Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers
Abstract
:1. Introduction
2. Results and Discussion
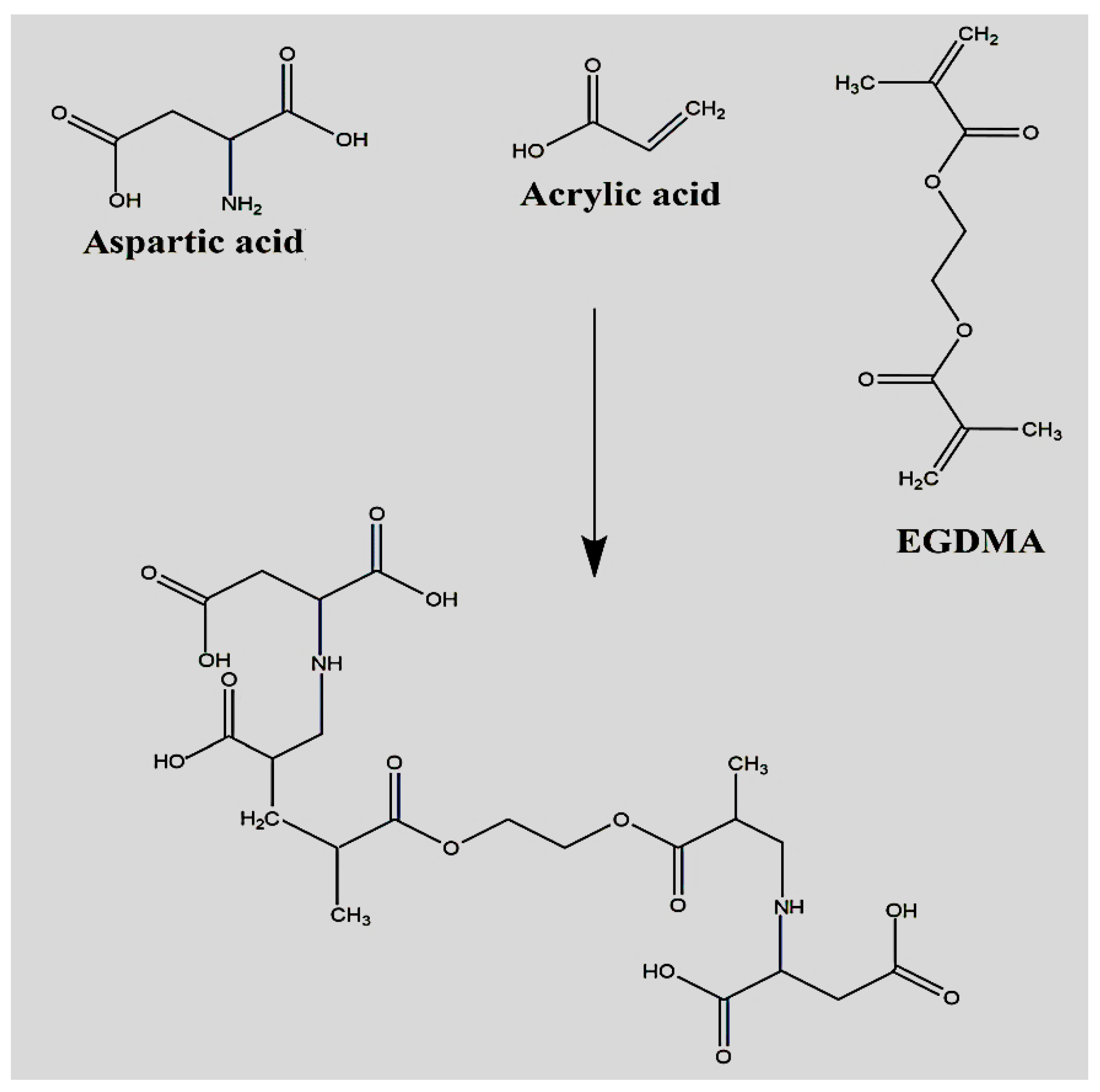
2.1. Synthesis of Polymeric Hydrogels
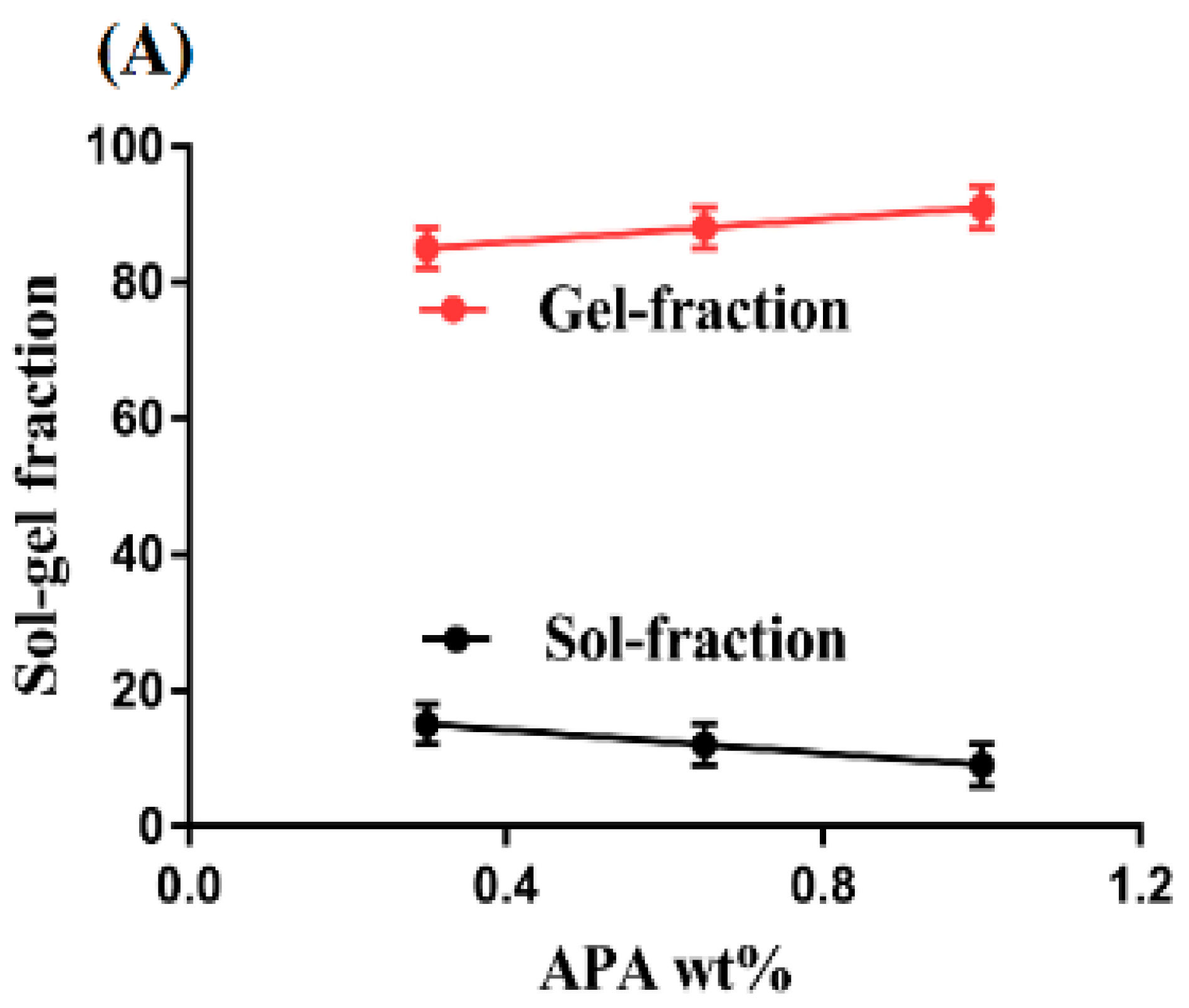
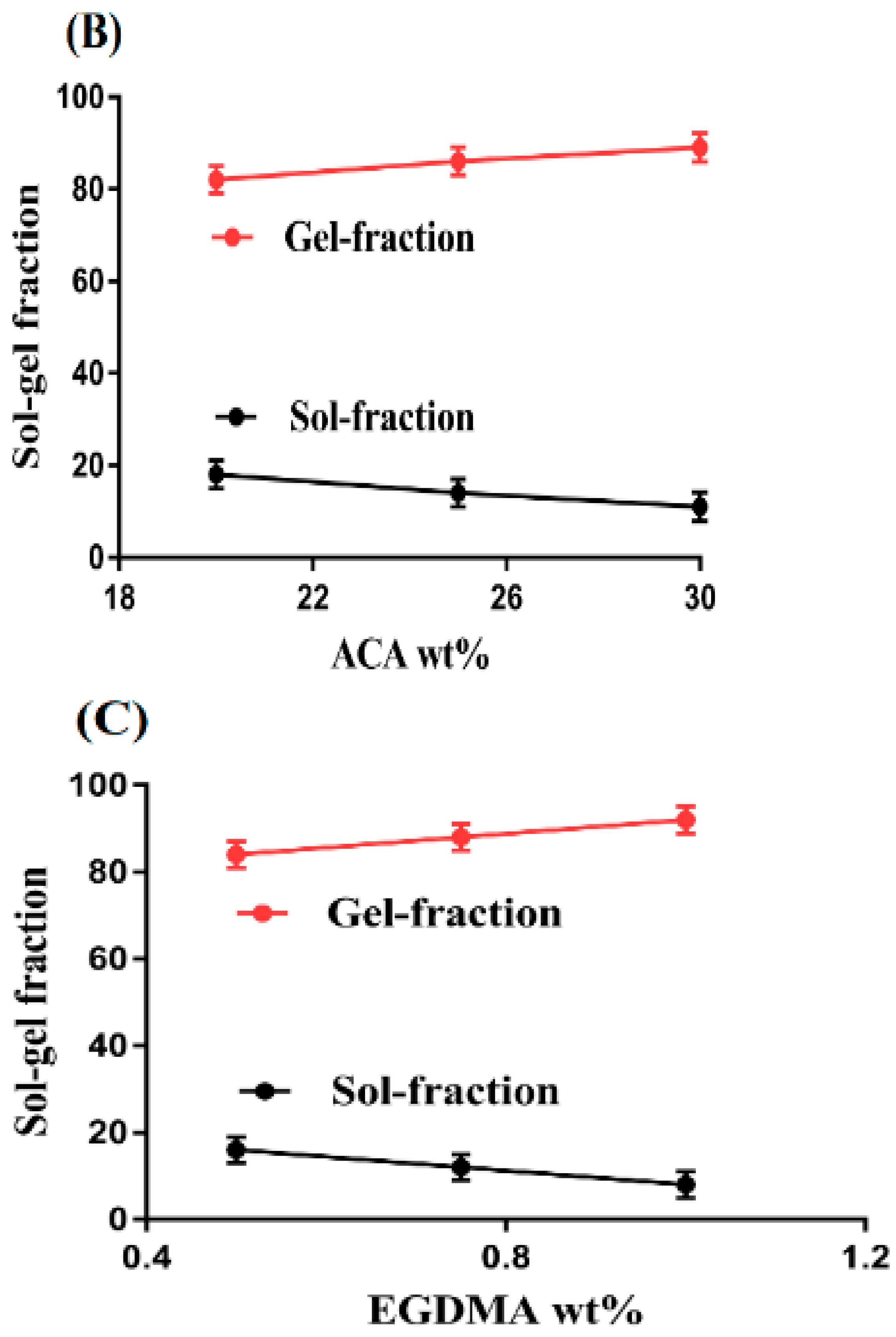
2.2. Sol-Gel Analysis
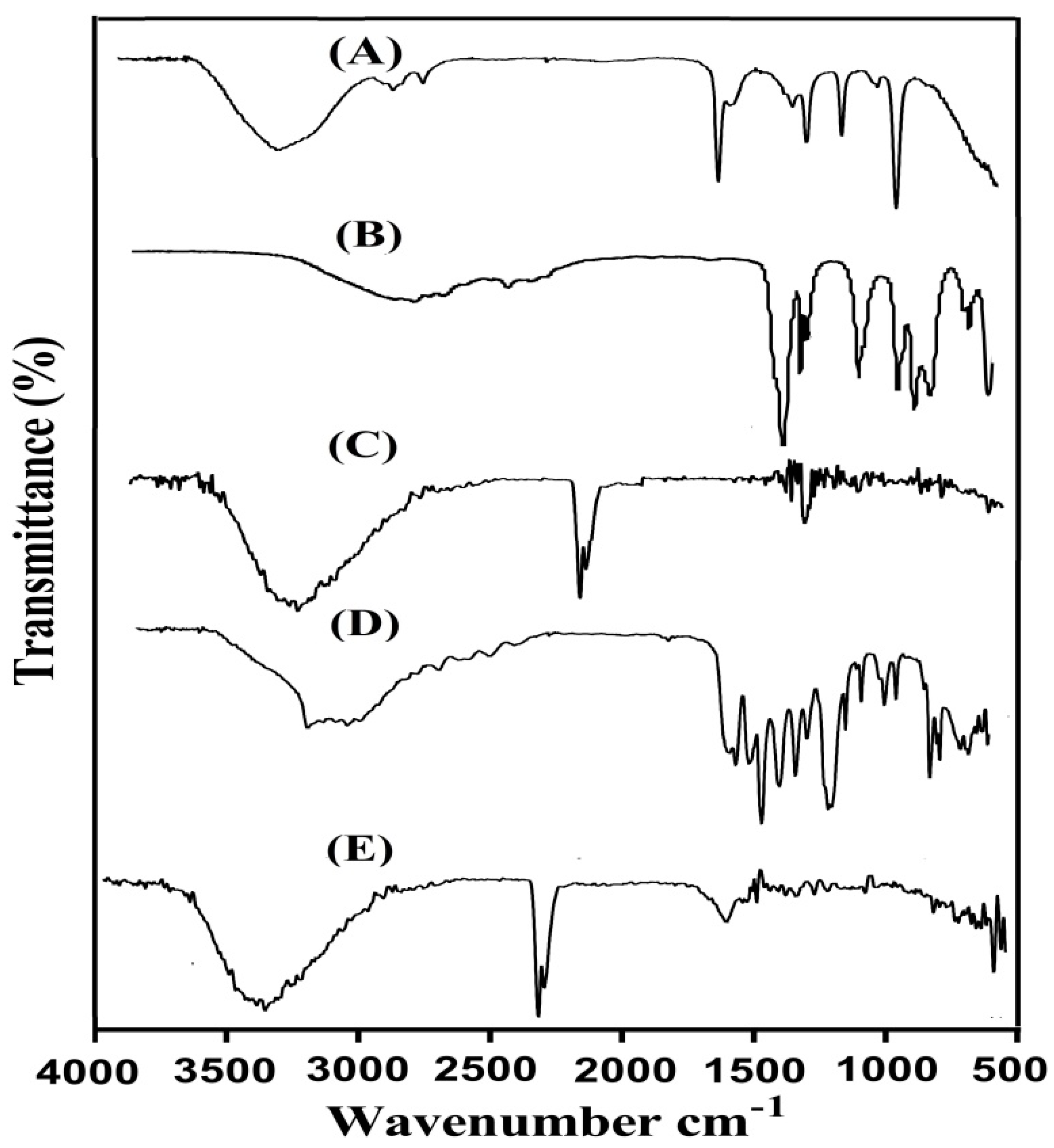
2.3. Fourier Transform Infrared (FTIR) Analysis
