1. Introduction
By 2050, the world’s population is expected to grow from 7.6 billion in 2017 to 9.4–10.2 billion people [
1]. Due to the increasing population as well as other factors such as rising living standards, often correlating with increasing meat consumption, the demand for food and agricultural products is expected to increase by 50% between 2012 and 2050. The adherence to increasing food requirements will not be achieved solely through the exploitation of new arable land as most of it is not developed, too remote from potential markets, susceptible to pest infestation, or competes with the conservation of important ecosystems. Furthermore, potential arable land is limited to a small number of countries. Rather, the increased demand for food must be met by increasing productivity and efficiency in agricultural production [
2], which leads to a greater need for nutrients, especially nitrogen fertilizers.
The Haber–Bosch process, which enables the synthesis of ammonia (NH
3) for fertilizer production, ensures the nutrition of half the world’s population [
3]. However, the production of NH
3 requires a high amount of energy [
4]. Dawson and Hilton [
5] calculated that 1.1% of the world’s energy consumption can be attributed to the production of fertilizers; 90% of this is due to the production of nitrogen fertilizers. Thus, partial substitution of the increasing demand for NH
3 may be achieved by recovering ammonium (NH
4+) from wastewater. For instance, sludge water from anaerobic digesters is a possible source of ammonium due to high ammonium-N (NH
4-N) concentrations of around 1000 mg/L and low volume flows which allow a partial flow treatment.
Recovery methods such as air stripping, bioelectrochemical systems, membrane separation, and ion exchange have been thoroughly investigated [
6]. The zeolite clinoptilolite (CLI) is known to be a very good ion exchanger, as it consists of a three-dimensional structure formed of AlO
4– and SiO
4 tetrahedral, which are connected by a common oxygen atom. The micropores formed by this structure are fine enough to allow cations and water molecules to enter and be exchanged [
7]. This ability is based on the substitution of SiO
4 by AlO
4−, creating a negative charge, which has to be compensated by exchangeable cations such as Na
+, K
+, Ca
2+, and Mg
2+ [
8].
As soon as the exchange capability for ammonium is exhausted, CLI is proposed to be utilized as a slow-release fertilizer in agriculture [
9] or regenerated by the use of sodium chloride, sodium carbonate, sodium bicarbonate, and sodium hydroxide solutions [
10,
11,
12,
13].
While the ability of ammonium adsorption by zeolites was the focus of recent investigations, most of these investigations were carried out with one type of CLI from one specific deposit only [
10,
14,
15,
16,
17,
18,
19,
20], focusing on the composition of the CLI, the adsorption/elimination of ammonium at different pH values, describing the adsorption process, the kinetics using different models, and evaluation of the thermodynamic properties of the adsorption process.
In most cases, the methods chosen differed significantly (e.g., investigated particle size, contact time, initial NH
4-N concentration), which is why a direct comparison of the investigated CLI, e.g., in terms of adsorption properties of ammonium from highly concentrated wastewater, is difficult. An overview of published data about CLI adsorption of ammonium is given in
Table 1, showing the large variety of parameters examined, such as particle size (0.063–1.43 mm), contact time (20 min–24 h), and initial NH
4-N concentration ranges (e.g., 25–150 mg/L vs. 10–5000 mg/L). However, it cannot be deduced from these investigations to what extent each parameter has an effect on the adsorption capacity. Hence, it becomes apparent that there is still a lack of detailed comparisons regarding the adsorption properties of CLI.
Using three CLIs from a mining area in Slovakia, from which CLI with a high adsorption has been reported [
10], this paper demonstrates in which way an analysis of adsorption properties from highly concentrated wastewater can be set up. The main aim of this research was to gain a deeper understanding of the adsorption process of ammonium from highly concentrated solutions, as well as to identify different properties (exchangeable cations, cation exchange capacity, and isoelectric pH) regarding the tested CLI. In further experiments, the effects of temperature (determination of isotherms by varying the sorbent mass at different temperatures) and contact time (determination of kinetics by varying the contact time at room temperature) on the adsorption process were investigated. All experiments were conducted as triplicates.
This procedure could be used as a uniform guideline for future studies comparing different CLIs in terms of adsorption properties to adsorb ammonium from highly concentrated wastewater.
2. Materials and Methods
2.1. Zeolite Samples, Solutions, and Chemicals
Three different Slovakian CLIs were obtained from the suppliers Zeocem (Bystré, Slovakia) and Labradorit GmbH (Berlin, Germany). According to the suppliers, the CLIs were ground and sieved to a particle size smaller than 200 µm (Micro 200) or 20 µm (EcoZeo 20 and CCP 20 (CCP = Carpathian clinoptilolite powder)). The CLIs were dried at 105 °C for 24 h before use. No other pretreatment was conducted. The composition of the examined CLIs is listed in
Table 2.
For all experiments, an ammonium solution with a concentration of 1000 mg NH4-N/L was prepared from ammonium chloride with an arbitrary pH of 5.3. NH4Cl (p.a.), NH4Ac (ammonium acetate, p.a.), KCl (p.a.), NaOH (p.a.), HF (47–51%, p.a.), HCl (32%, p.a.), and ethanol (99.5%) were obtained from VWR International (Radnor, Pennsylvania, USA). HNO3 (67–69%, p.a.) was obtained from Bernd Kraft GmbH (Duisburg, Germany).
