A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications
Abstract
1. Introduction
2. Gelling Agents
3. Conditions Necessary for the Formation of Gels
| Gelling Agent and Concentration | Source | Texture and Rheological Properties | Binding Blocks | Applications | Ref |
|---|---|---|---|---|---|
| Pectin (0.5–1%, w/w) | Heteropolysaccharides from higher terrestrial plant cell walls and fruits, such as citrus fruit, guava, and apple | Newtonian behavior | Partially esterified α-(1–4)-linked D-galactouronic and mannuronic acid; rhamnose, galactose, and arabinose can replace galacturonic acid | Jelly, jam, marmalade, fruit chews, and yogurt | [22] |
| Cellulose (0.5–2%, w/w) | Chemically modified plant cell walls | Shear thining crossmodelling and viscoelastic behavior | Homopolymer of D-glucose β-(1, 4) | Sweets and salad dressings | [22,23] |
| Agar (1–2%, w/w) | Red algae or seaweeds | Thermoreversible gels upon cooling; lowest viscosity at low shear rates; with increasing concentration of agarose, the particle size and particle surface decrease | Utilization of agarose and agaropectin together in a single product | Vegetarian gelatin and laxative | [22,24] |
| Guar gum (1–5%, w/w) | Endosperm | Although it is independent of electrolytes and has extremely high low-shear viscosity and high-shear thinning, it degrades and loses viscosity at high temperatures and low pH | Galactomannan molecular sequence | Sweets, yogurt, and various forms of liquid cheese, along with fillings for pastries | [25,26] |
| Carrageenan (0.5–3%, w/w) | Red seaweeds | High hardness, compressibility, adhesiveness, and cohesiveness | Sulfated D-galactose and L-anhydrogalactose | Desserts, cell/enzyme immobilization gel | [27,28] |
| Carob gum or locust bean gum (0.16–1.84%, w/w) | Carob tree seeds | High-shear thinning; very high low-shear viscosity, despite being degraded and losing viscosity regardless of the presence of electrolytes at high and low pH levels and high temperatures | Galactomannan | Glue | [29] |
| Xanthan gum (1–3%, w/w) | Xanthomonas campestris is responsible for the process of fermenting glucose, as well as sucrose | High-shear thinnability; maintains viscosity in the presence of electrolytes, high temperatures, and a wide pH range | Two -D-glucose units are linked at positions 1 and 4 along the polysaccharide chain; the primary structure has two mannose and one glucouronic acid, forming repeating modules of five sugar molecules | As emulsifiers, texture modifiers, fruit salads, and sauces, colloidal oil and solid ingredients are kept from turning into a cream | [30,31] |
| Alginate (1–2%, w/w) | Brown seaweeds | Pseudoplastic non-Newtonian and viscoelastic behavior | Different arrangements of (1-4)-linked -D-mannuronate and its C-5 epimer α-L-guluronate residues form a linear copolymer | Appetite-suppressant jellies, divalent ionic gelation, cell immobilization, and encapsulation | [32,33] |
| Gum Arabic (1–5%, w/w) | Acacia senegal and Acacia seyal sap | Low-viscosity gum; shear thinning occurs at low shear rates below 10/s, and near-Newtonian behavior occurs above shear rates of 100/s | Combination of saccharides and glycoproteins | Hard gummies, chocolates, and gums | [34,35] |
| Gelling Agents | Source | Binding Blocks | Applications | Ref |
|---|---|---|---|---|
| Whey protein | Acid/sweet dairy whey | β-lactoglobulin and α-lactalbumin | Thickener and gelling agent | [36] |
| Soya proteins | Soybeans | Glycinin and β-conglycinin interact | Heat-set gel | [37] |
| Egg proteins | Egg | Albumen is made up of almost 70% globular proteins with ovomucin fibers and 30% egg white | Confectionery gelling and bulking agent | [38] |
| Zein | Corn | Peptide chain (prolamine) | Encapsulations in candies, nuts, fruit, and pills, along with other foods and baked products | [39] |
| Gelatin | Animal skin and bones | Glycine–proline protein | Jelly, jam, yogurt, and margarine | [40] |
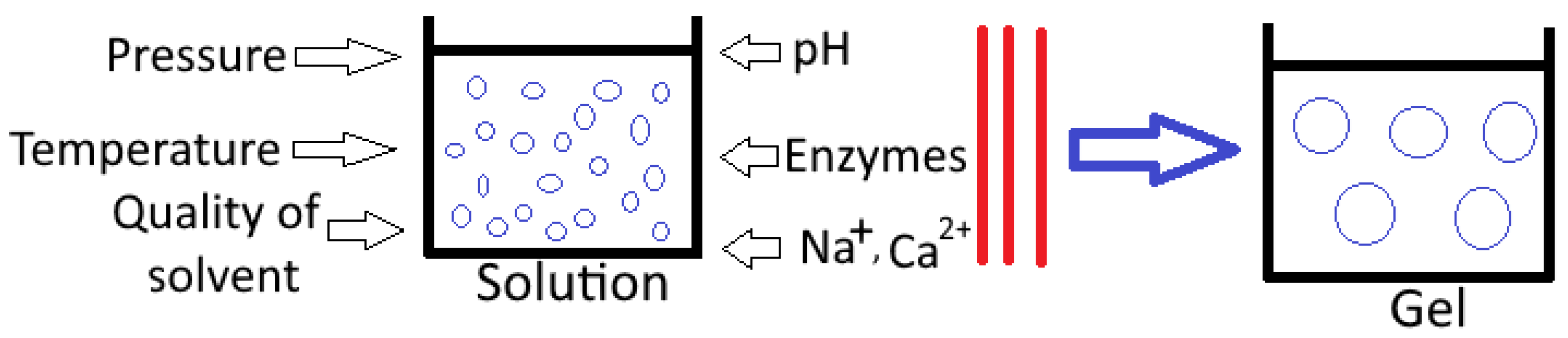
3.1. Pressure
3.2. pH
3.3. Ionic Strength
3.4. Temperature
3.5. Availability of Enzymes
3.6. Gelling Agent Concentration
3.7. Solvent Quality
