The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Results
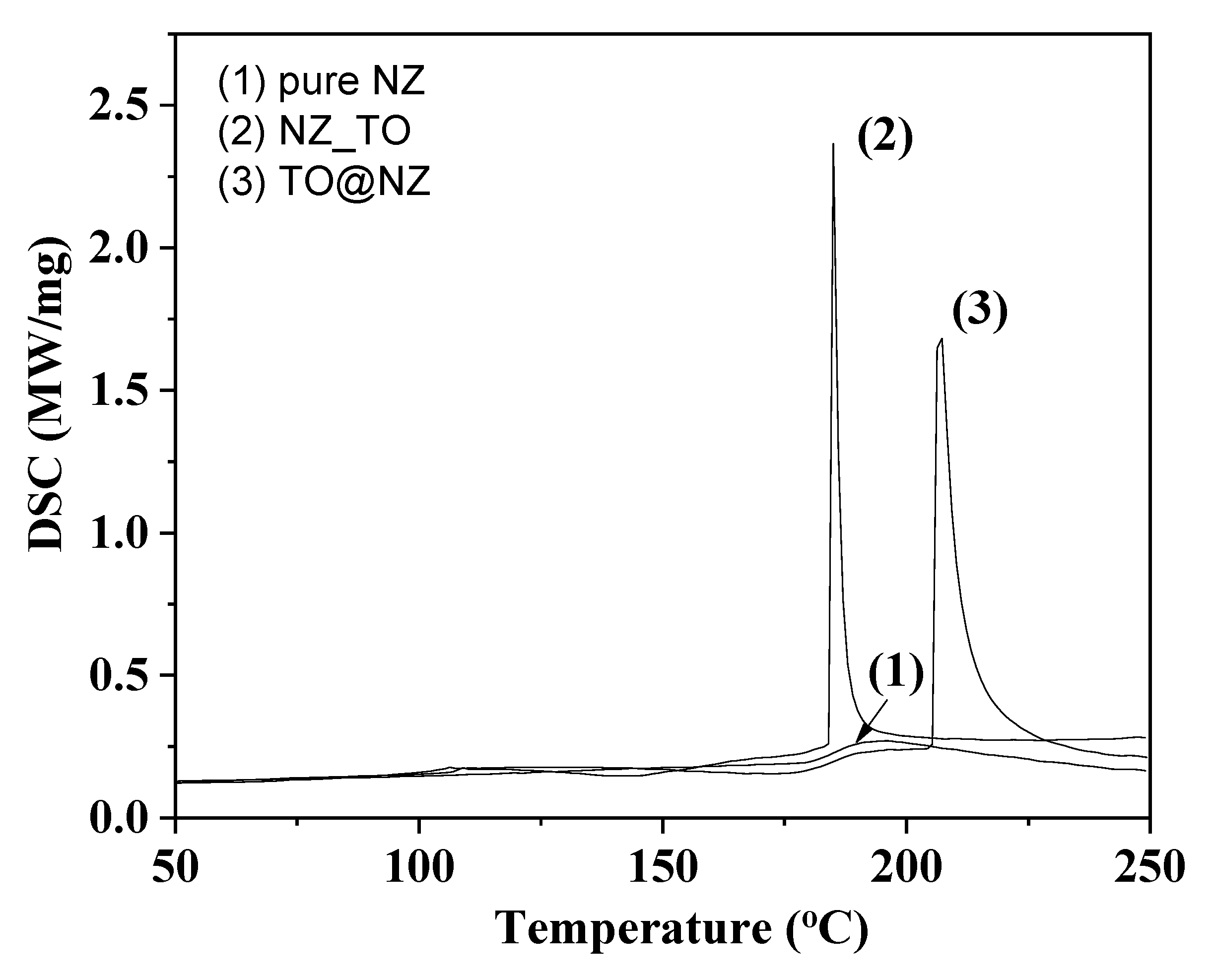
2.2. DSC Results
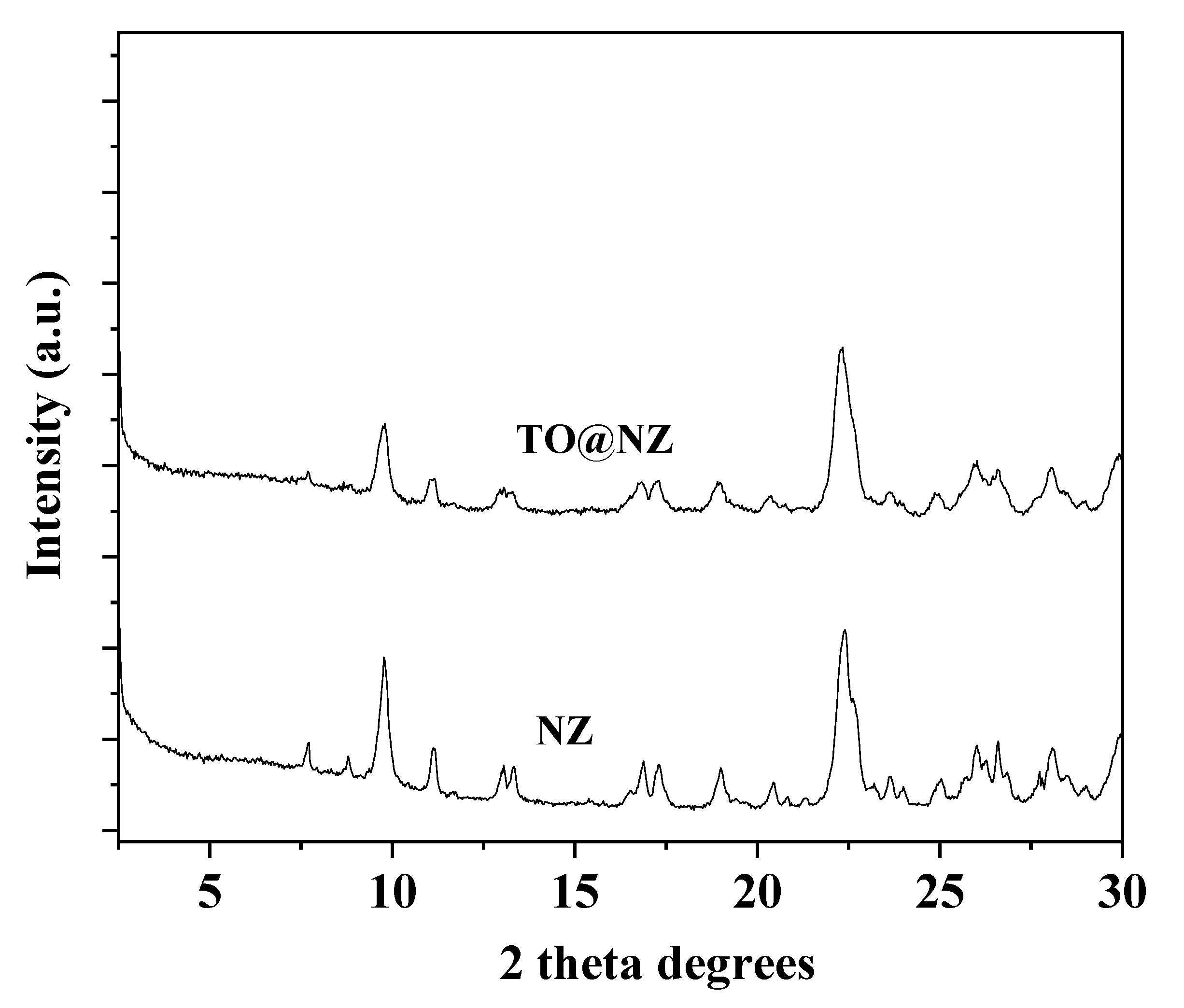
2.3. XRD Analysis
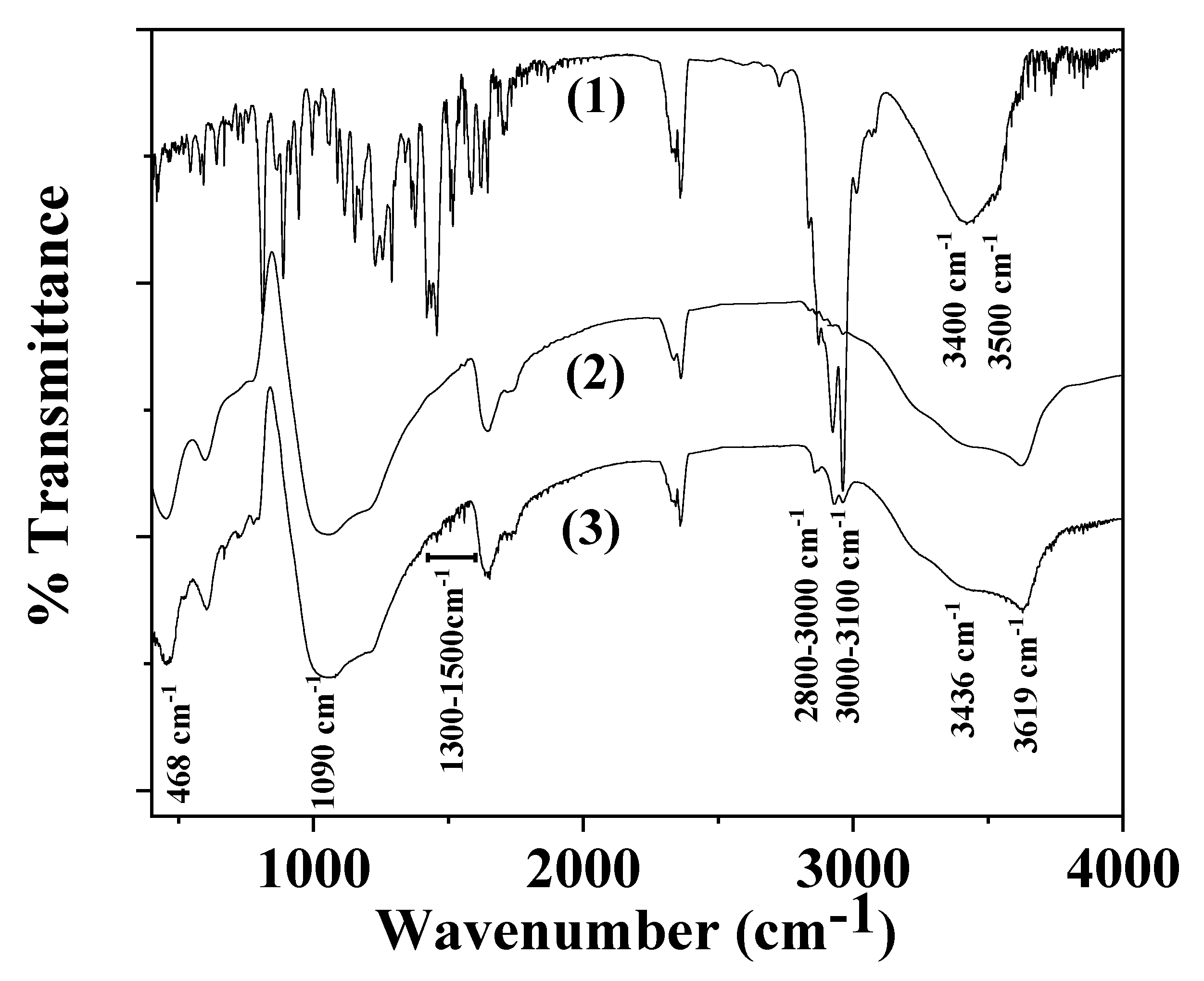
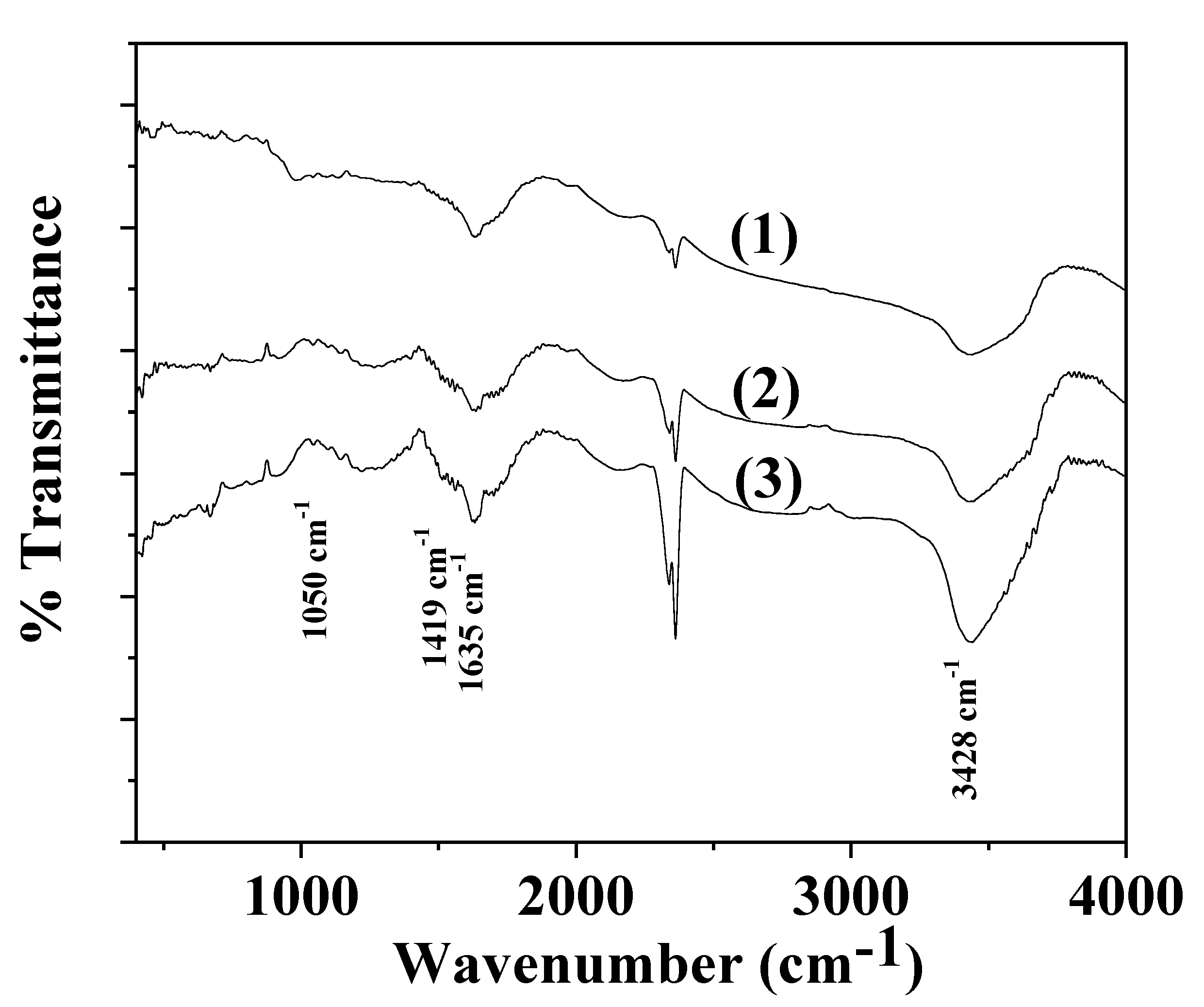
2.4. FTIR Spectroscopy
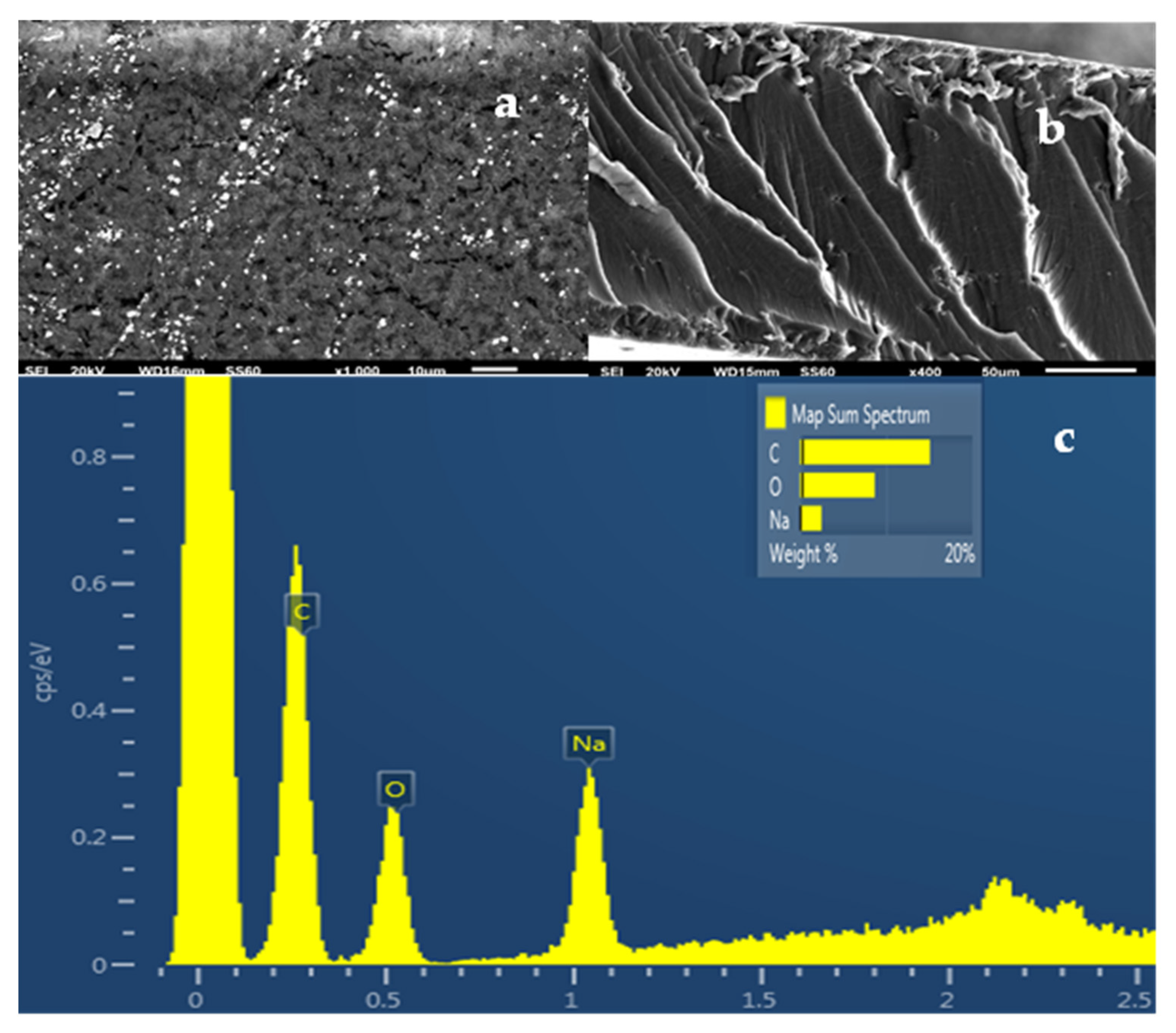
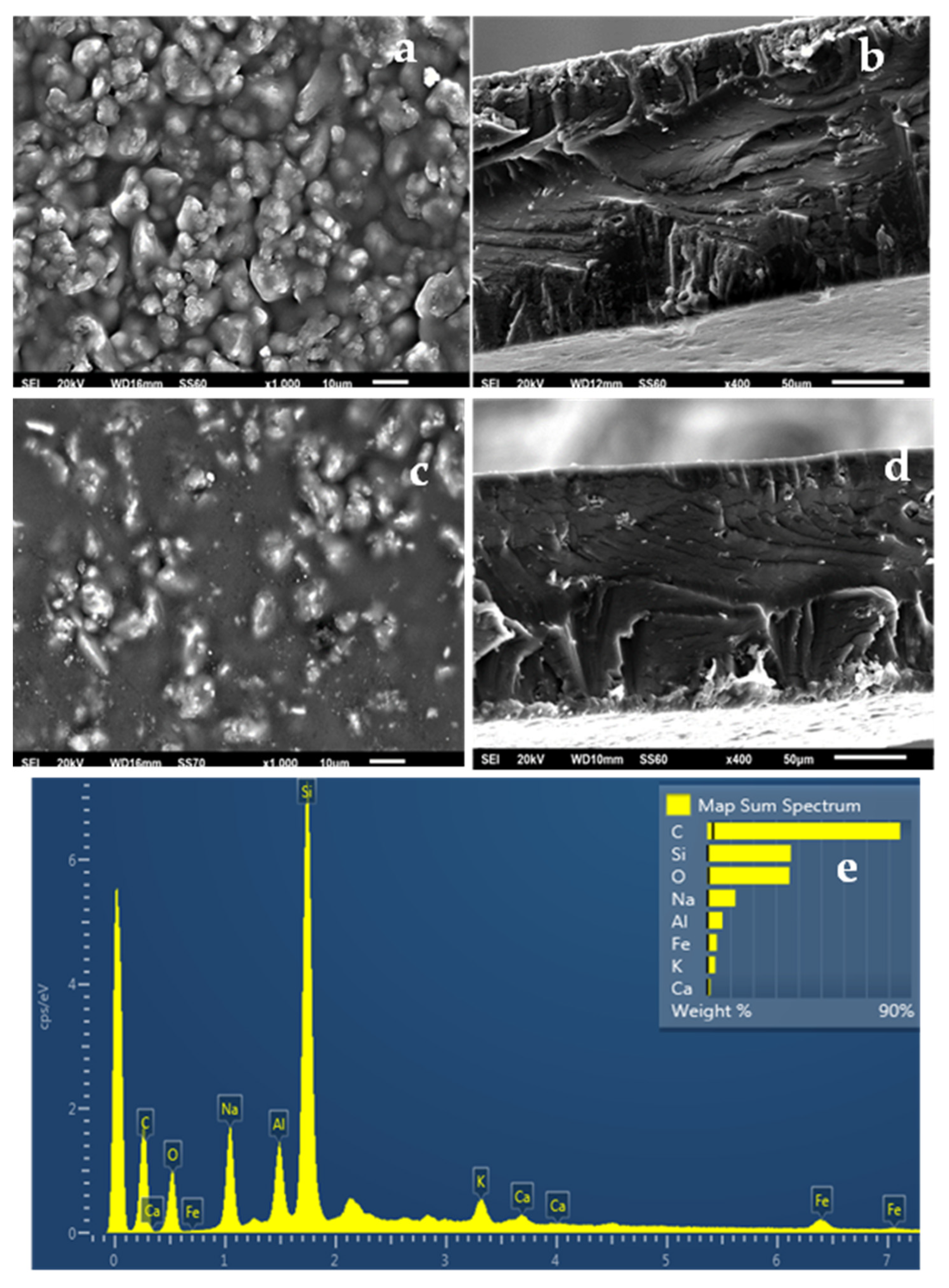
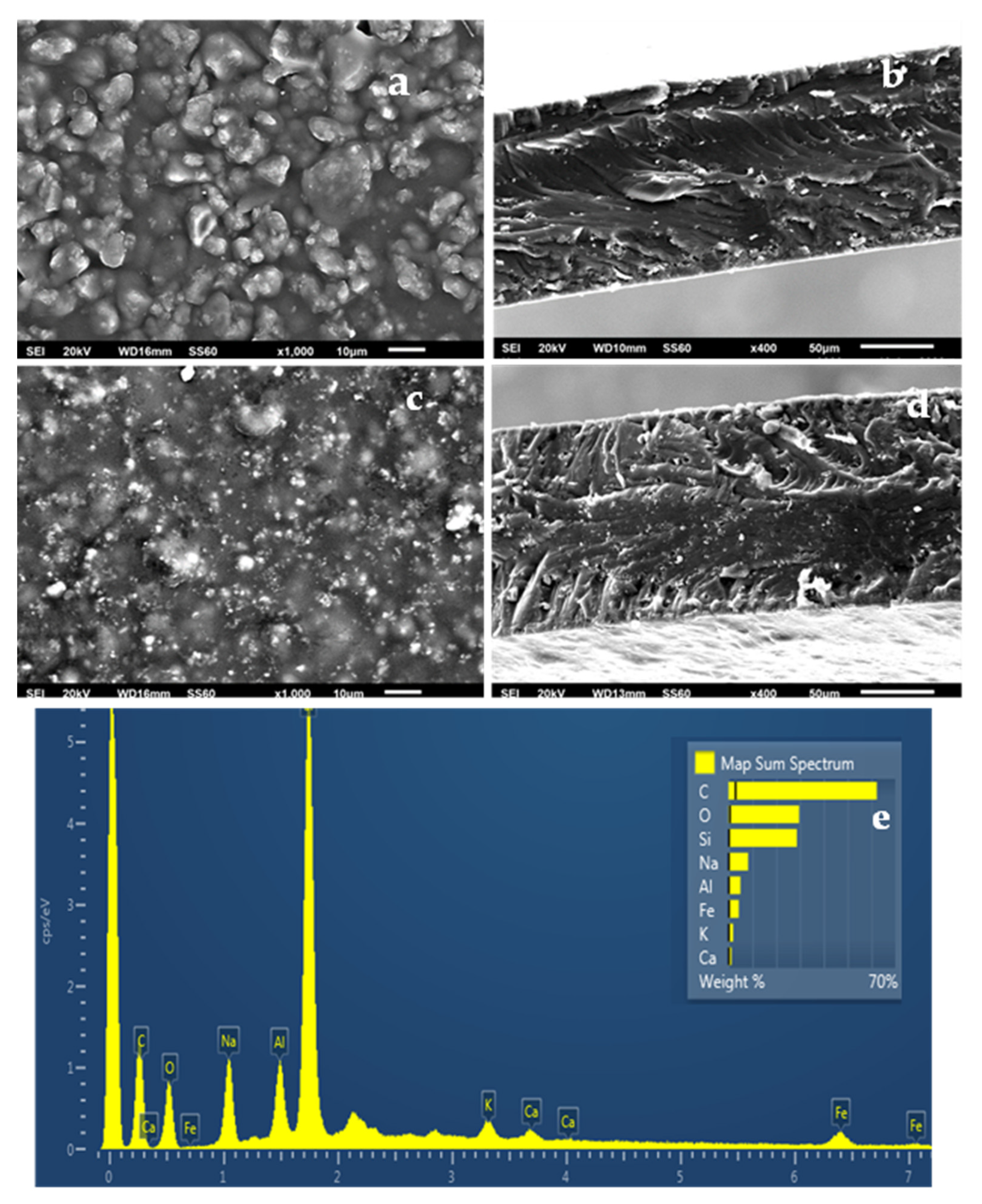
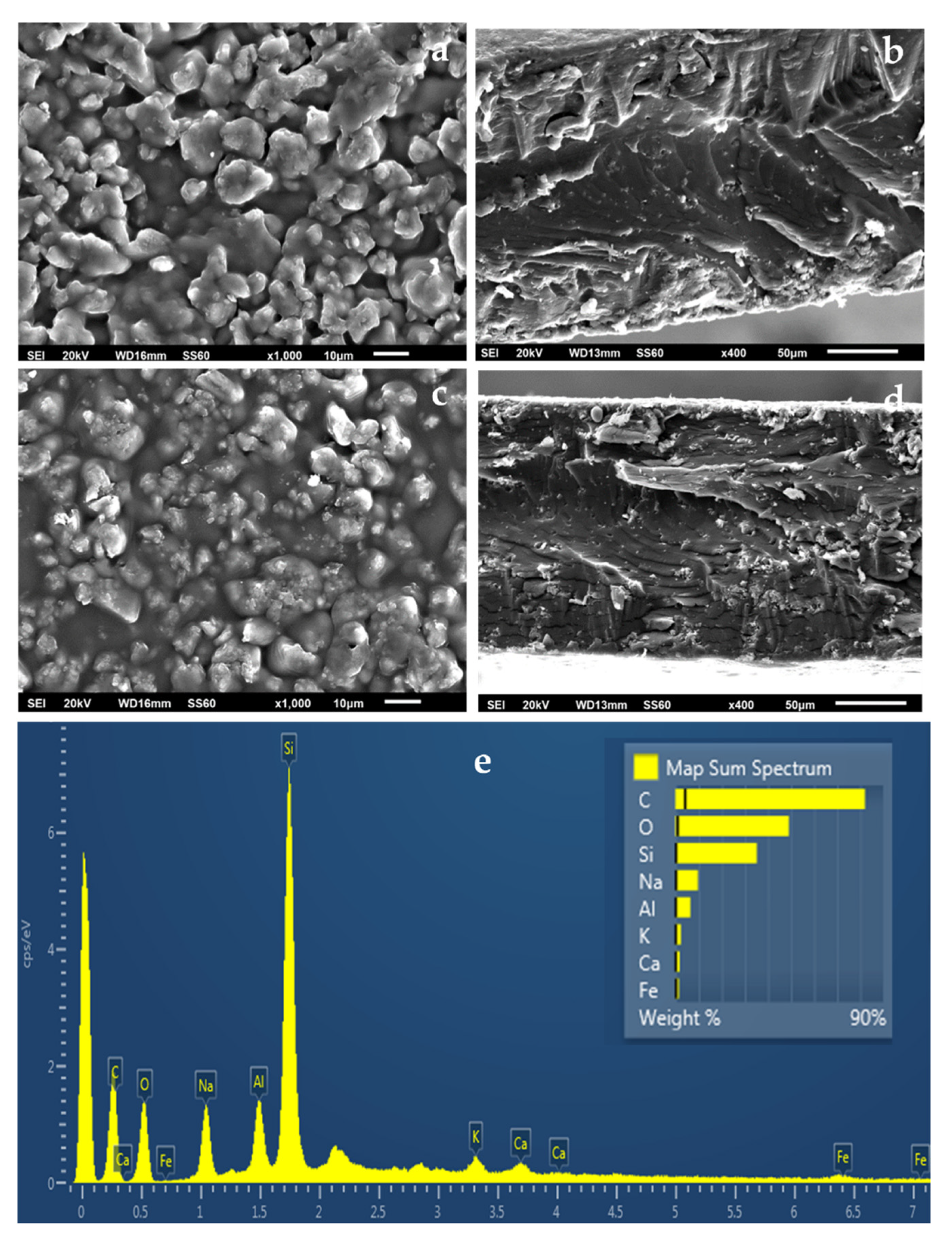
2.5. SEM Images
2.6. Tensile Properties
2.7. UV-vis Transmittance of Films
2.8. Water-Oxygen Barrier Properties
2.9. Antioxidant Activity of Films
2.10. Antimicrobial Tests
2.10.1. MICs and MBCs Determination of TO@NZ Hybrid Nanostructure against LAB and Pathogen Bacteria
2.10.2. Antimicrobial Activity of Active Films Application on Cheese against S. aureus
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Thyme oil GC-MS Analysis
4.3. Preparation of TO@NZ Hybrid Nanostructures
4.4. Preparation of ALG/G/NZ and ALG/G/TO@NZ Active Films
4.5. DSC Measurements
4.6. XRD Analysis
4.7. FTIR Spectrometry
4.8. SEM Images