2.2. Experimental Design
2.2.1. Cation Exchange Capacity (CEC)
To determine the amount of interchangeable cations, 1 g of dried CLI was weighed into centrifuge tubes and mixed with 30 mL of 1 M NH4Ac solution for 30 minutes in a rotator (uniROTATOR2, LLG Labware, Meckenheim, Germany) at 120 rpm and 22 °C (no pH adjustment). After centrifugation at 4000 rpm for 10 min, the supernatant was decanted and collected. The remaining CLI in the centrifuge tube was again mixed with fresh 30 mL of 1 M NH4Ac solution and treated according to the described procedure. In total, the procedure was carried out three times and the arising three supernatants were mixed and collected. Subsequently, the mixtures were analyzed for desorbed alkali and alkaline earth metals, which correspond to the cations exchanged. Afterwards, the CLI was washed with 30 mL 80% ethanol solution three times to remove unadsorbed ammonium, following the same procedure until no ammonium in the supernatant was detected. The loaded CLI was then regenerated three times with 30 mL 1 M KCl solution using the same procedure. The resulting supernatant was collected and analyzed for ammonium. The cation exchange capacity corresponds to the cations that were exchanged by ammonium.
2.2.2. Isoelectric State of CLI and pH-Dependent Adsorption
The NH4Cl solution investigated was previously adjusted to pH values ranging from 2 to 12 by HCl or NaOH. The specific sorbent mass was fixed at a ratio of 0.1 g CLI per mg NH4-N. Sorbent (20 g) and solution (200 mL) were stirred for 20 h on a magnetic stirrer (400 rpm) at room temperature (22 °C) in closed bottles, membrane-filtered (0.45 µm pore size), and subsequently the pH value as well as the ammonium concentration were determined. The initial pH values of the solutions were compared with those of the filtrates. The isoelectric state is the point at which both pH values are identical.
2.2.3. Isothermal Adsorption
The aim of the investigations was to determine the influence of temperature on CLI loading. Since wastewater with very high ammonium concentrations, e.g., sludge water, originates from the digestion tower operated mostly at a mesophilic temperature, the temperature of 34 °C (considering small heat losses) was chosen as the upper temperature limit. In addition, temperatures of 22 °C (room temperature) and 10 °C were tested.
Since in real wastewater the concentration of ammonium c0 (mg/L) cannot be changed without influencing its ionic composition, the sorbent mass m (g) was varied. Thus, different quantities ranging from 2 to 48 g sorbent were mixed with 200 mL adsorption solution Vp (mL) and stirred at a constant temperature (10 °C, 22 °C, and 34 °C) on a magnetic stirrer at 400 rpm. After 20 h, the residual ammonium concentration ceq (mg/L) as well as pH in the filtrate were determined. Since the pH barely varied between the different dosages and a competition adsorption by Na+ or H3O+ cations as well as a dilution due to the pH adjustment was to be avoided, a pH correction was not conducted. One experimental approach without sorbent for each examined pH was used to determine unwanted ammonium elimination, e.g., by stripping or adsorption onto parts of the glass apparatus. The ammonium concentration in the filtrate of that approach is expressed as cB (mg/L). The loading qeq (mg/g) of the sorbent mass was determined by Equation (1).
2.2.4. Adsorption Kinetics
CLI was mixed with the NH
4Cl solution (1.5 L) at a specific sorbent ratio of 0.1 g CLI per mg NH
4-N on a magnetic stirrer at 22 °C. At periodic intervals, an aliquot (10 mL) was taken and immediately membrane-filtered (0.45 µm pore size) to prevent further contact between sorbent and sample. Subsequently, the ammonium concentration was measured in the filtrate and the time-dependent loading of the sorbent q
t (mg/g) was calculated. Since it is known from published studies that the adsorption kinetics strongly depend on the stirring speed [
15,
21,
22], a high rotation frequency of 800 rpm was chosen to determine the maximum possible adsorption kinetic values. During the test, due to sampling, the total volume was continuously reduced. However, it can be assumed that during the sampling no change in the ratio of the sorbent mass to the volume of the adsorption solution occurred due to the homogeneously mixed conditions.
The arbitrary pH of the NH4Cl-soution was 5.3. As a consequence of the contact with zeolite, the pH value immediately rose to 6.6 and increased to 7.1 with increasing contact time.
2.3. Adsorption Models
2.3.1. Freundlich Model
The Freundlich model ([
23] in [
24]) is an empirical approach to describe the equilibrium of sorbent and sorbate. It describes the nonideal, reversible adsorption on a heterogeneous sorbent, whereby less adsorption can be achieved with increasing loading. However, it is not possible to calculate a complete loading, as the sorbent sites can be occupied in several layers. According to Freundlich, the loading of the sorbent q
eq,F (mg/g) can be calculated by exponentiation of the corresponding equilibrium concentration c
eq (mg/L) with the factor 1/n (-), as described by Equation (2).
Calculation methods for determining the constants K
F and 1/n with the help of nonlinear regression or linearization are given, e.g., by Ho et al. [
24]. In this study, the linearization is done by plotting log q
eq versus log c
eq. The gradient of the graph corresponds to n, while the tenth power of the intercept represents K
F.
2.3.2. Langmuir Model
The adsorption model of Langmuir [
25] assumes that the sorbent is loaded with a monomolecular layer of adsorbate. Accordingly, the properties of the sorbent sites are identical and equivalent, so that a determination of the maximum adsorption capacity is possible. The loading of sorbent is calculated according to Equation (3), where K
L (L/mg) is the Langmuir constant and q
max (mg/g) the maximum capacity.
The constants can either be calculated from linear or nonlinear regression based on measurement results. By plotting c
eq/q
eq vs. c
eq, 1/q
eq vs. 1/c
eq, q
eq vs. q
eq/c
eq, or q
eq/c
eq vs. q
eq, a linear relationship for Equation (3) can be deduced [
26].
Table 3 lists the four possible linear forms for determining Langmuir constants. In this study, only the type of isotherm with the highest coefficient of determination r² is listed. The correlation coefficient r² of the nonlinear form of the Langmuir isotherm and the experimentally determined loads q
eq and the arithmetical average loads
was calculated according to Equation (4).