4. Gel Types and Mechanism of Gel Formation
4.1. Polysaccharide Gels
4.1.1. Alginate
4.1.2. Pectin
4.1.3. Agar
4.1.4. Starch
4.1.5. Carrageenan
4.1.6. Gellan Gum
4.1.7. Cellulose Derivative Gels
Carboxymethyl Cellulose (CMC)
Hydroxypropyl Methyl Cellulose (HPMC)
Hydroxypropyl Cellulose (HPC)
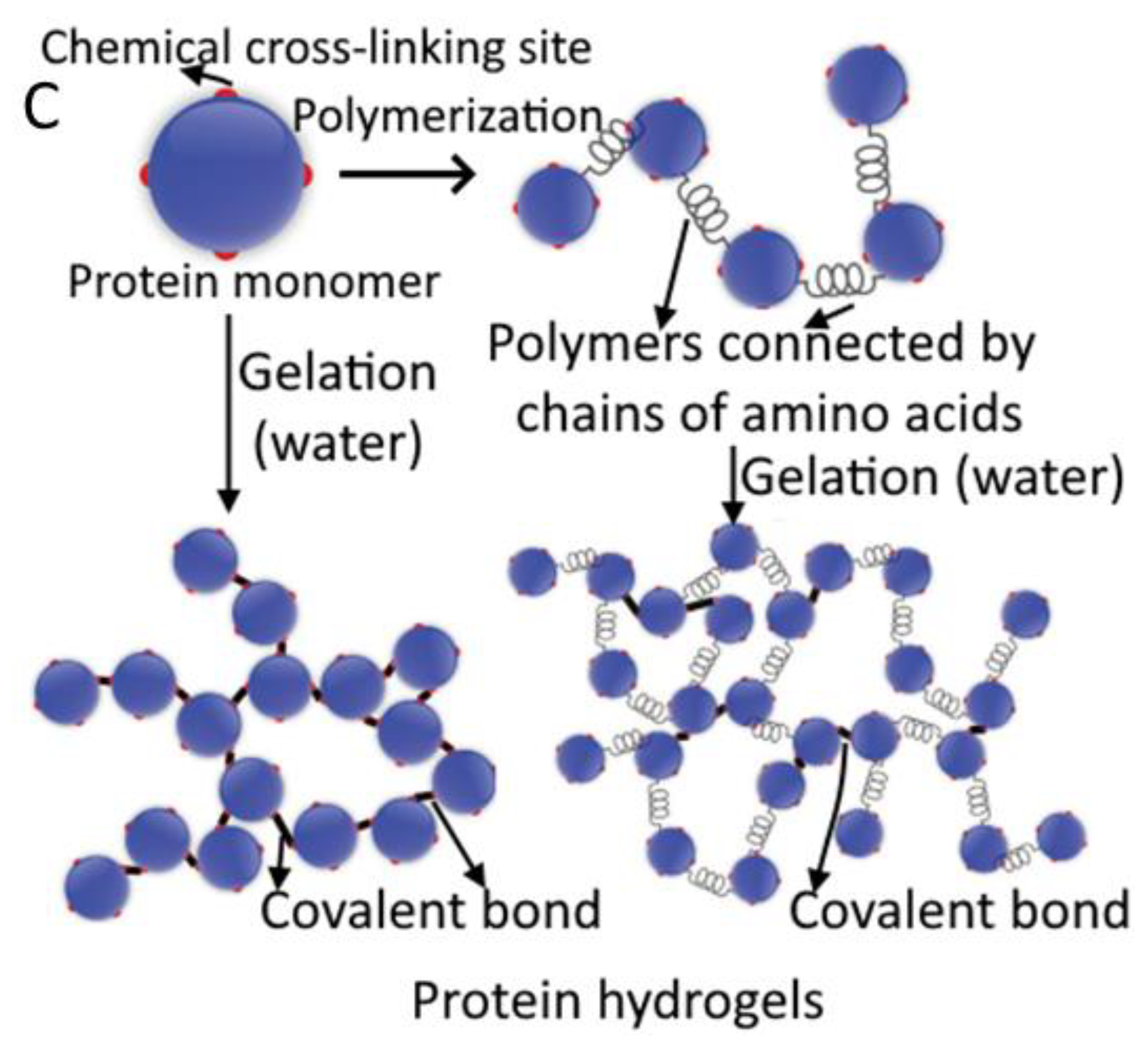
4.2. Protein Gels
4.2.1. Gelatin

4.2.2. Whey Proteins
4.2.3. Egg Albumin
4.2.4. Soy Proteins
4.2.5. Milk Proteins
5. Rheological Characterization of Gels
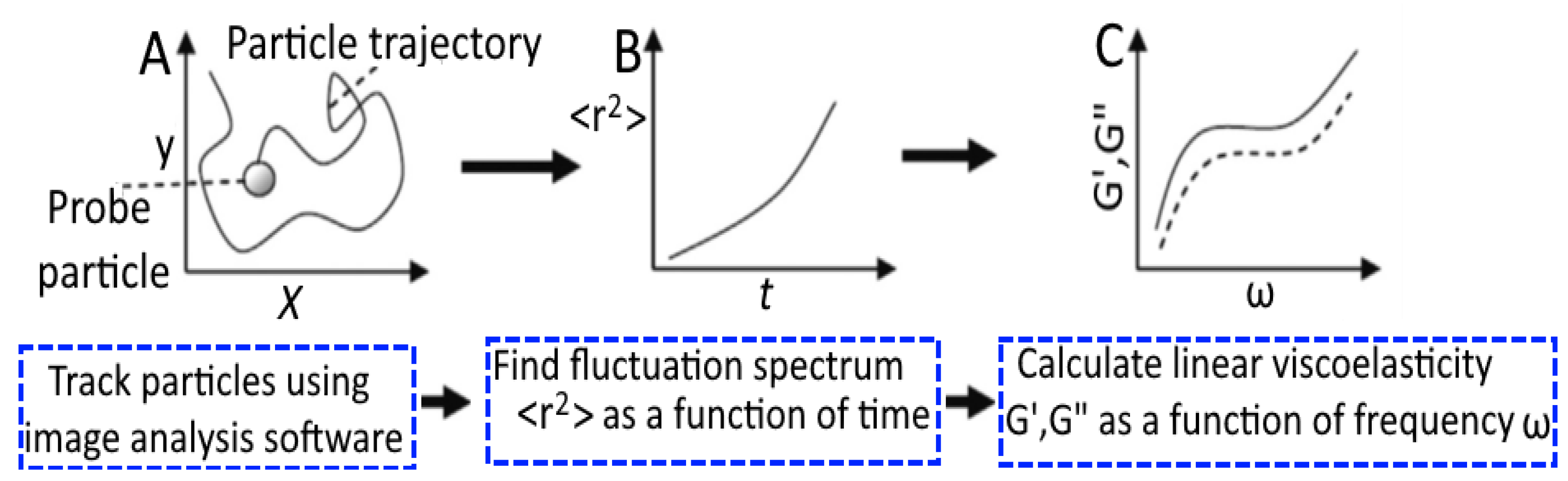
5.1. Microrheology of Gels
| for viscous medium | |
| for an elastic medium |
5.2. Oral Processing and Texture Perception of Gels
6. Network Structure and Strength
6.1. Interconnected Polymeric Networks
6.2. Strengthening Polymeric Networks
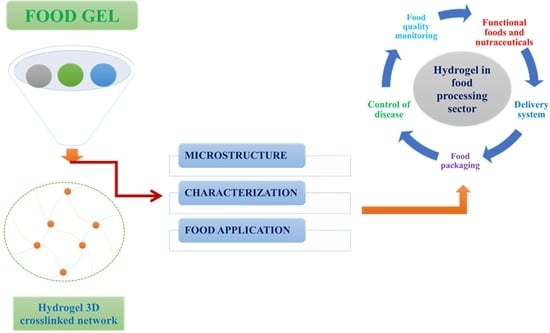
7. Application of Hydrogel-Based Formulations in the Food Industry
7.1. Fat Replacers
7.2. Encapsulation of Bioactive Compounds: Gel-Based Formulations
7.3. Delivery System
7.4. Calorie Control
7.5. Food Texture Perception
7.6. Risk Monitoring
7.7. Food Packaging Materials
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Siddiqui, S.A.; Nagdalian, A.A.; Blinova, A.A.; Gvozdenko, A.A.; Raffa, V.V.; Oboturova, N.P.; Golik, A.B.; Maglakelidze, D.G.; Ibrahim, S.A. Investigation of the influence of Zinc-containing compounds on the components of the colloidal phase of milk. Arab. J. Chem. 2021, 14, 103229. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme mimicry for combating bacteria and biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Singh, N.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Gao, Y.; Wang, D.; Jia, G.; Zhang, P.; Prakash, S.; Wang, Z.; Han, J. Understanding the rheological properties of a novel composite salecan/gellan hydrogels. Food Hydrocoll. 2022, 123, 107162. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Nazir, A.; Asghar, A.; Maan, A.A. Food gels: Gelling process and new applications. In Advances in Food Rheology and Its Applications; Ahmed, J., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 335–353. [Google Scholar]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Sala, G.; Stieger, M.; van de Velde, F. Serum release boosts sweetness intensity in gels. Food Hydrocoll. 2010, 24, 494–501. [Google Scholar] [CrossRef]
- Stieger, M.; van de Velde, F. Microstructure, texture and oral processing: New ways to reduce sugar and salt in foods. Curr. Opin. Colloid Interface Sci. 2013, 18, 334–348. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Tran, P.H.; Tran, T.T. A single-layer film coating for colon-targeted oral delivery. Int. J. Pharm. 2019, 559, 402–409. [Google Scholar] [CrossRef]
- Munir, N.; Hasnain, M.; Waqif, H.; Adetuyi, B.O.; Egbuna, C.; Olisah, M.C.; Chikwendu, C.J.; Uche, C.Z.; C Patrick-Iwuanyanwu, K.; El Sayed, A.M.A. Gelling agents, micro and nanogels in food system applications. In Application of Nanotechnology in Food Science, Processing and Packaging; Egbuna, C., Jeevanandam, J., Patrick-Iwuanyanwu, K.C., Onyeike, E.N., Eds.; Springer: Manhattan, NY, USA, 2022; pp. 153–167. [Google Scholar]
- Zhu, P.; Huang, W.; Chen, L. Develop and characterize thermally reversible transparent gels from pea protein isolate and study the gel formation mechanisms. Food Hydrocoll. 2022, 125, 107373. [Google Scholar] [CrossRef]
- Catauro, M.; Ciprioti, S.V. Characterization of hybrid materials prepared by sol-gel method for biomedical implementations. A critical review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- dos Santos Carvalho, J.D.; Rabelo, R.S.; Hubinger, M.D. Thermo-rheological properties of chitosan hydrogels with hydroxypropyl methylcellulose and methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef]
- Kornet, R.; Sridharan, S.; Venema, P.; Sagis, L.M.; Nikiforidis, C.V.; van der Goot, A.J.; Meinders, M.B.; van der Linden, E. Fractionation methods affect the gelling properties of pea proteins in emulsion-filled gels. Food Hydrocoll. 2022, 125, 107427. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Sun, Y.; Phuhongsung, P. Improving 3D/4D printing characteristics of natural food gels by novel additives: A review. Food Hydrocoll. 2022, 123, 107160. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Gousse, C.; Chanzy, H.; Cerrada, M.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 587–597. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [PubMed]