4.9. Tensile Properties
4.10. UV-vis Transmittance of Films
4.11. Water Vapor Diffusivity
4.12. Oxygen Permeability
4.13. Antioxidant Activity
4.14. Antimicrobial Activity Tests
4.14.1. Antimicrobial Activity of TO@NZ Bioactive Hybrid Nanostructure
4.14.2. Antimicrobial Activity of Active Films Application on Cheese against S. aureus
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, S.; Strenger, M.; Maier-Nöth, A.; Schmid, M. Food Packaging and sustainability—Consumer perception vs. correlated scientific facts: A review. J. Clean. Prod. 2021, 298, 126733. [Google Scholar] [CrossRef]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability governance and contested plastic food packaging—An integrative review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Biopolymer Hybrid Materials: Development, Characterization, and Food Packaging Applications|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2214289421000442?token=88623980430759D15A97DA076B67FCA8D2C2F53B55226D28DF548BEDE7B959F217E9E60E6EB57ADFDB2BC71882737DF3&originRegion=eu-west-1&originCreation=20220125174316 (accessed on 25 January 2022).
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel Food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in nanotechnology for food science and industry. Food Front. 2021, 3, 56–82. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018, e5427978. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A novel method for the preparation of inorganic and organo-modified montmorillonite essential oil hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The effect of different preparation methods on the development of chitosan/thyme oil/montmorillonite nanocomposite active packaging films. J. Food Process. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with essential oils as antimicrobial agents, packaging, repellents, and insecticides: An overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled release of essential oils using laminar nanoclay and porous halloysite/essential oil composites in a multilayer film reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Applications