Gel Carriers for Plant Extracts and Synthetic Pesticides in Rodent and Arthropod Pest Control: An Overview
Abstract
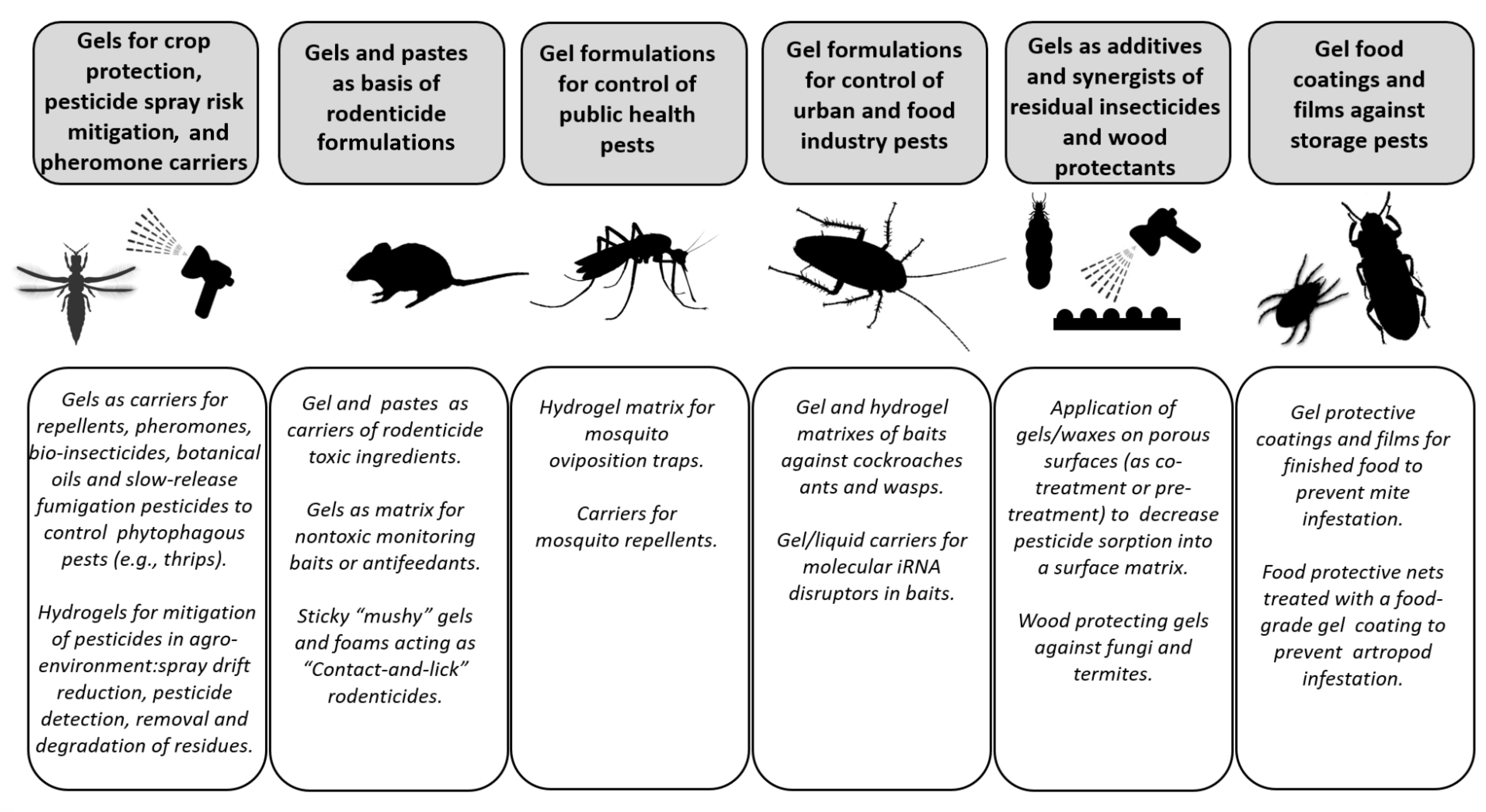
:1. Introduction
2. Overview of Pest Control Areas Regarding Usage Gel Formulations
3. Gels in Plant Protection and Pesticide Sprays Risk Mitigation
3.1. Gels as Carriers for Bioinsecticides and Slow-Release Pesticides or Pheromones in Crop Protection
3.2. Hydrogels for Mitigation of Pesticides in Agroenvironment (Spray Drift Reduction, Pesticide Detection, Removal and Degradation of Residues)
4. Rodenticide Gels and Pastes
4.1. “Contact-and-Lick” Rodenticide Sticky Gels and Foams
4.2. Toxic Bait Gels and Pastes (Soft Baits)
4.3. Nontoxic Monitoring Gel Baits and Antifeedants
5. Gels for Risk Mitigation of Mosquitoes
6. Gels Baits for Control of Ants, Cockroaches and Other Urban Pests
7. Gels as Additives of Residual Insecticides and Wood Protectants
8. Gel Coatings for Finished Food Protection against Mites
9. Gel Formulations with Natural Plant Extracts as Pesticides and Repellents
9.1. Hydrogels as Matrix for Essential Oils Based Baits or Fumigation Pesticides
9.2. Hydrogels Used as Carriers for Natural (EOs) Repellents
10. Outlooks and Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prathap, A.; Sureshan, K.M. Sugar-based organogelators for various applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, A.; Hou, J.-T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Tay, J.W.; Choe, D.H.; Mulchandani, A.; Rust, M.K. Hydrogels: From controlled release to a new bait delivery for insect pest management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Işıklan, N. Controlled release study of carbaryl insecticide from calcium alginate and nickel alginate hydrogel beads. J. Appl. Polym. Sci. 2007, 105, 718–725. [Google Scholar] [CrossRef]
- Baloch, F.E.; Afzali, D.; Fathirad, F. Design of acrylic acid/nanoclay grafted polysaccharide hydrogels as superabsorbent for controlled release of chlorpyrifos. Appl. Clay Sci. 2021, 211, 106194. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.J.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, Z.; Cui, H.; Verheggen, F. Polymer-based nanoinsecticides: Current developments, environmental risks and future challenges. A review. Biotechnol. Agron. Soc. Environ. 2020, 24, 59–69. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest. Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef] [Green Version]
- Nansen, C.; Hinson, B.; Davidson, D.; Vaughn, K.; Hosseini, A. Novel approaches to application and performance assessment of insecticide applications to crop leaves. J. Econ. Entomol. 2009, 103, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.N.; Harper, B.; Harper, S.L. The influence of size on the toxicity of an encapsulated pesticide: A comparison of micron-and nano-sized capsules. Environ. Int. 2016, 86, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Greaves, J.H.; Rowe, F.P.; Redfern, R.; Ayres, P. Microencapsulation of rodenticides. Nature 1968, 219, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, J.; Friesen, A.; Wieck, S.; Kümmerer, K. In search of the Holy Grail of Rodent control: Step-by-step implementation of safe and sustainable-by-design principles on the example of rodenticides. Sustain. Chem. Pharm. 2022, 25, 100602. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Efficacy of two formulations of pirimiphos-methyl as surface treatment against Sitophilus granarius, Rhyzopertha dominica, and Tribolium confusum. J. Pest Sci. 2014, 87, 507–519. [Google Scholar] [CrossRef]
- Marsh, R.E. Microencapsulation of rodenticides. Proc. Vertebr. Pest Conf. 1990, 14, 62–64. [Google Scholar]
- Stejskal, V.; Aulicky, R.; Pekar, S. Brief exposure of Blattella germanica (Blattodea) to insecticides formulated in various microcapsule sizes and applied on porous and non-porous surfaces. Pest Manag. Sci. 2009, 65, 93–98. [Google Scholar] [CrossRef]