- Bashir, A.; Sharma, P.K.; Warsi, M.H. Extraction and characterization of xyloglucan (tamarind seed polysaccharide) as pharmaceutical excipient. WJPPS 2016, 6, 2209–2220. [Google Scholar]
- Torres, M.D.; Flórez-Fernández, N.; Dominguez, H. Ultrasound-assisted water extraction of mastocarpus stellatus carrageenan with adequate mechanical and antiproliferative properties. Mar. Drugs 2021, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Liu, Y.; Wu, T.; Zhang, M. Fabricating soy protein hydrolysate/xanthan gum as fat replacer in ice cream by combined enzymatic and heat-shearing treatment. Food Hydrocoll. 2018, 81, 39–47. [Google Scholar] [CrossRef]
- Singhvi, G.; Hans, N.; Shiva, N.; Dubey, S.K. Xanthan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 121–144. [Google Scholar]
- Jindal, N.; Khattar, J.S. Microbial polysaccharides in food industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 95–123. [Google Scholar]
- Lee, H.W.; Karim, M.R.; Ji, H.M.; Choi, J.H.; Ghim, H.D.; Park, S.M.; Oh, W.; Yeum, J.H. Electrospinning fabrication and characterization of poly (vinyl alcohol)/montmorillonite nanofiber mats. J. Appl. Polym. Sci. 2009, 113, 1860–1867. [Google Scholar] [CrossRef]
- Roopa, B.; Bhattacharya, S. Alginate gels: I. Characterization of textural attributes. J. Food Eng. 2008, 85, 123–131. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Xiao, L.; Ould Eleya, M. Whey protein concentrate hydrogels as bioactive carriers. J. Appl. Polym. Sci. 2006, 99, 2470–2476. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Jena, R. Gelling behavior of defatted soybean flour dispersions due to microwave treatment: Textural, oscillatory, microstructural and sensory properties. J. Food Eng. 2007, 78, 1305–1314. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor made protein based aerogel particles from egg white protein, whey protein isolate and sodium caseinate: Influence of the preceding hydrogel characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar]
- Dissanayake, M.; Vasiljevic, T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Sci. 2009, 92, 1387–1397. [Google Scholar] [CrossRef]
- Lucey, J.; Singh, H. Formation and physical properties of acid milk gels: A review. Food Res. Int. 1997, 30, 529–542. [Google Scholar]
- Bryant, C.; McClements, D. Molecular basis of cold-setting whey protein ingredients. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar]
- Alting, A.C.; de Jongh, H.H.; Visschers, R.W.; Simons, J.-W.F. Physical and chemical interactions in cold gelation of food proteins. J. Agric. Food Chem. 2002, 50, 4682–4689. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Kessler, H.G. Properties of mixed and filled-type dairy gels. J. Food Sci. 1989, 54, 1213–1217. [Google Scholar] [CrossRef]
- Heinen, L.; Heuser, T.; Steinschulte, A.; Walther, A. Antagonistic enzymes in a biocatalytic pH feedback system program autonomous DNA hydrogel life cycles. Nano Lett. 2017, 17, 4989–4995. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Chronakis, I.S. Crosslinking of milk proteins by microbial transglutaminase: Utilization in functional yogurt products. Food Chem. 2018, 245, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Reddy, C.K.; Huang, K.; Chen, L.; Xu, B. Hydrocolloidal properties of flaxseed gum/konjac glucomannan compound gel. Int. J. Biol. Macromol. 2019, 133, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Burey, P.; Bhandari, B.; Howes, T.; Gidley, M. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Richter, S. Recent gelation studies on irreversible and reversible systems with dynamic light scattering and rheology—A concise summary. Macromol. Chem. Phys. 2007, 208, 1495–1502. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Mustafa, S.; Che Man, Y.B. Microbial polysaccharides and their modification approaches: A review. Int. J. Food Prop. 2015, 18, 332–347. [Google Scholar] [CrossRef]
