Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling
Abstract
:1. Introduction
2. Research Progress of Functional Gel Materials and Their Applications
2.1. Research Progress of Gels for Profile Control and Water Shutoff Purposes
2.2. Research Progress of Gels for Lost Circulation Prevention and Control
2.3. Research Progress of Gels for Hydraulic Fracturing
3. Research Progress on Improving the Temperature Resistance of Gels and Their Applications
4. Research Progress on High Temperature Resistant Gel Temporary Plugging Materials and Their Applications
5. Research Progress on Gel Breaking Methods and Applications
5.1. Oxidizing Gel Breaking Method
5.2. Enzyme Gel Breaking Method
5.3. Acid Gel Breaking Method
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Yang, X.; Liu, C.; Kang, Y.; Bai, Y.; You, Z. Dynamic fracture width prediction for lost circulation control and formation damage prevention in ultra-deep fractured tight reservoir. Fuel 2022, 307, 121770. [Google Scholar] [CrossRef]
- Sun, J.; Bai, Y.; Cheng, R.; Lyu, K.; Liu, F.; Feng, J.; Lei, S.; Zhang, J.; Hao, H. Research progress and prospect of plugging technologies for fractured formation with severe lost circulation. Pet. Explor. Dev. 2021, 48, 732–743. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Kang, Y.; Zhang, J.; Bai, Y.; Zhang, J.; You, Z. Physical plugging of lost circulation fractures at microscopic level. Fuel 2022, 317, 123477. [Google Scholar] [CrossRef]
- Lei, S.; Sun, J.; Bai, Y.; Lv, K.; Zhang, S.; Liu, F.; Zhang, J. Plugging performance and mechanism of temperature-responsive adhesive lost circulation material. J. Pet. Sci. Eng. 2022, 217, 110771. [Google Scholar] [CrossRef]
- Yan, X.; Kang, Y.; Xu, C.; Xu, F.; Shang, X.; Bai, Y.; Jing, H. Impact of friction coefficient on the mesoscale structure evolution under shearing of granular plugging zone. Powder Technol. 2021, 394, 133–148. [Google Scholar] [CrossRef]
- He, Y.; Xiong, S.; Yang, Z.; Ruan, X.; Gong, Y. The Research on Cross-linking Polymer Gel as In-Depth Profile Control Agent. Pet. Sci. Technol. 2009, 27, 1300–1311. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, H.; Li, J.; Cheng, R.; Wang, M.; Bai, X.; Liu, Y.; Sun, Y.; Dai, C. Experimental study of acrylamide monomer polymer gel for water plugging in low temperature and high salinity reservoir. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2948–2959. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.; Wei, Y.; Jie, F.; Huang, M.; Huang, Y.; Xu, J.; Wang, L.; Xu, L. An autonomously self-gel-breaking, highly reservoir protective completion fluid tailored for gel-free processing in multi-lateral well construction. J. Pet. Sci. Eng. 2022, 211, 110136. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Y.; Chen, P.; Sun, L.; Liu, Y.; Gao, C. A double network conductive gel with robust mechanical properties based on polymerizable deep eutectic solvent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130349. [Google Scholar] [CrossRef]
- Jia, H.; Ren, Q.; Zhao, J. Swelling Mechanism Investigation of Microgel with Double-Cross-Linking Structures. Energy Fuels 2014, 28, 6735–6744. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, E.; Cui, X. Study on Profile Control and Water Shut-Off Performance of Interpenetrating Network Polymer Gel Composite System in Shallow Low Temperature Fractured Oil Layer. ChemistrySelect 2019, 4, 8158–8164. [Google Scholar] [CrossRef]
- Magzoub, M.I.; Salehi, S.; Hussein, I.A.; Nasser, M.S. Loss circulation in drilling and well construction: The significance of applications of crosslinked polymers in wellbore strengthening: A review. J. Pet. Sci. Eng. 2020, 185, 106653. [Google Scholar] [CrossRef]