- Ali, S.; Akram, W.; Sajjad, A.; Shakeel, Q.; Ullah, M.I. Aerogels as Pesticides. In Aerogels II: Preparation, Properties and Applications; Inamudin, Mobin, R., Ahamed, M.I., Altalhi, T., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2021; pp. 168–182. [Google Scholar]
- Benelli, G.; Pavela, R. Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Ind. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Cornwell, P.B. Studies in microencapsulation of rodenticides. Proc. Vertebr. Pest Conf. 1970, 4, 83–97. [Google Scholar]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Thombare, N.; Ali, M.; Swami, S. Applications of Biopolymeric gels in Agricultural Sector. In Polymer Gels: Perspectives and Applications; Thakur, V.K., Thakur, M.K., Voicu, S.I., Eds.; Springer: Singapore, 2018; pp. 185–228. [Google Scholar]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Campea, M.A.; Majcher, M.J.; Lofts, A.; Hoare, T. A review of design and fabrication methods for nanoparticle network hydrogels for biomedical, environmental, and industrial applications. Adv. Funct. Mater. 2021, 31, 2102355. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y.C. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Księżak, J. The influence of different doses of hydrogel on the quality of seeds and the yield of faba beans. Pol. J. Agron. 2018, 33, 8–15. [Google Scholar]
- Sarvas, M.; Pavlenda, P.; Takacova, E. Effect of hydrogel application on survival and growth of pine seedlings in reclamations. J. For. Sci. 2007, 53, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Hayat, R.; Ali, S. Water absorption by synthetic polymer (Aquasorb) and its effect on soil properties and tomato yield. Int. J. Agric. Biol. 2004, 6, 998–1200. [Google Scholar]
- Letey, J.; Clark, P.R.; Amrhein, C. Water-sorbing polymers do not conserve water. Calif. Agric. 1992, 46, 9–10. [Google Scholar] [CrossRef]
- Fonteno, W.C.; Bilderback, T.E. Impact of hydrogel on physical properties of coarse-structured horticultural substrates. J. Am. Soc. Hortic. Sci. 1993, 118, 217–222. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Des. Monom. Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Chipuk, T.; Dave, A.M.; Kumbar, S.G.; Aminabhavi, T.M. PH-sensitive acrylic-based copolymeric hydrogels for the controlled release of a pesticide and a micronutrient. J. Appl. Polym. Sci. 2003, 87, 394–403. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R.; Mattoso, L.H.C. Biodegradable Hydrogel as Delivery Vehicle for the Controlled Release of Pesticide. In Pesticides-Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-532-7. [Google Scholar]
- Bhagat, D.; Samanta, S.K.; Bhattacharyya, S. Efficient management of fruit pests by pheromone nanogels. Sci. Rep. 2013, 3, 1294. [Google Scholar] [CrossRef]
- He, F.; Zhou, Q.; Wang, L.; Yu, G.; Li, J.; Feng, Y. Fabrication of a sustained release delivery system for pesticides using interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Appl. Clay Sci. 2019, 183, 105347. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chen, C.; Liu, B.; Cai, D.; Wu, Z. Fabrication of a pH-responsively controlled-release pesticide using an attapulgite-based hydrogel. ACS Sustain. Chem. Eng. 2018, 6, 1192–1201. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Gupta, A. Controlled Release of thiram fungicide from starch-based hydrogels. J. Environ. Sci. Health B 2007, 42, 677–695. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.; Gupta, A. In vitro release dynamics of thiram fungicide from starch and poly(methacrylic acid)-based hydrogels. J. Hazard. Mater. 2008, 154, 278–286. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P. Starch-chitosan hydrogels for the controlled-release of herbicide in agricultural applications: A study on the effect of the concentration of raw materials and crosslinkers. J. Polym. Environ. 2022, 30, 2448–2461. [Google Scholar] [CrossRef]