2.3.3. Temkin Model
The isothermal loading of sorbents according to Temkin ([
28] in [
29]) is extended by the temperature parameter. Accordingly, the adsorption enthalpy is linearly proportional to the loading on the sorbent [
30]. The form of the isotherm used in this work is taken from Ho et al. [
24] (Equation (5)), where R is the universal gas constant (8.314459 J/(mol K)), T the temperature (K), b
T (1/mol), and A
T (L/mg) the Temkin isothermal constants.
The linearized form of the Temkin isotherm is shown in Equation (6).
For the determination of bT and AT, ln ceq vs. qeq is plotted; the slope represents the term RT/bT, the intersection with the ordinate the term RT ln(AT)/bT.
2.3.4. Thermodynamic Calculations
During the adsorption process, thermodynamic effects, shown in the form of energy adsorption or release, i.e., a temperature increase or decrease, can be observed. The standard free energy ∆G
0 (kJ/mol) can be calculated according to the following Equation (7)
where K
d is the thermodynamic equilibrium constant, here the Freundlich constant (L/g). According to Milonjic [
31], it should be noted that K
d must be dimensionless. Therefore, the use of the temperature-dependent equilibrium constant K
F must be corrected by a factor of 1000 g/L (density of water) in its dimensionless form. The relationship of the other thermodynamic parameters such as change in enthalpy ∆H
0 (kJ/mol) and change in standard entropy ∆S
0 (J/(mol K)) can be derived by means of the Gibbs–Helmholtz equation (8).
A linear correlation, which enables the determination of the change in standard entropy ∆S0 and free standard enthalpy ∆H0, is shown in a diagram in which the logarithmic equilibrium constant Kd is plotted against the reciprocal value of the temperature 1/T (Van’t–Hoff diagram). Here, the gradient corresponds to the quotient of the negative change in the free standard enthalpy ∆H0 and the universal gas constant R. Furthermore, the quotient of the change of the free molar standard entropy ∆S0 and the universal gas constant can be read from the axis section.
Endothermic adsorption is described by a positive value of ∆H0, meaning energy is absorbed by the adsorption process. A negative value indicates exothermic adsorption, meaning energy is being released. A spontaneous (exergonic) adsorption is described by negative values of ∆G0, while negative values of ∆S0 indicate a random adsorption behavior.
2.4. Kinetic Models
2.4.1. Intraparticle Diffusion
According to Weber and Morris ([
32] in [
33]), a mathematical description of the diffusion process is provided by the intraparticle diffusion model (ID), which constitutes a correlation between the loading rate k
ID (mg/(g min
0.5)) and the square root of the contact time t (min). McKay et al. [
34] extend this model by the constant C (mg/g), which is proportional to the thickness of the boundary layer as well as the initial adsorption by it. The time-dependent loading of the sorbent q
t,ID (mg/g) can be calculated by Equation (9).
To determine the loading rate kID, qt versus t0.5 is plotted. The slope of the resulting graph corresponds to kID while the intersection with the ordinate corresponds to C. Sole intraparticle diffusion occurs when the graph intersects the origin (C = 0). If a multistage diffusion process is present, two or more partial lines passing into each other can be approximated to the existing empirical measuring points of qt.
2.4.2. Pseudo-Second-Order
A mathematical model describing the time-dependent loading of the sorbent is possible with the pseudo-second-order (PSO) model according to Ho and McKay [
35]. However, it is not possible to make statements about the prevailing adsorption kinetic processes when using this model. Rather, it employs a macroscopic view of the adsorption process. The model is based on the assumption that the adsorption rate is dependent on the loading of the ion exchange material at a certain point in time and its equilibrium state. Equation (10) shows the PSO in its differential form, i.e., as the differential of the load q
t (mg/g) at any time t
where k
2 is the pseudo-second-order rate (mg/(g min)) and q
e (mg/g) the load at equilibrium. From the integration of Equation (9) with the boundary conditions q
t = 0 at t = 0 and q
t = q
t at t = t, four different linear forms of the PSO model can be obtained (
Table 4).
In this study, only the type with the highest coefficient of determination r2 (Equation (4)) is listed.
2.5. Analytical Methods
Ammonium was measured according to German standard DIN 38406-5 [
37]. At a pH of about 12.6, ammonium cations and ammonia contained in the sample react with hypochlorite ions and salicylate ions in the presence of sodium pentacyanonitrosylferrate (2-)(nitroprusside sodium) as a catalyst to form a blue dye. The required hypochlorite ions are formed in the alkaline medium by hydrolysis of the dichloroisocyanuric acid ions. The absorbance of the blue dye at 655 nm wavelength is linearly proportional to the ammonium concentration.
For determination of pH, probes (SenTix 950 + Multi 3430) (WTW, Weilheim, Germany) were used.
To determine the chemical elements of the zeolite, 0.3 to 0.5 g of the CLI were weighed and mixed with 6 mL HNO3 (65%), 4 mL HF, and 2 mL HCl. The mixture was digested by microwave (Start, MLS GmbH, Leutkirch, Germany) with a selected program run of 10 min at 110 °C, then 5 min at 140 °C, and finally 9 min at 190 °C. Together with the cooling phase, the digestion lasted 64 min. Heavy metals were analyzed by inductively coupled plasma mass spectrometry (Nexion 2000, Perkin Elmer, Waltham, MA, USA).
3. Results and Discussion
3.1. Cation Exchange Capacity
The amount of exchanged cations is given in
Table 5. All investigated CLIs adsorbed an almost equal amount of ammonium (121–137 meq/100g) and desorbed a similar amount of alkali and alkaline earth cations, indicating an electro-neutral ion exchange. In terms of ammonium, CCP 20 adsorbed the largest amount of around 137 meq/100g, while Micro 200 and EcoZeo 20 adsorbed 125 and 121 meq/100g, respectively. Thus, no correlation to the material size could be found. During the ammonium adsorption, similar equivalent amounts of alkaline earth metal cations were desorbed from all three CLIs (119–139 meq/100g). In all cases, calcium was exchanged with the highest amount (63–67 meq/100g), followed by potassium (45–49 meq/100g), while magnesium was exchanged in a similar order of magnitude from all three materials, and the exchange of sodium differed significantly between around 3 and 21 meq/100g.