- Pu, L.; Xu, P.; Xu, M.; Song, J.; He, M. Lost circulation materials for deep and ultra-deep wells: A review. J. Pet. Sci. Eng. 2022, 214, 110404. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Song, Z.; Zheng, B.; Zhang, K. A review on mechanism and adaptive materials of temporary plugging agent for chemical diverting fracturing. J. Pet. Sci. Eng. 2022, 212, 110256. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure-property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Zuo, Y.; Wei, M.; Wang, H.; Chen, Z.; Shang, N.; Pei, P. A self-designed double cross-linked gel for flexible zinc-air battery with extreme conditions adaptability. Chem. Eng. J. 2023, 451, 138622. [Google Scholar] [CrossRef]
- Xiong, Z.; Fu, F.; Zou, Z.; Li, X.; Tao, S.; Li, Y. Development and application of guar gum crosslinked gel with adjustable gelation time for total loss treatment. Petroleum, 2022; in press. [Google Scholar]
- Nie, X.; Luo, P.; Wang, P.; Zhang, X.; Yang, L. Rheology of a New Gel Used for Severe Lost Circulation Control. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010; p. 132136. [Google Scholar]
- Yin, H.; Yin, X.; Cao, R.; Zeng, P.; Wang, J.; Wu, D.; Luo, X.; Zhu, Y.; Zheng, Z.; Feng, Y. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 2022, 432, 134350. [Google Scholar] [CrossRef]
- Pirgalıoğlu, S.; Özbelge, T.A.; Özbelge, H.Ö.; Bicak, N. Crosslinked polyDADMAC gels as highly selective and reusable arsenate binding materials. Chem. Eng. J. 2015, 262, 607–615. [Google Scholar] [CrossRef]
- Du, D.-J.; Pu, W.-F.; Tan, X.; Liu, R. Experimental study of secondary crosslinking core-shell hyperbranched associative polymer gel and its profile control performance in low-temperature fractured conglomerate reservoir. J. Pet. Sci. Eng. 2019, 179, 912–920. [Google Scholar] [CrossRef]
- Shi, X.; Yue, X.A. Migration and plugging mechanisms of self-aggregated microspheres as a novel profile control. J. Pet. Sci. Eng. 2020, 184, 106458. [Google Scholar] [CrossRef]
- Zhao, G.; You, Q.; Tao, J.; Gu, C.; Aziz, H.; Ma, L.; Dai, C. Preparation and application of a novel phenolic resin dispersed particle gel for in-depth profile control in low permeability reservoirs. J. Pet. Sci. Eng. 2018, 161, 703–714. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Gao, M.; Yang, Z.; Fang, J.; Wu, Y. Investigation on Preparation and Profile Control Mechanisms of the Dispersed Particle Gels (DPG) Formed from Phenol–Formaldehyde Cross-linked Polymer Gel. Ind. Eng. Chem. Res. 2016, 55, 6284–6292. [Google Scholar] [CrossRef]
- Han, X.; Liguo, Z.; Liu, Y.; Wang, Q.; Wang, C. Experimental study of polyacrylamide polymer gel for profile controlling in high-temperature offshore reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Cao, W.; Xie, K.; Wang, X.; Lu, X.; He, X.; Xu, G.; Li, X. Starch graft copolymer and polymer gel applied in Bohai oilfield for water plugging and profile control and their mechanisms. Geosyst. Eng. 2020, 23, 197–204. [Google Scholar] [CrossRef]
- Bai, Y.; Shang, X.; Wang, Z.; Zhao, X. Experimental study of low molecular weight polymer/nanoparticle dispersed gel for water plugging in fractures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 95–107. [Google Scholar] [CrossRef]