- Rehab, A.; Akelah, A.; Issa, R.; D’Antone, S.; Solaro, R.; Chiellini, E. Controlled release of herbicides supported on polysaccharide based hydrogels. J. Bioact. Compat. Polym. 1991, 6, 52–63. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Dong, H. Controlled release of herbicide acetochlor from clay/carboxylmethylcellulose gel formulations. J. Agric. Food Chem. 2008, 56, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, K.; Tay, J.W. Fumigant toxicity of essential oils against Frankliniella occidentalis and F. insularis (Thysanoptera: Thripidae) as affected by polymer release and adjuvants. Insects 2022, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Ropek, D.; Kulikowski, E. Potential of hydrogel application for plant protection. Ecol. Chem. Eng. A 2009, 16, 1191–1198. [Google Scholar]
- Galhardi, J.A.; de Oliveira, J.L.; Ghoshal, S.; Fraceto, L.F. Soil enzyme responses to polymeric nanopesticides: An ecological risk analysis approach to promote sustainable agriculture. ACS Agric. Sci. Technol. 2022, 3, 443–452. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, F.; Cao, C.; Cao, L.; Li, F.; Zhao, P.; Huang, Q. Reducing pesticide spraying drift by folate/Zn2+ supramolecular hydrogels. Pest Manag. Sci. 2021, 77, 5278–5285. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Yang, J.; Bai, C.; Tang, S.; Ye, Q.; Wang, H. Superabsorbent hydrogels coating increased degradation and decreased bound residues formation of carbendazim in soil. Sci. Total Environ. 2018, 630, 1133–1142. [Google Scholar] [CrossRef]
- Aouada, F.A.; Pan, Z.; Orts, W.J.; Mattoso, L.H. Removal of paraquat pesticide from aqueous solutions using a novel adsorbent material based on polyacrylamide and methylcellulose hydrogels. J. Appl. Polym. Sci. 2009, 114, 2139–2148. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Gosset, A.; Oestreicher, V.; Perullini, M.; Bilmes, S.A.; Jobbágy, M.; Dulhoste, S.; Bayard, R.; Durrieu, C. Optimization of sensors based on encapsulated algae for pesticide detection in water. Anal. Methods 2019, 11, 6193–6203. [Google Scholar] [CrossRef]
- Jia, W.; Fan, R.; Zhang, J.; Zhu, K.; Gai, S.; Yin, Y.; Yang, Y. Smart MOF-on-MOF hydrogel as a simple rod-shaped core for visual detection and effective removal of pesticides. Small 2022, 18, 2201510. [Google Scholar] [CrossRef] [PubMed]
- Aulicky, R.; Tkadlec, E.; Suchomel, J.; Frankova, M.; Heroldová, M.; Stejskal, V. Management of the common vole in the Czech lands: Historical and current perspectives. Agronomy 2022, 12, 1629. [Google Scholar] [CrossRef]
- Buckle, A.P.; Smith, R. Rodent Pests and Their Control; CABI: Wallingford, UK, 2014. [Google Scholar]
- Frankova, M.; Kaftanova, B.; Aulicky, R.; Rodl, P.; Frynta, D.; Stejskal, V. Temporal production of coloured faeces in wild roof rats (Rattus rattus) following consumption of fluorescent non-toxic bait and a comparison with wild R. norvegicus and Mus musculus. J. Stored Prod. Res. 2019, 81, 7–10. [Google Scholar] [CrossRef]
- Frankova, M.; Stejskal, V.; Aulicky, R. Suppression of food intake by house mouse (Mus musculus) following ingestion of brodifacoum-based rodenticide bait. Crop. Prot. 2017, 100, 134–137. [Google Scholar] [CrossRef]
- Frankova, M.; Stejskal, V.; Aulicky, R. Efficacy of rodenticide baits with decreased concentrations of brodifacoum: Validation of the impact of the new EU anticoagulant regulation. Sci. Rep. 2019, 9, 16779. [Google Scholar] [CrossRef] [Green Version]
- Grulich, I. Boj proti hrabosi polnímu. In Common Vole (Microtus arvalis), 1st ed.; Kratochvíl, J., Balát, F., Eds.; CAV Edition: Prague, Czech Republic, 1959; pp. 285–316. (In Czech) [Google Scholar]
- Wilson, G. The Evolution of Rodenticides. Professional Pest Manager. 2018. Available online: https://professionalpestmanager.com/the-evolution-of-rodenticides/ (accessed on 8 July 2022).