In the case of CCP 20, the largest amount of ammonium was exchanged for the largest amount of cations. Malekian et al. [
38] reported a similar Cation Exchange Capacity (CEC) of 140–165 cmol/kg (=140–165 meq/100g) for millimeter- and nanometer-sized particles of Iranian zeolite.
3.2. Isoelectric State and pH-Dependent Adsorption
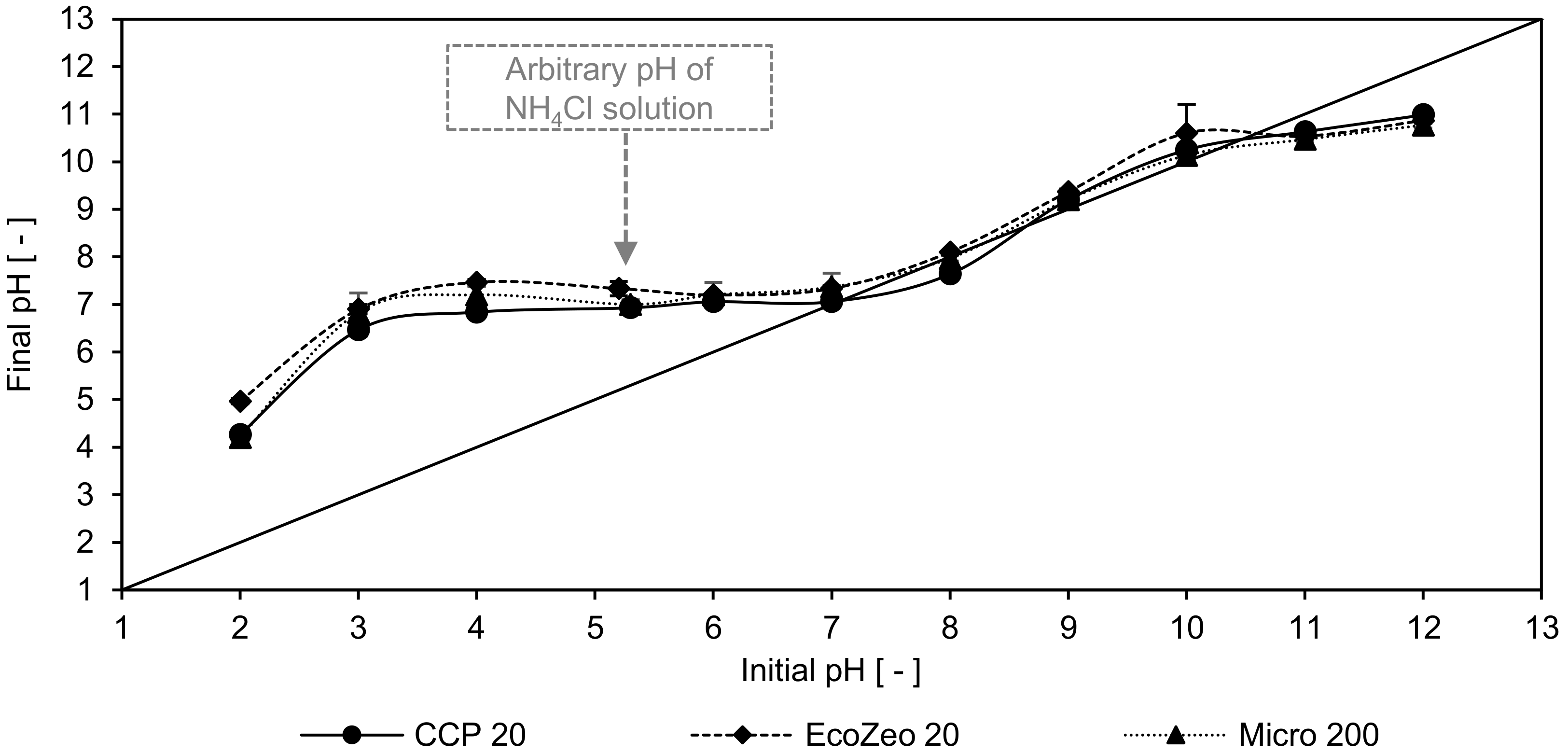
In
Figure 1, the final pH values of the filtrates are plotted against the pH values of the NH
4Cl solution after pH adjustment. The increase of the pH values in the range of 2 to 7 can be attributed to the removal of NH
4+ leaving an increasing amount of OH
−, as well as leaching of cations (e.g., K
+, Na
+, Ca
2+, Mg
2+). At higher pH values, especially in the alkaline range, no change in the pH could be observed. This can be attributed to the decrease in adsorption, since uncharged NH
3 which occurs at pH > 9 cannot be adsorbed by CLI and, therefore, no more cations that influence the pH can be released.
The isoelectric state of CCP 20 was determined between pH 7.9 and 10.5, while EcoZeo 20 and Micro 200 had their equilibrium between pH 8 and 10.5. Compared to the results of Zhang and Bi [
39], who determined an isoeletric state of 5.3 for natural CLI, the CLIs of this study had an increased negative charge, resulting in a higher isoeletric state. An even lower isoeletric state of 4.5 was reported by Guaya et al. [
10] who investigated modified Slovakian CLI. However, the authors observed that at a pH of 9 the maximum ammonium is adsorbed.