of Halloysite Nanotubes in Food Packaging for Improving Film Performance and Food Preservation—Science Direct. Available online: https://www.sciencedirect.com/science/article/pii/S0956713521000141?via%3Dihub (accessed on 26 January 2022).
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of clay-polymer nanocomposites containing bioactive compounds for food packaging applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Giannakas, A. Na-montmorillonite vs. organically modified montmorillonite as essential oil nanocarriers for melt-extruded low-density poly-ethylene nanocomposite active packaging films with a controllable and long-life antioxidant activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Karydis-Messinis, A.; Moschovas, D.; Kollia, E.; Tsigkou, V.; Proestos, C.; Avgeropoulos, A.; Zafeiropoulos, N.E. Nanoclay and polystyrene type efficiency on the development of polystyrene/montmorillonite/oregano oil antioxidant active packaging nanocomposite films. Appl. Sci. 2021, 11, 9364. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food applications: A review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Dogan, H.; Koral, M.; İnan, T.Y. Ag/Zn zeolite containing antibacterial coating for food-packaging substrates. J. Plast. Film Sheeting 2009, 25, 207–220. [Google Scholar] [CrossRef]
- Eimontas, J.; Striūgas, N.; Abdelnaby, M.A.; Yousef, S. Catalytic pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of metallized food packaging plastics with different concentrations of ZSM-5 zeolite catalyst. Polymers 2021, 13, 702. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.-H.; Jones, K.; Sharek, E.; Pascall, M.A. Antimicrobial packaging of raw beef, pork and turkey using silver-zeolite incorporated into the material. Int. J. Food Sci. Technol. 2011, 46, 2382–2386. [Google Scholar] [CrossRef]
- Rešček, A.; Katančić, Z.; Kratofil Krehula, L.; Ščetar, M.; Hrnjak-Murgić, Z.; Galić, K. Development of double-layered PE/PCL films for food packaging modified with zeolite and magnetite nanoparticles. Adv. Polym. Technol. 2018, 37, 837–842. [Google Scholar] [CrossRef]
- Youssef, H.F.; El-Naggar, M.E.; Fouda, F.K.; Youssef, A.M. Antimicrobial packaging film based on biodegradable CMC/PVA-zeolite doped with noble metal cations. Food Packag. Shelf Life 2019, 22, 100378. [Google Scholar] [CrossRef]
- Do Nascimento Sousa, S.D.; Santiago, R.G.; Soares Maia, D.A.; de Oliveira Silva, E.; Vieira, R.S.; Bastos-Neto, M. Ethylene adsorption on chitosan/zeolite composite films for packaging applications. Food Packag. Shelf Life 2020, 26, 100584. [Google Scholar] [CrossRef]
- Makhal, S.; Kanawjia, S.K.; Giri, A. Effectiveness of thymol in extending keeping quality of cottage cheese. J. Food Sci. Technol. 2014, 51, 2022–2029. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Kasapidou, E.; Mitlianga, P.; Mataragas, M.; Pappa, E.; Kondyli, E.; Bosnea, L. Production, characteristics and application of whey protein films activated with rosemary and sage extract in preserving soft cheese. LWT 2022, 155, 112996. [Google Scholar] [CrossRef]
- PubChem. P-Cymene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7463 (accessed on 8 May 2022).