- Vuksa, M.; Djedovic, S.; Jokic, G.; Stojnic, B. Palatability and efficacy of RB soft bag formulated baits in controlling house mouse and Norway rat in mills and storage facilities of agricultural products. J. Process. Energy Agric. 2011, 15, 267–269. [Google Scholar]
- Vukša, M.; Dedovic, S.; Jokic, G.; Stojnic, B. Palatability and efficacy of RB soft bag formulated baits in controlling house mouse and Norway rat in animal food blender facilities and pig farm. Biotechnol. Anim. Husb. 2011, 27, 1801–1810. [Google Scholar] [CrossRef]
- Jordan, K.K.; Riegel, C.; Bauder, F.M.; Smith, P.L. Urban Field Efficacy of a New Cholecalciferol-based Soft Bait on Commensal Rats in New Orleans, Louisiana, USA. In Proceedings of the 28th Vertebrate Pest Conference, Rohnert Park, CA, USA, 26 February–1 March 2018; Woods, D.M., Ed.; Independently published: Davis, CA, USA, 2018; pp. 23–32. [Google Scholar]
- Sked, S.; Abbar, S.; Cooper, R.; Corrigan, R.; Pan, X.; Ranabhat, S.; Wang, C. Monitoring and controlling house mouse, Mus musculus domesticus, Infestations in low-income multi-family dwellings. Animals 2021, 11, 648. [Google Scholar] [CrossRef]
- Kappes, P.J.; Siers, S.R. Relative acceptance of brodifacoum pellets and soft bait sachets by Polynesian rats (Rattus exulans) on Wake Atoll. Manag. Biol. Invasions 2021, 12, 685–699. [Google Scholar] [CrossRef]
- Sachdeva, S.; Singla, N. Antifeedant and repellent potential of alginate based microcapsules containing eucalyptus oil against house rat, Rattus rattus. J. Entomol. Zool. Stud. 2018, 6, 608–617. [Google Scholar]
- Frynta, D.; Eliasova, B.; Frankova, M.; Aulicky, R.; Rodl, P.; Stejskal, V. Production of UV-light-detectable faeces from house mice (Mus musculus domesticus) after consumption of encapsulted fluorescent pigment in monitoring bait. Pest Manag. Sci. 2012, 68, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Frankova, M.; Kaftanova-Eliasova, B.; Rodl, P.; Aulicky, R.; Frynta, D.; Stejskal, V. Monitoring of Rattus norvegicus based on non-toxic bait containing encapsulated fluorescent dye: Laboratory and semi-field validation study. J. Stored Prod. Res. 2015, 64, 103–108. [Google Scholar] [CrossRef]
- Wales, K.N.; Meinerz, R.; Baldwin, R.A. Assessing the Attractiveness of Three Baits for Roof Rats in California Citrus Orchards. Agronomy 2021, 11, 2417. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeira, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Coats, J. Insect repellents—Past, present and future. Pestic. Outlook 2001, 12, 154–158. [Google Scholar] [CrossRef]
- Hazarika, H.; Krishnatreyya, H.; Tyagi, V.; Islam, J.; Gogoi, N.; Goyary, D.; Chattopadhyay, P.; Zaman, K. The fabrication and assessment of mosquito repellent cream for outdoor protection. Sci. Rep. 2022, 12, 2180. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Junior, E.R.; Mansur, C.R.E. Polymer-based drug delivery systems applied to insects repellents devices: A review. Curr. Drug Deliv. 2016, 13, 221–235. [Google Scholar] [CrossRef]
- Milutinović, R.; Vuleta, G.; Milić, J.; Stajković, N. Assessment of efficiency of repellent formulations with N, N-diethyl-m-toluamide in laboratory conditions. Int. J. Cosmet. Sci. 1999, 21, 7–14. [Google Scholar] [CrossRef]
- Pinto, I.C.; Cerqueira-Coutinho, C.S.; Santos, E.P.; Carmo, F.A.; Ricci-Junior, E. Development and characterization of repellent formulations based on nanostructured hydrogels. Drug Dev. Ind. Pharm. 2017, 43, 67–73. [Google Scholar] [CrossRef]
- Delong, W.; Weibin, M.; Mingchen, J.; Zhonglin, Y.; Juntao, F.; Xing, Z. pHEMA hydrogels with pendant triazinyl-β-cyclodextrin as an efficient and recyclable reservoir for loading and release of plant-based mosquito repellents: A new aqueous mosquito repellent formulation. RSC Adv. 2016, 6, 27301–27312. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Singh, S.P.; Rao, R. Enhanced protective potential of novel citronella essential oil microsponge hydrogel against Anopheles stephensi mosquito. J. Asia Pac. Entomol. 2021, 24, 61–69. [Google Scholar] [CrossRef]
- Rogerio, C.B.; Abrantes, D.C.; De Oliveira, J.L.; de Araújo, D.R.; da Costa, T.G.; De Lima, R.; Fraceto, L.F. Cellulose hydrogels containing geraniol and icaridin encapsulated in zein nanoparticles for arbovirus control. ACS Appl. Bio Mater. 2022, 5, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Conti, B. Eco-friendly control strategies against the asian tiger mosquito, Aedes albopictus (Diptera: Culicidae): Repellency and toxic activity of plant essential oils and extracts. Pharmacologyonline 2014, 1, 44–51. [Google Scholar]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crops Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [Green Version]