- Yang, H.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.; Tang, X.; Kang, W. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.; Zhou, Q.; Fu, C.; He, S. Preparation and Experimental Study of a Low-Initial-Viscosity Gel Plugging Agent. ACS Omega 2020, 5, 15715–15727. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Zhang, Y.; Wang, Z.; Wang, Z.; Liu, C. Successful Applications of a Novel Compound Lost Circulation Additive with Variable Structure. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2018; p. 192403. [Google Scholar]
- Hamza, A.; Shamlooh, M.; Hussein, I.A.; Nasser, M.; Salehi, S. Polymeric formulations used for loss circulation materials and wellbore strengthening applications in oil and gas wells: A review. J. Pet. Sci. Eng. 2019, 180, 197–214. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, M.; Qin, J.; Sun, H.; Zhang, H.; Zhi, K.; Zhu, D. Mechanism study of water control and oil recovery improvement by polymer gels based on nuclear magnetic resonance. J. Pet. Sci. Eng. 2022, 209, 109881. [Google Scholar] [CrossRef]
- Jiang, G.; Deng, Z.; He, Y.; Li, Z.; Ni, X. Cross-linked polyacrylamide gel as loss circulation materials for combating lost circulation in high temperature well drilling operation. J. Pet. Sci. Eng. 2019, 181, 106250. [Google Scholar] [CrossRef]
- Liu, X.; Aughenbaugh, K.; Lee, H.; Nair, S.; Oort, E.V. Geopolymer—Synthetic Based Mud Hybrid Cements for Primary Cementing and Lost Circulation Control. In Proceedings of the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 January 2017; p. D011S002R001. [Google Scholar]
- Johnson, L.; Murphy, P.; Arsanious, K. Improvements in Lost-Circulation Control During Drilling Using Shear-Sensitive Fluids. In Proceedings of the Canadian International Petroleum Conference, Calgary, AL, Canada, 1 June 2000; p. 2062. [Google Scholar]
- Quinn, D.; Sunde, E.; Baret, J.F. Mechanism of a Novel Shear-Sensitive Plugging Fluid to Cure Lost Circulation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1999; p. 50722. [Google Scholar]
- Liu, D.; Fan, M.; Yao, L.; Zhao, X.; Wang, Y. A new fracturing fluid with combination of single phase microemulsion and gelable polymer system. J. Pet. Sci. Eng. 2010, 73, 267–271. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Guo, J.; Lai, J.; Wang, D.; He, L.; Qin, Y. A study of relation between suspension behavior and microstructure and viscoelastic property of guar gum fracturing fluid. J. Pet. Sci. Eng. 2014, 124, 432–435. [Google Scholar] [CrossRef]
- Song, J.; Fan, W.; Long, X.; Zhou, L.; Wang, C.; Li, G. Rheological behaviors of fluorinated hydrophobically associating cationic guar gum fracturing gel. J. Pet. Sci. Eng. 2016, 146, 999–1005. [Google Scholar] [CrossRef]
- Hurnaus, T.; Plank, J. Synthesis, characterization and performance of a novel phosphate-modified fluid loss additive useful in oil well cementing. J. Nat. Gas Sci. Eng. 2016, 36, 165–174. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, G.; Wang, C.; Zhu, Q.; Yang, L.; Wang, L.; Zhang, X.; Liu, C. A New High-Temperature Shear-Tolerant Supramolecular Viscoelastic Fracturing Fluid. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Singapore, 22–24 August 2016; p. D021S013R004. [Google Scholar]
- Jiang, G.; Dong, T.; Cui, K.; He, Y.; Quan, X.; Yang, L.; Fu, Y. Research status and development directions of intelligent drilling fluid technologies. Pet. Explor. Dev. 2022, 49, 660–670. [Google Scholar] [CrossRef]
- Bai, B.; Leng, J.; Wei, M. A comprehensive review of in-situ polymer gel simulation for conformance control. Pet. Sci. 2022, 19, 189–202. [Google Scholar] [CrossRef]
- Hakiki, F.; Arifurrahman, F. Cross-Linked and Responsive Polymer: Gelation Model and Review. J. Ind. Eng. Chem. 2022; in press. [Google Scholar]