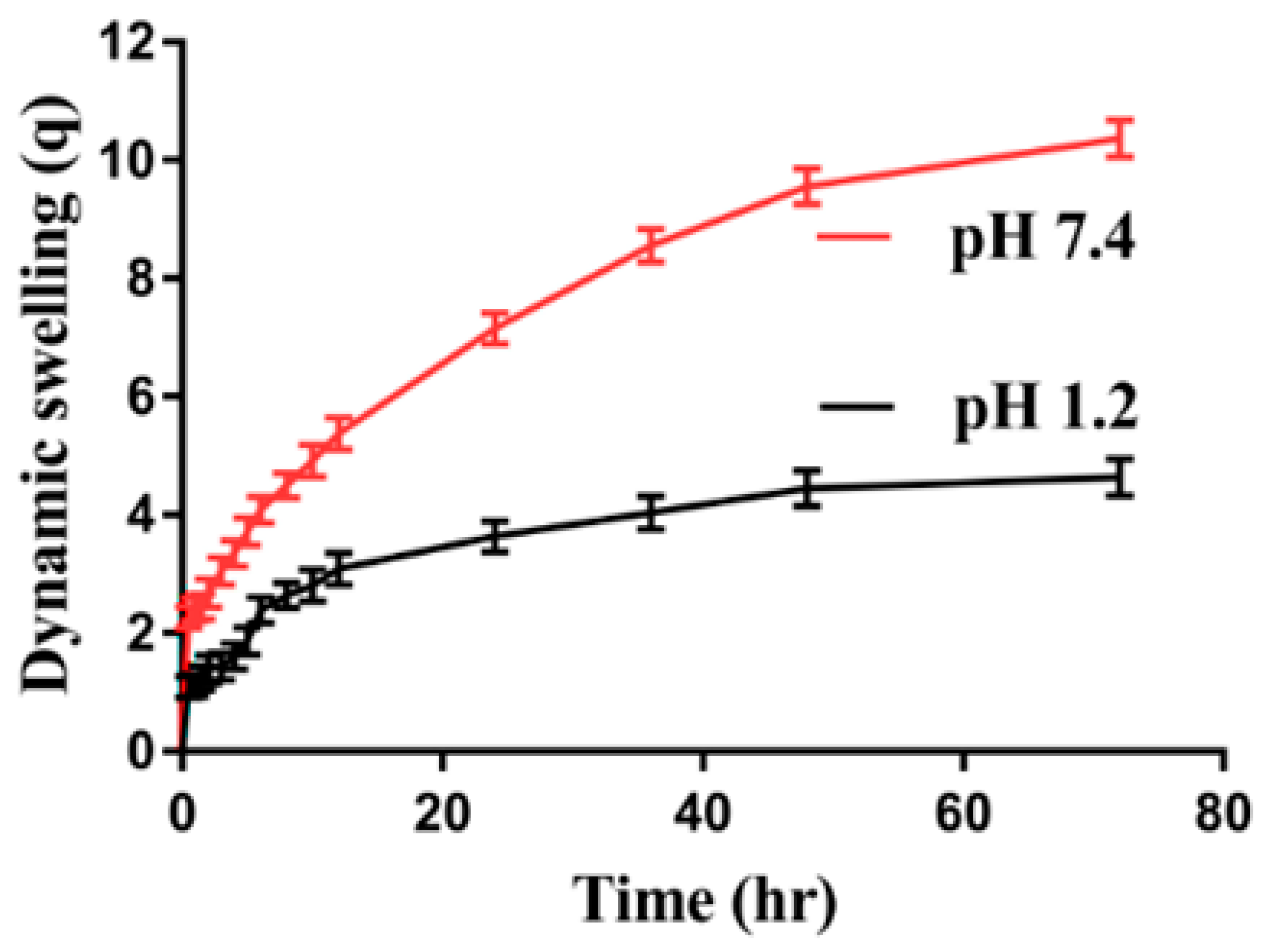
2.4. Dynamic Swelling Studies
2.4.1. Effect of pH on Swelling
2.4.2. Effect of APA/ACA/and EGDMA on Swelling
2.5. Polymer Volume Fraction
2.6. Thermogravimetric Analysis (TGA)
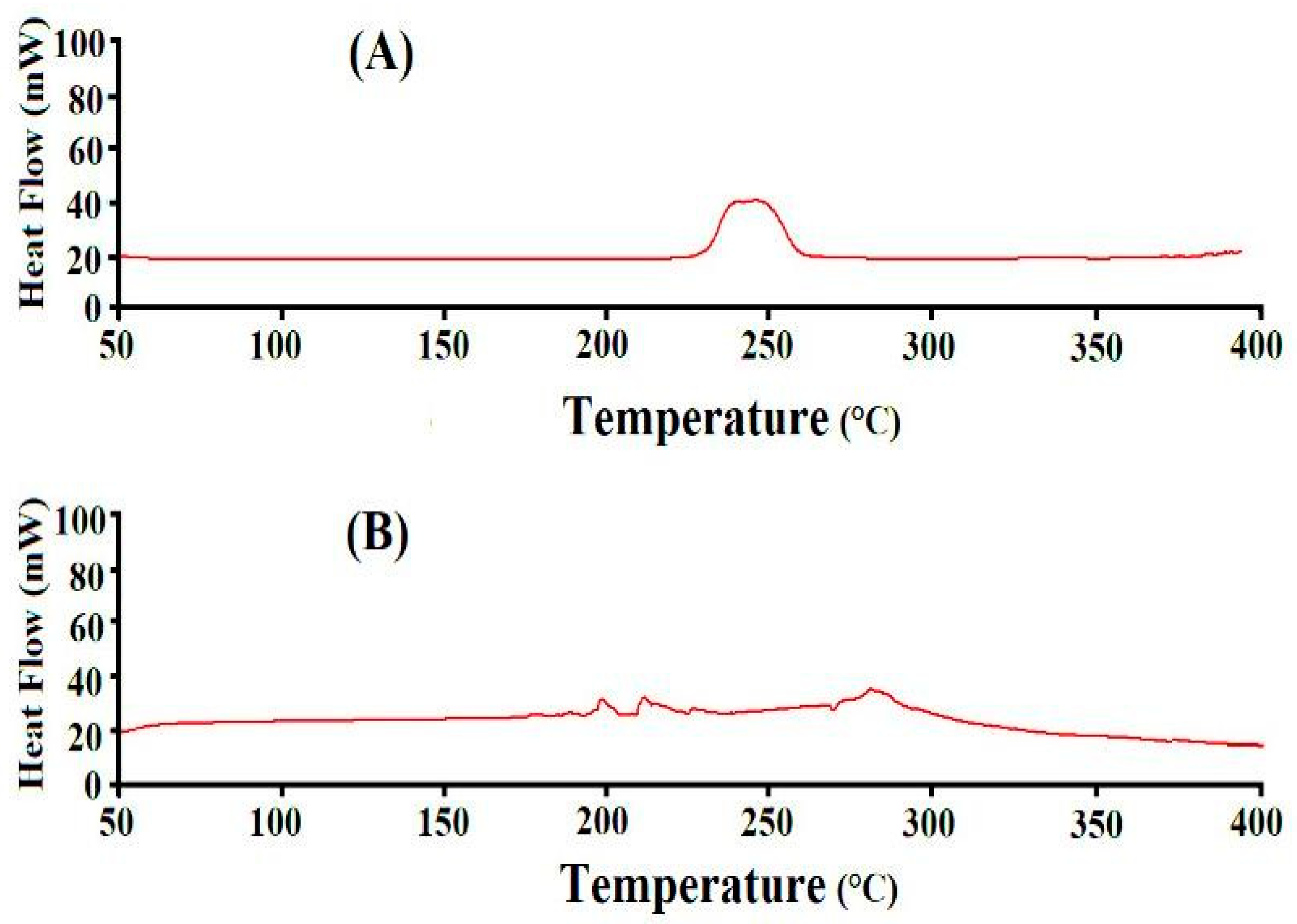
2.7. Differential Scanning Calorimetry (DSC) Analysis
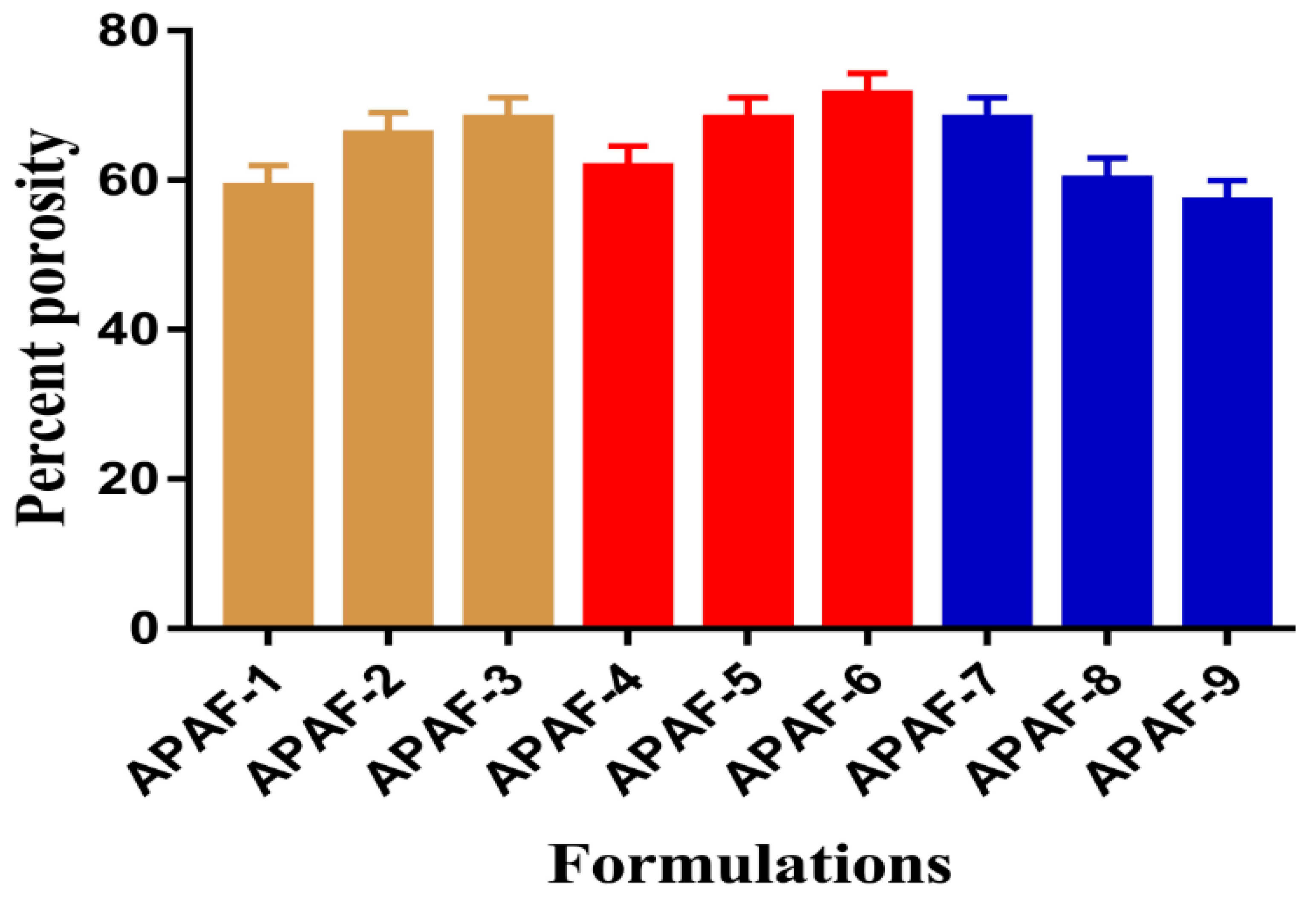
2.8. Percent Porosity