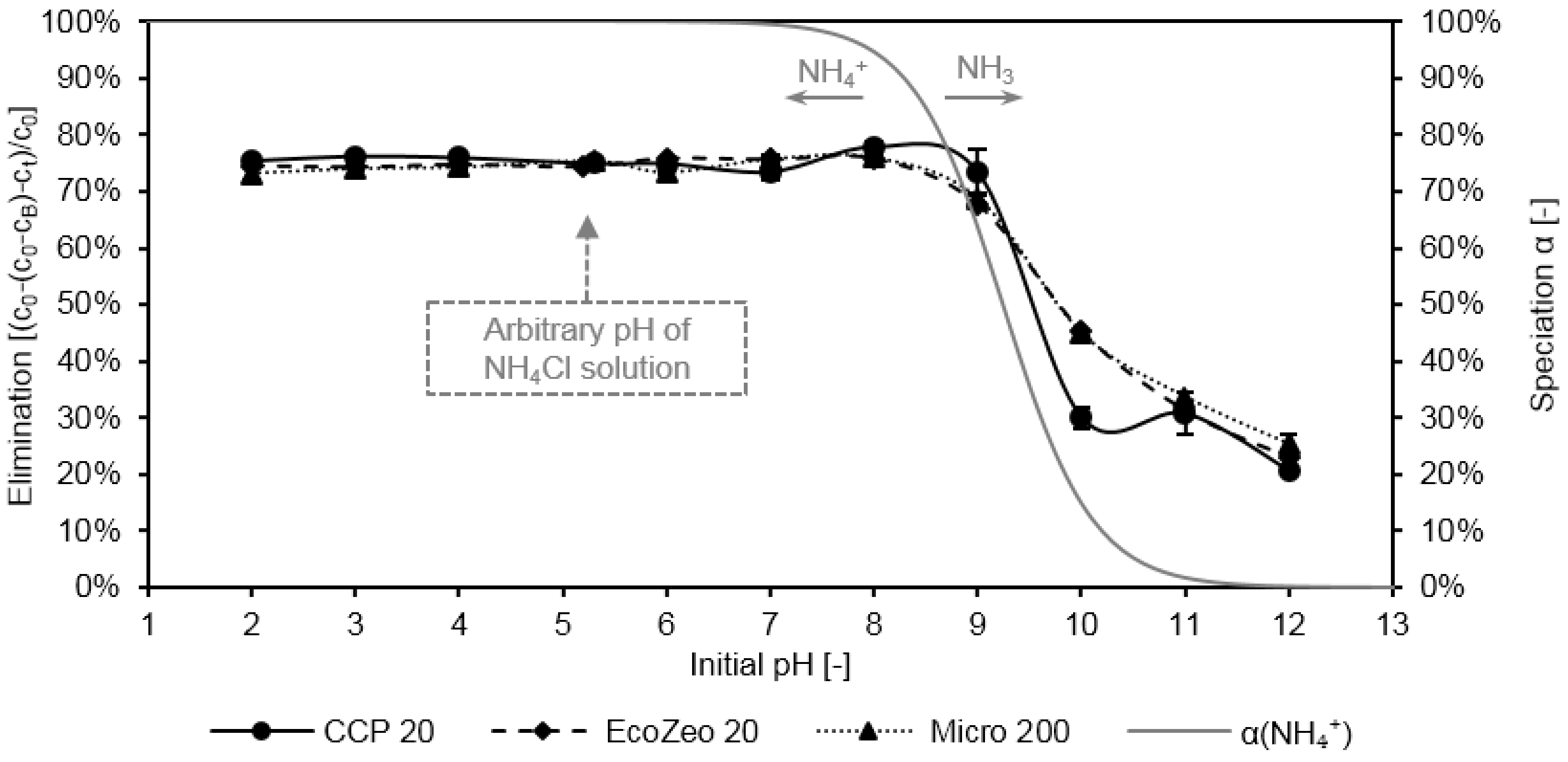
In
Figure 2, the ammonium elimination by CCP 20, Micro 200, and EcoZeo 20 is depicted as a function of pH. The standard deviation around the mean value is represented by black bars. Additionally, the pH-dependent level of speciation α of ammonium is shown by the secondary axis.
All three evaluated CLIs achieved a high elimination in a pH range from 2 to 8. In this pH range, the loading of the sorbents was ~8 mg NH4-N/g (loading not shown in the figure). The arbitrary pH value of the NH4Cl solution is indicated in the figure and amounted to 5.3. With pH values higher than 9, a significant decrease of elimination could be observed for all CLIs. CCP 20 eliminated 73% at pH 9, whereas at pH 10 only 30% of ammonium were removed. Within the same pH range, elimination by Micro 200 and EcoZeo 20 decreased from 69% to 45%. At higher pH values, with all three sorbents only a low elimination of 20% was achieved.
These observations are consistent with the results published by Li et al. [
40] and Guo et al. [
41]. The authors investigated the elimination of ammonium by CLI from China and USA and stated that there is no change in the elimination or loading in a pH range of 5 or 6 to 9. However, with other zeolites, specific pH values were also found at which maximum elimination extents were achieved (pH 8: [
42]; pH 7: [
11,
14,
43]; pH 6: [
13,
16,
44]; pH 4: [
20]). The decrease in elimination of ammonium was in the same pH range where ammonium dissociates to uncharged ammonia, which was, thus, no longer available for ion exchange. This finding was also shared by Alshameri et al. [
42], who also attributed the decrease in elimination at high pH to the dissociation of ammonium.
