.; Wang, S.; Wei, B.; Yuan, C.; Li, Y. Investigation into the Migration of Polymer Microspheres (PMs) in Porous Media: Implications for Profile Control and Oil Displacement. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 540, 265–275. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z. Microgel Reinforced Composite Hydrogels with PH-Responsive, Self-Healing Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 468, 327–332. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, C.; Sang, H.; Zhao, Q. Mechanism Study of the Cross-Linking Reaction of Hydrolyzed Polyacrylamide/Ac3Cr in Formation Water. Energy Fuels 2015, 29, 4701–4710. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative Study of Using Nanoparticles for Enhanced Oil Recovery: Wettability Alteration of Carbonate Rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Han, Z.; Ram, M.K.; Kamal, R.; Alamro, T.; Goswami, D.Y.; Jotshi, C. Characterization of Molten Salt Doped with Different Size Nanoparticles of Al2O3. Int. J. Energy Res. 2019, 43, 3732–3745. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Li, M.; Xie, G. Synthesis of Core–Shell Micro/Nanoparticles and Their Tribological Application: A Review. Materials 2020, 13, 4590. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Li, X.; Dai, L.; Li, H.; Xue, B.; He, X.; Cao, W.; Lu, X. Experimental Study on Injection and Plugging Effect of Core-Shell Polymer Microspheres: Take Bohai Oil Reservoir as an Example. ACS Omega 2020, 5, 32112–32122. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Luo, P.; Sun, Y.; Pan, Y.; Sun, L. Experimental Study on the Optimization of Multi-Level Nano-Microsphere Deep Profile Control in the Process of Gas Injection in Fracture-Type Buried-Hill Reservoirs. ACS Omega 2021, 6, 24185–24195. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wu, X.; Kang, X.; Guan, B.; Li, X.; Ye, Y.; Xiao, P.; Wang, X.; Li, S. Experimental Investigation on a Novel Particle Polymer for Enhanced Oil Recovery in High Temperature and High Salinity Reservoirs. J. Chem. 2021, 2021, 5593038. [Google Scholar] [CrossRef]
- Lu, G.H.; Schneider, A.F.; Vanderpol, M.; Lu, E.K.; Wong, M.Y.; Brook, M.A. Tunable, Catalyst-Free Preparation of Silicone Gels. Ind. Eng. Chem. Res. 2021, 60, 15019–15026. [Google Scholar] [CrossRef]
- Zhang, K.; Schlottig, G.; Mengotti, E.; Quittard, O.; Lannuzzo, F. Study of moisture transport in silicone gel for IGBT modules. Microelectron. Reliab. 2020, 114, 113773. [Google Scholar] [CrossRef]
- Mitsumata, T.; Sugitani, K.; Koyama, K. Electrorheological response of swollen silicone gels containing barium titanate. Polymer 2004, 45, 6647–6654. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, B.; Chen, C.; Sarsenbekuly, B.; Yang, H.; Kang, W. Regulation of Polymerizable Modification Degree of Nano-SiO2 and the Effects on Performance of Composite Microsphere for Conformance Control. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124100. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Lv, K.; Liu, J.; Shen, H.; Zhang, F. Application of Core-Shell Structural Acrylic Resin/Nano-SiO2 Composite in Water Based Drilling Fluid to Plug Shale Pores. J. Nat. Gas Sci. Eng. 2018, 55, 418–425. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.X.; Lu, W. Study on the stability of foamed gel and its structural evolution characteristics. Combust. Sci. Technol. 2022, 2045283. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Zheng, W. Synthetic Copolymer (AM/AMPS/DMDAAC/SSS) as Rheology Modifier and Fluid Loss Additive at HTHP for Water-Based Drilling Fluids. J. Appl. Polym. Sci. 2019, 136, 47813. [Google Scholar] [CrossRef]
- Tian, X.; Song, N.; Yang, G.; Zhou, C. Organic-Sulfonate Functionalized Graphene as a High Temperature Lubricant for Efficient Antifriction and Antiwear in Water Based Drilling Fluid. Materials 2022, 70, 32. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, C.; Qiu, Z.; Leong, Y.K.; Zhong, H.; Cao, J. Synthesis, Characterization and Evaluation of a Quadripolymer with Low Molecular Weight as a Water Based Drilling Fluid Viscosity Reducer at High Temperature (245 °C). Polym. Int. 2015, 64, 1352–1360. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, Z.; Wang, P.; Luo, P.; Lin, Y. Fe3O4/Poly(Acrylic Acid) Hybrid Nanoparticles for Water-Based Drilling Fluids. J. Appl. Polym. Sci. 2016, 133, 4–9. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Wang, Y.; Dong, Y. Grafting Lignite with Sulformethal Phenoldehy Resin and Their Performance in Controlling Rheological and Filtration Properties of Water-Bentonite Suspensions at High Temperatures. J. Pet. Sci. Eng. 2016, 144, 84–90. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Ge, J.; Guo, H.; Wu, Q.; Mao, Y. Phenolic Resin Gel Suitable for Medium-Temperature and High-Salinity Reservoirs. J. Mol. Liq. 2022, 364, 119887. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Chen, Z.; Chen, W. Synthesis and Characterization of High-Temperature Self-Crosslinking Polymer Latexes and Their Application in Water-Based Drilling Fluid. Powder Technol. 2021, 389, 392–405. [Google Scholar] [CrossRef]
- Du, Y.P.; Luo, Z.H.; Yang, Y.; Yang, Y.; Yuan, W.J.; Li, H.; Hong, Y.Q.; Dai, Z.; Zhang, P.X.; Zhao, T. Theoretical and Experimental Investigations into the Pyrolysis Mechanisms of Silicon-Modified Phenolic Resin under High Temperatures. Carbon 2023, 201, 504–519. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; Zhang, M. Synthesis and Properties of a Betaine Type Copolymer Filtrate Reducer. Chem. Eng. Process.-Process Intensif. 2020, 153, 107953. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, J.; Wang, Y. A Polymer-Based Drilling Fluid with High Temperature, Salt and Calcium Resistance Property. IOP Conf. Ser. Earth Environ. Sci. 2019, 237, 052058. [Google Scholar] [CrossRef]
- Sheng, W.; Chuan, Z.; Chaopeng, Y.; Liyi, C. Rheological Properties of Polymer Drilling Fluid Developed for Permafrost Natural Gas Hydrate Drilling. Chem. Technol. Fuels Oils 2017, 53, 274–285. [Google Scholar] [CrossRef]