- Mapossa, A.B.; Focke, W.W.; Tewo, R.K.; Androsch, R.; Kruger, T. Mosquito-repellent controlled-release formulations for fighting infectious diseases. Malar. J. 2021, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, S.M.; Park, I.K.; Hyun, J. Larvicidal composite alginate hydrogel combined with a pickering emulsion of essential oil. Carbohydr. Polym. 2021, 254, 117381. [Google Scholar] [CrossRef]
- Johnson, B.J.; Ritchie, S.A.; Fonseca, D.M. The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Friuli, M.; Cafarchia, C.; Lia, R.P.; Otranto, D.; Pombi, M.; Demitri, C. From tissue engineering to mosquitoes: Biopolymers as tools for developing a novel biomimetic approach to pest management/vector control. Parasites Vectors 2022, 15, 79. [Google Scholar] [CrossRef]
- Friuli, M.; Cafarchia, C.; Cataldo, A.; Lia, R.P.; Otranto, D.; Pombi, M.; Demitri, C. Proof of concept of biopolymer based hydrogels as biomimetic oviposition substrate to develop tiger mosquitoes (Aedes albopictus) cost-effective lure and kill ovitraps. Bioengineering 2022, 9, 267. [Google Scholar] [CrossRef]
- Rust, M.K.; Owens, J.M.; Reierson, D.A. Understanding and Controlling the German Cockroach; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Tee, H.; Lee, C. Sustainable cockroach management using insecticidal baits: Formulations, behavioral responses and issues. In Urban Insect Pests-Sustainable Management Strategies; Dhang, P., Ed.; CAB International: Oxfordshire, UK; Boston, MA, USA, 2014; pp. 65–85. [Google Scholar]
- Stejskal, V.; Vendl, T.; Li, Z.; Aulicky, R. Minimal thermal requirements for development and activity of stored product and food industry pests (Acari, Coleoptera, Lepidoptera, Psocoptera, Diptera and Blattodea): A review. Insects 2019, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Appel, A.G. Laboratory and field performance of an indoxacarb bait against German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 2003, 96, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.B. Amidinohydrazones—A new class of insecticides. In Proceedings of the 10th British Crop Protection Conference-Pests and Diseases, Brighton, UK, 19–22 November 1979; British Crop Protection Council: Cambridge, UK, 1979; pp. 575–582. [Google Scholar]
- Oladipupo, S.O.; Hu, X.P.; Appel, A.G. Essential oils in urban insect management—A review. J. Econ. Entomol. 2022, toac083. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Liu, Y.; Lin, Y.H.; Belles, X.; Lee, H.J. Practical use of RNA interference: Oral delivery of double-stranded RNA in liposome carriers for cockroaches. J. Vis. Exp. 2018, 135, e57385. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Miao, S.; Yang, B.; Wang, Z.; Liu, Q.; Wang, R.; Du, X.; Ren, Y.; Lu, Y. Initial characterization of the vitellogenin receptor from a Psocoptera insect: Function analysis and RNA interference in Liposcelis entomophila (Enderlein). J. Stored Prod. Res. 2021, 92, 101803. [Google Scholar] [CrossRef]
- Markin, G.P.; OHill, S. Microencapsulated oil bait for control of the imported fire ant. J. Econ. Entomol. 1971, 64, 193–196. [Google Scholar] [CrossRef]
- Oladipupo, S.O.; Hu, X.P.; Appel, A.G. Essential oil components in superabsorbent polymer gel modify reproduction of Blattella germanica (Blattodea: Ectobiidae). J. Econ. Entomol. 2020, 113, 2436–2447. [Google Scholar] [CrossRef]
- Buczkowski, G.; Roper, E.; Chin, D. Polyacrylamide hydrogels: An effective tool for delivering liquid baits to pest ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2014, 107, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.W.; Hoddle, M.S.; Mulchandani, A.; Choe, D.H. Development of an alginate hydrogel to deliver aqueous bait for pest ant management. Pest Manag. Sci. 2017, 73, 2028–2038. [Google Scholar] [CrossRef]
- Rust, M.K.; Soeprono, A.; Wright, S.; Greenberg, L.; Choe, D.-H.; Boser, C.L.; Cory, C.; Hanna, C. Laboratory and field evaluations of polyacrylamide hydrogel baits against Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2015, 108, 1228–1236. [Google Scholar] [CrossRef]
- Choe, D.H.; Campbell, K.; Hoddle, M.S.; Kabashima, J.; Dimson, M.; Rust, M.K. Evaluation of a hydrogel matrix for baiting western yellowjacket (Vespidae: Hymenoptera). J. Econ. Entomol. 2018, 111, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Boser, C.L.; Hanna, C.; Holway, D.A.; Faulkner, K.R.; Naughton, I.; Merrill, K.; Randall, J.M.; Cory, C.; Choe, D.H.; Morrison, S.A. Protocols for argentine ant eradication in conservation areas. J. Appl. Entomol. 2017, 141, 540–550. [Google Scholar] [CrossRef]
- Merrill, K.C.; Boser, C.L.; Hanna, C.; Holway, D.A.; Naughton, I.; Choe, D.-H.; Rankin, E.E.W. Argentine ant (Linepithema humile, Mayr) eradication efforts on San Clemente Island, California, USA. West. N. Am. Nat. 2019, 78, 829. [Google Scholar] [CrossRef]
- Klotz, J.H.; Shorey, H. Low-Toxic Control of Argentine Ants Using Pheromone-Enhanced Liquid Baits; California Department of Consumer Affairs: Sacramento, CA, USA, 2000; p. 35.