- Vilgis, T.A. Gels: Model systems for soft matter food physics. Curr. Opin. Food Sci. 2015, 3, 71–84. [Google Scholar] [CrossRef]
- Nishinari, K.; Zhang, H. Recent advances in the understanding of heat set gelling polysaccharides. Trends Food Sci. Technol. 2004, 15, 305–312. [Google Scholar] [CrossRef]
- McClements, D.J. Non-covalent interactions between proteins and polysaccharides. Biotechnol. Adv. 2006, 24, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 3–26. [Google Scholar]
- Lapomarda, A.; Pulidori, E.; Cerqueni, G.; Chiesa, I.; De Blasi, M.; Geven, M.A.; Montemurro, F.; Duce, C.; Mattioli-Belmonte, M.; Tiné, M.R. Pectin as rheology modifier of a gelatin-based biomaterial ink. Materials 2021, 14, 3109. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar]
- Chan, S.Y.; Owh, C.; Choo, W.S.; Young, D.J.; Loh, X.J.; Li, P.; Huang, W. Pectin as a rheology modifier: Chemistry, production, and rheology. Mater. Sci. Technol. 2006, 24, 621–625. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biotechnology of microbial polysaccharides in food. In FoodBiotechnology, 1st ed.; Pometto, A., Shetty, K., Paliyath, G., Levin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 220–247. [Google Scholar]
- Mizrahi, S. Syneresis in food gels and its implications for food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 324–348. [Google Scholar]
- Gallant, D.; Bouchet, B.; Buleon, A.; Perez, S. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur. J. Clin. Nutr. 1992, 46, S3–S16. [Google Scholar] [PubMed]
- Wang, Q.; Rademacher, B.; Sedlmeyer, F.; Kulozik, U. Gelation behaviour of aqueous solutions of different types of carrageenan investigated by low-intensity-ultrasound measurements and comparison to rheological measurements. Innov. Food Sci. Emerg. Technol. 2005, 6, 465–472. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Robinson, G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef]
- Viebke, C.; Piculell, L.; Nilsson, S. On the mechanism of gelation of helix-forming biopolymers. Macromolecules 1994, 27, 4160–4166. [Google Scholar] [CrossRef]
- Babbar, S.; Jain, R.; Walia, N. Guar gum as a gelling agent for plant tissue culture media. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 258–261. [Google Scholar] [CrossRef]
- Jain, R.; Babbar, S.B. Guar gum and isubgol as cost-effective alternative gelling agents for in vitro multiplication of an orchid, Dendrobium chrysotoxum. Curr. Sci. 2005, 88, 292–295. [Google Scholar]
- Astrini, N.; Anah, L.; Haryono, A. Crosslinking parameter on the preparation of cellulose based hydrogel with divynilsulfone. Procedia Chem. 2012, 4, 275–281. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Eladl, M.; Abou-Baker, N.H. Lignocellulosic biomass for the preparation of cellulose-based hydrogel and its use for optimizing water resources in agriculture. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Motamedi, E.; Sadeghian Motahar, S.F.; Maleki, M.; Kavousi, K.; Ariaeenejad, S.; Moosavi-Movahedi, A.A.; Hosseini Salekdeh, G. Upgrading the enzymatic hydrolysis of lignocellulosic biomass by immobilization of metagenome-derived novel halotolerant cellulase on the carboxymethyl cellulose-based hydrogel. Cellulose 2021, 28, 3485–3503. [Google Scholar]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [PubMed]
- Mali, K.; Dhawale, S.; Dias, R.; Dhane, N.; Ghorpade, V. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Baiya, C.; Nannuan, L.; Tassanapukdee, Y.; Chailapakul, O.; Songsrirote, K. The synthesis of carboxymethyl cellulose-based hydrogel from sugarcane bagasse using microwave-assisted irradiation for selective adsorption of copper (II) ions. Environ. Prog. Sustain. Energy 2019, 38, S157–S165. [Google Scholar] [CrossRef]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl cellulose-based hydrogel: Dielectric study, antimicrobial activity and biocompatibility. Arab. J. Sci. Eng. 2021, 46, 17–30. [Google Scholar]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar]