- Kim, Y.; Kim, S.; Noh, S.; Kim, S.; Park, G.; Le, T.-H.; Han, H.; Kim, Y.A.; Yoon, H. Single-walled carbon nanotube-mediated physical gelation of binary polymer blends: An efficient route to versatile porous carbon electrode materials. Chem. Eng. J. 2018, 353, 849–857. [Google Scholar] [CrossRef]
- Long, Y.; Yu, B.; Zhu, C. Conformance Improvement for Ultra-High-Temperature Reservoir: A Comparative Study between Hydrostable and Conventional Preformed Particle Gel. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018; p. D041S099R001. [Google Scholar]
- Chen, L.; Zhu, X.; Fu, M.; Zhao, H.; Li, G.; Zuo, J. Experimental study of calcium-enhancing terpolymer hydrogel for improved oil recovery in ultrodeep carbonate reservoir. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 251–259. [Google Scholar] [CrossRef]
- Chang, F.F.; Ali, S.A.; Cromb, J.; Bowman, M.; Parlar, M. Development of a New Crosslinked-HEC Fluid Loss Control Pill for Highly-Overbalanced, High-Permeability and/or High Temperature Formations. In Proceedings of the SPE Formation Damage Control Conference, Lafayette, LA, USA, 18–19 February 1998; p. 39438. [Google Scholar]
- Zhuang, Y.; Pandey, S.N.; McCool, C.S.; Willhite, G.P. Permeability Modification with Sulfomethylated Resorcinol-Formaldehyde Gel System. SPE Reserv. Eval. Eng. 2000, 3, 386–393. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, F.; Pu, C. Mechanism of formation of chromium acetate(Cr3+)/phenol-formaldehyde resin prepolymer (PRP) complex and its compound cross-linking reaction with polymer for conformance control. J. Pet. Sci. Eng. 2019, 179, 675–683. [Google Scholar] [CrossRef]
- Almoshin, A.M.; Alsharaeh, E.; Fathima, A.; Bataweel, M. A Novel Polymer Nanocomposite Graphene Based Gel for High Temperature Water Shutoff Applications. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2018; p. 192358. [Google Scholar]
- Jia, H.; Niu, C.-C.; Yang, X.-Y. Improved understanding nanocomposite gel working mechanisms: From laboratory investigation to wellbore plugging application. J. Pet. Sci. Eng. 2020, 191, 107214. [Google Scholar] [CrossRef]
- Wu, H.; Ge, J.; Yang, L.; Yang, Y.; Zhang, T.; Guo, H. Developments of polymer gel plug for temporary blocking in SAGD wells. J. Pet. Sci. Eng. 2022, 208, 109650. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhu, D.-Y.; Gong, Y.-L.; Qin, J.-H.; Liu, X.-N.; Pi, Y.-H.; Zhao, Q.; Luo, R.-T.; Wang, W.-S.; Zhi, K.-K.; et al. Degradable preformed particle gel as temporary plugging agent for low-temperature unconventional petroleum reservoirs: Effect of molecular weight of the cross-linking agent. Pet. Sci. 2022, 19, 3182–3193. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, H.; Feng, Y.; Li, J.; Yang, Y.; She, J. A composite temporary plugging technology for hydraulic fracture diverting treatment in gas shales: Using degradable particle/powder gels (DPGs) and proppants as temporary plugging agents. J. Pet. Sci. Eng. 2022, 216, 110851. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Z.-H.; Wang, G.-Z.; Xiong, Y.-L.; Lv, Q.-C.; Cao, Q.; Niu, Q.-Q.; Wang, Y.-B.; Dong, Z.-X. 2D Janus polymer nanosheets for enhancing oil recovery: From material preparation to property evaluation. Pet. Sci. 2022; in press. [Google Scholar]