- Hewlett, P.S. The formation of insecticidal films on building materials. Ann. Appl. Biol. 1948, 35, 228–232. [Google Scholar] [CrossRef]
- Parkin, E.; Hewlett, S. The formation of insecticidal films on building materials. I. Preliminary experiments with films of pyrethrum and D.D.T. in a heavy oil. Ann. Appl. Biol. 1946, 33, 381. [Google Scholar] [CrossRef]
- Hewlett, P.S. The toxicities of three petroleum oils to the grain weevils. Ann. Appl. Biol. 1947, 34, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Tyler, P.S.; Rowlands, D.G. Sodium carboxymethyl cellulose as a stabilizer for malathion formulations. J. Stored Prod. Res. 1967, 3, 109–115. [Google Scholar] [CrossRef]
- Gudrups, I.; Harris, A.H.; Dales, M.J. Are residual insecticide applications to store surfaces worth using? In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Wallingford, UK, 1994; pp. 785–789. [Google Scholar]
- Obounou-Akong, F.; Gérardin, P.; Thévenon, M.F.; Gérardin-Charbonnier, C. Hydrogel-based boron salt formulations for wood preservation. Wood Sci. Technol. 2015, 49, 443–456. [Google Scholar] [CrossRef]
- Krizkova-Kudlikova, I.; Stejskal, V.; Hubert, J. Comparison of detection methods for Acarus siro (Acari: Acaridida: Acarididae) contamination in grain. J. Econ. Entomol. 2007, 100, 1928–1937. [Google Scholar] [CrossRef]
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol. 2018, 63, 553–573. [Google Scholar] [CrossRef]
- Hubert, J.; Erban, T.; Nesvorna, M.; Stejskal, V. Emerging risk of infestation and contamination of dried fruits by mites in the Czech Republic. Food Addit. Contam. Part A 2011, 28, 1129–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskal, V.; Bostlova, M.; Nesvorna, M.; Volek, V.; Dolezal, V.; Hubert, J. Comparison of the resistance of mono-and multilayer packaging films to stored-product insects in a laboratory test. Food Control 2017, 73, 566–573. [Google Scholar] [CrossRef]
- Aulicky, R.; Vendl, T.; Stejskal, V. Evaluation of contamination of packages containing cereal-fruit bars by eggs of the pest Indian meal moth (Plodia interpunctella, Lepidoptera) due to perforations in their polypropylene foil packaging. J. Food Sci. Technol. 2019, 56, 3293–3299. [Google Scholar] [CrossRef]
- Vendl, T.; Stejskal, V.; Kadlec, J.; Aulicky, R. New approach for evaluating the repellent activity of essential oils against storage pests using a miniaturized model of stored-commodity packaging and a wooden transport pallet. Ind. Crops Prod. 2021, 172, 114024. [Google Scholar] [CrossRef]
- Riudavets, J.; Castane, C.; Alomar, O.; Pons, M.J.; Gabarra, R. Modified atmosphere packaging (MAP) as an alternative measure for controlling ten pests that attack processed food products. J. Stored Prod. Res. 2009, 45, 91–96. [Google Scholar] [CrossRef]
- Kucerova, Z.; Kyhos, K.; Aulicky, R.; Stejskal, V. Low pressure treatment to control food-infesting pests (Tribolium castaneum, Sitophilus granarius) using a vacuum packing machine. Czech J. Food Sci. 2013, 31, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, Z.; Kyhos, K.; Aulicky, R.; Lukas, J.; Stejskal, V. Laboratory experiments of vacuum treatment in combination with an O2 absorber for the suppression of Sitophilus granaries infestations in stored grain samples. Crop Prot. 2014, 61, 79–83. [Google Scholar] [CrossRef]
- Golob, P.; Cox, J.R.; Kilminster, K. Evaluation of insecticide dips as protectants of stored dried fish from dermestid beetle infestation. J. Stored Prod. Res. 1987, 23, 47–56. [Google Scholar] [CrossRef]
- Stara, J.; Stejskal, V.; Nesvorna, M.; Plachy, J.; Hubert, J. Efficacy of selected pesticides against synanthropic mites under laboratory assay. Pest. Manag. Sci. 2011, 67, 446–457. [Google Scholar] [CrossRef]
- Rogers, W.; Campbell, Y.L.; Zhang, X.; Shao, W.; White, S.; Phillips, T.W.; Schilling, M.W. The application of food grade short chain fatty acids to prevent infestation of Tyrophagus putrescentiae on dry cured ham and the effects on sensory properties. J. Stored Prod. Res. 2020, 88, 101684. [Google Scholar] [CrossRef]
- Shao, W.; Campbell, Y.L.; Phillips, T.W.; Freeman, C.; Kundu, S.; Crist, C.A.; Williams, J.B.; Schilling, M.W. The application of chitosan in food-grade coatings to control Tyrophagus putrescentiae on dry-cured hams and the effects on sensory properties. J. Stored Prod. Res. 2021, 94, 101899. [Google Scholar] [CrossRef]
- Zhao, Y.; Abbar, S.; Phillips, T.W.; Williams, J.B.; Smith, B.S.; Schilling, M.W. Developing food-grade coatings for dry-cured hams to protect against ham mite infestation. Meat Sci. 2016, 113, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Campbell, Y.; Shao, W.; Dinh, T.; To, K.; Rogers, W.; Zhang, X.; Phillips, T.; Schilling, W. Use of nets treated with food grade coatings on controlling mold growth and mite infestation in dry-cured ham aging facilities. J. Stored Prod. Res. 2020, 89, 101716. [Google Scholar] [CrossRef]