- Sethi, S.; Kaith, B.; Kumar, V. Fabrication and characterization of microwave assisted carboxymethyl cellulose-gelatin silver nanoparticles imbibed hydrogel: Its evaluation as dye degradation. React. Funct. Polym. 2019, 142, 134–146. [Google Scholar] [CrossRef]
- Sharmeen, S.; Rahman, M.S.; Islam, M.M.; Islam, M.S.; Shahruzzaman, M.; Mallik, A.K.; Haque, P.; Rahman, M.M. Application of polysaccharides in enzyme immobilization. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 357–395. [Google Scholar]
- Kukrety, A.; Singh, R.K.; Singh, P.; Ray, S.S. Comprehension on the synthesis of carboxymethylcellulose (CMC) utilizing various cellulose rich waste biomass resources. Waste Biomass Valorization 2018, 9, 1587–1595. [Google Scholar]
- Koh, M.H. Preparation and Characterization of Carboxymethyl Cellulose from Sugarcane Bagasse. Ph.D. Thesis, Universiti Tunku Abdul Rahman, Petaling Jaya, Malaysia, 2013. Available online: http://eprints.utar.edu.my/895/1/CE-2013-1004861.pdf (accessed on 21 October 2022).
- Haqiqi, M.T.; Bankeeree, W.; Lotrakul, P.; Pattananuwat, P.; Punnapayak, H.; Ramadhan, R.; Kobayashi, T.; Amirta, R.; Prasongsuk, S. Antioxidant and UV-blocking properties of a carboxymethyl cellulose–lignin composite film produced from oil palm empty fruit bunch. ACS Omega 2021, 6, 9653–9666. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.; Aruan, H.K.; Herfananda, A.L. Hydrogel and effects of crosslinking agent on cellulose-based hydrogels: A review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Gårdebjer, S.; Larsson, M.; Gebäck, T.; Skepö, M.; Larsson, A. An overview of the transport of liquid molecules through structured polymer films, barriers and composites–Experiments correlated to structure-based simulations. Adv. Colloid Interface Sci. 2018, 256, 48–64. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Tassin, J.-F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Nanocellulose in biotechnology and medicine: Focus on skin tissue engineering and wound healing. Nanomateirals 2019, 9, 164. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of flavours and fragrances into polymeric capsules and cyclodextrins inclusion complexes: An update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of radiation-induced cross-linked hydroxypropylcellulose. Macromol. Mater. Eng. 2002, 287, 285–295. [Google Scholar] [CrossRef]
- Lu, C.; Wang, C.; Zhang, D.; Wang, J.; Yong, Q.; Chu, F. Ultra-strong hydroxypropyl cellulose/polyvinyl alcohol composite hydrogel by combination of triple-network and mechanical training. Int. J. Biol. Macromol. 2021, 184, 200–208. [Google Scholar] [CrossRef]
- Van den Berg, L.; Rosenberg, Y.; Van Boekel, M.A.; Rosenberg, M.; Van de Velde, F. Microstructural features of composite whey protein/polysaccharide gels characterized at different length scales. Food Hydrocoll. 2009, 23, 1288–1298. [Google Scholar] [CrossRef]
- He, H.; Cao, X.; Dong, H.; Ma, T.; Payne, G.F. Reversible programing of soft matter with reconfigurable mechanical properties. Adv. Funct. Mater. 2017, 27, 1605665. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, S.; Han, D.; Ding, Z.; Zeng, S.; Xiao, X. Construction and evaluation of the hydroxypropyl methyl cellulose-sodium alginate composite hydrogel system for sustained drug release. J. Polym. Res. 2018, 25, 148. [Google Scholar] [CrossRef]
- Hanson, B.S.; Brown, C.P.; Laurent, H.; Hughes, M.D.G.; Dougan, L. Hierarchical biomechanics: Student engagement activities with a focus on biological physics. Phys. Educ. 2020, 55, 025015. [Google Scholar] [CrossRef]
- De Jong, S.; Klok, H.J.; Van de Velde, F. The mechanism behind microstructure formation in mixed whey protein–polysaccharide cold-set gels. Food Hydrocoll. 2009, 23, 755–764. [Google Scholar] [CrossRef]
- Laneuville, S.I.; Turgeon, S.L. Microstructure and stability of skim milk acid gels containing an anionic bacterial exopolysaccharide and commercial polysaccharides. Int. Dairy J. 2014, 37, 5–15. [Google Scholar] [CrossRef]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef]
- Fertsch, B.; Müller, M.; Hinrichs, J. Firmness of pressure-induced casein and whey protein gels modulated by holding time and rate of pressure release. Innov. Food Sci. Emerg. Technol. 2003, 4, 143–150. [Google Scholar] [CrossRef]