- Du, J.; Liu, J.; Zhao, L.; Liu, P.; Chen, X.; Wang, Q.; Yu, M. Water-soluble polymers for high-temperature resistant hydraulic fracturing: A review. J. Nat. Gas Sci. Eng. 2022, 104, 104673. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, D.; Bai, B. Experimental study of degradable preformed particle gel (DPPG) as temporary plugging agent for carbonate reservoir matrix acidizing to improve oil recovery. J. Pet. Sci. Eng. 2021, 205, 108760. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Liao, R.; Zhang, G.; Zhang, M.; Li, X. Study of adhesive self-degrading gel for wellbore sealing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129567. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2021, 202, 108416. [Google Scholar] [CrossRef]
- Urdahl, H.; Kettl, F.C.; Crossley, E.G.; Williams, T.A. Experience With Temporarily Sealing Leaking Tubing Annuli With Extended-Life Polymer Gel Plugs in the Greater Ekofisk Area. In Proceedings of the European Petroleum Conference, Stavanger, Norway, 24–27 May 1992; p. 24982. [Google Scholar]
- Cole, R.C.; Ali, S.A.; Foley, K.A. A New Environmentally Safe Crosslinked Polymer for Fluid-Loss Control. In Proceedings of the SPE Production Operations Symposium, Tulsa, OK, USA, 22–24 April 1995; p. 29525. [Google Scholar]
- Evans, B. Fluid Loss Control—Improvement for HTHP Wells. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 9–11 March 2003; p. 80946. [Google Scholar]
- Khater, M.R.; Uddin, S.; Al-Rubaiyea, J.A.; Rai, A.R.; Gazi, N. Isolation of a Horizontal Hole Section in an Openhole Well Using a NonDamaging Temporary Gel Plug to Facilitate Hydrocarbon Production from the Remaining Lateral Section—A Case History from Kuwait. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference, Abu Dhabi, United Arab Emirates, 20–22 October 2001; p. 72291. [Google Scholar]
- Wilson, A. Novel, Thermally Stable Fluid-Loss Pill Performs Better Than Guar-Based Gels. J. Pet. Technol. 2013, 65, 134–136. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Gao, Z.; Wu, Y.; Dai, C. The spontaneous imbibition mechanisms for enhanced oil recovery by gel breaking fluid of clean fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129568. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, G.; Long, X.; Wang, T.; Zhang, X.; Liang, L.; Bai, J.; Li, Z.; Qiu, L.; Yang, X.; et al. Hooked gemini viscoelastic surfactant based on linolenic oil for oil recovery and its various gel-breaking mechanisms. J. Pet. Sci. Eng. 2021, 204, 108717. [Google Scholar] [CrossRef]
- Qiu, L.; Shen, Y.; Wang, C.; Yang, X. Scanning electron microscopy analysis of guar gum in the dissolution, gelation and gel-breaking process. Polym. Test. 2018, 68, 95–99. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, X.; Ma, C.; Li, Y.; He, X. Controlled-release chemicals in oilfield application: A review. J. Pet. Sci. Eng. 2022, 215, 110616. [Google Scholar] [CrossRef]
- Zuo, M.; Liu, T.; Han, J.; Tang, Y.; Yao, F.; Yuan, Y.; Qian, Z. Preparation and characterization of microcapsules containing ammonium persulfate as core by in situ polymerization. Chem. Eng. J. 2014, 249, 27–33. [Google Scholar] [CrossRef]
- Yang, J.-B.; Sun, J.-S.; Bai, Y.-R.; Lv, K.-H.; Wang, Z.-Y.; Xu, C.-Y.; Dai, L.-Y.; Wang, R. Review of the application of environmentally responsive gels in drilling and oil recovery engineering: Synthetic materials, mechanism, and application prospect. J. Pet. Sci. Eng. 2022, 215, 110581. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Zhang, Z.; Yang, B.; Zhang, W.; Zhang, H. Preparation of a novel ultra-high temperature low-damage fracturing fluid system using dynamic crosslinking strategy. Chem. Eng. J. 2018, 354, 913–921. [Google Scholar] [CrossRef]
- Ma, X.; Song, P.; Liu, L.; Da, Q.A.; Lei, G.; Yao, C.; Shor, L.M. Low-temperature pH-regulable gel-breaking of galactomannan-based fracturing fluids by the mannanase from Bacillus aerius. Int. Biodeterior. Biodegrad. 2021, 160, 105226. [Google Scholar] [CrossRef]