3.3. Isothermal Adsorption
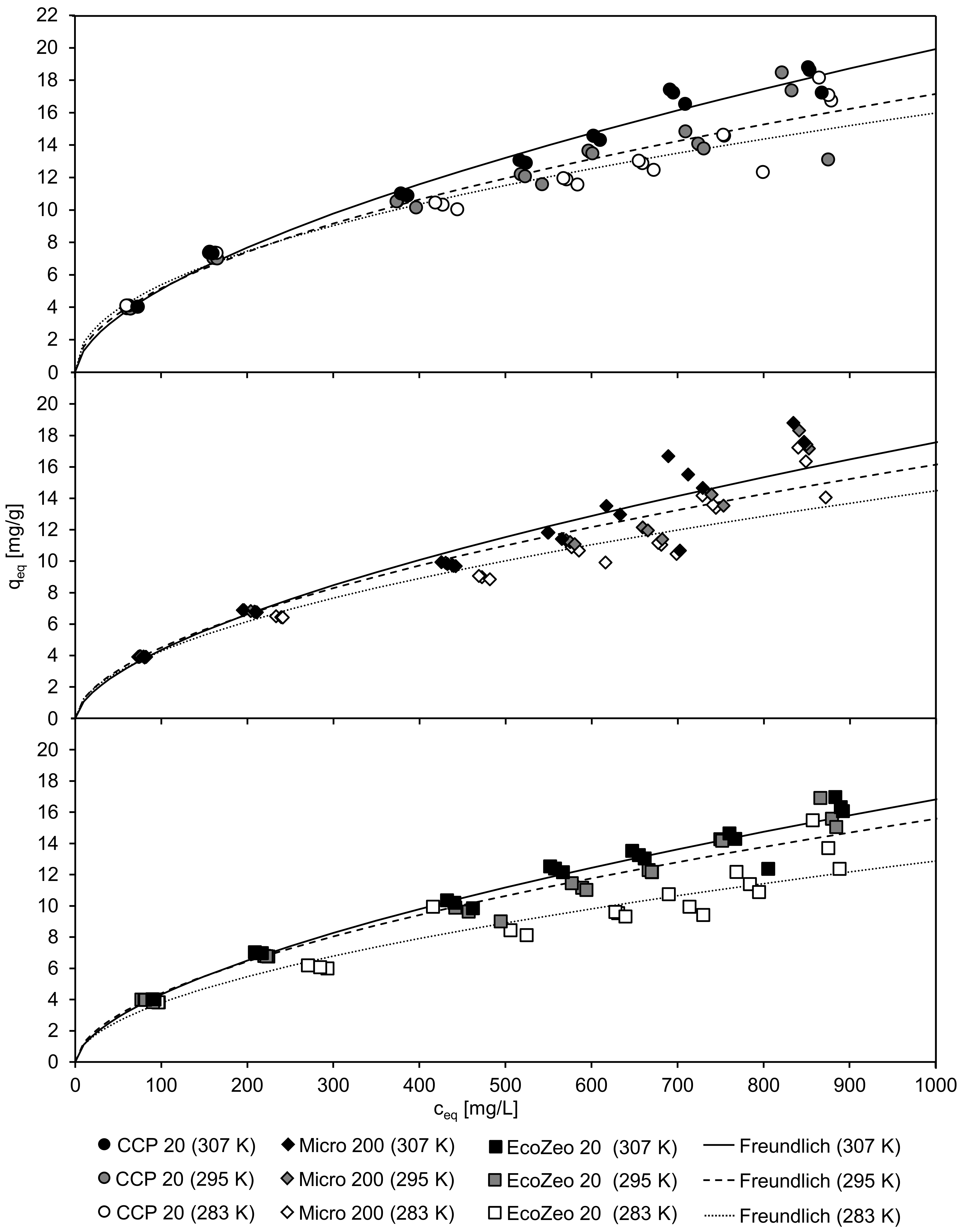
Figure 3 shows the equilibrium concentration c
eq and the associated loading of the CLI q
eq after 20 h contact time of the ammonium solution at different temperatures. The lines represent the Freundlich isotherm, of which the coefficients of determination were the highest of all the isotherm models (Freundlich, Langmuir, Temkin) tested. From the high correlation with the Freundlich isotherm it can be deduced that CLI has a heterogeneous surface which allows a nonideal adsorption. With increasing load of the CLI, less adsorption of ammonium can be achieved.
Table 6 lists the coefficients of the isothermal fit according to Freundlich, Langmuir, and Temkin as well as the coefficients of determination. The final pH was found to be between 6.4 and 7.3 whereby the latter value was reached with the largest amount of CLI.
Unaffected by temperature, CLI had a low qeq when a low ceq remained in the solution. At a temperature of 34 °C (307 K), all CLIs achieved the highest loading. The highest loading of qeq = 18.8 mg/g was achieved with CCP 20 and Micro 200, followed by EcoZeo 20 with qeq = 16.3 mg/g. Due to higher temperature, there is a lower viscosity of the solution. As a result, the NH4+ cations can penetrate deeper into the lattice, resulting in higher loading of the CLI.
An increase in adsorption capacity with higher temperatures was also reported by Alshameri et al. [
14] and Tosun [
45]. The authors observed a maximum adsorption capacity of 9.794 mg/g at 318 K (investigated range: 298–318 K) and 16.305 mg/g at 313 K (investigated range: 283–313 K), respectively. However, Gunay [
46] reported that with their zeolite used at 308 K, the highest adsorption capacity (q = 15.419 mg/g) was achieved and higher temperatures (investigated range: 296–343 K) led to a decrease in adsorption. Although the results show a large variation with regard to the maximum ammonium adsorption at the temperatures examined, it can nevertheless be seen that in the range of 308–318 K, the different CLIs adsorb the most ammonium.
3.4. Thermodynamic Properties
In
Table 7, the coefficients of isothermal adaptation and the determined thermodynamic state variables, free enthalpy of reaction, free standard enthalpy, and molar standard entropy, are shown.