- Keogh, M.K.; Lainé, K.I.; O’connor, J.F. Rheology of sodium caseinate-carrageenan mixtures. J. Texture Stud. 1996, 26, 635–652. [Google Scholar] [CrossRef]
- Foegeding, E.A. Rheology and sensory texture of biopolymer gels. Curr. Opin. Colloid Interface Sci. 2007, 12, 242–250. [Google Scholar] [CrossRef]
- Herranz, B.; Tovar, C.A.; Solo-de-Zaldívar, B.; Borderias, A.J. Effect of alkalis on konjac glucomannan gels for use as potential gelling agents in restructured seafood products. Food Hydrocoll. 2012, 27, 145–153. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C. The effect of egg albumen on the viscoelasticity of crab sticks made from Alaska Pollock and Pacific Whiting surimi. Food Hydrocoll. 2009, 23, 1641–1646. [Google Scholar] [CrossRef]
- Moreno, H.M.; Dominguez-Timon, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of gels made with different commercial pea protein isolate: Rheological, structural and functional properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]
- Borderías, A.J.; Tovar, C.A.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by pea protein isolates. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walter, K. An Introduction to Rheology, 1st ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1989; pp. 1–201. [Google Scholar]
- Moschakis, T. Microrheology and particle tracking in food gels and emulsions. Curr. Opin. Colloid Interface Sci. 2013, 18, 311–323. [Google Scholar] [CrossRef]
- Grimm, M.; Jeney, S.; Franosch, T. Brownian motion in a Maxwell fluid. Soft Matter 2011, 7, 2076–2084. [Google Scholar] [CrossRef]
- Ishihara, S.; Nakauma, M.; Funami, T.; Tanaka, T.; Nishinari, K.; Kohyama, K. Electromyography during oral processing in relation to mechanical and sensory properties of soft gels. J. Texture Stud. 2011, 42, 254–267. [Google Scholar] [CrossRef]
- Kohyama, K.; Mioche, L.; Bourdio3, P. Influence of age and dental status on chewing behaviour studied by EMG recordings during consumption of various food samples. Gerodontology 2003, 20, 15–23. [Google Scholar] [CrossRef]
- Pascua, Y.; Koç, H.; Foegeding, E.A. Food structure: Roles of mechanical properties and oral processing in determining sensory texture of soft materials. Curr. Opin. Colloid Interface Sci. 2013, 18, 324–333. [Google Scholar] [CrossRef]
- De Silva, D.A. Characterization of Single Network and Interpenetrating Network Hydrogels of Natural and Synthetic Polymers. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2013. Available online: https://ro.uow.edu.au/theses/4042 (accessed on 10 November 2022).
- Liu, K.; Li, Q.-M.; Zha, X.-Q.; Pan, L.-H.; Bao, L.-J.; Zhang, H.-L.; Luo, J.-P. Effects of calcium or sodium ions on the properties of whey protein isolate-lotus root amylopectin composite gel. Food Hydrocoll. 2019, 87, 629–636. [Google Scholar] [CrossRef]
- Sorde, K.L.; Ananthanarayan, L. Effect of transglutaminase treatment on properties of coconut protein-guar gum composite film. LWT 2019, 115, 108422. [Google Scholar] [CrossRef]
- Fan, M.; Hu, T.; Zhao, S.; Xiong, S.; Xie, J.; Huang, Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chem. 2017, 218, 221–230. [Google Scholar] [CrossRef]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Wu, H.; Wu, L.; Wu, Y.; Jia, L.; Pang, J.; Sun, Y.-m. Effect of molecular characteristics of Konjac glucomannan on gelling and rheological properties of Tilapia myofibrillar protein. Carbohydr. Polym. 2016, 150, 21–31. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Yang, X.; Gong, T.; Lu, Y.-h.; Li, A.; Sun, L.; Guo, Y. Compatibility of sodium alginate and konjac glucomannan and their applications in fabricating low-fat mayonnaise-like emulsion gels. Carbohydr. Polym. 2020, 229, 115468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zeng, R.; Zhang, F.; Kan, J. Effects of sodium carboxymethyl cellulose on rheological properties and gelation behaviors of sodium alginate induced by calcium ions. LWT 2019, 103, 131–138. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Liang, B.; Wu, T.; Sui, W.; Zhang, M. Microparticulated whey protein-pectin complex: A texture-controllable gel for low-fat mayonnaise. Food Res. Int. 2018, 108, 151–160. [Google Scholar] [CrossRef]