- Gupta, D.V.S.; Pakulski, M.K.; Prasek, B.; Franklin, V.F. High-pH-Tolerant Enzyme Breaker for Oilfield Applications. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 27–29 March 1992; p. 23986. [Google Scholar]
- Reddy, B.R. Laboratory Characterization of Gel Filter Cake and Development of Non-Oxidizing Gel Breakers for Zirconium Crosslinked Fracturing Fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Woodlands, TX, USA, 8–10 April 2013; p. 64116. [Google Scholar]
- Battistel, E.; Bianchi, D.; Fornaroli, M.; Cobianco, S. Enzymes breakers for viscosity enhancing polymers. J. Pet. Sci. Eng. 2011, 77, 10–17. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.Y. Optimization of breaker schedules for stimulation of tight gas reservoirs. J. Nat. Gas Sci. Eng. 2014, 16, 31–43. [Google Scholar] [CrossRef]
- Xue, S.; Li, X.; Li, S.; Chen, N.; Zhan, Q.; Long, L.; Zhao, J.; Hou, X.; Yuan, X. Bone fracture microenvironment responsive hydrogel for timing sequential release of cargoes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127413. [Google Scholar] [CrossRef]
- Aggarwal, P.; Dollimore, D. A Method of Comparison Between Corn Starch and Its Products Using Thermal Analysis. Instrum. Sci. Technol. 1999, 27, 191–197. [Google Scholar] [CrossRef]
- Brannon, H.D. Biotechnological Breakthrough Improves Performance of Moderate to High-Temperature Fracturing Applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994; p. 28513. [Google Scholar]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some Aspects of the Properties and Degradation of Polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef] [PubMed]
- Al-Muntasheri, G.A.; Hussein, I.A.; Nasr-El-Din, H.A.; Amin, M.B. Viscoelastic properties of a high temperature cross-linked water shut-off polymeric gel. J. Pet. Sci. Eng. 2007, 55, 56–66. [Google Scholar] [CrossRef]
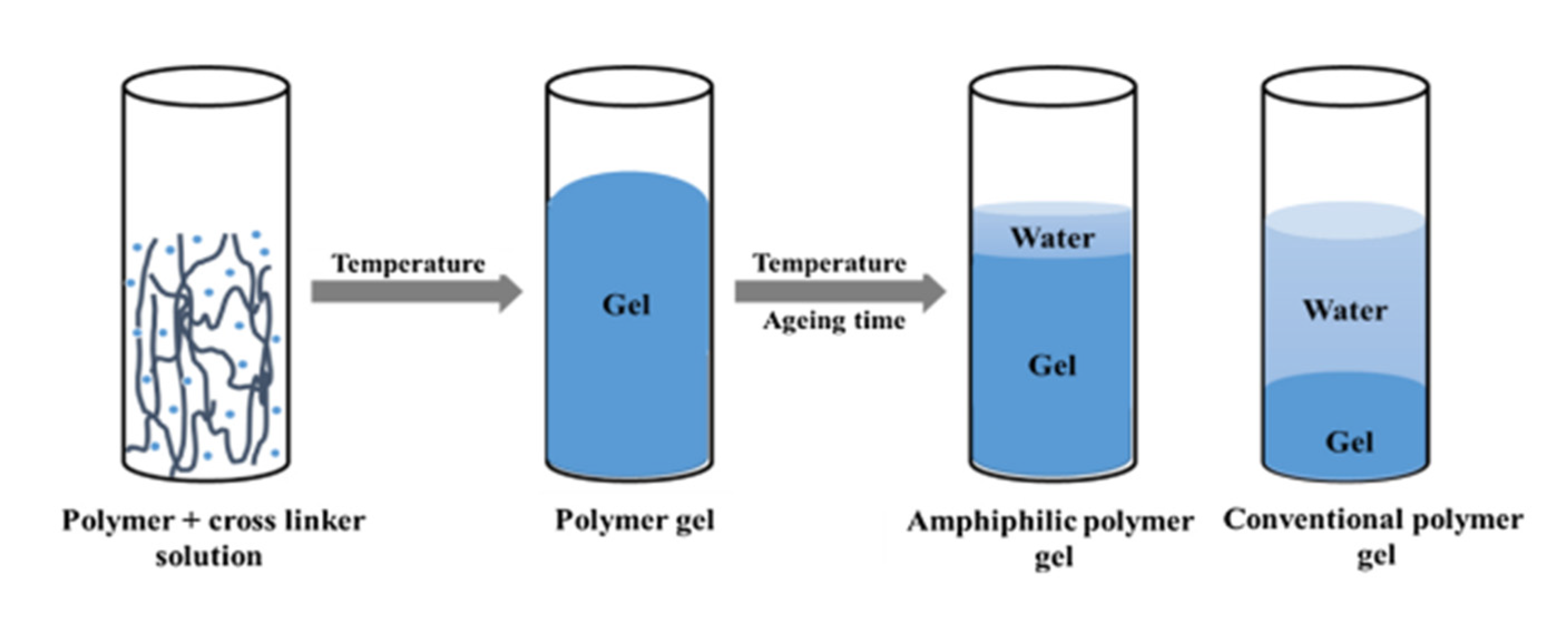

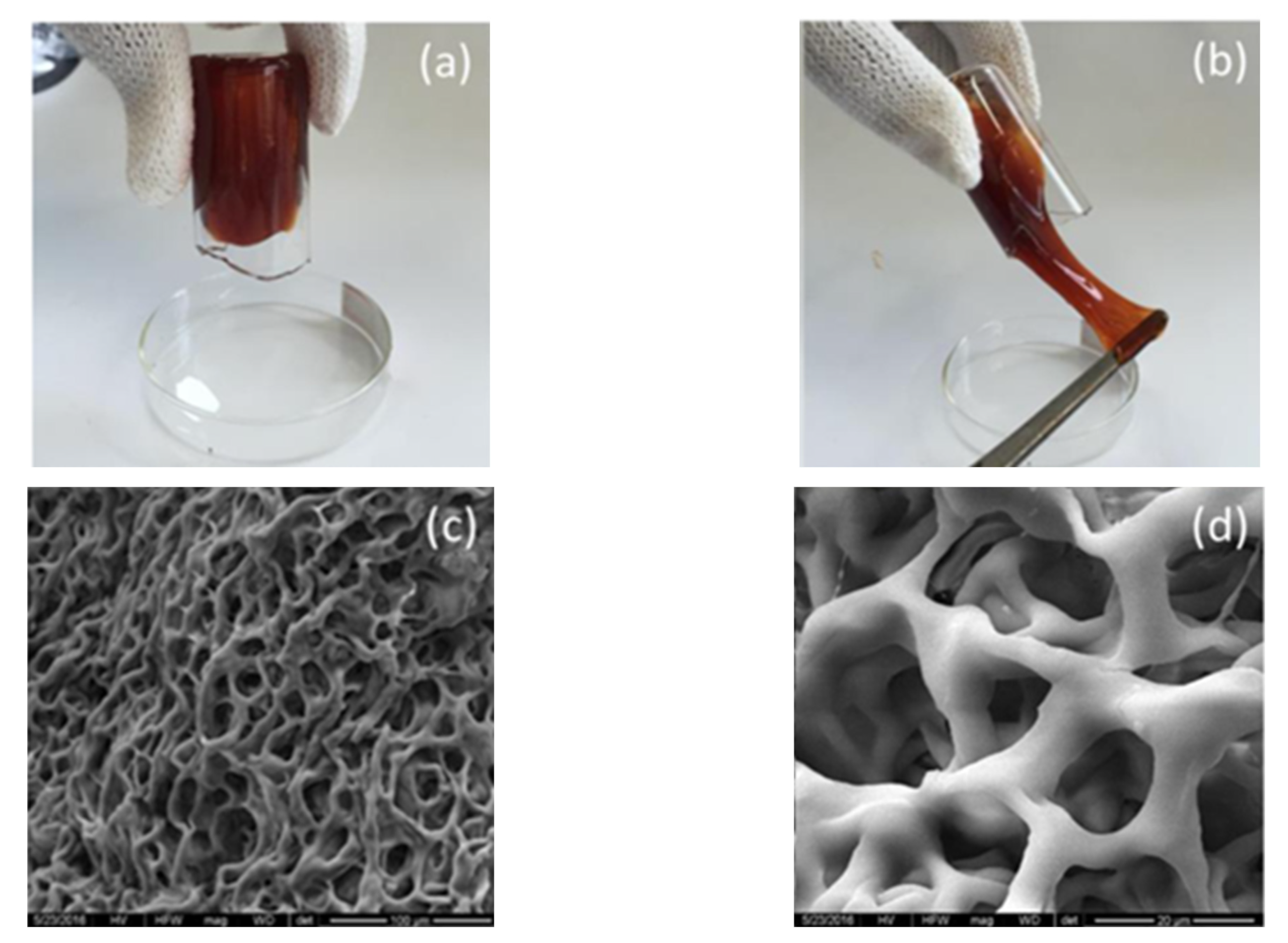
| Company/Institute | Technology | Core Materials | Technology Characteristics |
|---|---|---|---|
| Schlumberger | CACP chemical crosslinking lost circulation control technology [2] | Crosslinked polymer, biomacromolecule, crosslinker, retarder, fiber | Suitable for water-based and oil-based drilling fluids, with rapid downhole gelation, strong gel structure, good water-plugging effect, and resistance to thermal degradation |
| Baker Hughes | Crosslinking lost circulation control technology [13] | Crosslinked polymer, acid-soluble bridging particle, organic crosslinker, retarder | Crosslinking time can be tuned by adjusting the crosslinker, retarder, and downhole temperature; self-breaking occurs in long-term high-temperature environments |
| Halliburton | Chemical plugging technology CS-LCM [14] | Tackifier, clay, reactant, activator | Direct gelation feature, ability to plug 31 mm circular hole, pressure bearing capacity of 6.8 MPa, good pumping performance, 100% acid soluble |
| Sinopec Research Institute | Downhole crosslinked gel SF-1 [15] | Chitosan, cationic polyacrylamide, HDL curing material | Temperature resistance of 120 °C, curing upon contact with chemical consolidation plugging slurry, compressive strength of up to 15 MPa |
| Sinopec Research Institute | Controllable smart crosslinked gel [16] | Polyacrylamide, inorganic Cr3+ crosslinker | Delayed crosslinking achieved by coating crosslinker with polyvinyl alcohol, crosslinking time of 9–12 h, temperature resistance of 60–90 °C |