- Campbell, Y.; Zhang, X.; Shao, W.; Williams, J.B.; Kim, T.; Goddard, J.; Abbar, S.; Phillips, T.; Schilling, M.W. Use of nets treated with food-grade coatings on dry-cured ham to control Tyrophagus putrescentiae infestations without impacting sensory properties. J. Stored Prod. Res. 2018, 76, 30–36. [Google Scholar] [CrossRef]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Nikolaou, P.; Marciniak, P.; Adamski, Z.; Ntalli, N. Controlling stored products’ pests with plant secondary metabolites: A review. Agriculture 2021, 11, 879. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Giunti, G.; Laudani, F.; Lo Presti, E.; Bacchi, M.; Palmeri, V.; Campolo, O. Contact toxicity and ovideterrent activity of three essential oil-based nano-emulsions against the olive fruit fly Bactrocera oleae. Horticulturae 2022, 8, 240. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.; Castañé, C.; Martín, F.; López, M.; Guirao, P.; Riudavets, J. (E)-Anethole microspheres as an alternative insecticide in funnel traps. J. Stored Prod. Res. 2021, 93, 101862. [Google Scholar] [CrossRef]
- Camara, M.C.; Monteiro, R.A.; Carvalho, L.B.; Oliveira, J.L.; Fraceto, L.F. Enzyme stimuli-responsive nanoparticles for bioinsecticides: An emerging approach for uses in crop protection. ACS Sustain. Chem. Eng. 2021, 9, 106–112. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.; Camara, M.C.; Della Vechia, J.F.; de Matos, S.T.S.; de Andrade, D.J.; Goncalves, K.C.; Nascimento, J.D.; Polanczyk, R.A.; de Araujo, D.R.; et al. Hydrogels containing botanical repellents encapsulated in zein nanoparticles for crop protection. ACS Appl. Nano Mater. 2019, 3, 207–217. [Google Scholar] [CrossRef]
- Kelany, Y.; Ibrahim, A.; Hegazy, M. Improving the efficiency of two local baits used for the control of the German cockroach, Blattella germanica (L.), (Dictyoptera: Blattellidae). Int. J. Pharm. Biol. Sci. 2017, 12, 59–66. [Google Scholar]
- Teli, M.D.; Chavan, P.P. Application of gelatine based microcapsules containing mosquito repellent oils on cellulosic biopolymer. J. Bionanosci. 2016, 10, 390–395. [Google Scholar] [CrossRef]
- Rana, M.; Singh, S.S.J.; Yadav, S. Effect of microencapsulated plant extracts on mosquito repellency. J. Appl. Nat. Sci. 2017, 9, 2127–2131. [Google Scholar] [CrossRef]
- Lucia, A.; Toloza, A.C.; Guzmán, E.; Ortega, F.; Rubio, R.G. Novel polymeric micelles for insect pest control: Encapsulation of essential oil monoterpenes inside a triblock copolymer shell for head lice control. PeerJ 2017, 5, e3171. [Google Scholar] [CrossRef]
- Kamari, A.; Yusoff, S.N.M.; Wong, S.T.S.; Fatimah, I. A mini review of materials used as improvers for insect and arthropod pest repellent textiles. Curr. Appl. Sci. Technol. 2022, 22, 18. [Google Scholar] [CrossRef]
- Oyedele, A.O.; Gbolade, A.A.; Sosan, M.B.; Adewoyin, F.B.; Soyelu, O.L.; Orafidiya, O.O. Formulation of an effective mosquito-repellent topical product from lemongrass oil. Phytomedicine 2002, 9, 259–262. [Google Scholar] [CrossRef]
- Abd El-Bar, M.; Fawki, S. Fumigant activity and chemical composition of three essential oils used in gelatin capsules for the control of Acanthoscelides obtectus (Say) (Coleoptera: Chrysomelidae) in Egypt. Afr. Entomol. 2021, 29, 534–546. [Google Scholar] [CrossRef]
- Navarro, S.; Zehavi, D.; Angel, S.; Finkelman, S. Natural nontoxic insect repellent packaging materials. In Intelligent and Active Packaging for Fruits and Vegetables; Wilson, C.L., Ed.; CRC Press: New York, NY, USA, 2007; pp. 201–236. [Google Scholar]
- Milićević, Z.; Krnjajić, S.; Stević, M.; Ćirković, J.; Jelušić, A.; Pucarević, M.; Popović, T. Encapsulated clove bud essential oil: A new perspective as an eco-friendly biopesticide. Agriculture 2022, 12, 338. [Google Scholar] [CrossRef]
- Picard, I.; Hollingsworth, G.R.; Salmieri, S.; Lacroix, M. Repellency of essential oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as affected by type of oil and polymer release. J. Econ. Entom. 2012, 105, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Trematerra, P.; Athanassiou, C.; Stejskal, V.; Sciarretta, A.; Kavallieratos, N.; Palyvos, N. Large-scale mating disruption of Ephestia spp. and Plodia interpunctella in Czech Republic, Greece and Italy. J. Appl. Entomol. 2011, 135, 749–762. [Google Scholar] [CrossRef]