- Diamantino, V.R.; Costa, M.S.; Taboga, S.R.; Vilamaior, P.S.; Franco, C.M.; Penna, A.L.B. Starch as a potential fat replacer for application in cheese: Behaviour of different starches in casein/starch mixtures and in the casein matrix. Int. Dairy J. 2019, 89, 129–138. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Ge, Q.; Yu, H.; Xiong, Y.L. Rheology and microstructure of myofibrillar protein–starch composite gels: Comparison of native and modified starches. Int. J. Biol. Macromol. 2018, 118, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zha, X.-Q.; Shen, W.-D.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. The hydrogel of whey protein isolate coated by lotus root amylopectin enhance the stability and bioavailability of quercetin. Carbohydr. Polym. 2020, 236, 116009. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Gautam, M.; Santhiya, D. Pectin/PEG food grade hydrogel blend for the targeted oral co-delivery of nutrients. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 637–644. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Cargnin, M.A.; Gasparin, B.C.; dos Santos Rosa, D.; Paulino, A.T. Performance of lactase encapsulated in pectin-based hydrogels during lactose hydrolysis reactions. LWT 2021, 150, 111863. [Google Scholar] [CrossRef]
- Jin, W.; Xiang, L.; Peng, D.; Liu, G.; He, J.; Cheng, S.; Li, B.; Huang, Q. Study on the coupling progress of thermo-induced anthocyanins degradation and polysaccharides gelation. Food Hydrocoll. 2020, 105, 105822. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Soler, C.; Lopez-Rubio, A. Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll. 2016, 61, 128–138. [Google Scholar] [CrossRef]
- Zhou, T.; Li, J.; Liu, P. Ionically crosslinked alginate-based nanohydrogels for tumor-specific intracellular triggered release: Effect of chemical modification. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 180–186. [Google Scholar] [CrossRef]
- Wang, A.; Lin, J.; Zhong, Q. Enteric rice protein-shellac composite coating to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 2021, 113, 106469. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Ghauri, Y.M.; Alvi, T.; Amin, U.; Khan, M.I.; Nazir, A.; Saeed, F.; Aadil, R.M.; Nadeem, M.T.; Babu, I. Microwave assisted drying and extraction technique; kinetic modelling, energy consumption and influence on antioxidant compounds of fenugreek leaves. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, B.; Yadav, M.P.; Feng, L.; Yan, J.; Jia, X.; Yin, L. Corn fiber gum-soybean protein isolate double network hydrogel as oral delivery vehicles for thermosensitive bioactive compounds. Food Hydrocoll. 2020, 107, 105865. [Google Scholar] [CrossRef]
- Alvi, T.; Asif, Z.; Khan, M.K.I. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique—A review. Food Biosci. 2022, 46, 101580. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Adhikari, B.; Wang, M. Microencapsulation of Sichuan pepper essential oil in soybean protein isolate-Sichuan pepper seed soluble dietary fiber complex coacervates. Food Hydrocoll. 2022, 125, 107421. [Google Scholar] [CrossRef]
- Kandekar, U.; Ramdasi, S.; Thakurdesai, P. Fenugreek: Novel delivery technologies and versatile formulation excipients. In Fenugreek, 1st ed.; Ghosh, D., Thakurdesai, P., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 363–402. [Google Scholar]
- Yadav, H.; Agrawal, R.; Panday, A.; Patel, J.; Maiti, S. Polysaccharide-silicate composite hydrogels: Review on synthesis and drug delivery credentials. J. Drug Deliv. Sci. Technol. 2022, 74, 103573. [Google Scholar] [CrossRef]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. Fundamentals of composites containing fibrous materials and hydrogels: A review on design and development for food applications. Food Chem. 2021, 364, 130329. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef]
- Almagro, L.; Correa-Sabater, J.M.; Sabater-Jara, A.B.; Pedreño, M.Á. Biotechnological production of β-carotene using plant in vitro cultures. Planta 2022, 256, 41. [Google Scholar] [CrossRef]