| Northwest Oil Field Company | Downhole crosslinked gel [17] | Thiourea, temperature resistant polymer, hexamethylenetetramine, methylparaben, oxalic acid, fiber, baryte | Temperature resistance of 120 °C, pressure-bearing capacity of 2.5–11 MPa for 2–5 mm fractures, stable for 10 d under high temperature, automatic flowback after 15 d |
| Southwest Petroleum University | Special Gel ZND [18] | Polymer formed by hydrophobic association of macromolecules | Excellent shear dilution performance and anti-dilution properties, not suitable for high-temperature high-salinity environments |
| Gel type | Typical Products | Gelation Mechanism | Advantages | Disadvantages |
|---|---|---|---|---|
| In situ crosslinked gel [19] | monomer crosslinked gels, organic crosslinked gels, and metal crosslinked gels | Free radical-initiated polymerization, crosslinker interactions to form covalent and ionic bonds | Low initial viscosity, high mineralization resistance, good plugging effect in high permeability channels, high gel strength | Prone to shear influence, significant negative impact on low permeability layers, sensitive to oilfield environments |
| Ground pre-crosslinked gel [20] | Pre-crosslinked gel particles, polymer gel microspheres | Gel synthesized from monomers, dried and ground into particles; resulting particles are nanosized | Good thermal stability and low sensitivity to mineralization; improved viscoelasticity of polymer gel microspheres | Microsphere particles are small and not suitable for plugging in high permeability and fractured formations |
| Material System | Core Materials | Action Mechanism | Characteristics |
|---|---|---|---|
| High-temperature thickening crosslinked temporary plugging agent system [53] | Filler SDC-A, crosslinker SDC-B, ammonium persulfate, and gel CY-A27 | Ammonium persulfate is the gel breaker, forming an emulsion in the early stage after gel breaking | High-temperature resistance, high degree of gel breaking, strong pressure-bearing capacity of the temporary plugging layer |
| HPAM/phenolic/urea-formaldehyde crosslinked polymer gel temporary plugging agent [13] | HPAM, phenolic, and urea-formaldehyde crosslinked polymer | HPAM/phenolic/urea-formaldehyde crosslinked polymer compounding to form a temporary gel-plugging agent system | Gel temporary plugging agent is prepared at 75 °C for temporary plugging purposes in high-temperature reservoirs below 120 °C. |
| High-temperature resistant curable elastic blend gel temporary plugging agent system [57] | Acrylamide copolymer, polyethyleneimine, toughening material sodium-based montmorillonite, and thiourea | The gel is produced from the reaction of acrylamide copolymer, crosslinker polyethyleneimine, toughening material sodium-based montmorillonite, and thiourea. | The system is stable without dehydration for more than 10 d at 130–160 °C. |
| Degradable preformed particle gel (DPPG) temporary plugging agent system [58] | Acrylamide, 2-acrylamido-2-methylpropanesulfonic acid, PEGDA-250, and KPS | Introduction of acid-resistant functional groups and self-degradable crosslinked structures into the composition of conventional PPG | Good water-swelling property, self-degradation to fluid in acidic solutions, little damage to the reservoir. |
| Self-breaking gel temporary plugging system [59] | Acrylamide, monomer GXDF, crosslinker JF, and initiator YF | Formed from reactions between self-hydration degradable polymers and other auxiliary temporary plugging materials | Pressure-bearing capacity of up to 15 MPa, plugging efficiency of >90%, and permeability recovery rate of >90%. |