- Ortiz, A.; Porras, A.; Marti, J.; Tudela, A.; Rodríguez-González, Á.; Sambado, P. Mating disruption of the olive moth Prays oleae (Bernard) in olive groves using aerosol dispensers. Insects 2021, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, A.; Thévenon, M.-F.; Wozniak, E.; Gérardin, P. Effects of monoglycerides on leachability and efficacy of boron wood preservatives against decay and termites. Int. Biodeter. Biodegr. 2010, 64, 135–138. [Google Scholar] [CrossRef]
- Patachia, S.; Croitoru, C. Biopolymers for wood preservation. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–332. [Google Scholar]
- Alade, A.A.; Naghizadeh, Z.; Wessels, C.B.; Tyhoda, L. A review of the effects of wood preservative impregnation on adhesive bonding and joint performance. J. Adhes. Sci. Technol. 2021, 36, 1593–1617. [Google Scholar] [CrossRef]
- Wang, C.; Lee, C.Y.; Rust, M.K. Biology and Management of the German Cockroach, 1st ed.; CABI: Wallingford, UK, 2021; pp. 1–304. [Google Scholar]
- Welzel, K.F.; Choe, D.H. Development of a pheromone-assisted baiting technique for Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2016, 109, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- McCalla, K.A.; Tay, J.-W.; Mulchandani, A.; Choe, D.-H.; Hoddle, M.S. Biodegradable alginate hydrogel bait delivery system effectively controls high-density populations of Argentine ant in commercial citrus. J. Pest Sci. 2020, 93, 1031–1042. [Google Scholar] [CrossRef]
- Campbell, K.J.; Beek, J.; Eason, C.T.; Glen, A.S.; Godwin, J.; Gould, F.; Holmes, N.D.; Howald, G.R.; Madden, F.M.; Ponder, J.B. The next generation of rodent eradications: Innovative technologies and tools to improve species specificity and increase their feasibility on islands. Biol. Conserv. 2015, 185, 47–58. [Google Scholar] [CrossRef]
- Vendl, T.; Frankova, M.; Aulicky, R.; Stejskal, V. First record of the development of Sitophilus oryzae on two rodent bait formulations and literature overview of stored product arthropods infestations in rodent baits. J. Stored Prod. Res. 2020, 86, 101557. [Google Scholar] [CrossRef]
- Horak, K.E. RNAi: Applications in vertebrate pest management. Trends Biotechnol. 2020, 38, 1200–1202. [Google Scholar] [CrossRef]
- Brevik, K.; Schoville, S.; Mota-Sanchez, D.; Chena, Y. Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Manag. Sci. 2018, 74, 1953–1963. [Google Scholar] [CrossRef]
- Sanou, A.; Nelli, L.; Guelbéogo, W.M.; Cissé, F.; Tapsoba, M.; Ouédraogo, P.; Sagnon, N.; Ranson, H.; Matthiopoulos, J.; Ferguson, H.M. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci. Rep. 2021, 11, 17569. [Google Scholar] [CrossRef] [PubMed]
- Aulicky, R.; Stejskal, V.; Frydova, B.; Athanassiou, C.G. Susceptibility of two strains of the confused flour beetle (Coleoptera: Tenebrionidae) following phosphine structural mill fumigation: Effects of concentration, temperature, and flour deposits. J. Econ. Entomol. 2015, 108, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Aulicky, R.; Stejskal, V.; Frydova, B. Field validation of phosphine efficacy on the first recorded resistant strains of Sitophilus granarius and Tribolium castaneum from the Czech Republic. J. Stored Prod. Res. 2019, 81, 107–113. [Google Scholar] [CrossRef]
- Yang, Q.; Kucerova, Z.; Li, Z.; Kalinović, I.; Stejskal, V.; Opit, G.; Cao, Y. Diagnosis of Liposcelis entomophila (Insecta: Psocodea: Liposcelididae) based on morphological characteristics and DNA barcodes. J. Stored Prod. Res. 2012, 48, 120–125. [Google Scholar] [CrossRef]
- Chandrashekharaiah, M.; Kandakoor, S.B.; Gowda, G.B.; Kammar, V.; Chakravarthy, A.K. Nanomaterials: A review of their action and application in pest management and evolution of DNA-tagged particles. In New Horizons in Insect Science: Towards Sustainable Pest Management; Chakravarthy, A.K., Ed.; Springer: Berlin, Germany, 2015; pp. 113–126. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.A.; Vendl, T.; Aulicky, R.; Frankova, M.; Stejskal, V. Gel Carriers for Plant Extracts and Synthetic Pesticides in Rodent and Arthropod Pest Control: An Overview. Gels 2022, 8, 522. https://doi.org/10.3390/gels8080522
Shah JA, Vendl T, Aulicky R, Frankova M, Stejskal V. Gel Carriers for Plant Extracts and Synthetic Pesticides in Rodent and Arthropod Pest Control: An Overview. Gels. 2022; 8(8):522. https://doi.org/10.3390/gels8080522
Chicago/Turabian StyleShah, Jawad Ali, Tomas Vendl, Radek Aulicky, Marcela Frankova, and Vaclav Stejskal. 2022. "Gel Carriers for Plant Extracts and Synthetic Pesticides in Rodent and Arthropod Pest Control: An Overview" Gels 8, no. 8: 522. https://doi.org/10.3390/gels8080522
APA StyleShah, J. A., Vendl, T., Aulicky, R., Frankova, M., & Stejskal, V. (2022). Gel Carriers for Plant Extracts and Synthetic Pesticides in Rodent and Arthropod Pest Control: An Overview. Gels, 8(8), 522. https://doi.org/10.3390/gels8080522