- PubChem. Limonene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/22311 (accessed on 8 May 2022).
- PubChem. Thymol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6989 (accessed on 8 May 2022).
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Paşcalău, V.; Popescu, V.; Popescu, G.L.; Dudescu, M.C.; Borodi, G.; Dinescu, A.; Perhaiţa, I.; Paul, M. The alginate/k-Carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloys Compd. 2012, 536, S418–S423. [Google Scholar] [CrossRef]
- Kinninmonth, M.A.; Liauw, C.M.; Verran, J.; Taylor, R.; Edwards-Jones, V.; Shaw, D.; Webb, M. Investigation into the suitability of layered silicates as adsorption media for essential oils using FTIR and GC-MS. Appl. Clay Sci. 2013, 83–84, 415–425. [Google Scholar] [CrossRef]
- Elaiopoulos, K.; Perraki, T.; Grigoropoulou, E. Monitoring the effect of hydrothermal treatments on the structure of a natural zeolite through a combined XRD, FTIR, XRF, SEM and N2-porosimetry analysis. Microporous Mesoporous Mater. 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Eimontas, J.; Yousef, S.; Striūgas, N.; Abdelnaby, M.A. Catalytic pyrolysis kinetic behaviour and TG-FTIR-GC–MS analysis of waste fishing nets over ZSM-5 zeolite catalyst for caprolactam recovery. Renew. Energy 2021, 179, 1385–1403. [Google Scholar] [CrossRef]
- Olegario, E.M.; Mark Pelicano, C.; Cosiñero, H.S.; Sayson, L.V.; Chanlek, N.; Nakajima, H.; Santos, G.N. Facile synthesis and electrochemical characterization of novel metal oxide/philippine natural zeolite (MOPNZ) nanocomposites. Mater. Lett. 2021, 294, 129799. [Google Scholar] [CrossRef]
- Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum Turbinarioides Grunow|SpringerLink. Available online: https://link.springer.com/article/10.1007/s10811-009-9432-y (accessed on 31 January 2022).
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Keshavarzi, N.; Mashayekhy Rad, F.; Mace, A.; Ansari, F.; Akhtar, F.; Nilsson, U.; Berglund, L.; Bergström, L. Nanocellulose–Zeolite composite films for odor elimination. ACS Appl. Mater. Interfaces 2015, 7, 14254–14262. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0141813014006928 (accessed on 3 April 2022).
- Uning Rininingsih, E.M. Correlation Between Antioxidant Activity of Synthetic Zeolites Pillared Titanium Dioxide and Iron (III) Oxide with Adsorption DPPH. Masters’ Thesis, Bogor Agricultural University, Bogor City, Indonesia, 2014. [Google Scholar]
- Water|Free Full-Text|Evaluation of Different Clinoptilolite Zeolites as Adsorbent for Ammonium Removal from Highly Concentrated Synthetic Wastewater. Available online: https://www.mdpi.com/2073-4441/10/5/584 (accessed on 2 April 2022).
- A Review on Applications and Uses of Thymus in the Food Industry—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7464319/ (accessed on 3 April 2022).
- Comparative Inhibitory Effects of Thymus vulgaris L. Essential Oil against Staphylococcus aureus, Listeria Monocytogenes and Mesophilic Starter Co-Culture in Cheese-Mimicking Models—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0740002015001252?via%3Dihub (accessed on 3 April 2022).
- Antimicrobial Efficacy of an Array of Essential Oils Against Lactic Acid Bacteria—Dunn—2016—Journal of Food Science—Wiley Online Library. Available online: https://ift.onlinelibrary.wiley.com/doi/10.1111/1750-3841.13181 (accessed on 3 April 2022).