| High-temperature resistant copolymer gel temporary plugging system [60] | Acrylamide, 2-acrylamido-2-methylpropanesulfonic acid, terpolymer, crosslinker, and initiator | Achieving plugging of different lost circulation formations via tuning concentrations of terpolymer and crosslinker | The system forms a stable and continuous three-dimensional network structure at high temperatures (120–200 °C) with excellent long-term thermal stability. |
| Company/Institute | Technology | Core Materials | Technical Characteristics |
|---|---|---|---|
| Beijing Chemrein Energy Science Co., Ltd. | Controllable capsule gel breaker and its preparation method [69] | Temperature-resistant micro-permeable capsule core is a curing agent and epoxy resin; temperature-resistant waterproof polymer capsule coating is polyether, polyolefin copolymer, and polyester acid copolymer | Suitable for a variety of gel breaking objects, strong gel breaking ability; applicable to gel-breaking temperatures of 0–120 °C with good gel-breaking ability. |
| Yangzhou University | Preparation and performance study of microcapsules with controllable release of ammonium persulfate [70] | Preparation of slow-release microcapsules by in situ polymerization with solid ammonium persulfate particles as the core material and polypyrrole as the coating material | The release time can be regulated by adjusting either the thickness of the coating, the concentration of glycerol monomer in the coating, or the temperature. |
| Jidong Oilfield Company | Preparation of core-shell delayed gel breaker by sol-gel method [71] | With tetraethoxysilane (TEOS) as the precursor, core-shell delayed gel breaker with silica as the coating and ammonium persulfate as the core is prepared by sol-gel method | Delayed gel breaker fulfills the requirements of underbalanced completion operations at 120 °C and delayed gel breaking for 5 d. |
| Southwest Petroleum University | Preparation method of potassium persulfate microcapsule gel breaker [14] | Oxidant potassium persulfate as the core, gelatin as capsule coating solution, and sorbitan monooleate as the stabilizer | microcapsule gel breaker has advantages of good sphericity, uniform and controllable particle size distribution, high effective content, and slow release. Can be used for gel breaking of water-based fracturing fluids in oilfields. |
| China National Petroleum Corporation | Capsule gel breaker and its preparation method [72] | Encapsulation of solid gel breakers with amphiphilic modified starch or amphiphilic modified cellulose derivative grafted acrylic resin-ethylene copolymer as capsule coating | Prolonged release time and controllable slow release time of capsule gel breakers, achieving self-breaking of gels at the target time to complete gel flowback |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Zhang, X.; Li, L.; Zhang, J.; Shi, X.; Hu, G. Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels 2023, 9, 34. https://doi.org/10.3390/gels9010034
Fang J, Zhang X, Li L, Zhang J, Shi X, Hu G. Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels. 2023; 9(1):34. https://doi.org/10.3390/gels9010034
Chicago/Turabian StyleFang, Junwei, Xiong Zhang, Liang Li, Jianjun Zhang, Xin Shi, and Guangqiang Hu. 2023. "Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling" Gels 9, no. 1: 34. https://doi.org/10.3390/gels9010034
APA StyleFang, J., Zhang, X., Li, L., Zhang, J., Shi, X., & Hu, G. (2023). Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels, 9(1), 34. https://doi.org/10.3390/gels9010034