2.9. Morphology of Hydrogels
2.10. Drug Loading
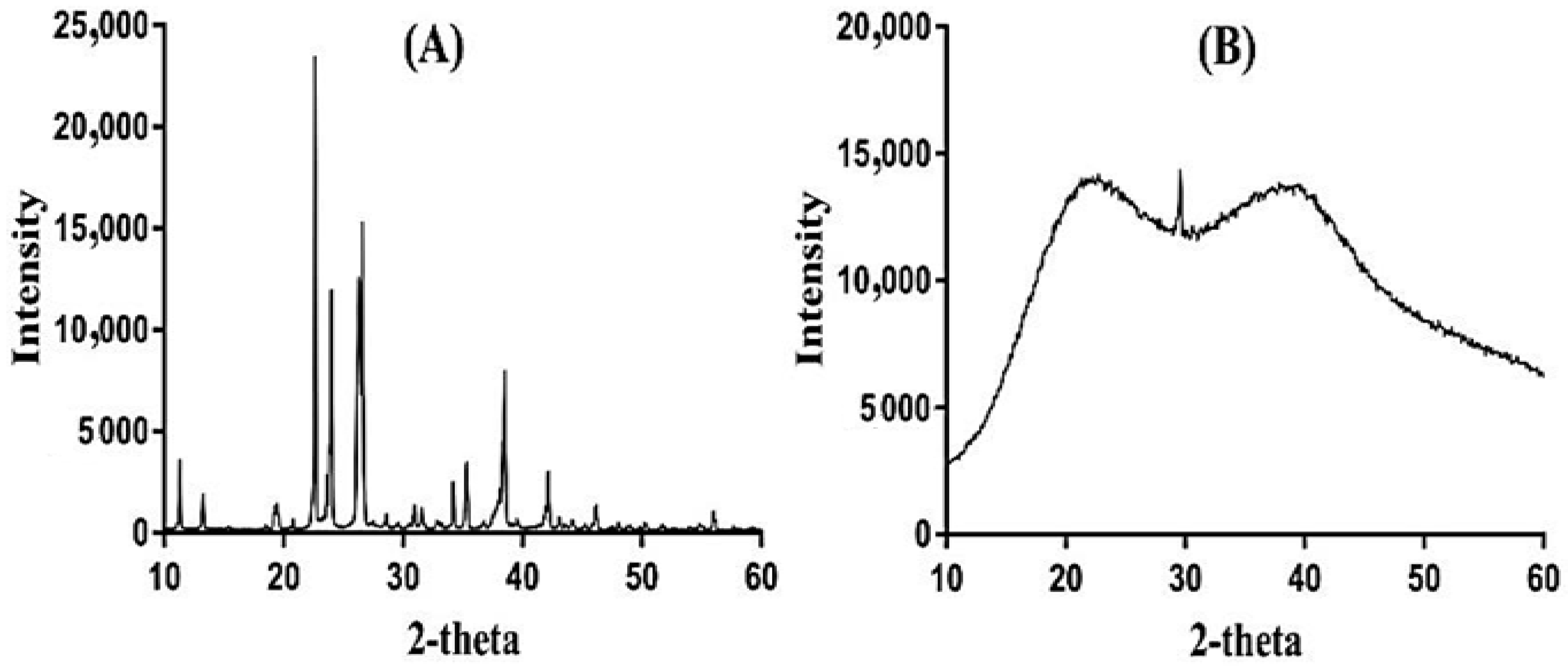
2.11. Powder X-ray Diffraction (PXRD) Study
2.12. In Vitro Drug Release Study and Kinetics
2.12.1. Effect of pH on Drug Release
2.12.2. Effect of APA/ACA/EGDMA and the Commercial Product (Acetaminophen) on Drug Release
2.12.3. Comparative Study of ACMP-Loaded APA-g-PACA Hydrogels with Other Delivery Systems for ACMP