- Liu, L.; Pu, X.; Tao, H.; Deng, Q.; Luo, A. Synthesis and Characterization of Comb-Shaped Copolymer as a Filtration Reducer and Comparison with Counterparts. RSC Adv. 2018, 8, 11424–11435. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, G.; Li, H. Selective Binding and Removal of Guests in a Microporous Metal–Organic Framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Hardian, R.; Liang, Z.W.; Zhang, X.L.; Szekely, G. Artificial intelligence: The silver bullet for sustainable materials development. Green Chem. 2020, 22, 7521. [Google Scholar] [CrossRef]
- Liu, L.; Gou, S.; Fang, S.; He, Y.; Tang, L. Organic-Inorganic Microspheres of Temperature-Controlled Size for Profile Control. J. Mol. Liq. 2020, 317, 113993. [Google Scholar] [CrossRef]
- Halouani, M.; Abdelhedi, M.; Dammak, M.; Audebrand, N.; Ktari, L. Structural Studies and Conductivity of [Fe(O3C4)(COO)]·H2O Based H4btec (H4btec = 1,2,4,5-Benzenetetracarboxylic Acid) Manel. Open J. Inorg. Chem. 2013, 3, 100–108. [Google Scholar] [CrossRef][Green Version]
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H.L. Metal-Organic Frameworks (MOFs) beyond Crystallinity: Amorphous MOFs, MOF Liquids and MOF Glasses. J. Mater. Chem. A 2021, 9, 10562–10611. [Google Scholar] [CrossRef]
- Choi, S.; Kim, T.; Ji, H.; Lee, H.J.; Oh, M. Isotropic and Anisotropic Growth of Metal-Organic Framework (MOF) on MOF: Logical Inference on MOF Structure Based on Growth Behavior and Morphological Feature. J. Am. Chem. Soc. 2016, 138, 14434–14440. [Google Scholar] [CrossRef] [PubMed]
- Ikigaki, K.; Okada, K.; Tokudome, Y.; Toyao, T.; Falcaro, P.; Doonan, C.J.; Takahashi, M. MOF-on-MOF: Oriented Growth of Multiple Layered Thin Films of Metal–Organic Frameworks. Angew. Chem.-Int. Ed. 2019, 58, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, R.; Yang, Z.; Chai, L.; Jin, L.; Alhassan, S.I.; Ren, L.; Wang, H.; Huang, L. Preparation of MOFs and MOFs Derived Materials and Their Catalytic Application in Air Pollution: A Review. Catal. Today 2021, 375, 10–29. [Google Scholar] [CrossRef]
- Akbarzadeh, M.J.; Hashemian, S.; Moktarian, N. Structural and Magnetic Properties of Zeolitic Imidazolate Framework Supported on Nickel Titanate. J. Mol. Struct. 2021, 1240, 130555. [Google Scholar] [CrossRef]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Andre Mkhoyan, K.; Tsapatsis, M. Zeolitic Imidazolate Framework Membranes Made by Ligand-Induced Permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [CrossRef]
- Hovestadt, M.; Vargas Schmitz, J.; Weissenberger, T.; Reif, F.; Kaspereit, M.; Schwieger, W.; Hartmann, M. Scale-up of the Synthesis of Zeolitic Imidazolate Framework ZIF-4. Chem.-Ing.-Tech. 2017, 89, 1374–1378. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Battisti, A.; Taioli, S.; Garberoglio, G. Zeolitic Imidazolate Frameworks for Separation of Binary Mixtures of CO2, CH4, N2 and H2: A Computer Simulation Investigation. Microporous Mesoporous Mater. 2011, 143, 46–53. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal Stability of ZIF-8 under Oxidative and Inert Environments: A Practical Perspective on Using ZIF-8 as a Catalyst Support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Dai, C.; Yang, N.; Lei, Z. Gas Adsorption in Shaped Zeolitic Imidazolate Framework-8. Chin. J. Chem. Eng. 2015, 23, 1275–1282. [Google Scholar] [CrossRef]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Xiong, T. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: Fabrication, applications and perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Hou, J.; Sutrisna, P.D.; Zhang, Y.; Chen, V. Formation of Ultrathin, Continuous Metal-Organic Framework Membranes on Flexible Polymer Substrates. Angew. Chem. Int. Ed. 2016, 55, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Ragwhu, A.V.; Gadaginamath, G.S.; Jawalkar, S.S.; Halligudi, S.B.; Halligud, T.M. Synthesis, characterization, and molecular modeling studies of novel polyurethanes based on 2,2′-[ethane-1,2-diylbis(nitrilomethylylidene)]diphenol and 2,2′-[hexane-1,6-diylbis(nitrilomethylylidene)] diphenol hard segments. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6032–6046. [Google Scholar] [CrossRef]



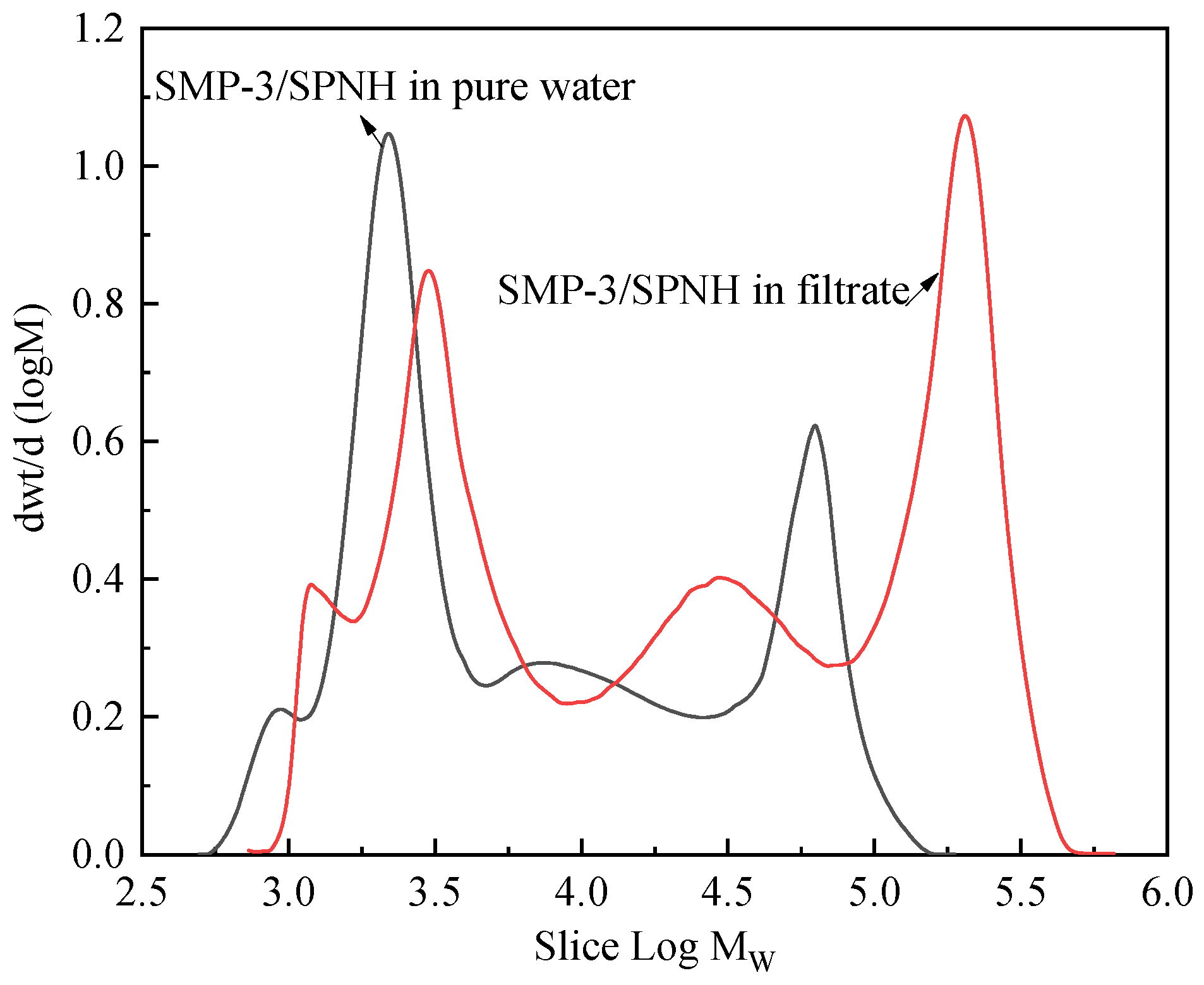

| Sample | State | Mn | Mw | Mp | Polydispersity |
|---|---|---|---|---|---|
| SMP-3/SPNH Mixture | Solution | 3897 | 36,544 | 2927 | 9.378 |
| Filtrate | 6651 | 71,235 | 187,374 | 10.712 |
| Drilling Fluid | Scenario | AV/(mPa·s) | PV/(mPa·s) | YP/Pa | FL(API)/mL | FL(HTHP)/mL |
|---|---|---|---|---|---|---|
| (1) Before replacement | Before aging | 56.5 | 43.0 | 13.5 | 2.6/0.5 mm | - |
| Aging for 16 h | 44.5 | 34.0 | 10.5 | 3.2/0.5 mm | 7.8/1.6 mm | |
| Aging for 32 h | 41.5 | 34.0 | 7.5 | 3.4/0.8 mm | 8.6/1.6 mm | |
| Aging for 48 h | 37.5 | 31.0 | 6.5 | 3.6/0.8 mm | 10.2/2.2 mm | |
| (1) After replacement | Before aging | 53.5 | 42.0 | 11.5 | 2.4/0.5 mm | - |
| Aging for 16 h | 51.5 | 40.5 | 11.0 | 2.4/0.6 mm | 7.0/1.2 mm | |
| Aging for 32 h | 53.5 | 41.0 | 12.5 | 2.2/0.6 mm | 7.4/1.4 mm | |
| Aging for 48 h | 56.5 | 43.0 | 13.5 | 2.4/0.6 mm | 7.4/1.4 mm | |
| (2) Before replacement | Before aging | 57.0 | 46.0 | 11.0 | 2.4/0.5 mm | - |
| Aging for 16 h | 44.5 | 35.5 | 9.0 | 3.2/0.7 mm | 7.6/1.4 mm | |
| Aging for 32 h | 42.5 | 35.0 | 7.5 | 3.4/0.8 mm | 8.8/1.8 mm | |
| Aging for 48 h | 39.5 | 33.0 | 6.5 | 3.8/0.8 mm | 10.8/2.2 mm | |
| (2) After replacement | Before aging | 56.5 | 43.0 | 13.5 | 2.4/0.5 mm | - |
| Aging for 16 h | 49.5 | 35.5 | 14.0 | 2.6/0.5 mm | 7.0/1.4 mm | |
| Aging for 32 h | 52.5 | 41.0 | 11.5 | 2.2/0.5 mm | 7.2/1.4 mm | |
| Aging for 48 h | 54.5 | 43.0 | 11.5 | 2.2/0.6 mm | 6.8/1.6 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Sun, J.; Luo, X. Preparation of MOF-Based Core-Shell Gel Particles with Catalytic Activity and Their Plugging Performance. Gels 2023, 9, 44. https://doi.org/10.3390/gels9010044
Liu F, Sun J, Luo X. Preparation of MOF-Based Core-Shell Gel Particles with Catalytic Activity and Their Plugging Performance. Gels. 2023; 9(1):44. https://doi.org/10.3390/gels9010044
Chicago/Turabian StyleLiu, Fengbao, Jinsheng Sun, and Xiao Luo. 2023. "Preparation of MOF-Based Core-Shell Gel Particles with Catalytic Activity and Their Plugging Performance" Gels 9, no. 1: 44. https://doi.org/10.3390/gels9010044
APA StyleLiu, F., Sun, J., & Luo, X. (2023). Preparation of MOF-Based Core-Shell Gel Particles with Catalytic Activity and Their Plugging Performance. Gels, 9(1), 44. https://doi.org/10.3390/gels9010044