From the thermodynamic parameters determined, it can be concluded that due to the negative free reaction enthalpy ΔG0 in the examined temperature range (283–307 K), the adsorption process of ammonium onto the investigated CLI was exergonic, i.e., a voluntary reaction. The free standard enthalpy ΔH0 of all three sorbents was negative, indicating an exothermic reaction. The standard molar entropy ΔS0, which was positive for Micro 200 and EcoZeo 20, indicates that the ammonium adsorption is a directional process, decreasing slightly as the temperature increases. However, the negative molar standard entropy of CCP 20 indicates, that the sorption process was random.
An exergonic reaction was also observed by Alshameri et al. [
14], Gunay [
46], and Karadag et al. [
19]. Their reported values of ΔG
0 ranged from −2.8662 to 0.224 kJ/mol [
14], −0.79 to 1.63 kJ/mol [
46], and −0.22 to 1.60 kJ/mol [
19], respectively. In this study, the values of ΔG
0 range from −15 to −13.7 kJ/mol. The much lower values regarding ΔG
0 of this study can be attributed to the smaller particle size and therefore short diffusion pathways of cations into the CLI compared to the ones of Alshameri et al. [
14], Gunay [
46], and Karadag et al. [
19], where zeolites with particle sizes of 0.063–0.074 mm, 0.3–0.6 mm, and 1.0–1.4 mm, respectively, were used. Furthermore, the values of ΔG
0 of the investigated CLIs decrease with increasing CEC, indicating that a high natural preload of the CLI with alkali and alkaline earth cations leads to a more exergonic adsorption. Similar to the results published by other researchers (ΔH
0: −49.384, −22.34, −5.43, −15.38 kJ/mol [
14,
19,
45,
46]), which indicate that adsorption of ammonium is exothermic, a slightly exothermic adsorption was found for the CLIs tested in this study (ΔH
0 ranging from 4.7 kJ/mol (EcoZeo 20) to 16.9 kJ/mol (CCP 20)). Furthermore, results reported with negative values of ΔS
0 (−156.1, −74.42, −43.03, −49.34, J/(K mol) [
14,
19,
45,
46]) indicate decreasing ammonium uptake due to increasing randomness. In contrast to this, a strongly directed adsorption process, as indicated by positive ΔS
0 values ranging between 32.7 J/(K mol) (EcoZeo 20) and 17.6 J/(K mol) (Micro 200), was achieved with the investigated materials of this study.
The results of studies by other researchers show that at higher temperatures not every zeolite shows a good adsorption performance with regard to ammonium. This means that zeolites must be chosen well to suit each individual situation, e.g., for the treatment of wastewater in typical temperature ranges (e.g., 283–307 K). All the investigated materials of this study showed best adsorption properties at temperatures of 34 °C, so they are obviously well suited for the application of ammonium extraction from wastewater of this temperature level, e.g., sludge water from anaerobic mesophilic digesters.
3.5. Kinetic Studies
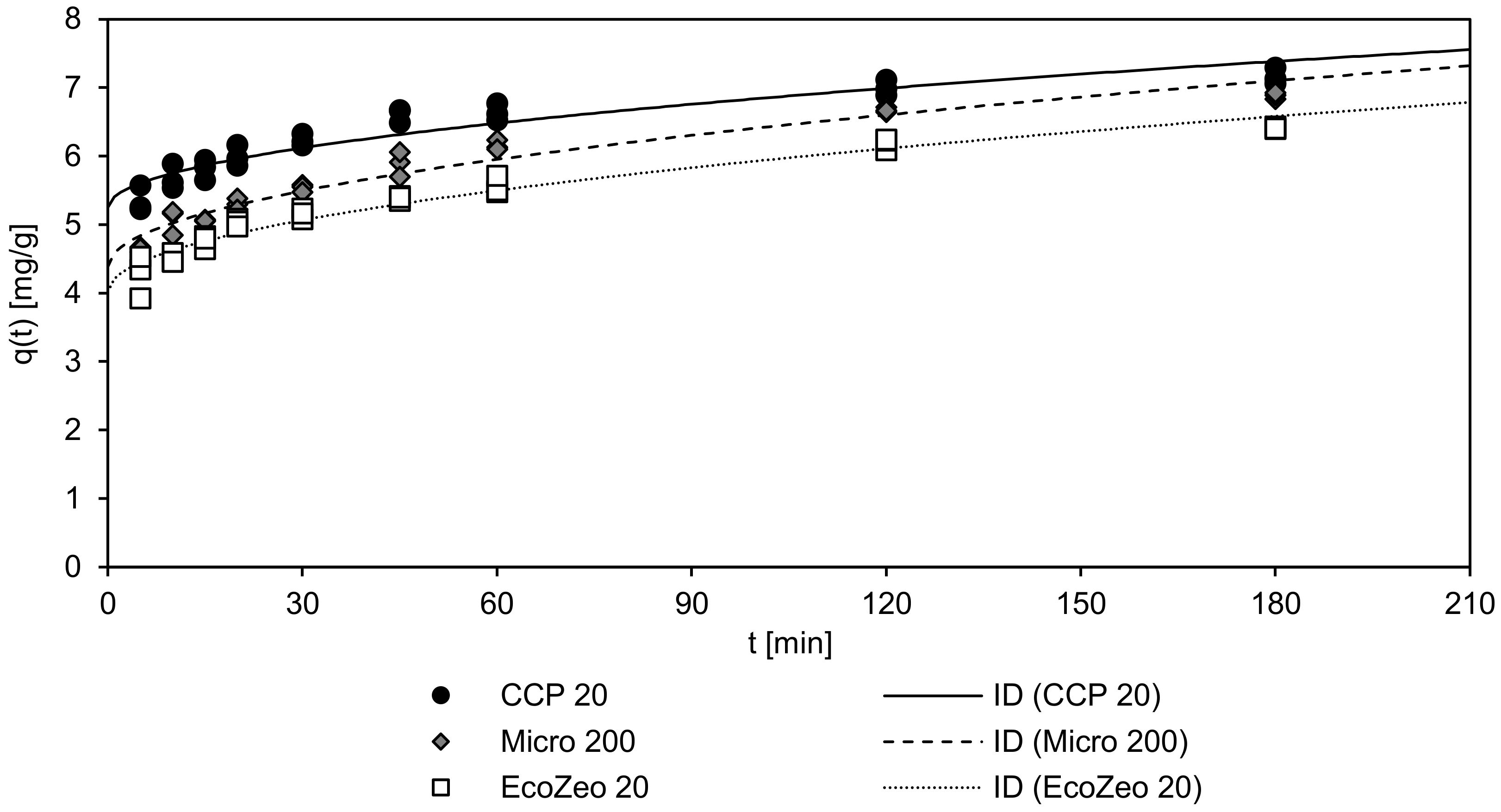
The results of the adsorption kinetics (loading q
t as a function of the contact time) of the three sorbents in NH
4Cl solution are depicted in
Figure 4. Furthermore, the fit to the ID model, which achieved higher r² values compared to the PSO model, is represented as lines.