- Celikel, N.; Kavas, G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. Czech J. Food Sci. 2008, 26, 174–181. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Gurbuz, O.; Sahan, Y.; Yigit, A.; Kacar, O.; Rouseff, R. Chemical characterization and antifungal activity of Origanum onites L. essential oils and extracts. J. Food Saf. 2009, 29, 144–161. [Google Scholar] [CrossRef]
- Kavas, G.; Kavas, N.; Saygili, D. The effects of thyme and clove essential oil fortified edible films on the physical, chemical and microbiological characteristics of kashar cheese. J. Food Qual. 2015, 38, 405–412. [Google Scholar] [CrossRef]
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Leandro, O.; da Ramos, S.; Nuno, R.; Pereira, C.; Martins, J.T.; Malcata, F.X. Edible packaging for dairy products. In Edible Food Packaging; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-37317-1. [Google Scholar]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical composition of essential oils of aromatic and medicinal herbs cultivated in Greece—Benefits and drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Sidira, M.; Skitsa, M.; Tsochantaridis, I.; Pappa, A.; Dimtsoudis, C.; Proestos, C.; Kourkoutas, Y. Assessment of the antimicrobial, antioxidant, and antiproliferative potential of Sideritis raeseri Subps. Raeseri essential oil. Foods 2020, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical, barrier and antimicrobial properties of chitosan/PVOH/clay nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of copper and titanium-exchanged montmorillonite nanostructures on the packaging performance of chitosan/poly-vinyl-alcohol-based active packaging nanocomposite films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Yasuda, H. Units of gas permeability constants. J. Appl. Polym. Sci. 1975, 19, 2529–2536. [Google Scholar] [CrossRef]



| Code Name | E-Elastic Modulus (MPa) | σuts (MPa) | %ε |
|---|---|---|---|
| ALG/G | 445.5 (63.8) | 15.2 (2.4) | 40.2 (4.7) |
| ALG/G/5NZ | 755.6 (67.3) | 22.7 (0.9) | 24.7 (12.4) |
| ALG/G/10NZ | 669.3 (24.3) | 21.1 (5.9) | 20.3 (2.7) |
| ALG/G/15NZ | 785.3 (146.6) | 23.1 (5.5) | 23.1 (2.5) |
| ALG/G/5TO@NZ | 739.4 (20.3) | 20.9 (3.5) | 28.4 (8.2) |
| ALG/G/10TO@ΝΖ | 651.5 (76.2) | 18.5 (2.9) | 28.3 (6.6) |
| ALG/G/15TO@NZ | 798.5 (177.5) | 22.6 (1.4) | 25.3 (2.5) |
| Code Name | Film Thickness (mm) | WVTR (10−6) (gr∙cm−2∙s−1) | DWV (10−4) (cm2∙s−1) | OTR (10−4) (ml∙cm−2∙day−1) | PeO2 (10−7) (cm2∙s−1) |
|---|---|---|---|---|---|
| ALG/G | 0.11 (0.01) | 2.45 (0.14) | 5.96 (0.45) | 111,939 (234) | 15.70 (0.21) |
| ALG/G/5NZ | 0.07 (0.01) | 3.00 (0.45) | 5.40 (0.11) | 70,476 (124) | 5.71 (0.10) |
| ALG/G/10NZ | 0.08 (0.01) | 2.31 (0.21) | 3.97 (0.42) | 85,456 (174) | 9.23 (0.19) |
| ALG/G/15NZ | 0.09 (0.01) | 2.47 (0.25) | 5.55 (0.67) | 127,556 (435) | 15.3 (0.52) |
| ALG/G/5TO@NZ | 0.08 (0.01) | 2.36 (0.21) | 4.06 (0.11) | 93,984 (205) | 9.06 (0.20) |
| ALG/G/10TO@NZ | 0.10 (0.01) | 2.26 (0.16) | 5.16 (0.23) | 90,549 (345) | 6.99 (0.27) |
| ALG/G/15TO@NZ | 0.13 (0.01) | 2.28 (0.27) | 6.89 (0.74) | 78,476 (234) | 11.8 (0.35) |
| Code Name | % Antioxidant Activity 24 h |
|---|---|
| ALG/G | 8.7 (0.6) |
| ALG/G/5NZ | 7.7 (2.7) |
| ALG/G/10NZ | 10.4 (1.9) |
| ALG/G/15NZ | 15.1 (2.0) |
| ALG/G/5TO@NZ | 17.3 (1.3) |
| ALG/G/10TO@NZ | 25.3 (3.9) |
| ALG/G/15TO@NZ | 46.4 (4.3) |
| Bacteria (106 cfu/mL) | MIC | MBC |
|---|---|---|
| Lactococcus lactis ssp. lacts ACA-DC127 | 0.025 (0.1) | 0.025 (0.1) |
| S. thermophilus ACA-DC112 | 0.025 (0.1) | 0.025 (0.1) |
| S. aureus ATCC1538 | 0.05 (0.4) | 0.05 (0.4) |
| L. monocytogenes NCTC10527 | 0.1 (0.2) | 0.1 (0.2) |
| E. faecalis EF1 | 0.1 (0.3) | 0.1 (0.4) |
| Code Name | ALG (g) | G (g) | NZ (g) | TO@NZ (g) |
|---|---|---|---|---|
| ALG/G | 2 | 1 | - | - |
| ALG/G/5NZ | 2 | 1 | 0.15 | - |
| ALG/G/10NZ | 2 | 1 | 0.30 | - |
| ALG/G/15NZ | 2 | 1 | 0.45 | - |
| ALG/G/5TO@NZ | 2 | 1 | - | 0.15 |
| ALG/G/10TO@NZ | 2 | 1 | - | 0.30 |
| ALG/G/15TO@NZ | 2 | 1 | - | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. https://doi.org/10.3390/gels8090539
Giannakas AE, Salmas CE, Moschovas D, Zaharioudakis K, Georgopoulos S, Asimakopoulos G, Aktypis A, Proestos C, Karakassides A, Avgeropoulos A, et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels. 2022; 8(9):539. https://doi.org/10.3390/gels8090539
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Dimitrios Moschovas, Konstantinos Zaharioudakis, Stavros Georgopoulos, Georgios Asimakopoulos, Anastasios Aktypis, Charalampos Proestos, Anastasios Karakassides, Apostolos Avgeropoulos, and et al. 2022. "The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures" Gels 8, no. 9: 539. https://doi.org/10.3390/gels8090539
APA StyleGiannakas, A. E., Salmas, C. E., Moschovas, D., Zaharioudakis, K., Georgopoulos, S., Asimakopoulos, G., Aktypis, A., Proestos, C., Karakassides, A., Avgeropoulos, A., Zafeiropoulos, N. E., & Nychas, G.-J. (2022). The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels, 8(9), 539. https://doi.org/10.3390/gels8090539