- Park, S.; Mun, S.; Kim, Y.-R. Effect of xanthan gum on lipid digestion and bioaccessibility of β-carotene-loaded rice starch-based filled hydrogels. Food Res. Int. 2018, 105, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Kim, Y.-R.; McClements, D.J. Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015, 173, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Chen, L.; Tong, Q.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Montes, C.; Villaseñor, M.J.; Rios, A. Analytical control of nanodelivery lipid-based systems for encapsulation of nutraceuticals: Achievements and challenges. Trends Food Sci. Technol. 2019, 90, 47–62. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Rayner, M.; Östbring, K.; Purhagen, J. Application of natural polymers in food. In Natural Polymers; Springer: Cham, Switzerland, 2016; pp. 115–161. [Google Scholar]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, S.D.; Paunov, V.N. Structuring and calorie control of bakery products by templating batter with ultra melt-resistant food-grade hydrogel beads. Food Funct. 2017, 8, 2967–2973. [Google Scholar] [CrossRef]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New insights into food hydrogels with reinforced mechanical properties: A review on innovative strategies. Adv. Colloid Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Stribiţcaia, E.; Krop, E.M.; Lewin, R.; Holmes, M.; Sarkar, A. Tribology and rheology of bead-layered hydrogels: Influence of bead size on sensory perception. Food Hydrocoll. 2020, 104, 105692. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar]
- Qi, F.; Meng, Z.; Xue, M.; Qiu, L. Recent advances in self-assemblies and sensing applications of colloidal photonic crystals. Anal. Chim. Acta 2020, 1123, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Meng, Z.; Xue, M.; Zhang, H.; Shea, K.J.; Kang, L. Detection of lysozyme in body fluid based on two-dimensional colloidal crystal sensor. Microchem. J. 2020, 157, 105073. [Google Scholar] [CrossRef]
- Nesic, A.R.; Seslija, S.I. The influence of nanofillers on physical–chemical properties of polysaccharide-based film intended for food packaging. In Food Packaging: Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2017; Volume 7, pp. 637–697. [Google Scholar]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Attaran, S.A.; Hassan, A.; Wahit, M.U. Materials for food packaging applications based on bio-based polymer nanocomposites: A review. J. Thermoplast. Compos. Mater. 2017, 30, 143–173. [Google Scholar] [CrossRef]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Véronique, C. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar]
- Kerry, J.; O’grady, M.; Hogan, S. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications–A review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
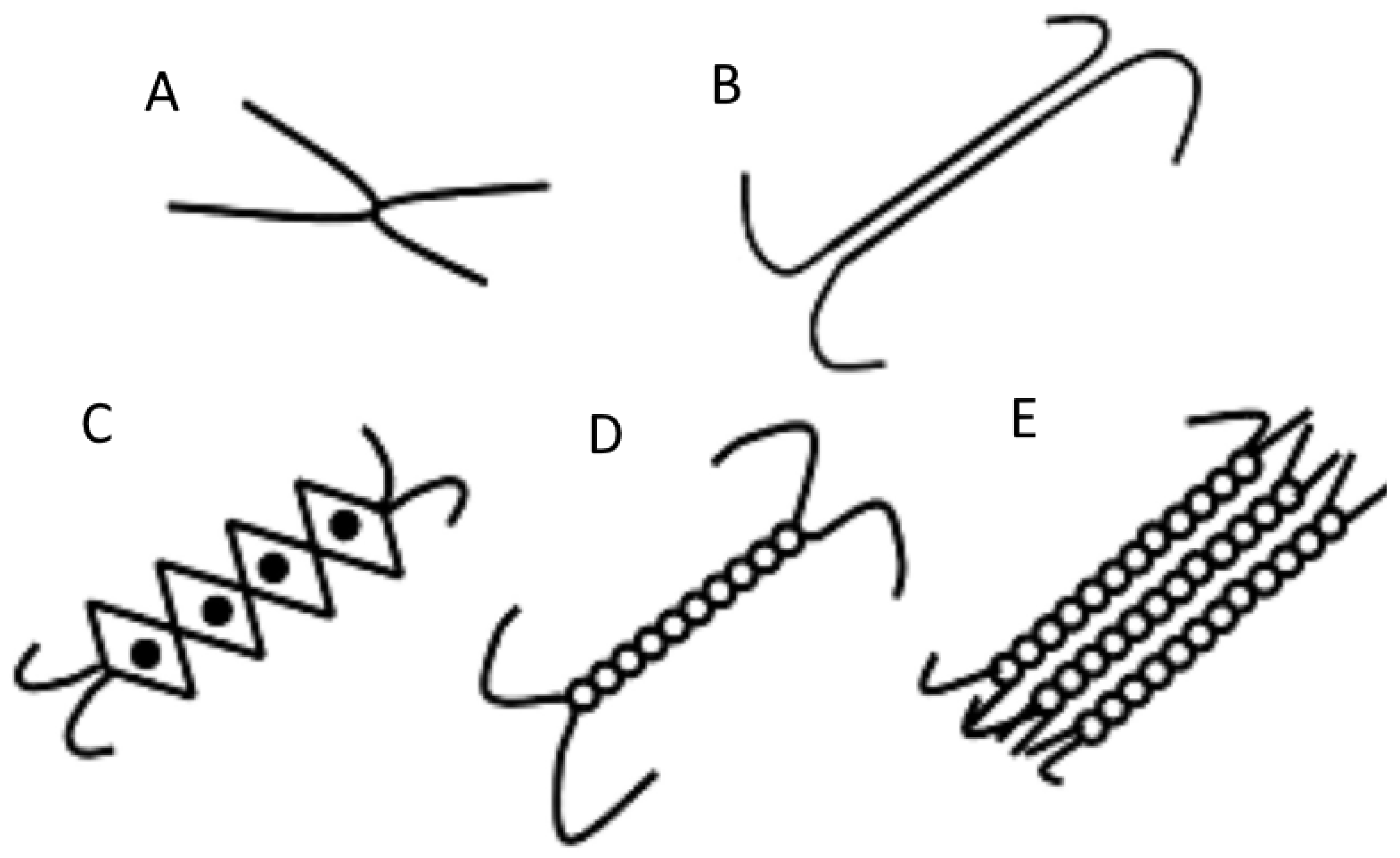
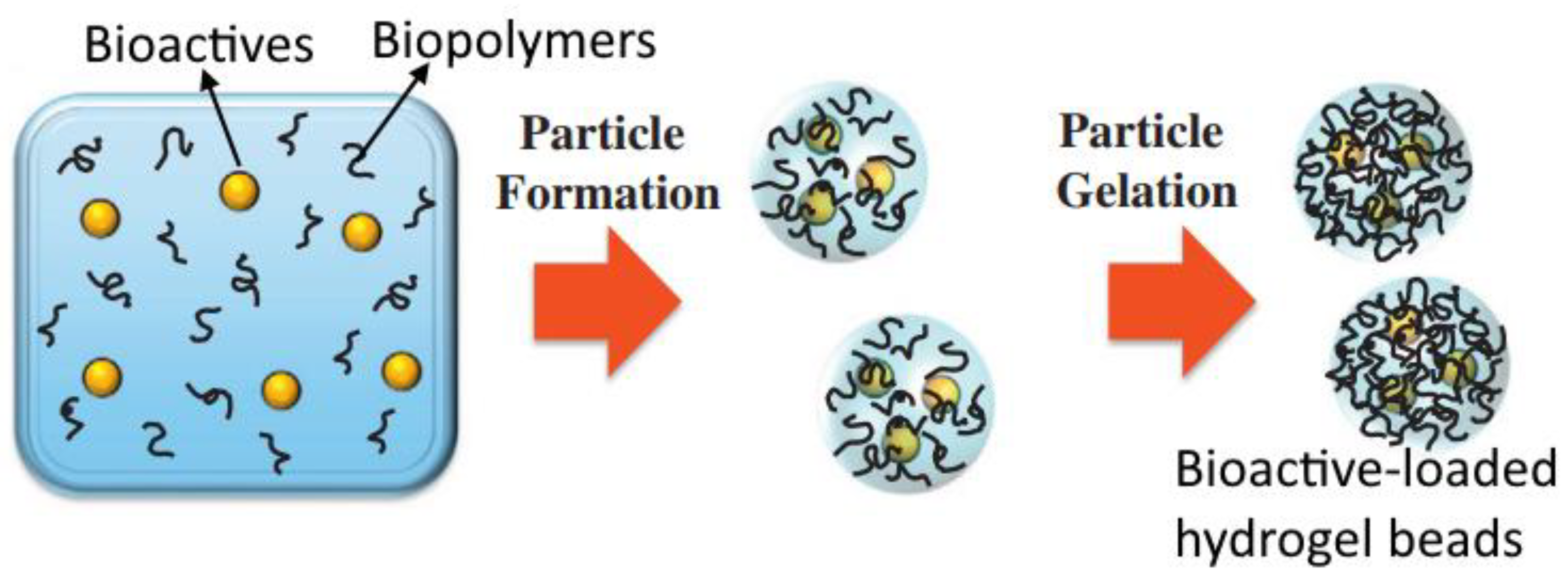
| Hydrogels | Form | Bioactive Compound | Encapsulation Efficiency (%) | Load Capacity | Ref. |
|---|---|---|---|---|---|
| Pectin-based | Core-shell hydrogel beads | Lactase | 72 | * | [129] |
| Xanthan gum | Biopolymer gels | Anthocyanin | * | * | [130] |
| Gelatin and chitosan | Gelation | Epigallocatechin gallate | 95 | * | [131] |
| Alginate-based nanohydrogel | Nanohydrogel | DOX (anticancer drug) | 90 | 35% | [132] |
| Rice-protein varnish | Nanocomposite | Apigenin | 91 | 92.5 mg·g−1 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2023, 9, 1. https://doi.org/10.3390/gels9010001
Nath PC, Debnath S, Sridhar K, Inbaraj BS, Nayak PK, Sharma M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels. 2023; 9(1):1. https://doi.org/10.3390/gels9010001
Chicago/Turabian StyleNath, Pinku Chandra, Shubhankar Debnath, Kandi Sridhar, Baskaran Stephen Inbaraj, Prakash Kumar Nayak, and Minaxi Sharma. 2023. "A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications" Gels 9, no. 1: 1. https://doi.org/10.3390/gels9010001
APA StyleNath, P. C., Debnath, S., Sridhar, K., Inbaraj, B. S., Nayak, P. K., & Sharma, M. (2023). A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels, 9(1), 1. https://doi.org/10.3390/gels9010001