As shown in
Figure 4, all CLIs are characterized by a rapid initial adsorption within the first minutes due to the thick boundary layer, which has a high capacity. Already after a contact time of 5 min, a load of 4.0 to 5.5 mg/g, which corresponds to 68% (100% was achieved after 180 min contact time) in the case of Micro 200 and EcoZeo 20 and 76% in the case of CCP 20, was achieved. After 60 min, Micro 200 and EcoZeo 20 reached 84% of their maximum load, while CCP 20 had already reached 88% at that time. As it was already shown in the isothermal studies (Chapter 3.3), CCP 20 achieved the highest loading in comparison to the other CLIs.
Table 8 summarizes the coefficients of the PSO model and ID model. The high coefficients of determination achieved by the ID model indicate that the adsorption speed is controlled by intraparticle diffusion. Fitting the obtained results to the PSO model reveals that CCP 20 achieved the highest adsorption rate (k
2 = 0.064 g/(mg min)), followed by EcoZeo 20 (k
2 = 0.048 g/(mg min)) and Micro 200 (k
2 = 0.046 g/(mg min)). Equilibrium loading q
e of CCP 20 was also highest (q
e = 6.99 mg/g), followed by Micro 200 (q
e = 6.62 mg/g) and EcoZeo 20 (q
e = 6.13 mg/g). According to the ID model, the intraparticle adsorption rate k
ID of Micro 200 was highest (0.203 mg/(min
0.5 g)), followed by EcoZeo 20 (0.191 mg/(min
0.5 g)) and CCP 20 (0.159 mg/(min
0.5 g)). Initial adsorption C, on the other hand, was the highest of CCP 20 (5.25 mg/g), followed by Micro 200 (4.38 mg/g) and EcoZeo 20 (4.02 mg/g). Thus, although the adsorption rates of Micro 200 and EcoZeo 20 were higher, neither CLIs reached the loading of CCP 20, which remained unattained due to the large initial adsorption by its thick boundary layer.
The results of the PSO model confirm already published conclusions according to which a high initial ammonium concentration leads to a high equilibrium load q
e but a slow adsorption rate k
2 [
22,
47,
48]. Thus, the adsorption velocities found in this study were slower than those reported by Karadag et al. [
47] (k
2 = 0.526 g/(mg min), c
0 = 100 mg/L) and Moussavi et al. [
48] (k
2 = 0.096 g/(mg min), c
0 = 100 mg/L), but exceeded those of Erdogan and Ülkü [
22] (k
2 = 0.6 × 10
−3 g/(mg min), c
0 = 300 mg/L) significantly.
Micro 200, the particle size distribution of which extends over a wider range, adsorbed ammonium more slowly than the other two CLIs. This confirms the results of Erdogan and Ülkü [
22] and Huang et al. [
49], who achieved the fastest adsorption velocity k
2 with the smallest particles. Therefore, sorbent particles should always be as small as practicable in order to achieve a high adsorption rate.
4. Conclusions
Within the scope of the investigations, the adsorption of ammonium by three different CLIs (CCP 20, Micro 200, and EcoZeo 20) at different pH values, different amounts of sorbent mass, different temperatures, and different contact times were systematically investigated. All three CLIs tested were very effective at adsorbing ammonium. In a pH range from 2 to 8, the CLIs were able to eliminate ammonium equally well, whereas higher pH values led to a significant drop in elimination. The CLI CCP 20 achieved the highest loading compared to Micro 200 and EcoZeo 20. This can be attributed to its small particle size, the highest CEC, and the highest natural preloading of the sorbent with K+ and Na+ cations of all CLIs tested. The isoelectric state of the CLIs was in the alkaline range, resulting from a large negative charge attracting cations as well as leaching of alkali and earth alkali cations influencing the pH. Furthermore, a contact time of 60 minutes was sufficient to achieve 84–88% of the maximum load. At a temperature of 34 °C, the highest loading was achieved (investigated range: 10–34 °C). The adsorption process of all CLIs was of exergonic and exothermic nature. Especially for those types of wastewater streams with high ammonium concentrations such as sludge water from anaerobic sludge digesters operated at mesophilic temperatures, CLI proved to be a suitable sorbent for the elimination of ammonium. Both the CEC determination and the determination of the isoelectric state are simple and fast methods to assess qualitatively the adsorption capacity of a CLI. In this study, CCP 20, which has the largest CEC and lowest isoelectric state, achieved the highest adsorption capacity. Depending on the research objective, the investigation of the CEC and the isoelectric point can provide sufficient information about the adsorption behavior of CLI.
Further investigations with real wastewater should be carried out.