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Polymeric Hydrogels
4.3. Sol-Gel Analysis
4.4. Fourier Transform Infrared (FTIR) Analysis
4.5. Dynamic Swelling Studies
4.6. Polymer Volume Fraction
4.7. Thermogravimetric Analysis (TGA)
4.8. Differential Scanning Calorimetry (DSC) Analysis
4.9. Percent Porosity
4.10. Morphology of Hydrogels
4.11. Drug Loading
4.12. Powder X-ray Diffraction (PXRD) Analysis
4.13. In Vitro Dissolution and Kinetics
Author Contributions
Funding
Conflicts of Interest
References
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliver. Rev. 2004, 56, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Soleimani, F.; Yarahmadi, M. Controlled release of acetaminophen from CMC-based hydrogels. Orient. J. Chem. 2011, 27, 895–902. [Google Scholar]
- Koo, H.; Jin, G.W.; Kang, H.; Lee, Y.; Nam, H.Y.; Jang, H.S.; Park, J.S. A new biodegradable crosslinked polyethylene oxide sulfide (PEOS) hydrogel for controlled drug release. Int. J. Pharm. 2009, 374, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Guigou, M.D.; Sobczak-Kupiec, A.; Mierzwiński, D.; Gajda, P.; Walter, J.; Tyliszczak, B. Multistep chemical processing of crickets leading to the extraction of chitosan used for synthesis of polymer drug carriers. Materials 2021, 14, 5070. [Google Scholar] [CrossRef]
- Kakinoki, S.; Taguchi, T.; Saito, H.; Tanaka, J.; Tateishi, T. Injectable in situ forming drug delivery system for cancer chemotherapy using a novel tissue adhesive: Characterization and in vitro evaluation. Eur. J. Pharm. Biopharm. 2007, 66, 383–390. [Google Scholar] [CrossRef]
- Głąb, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Krzan, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Modified with Equisetum arvense L.(Horsetail) Extract in View of Their Usefulness as Innovative Dressing Materials. Materials 2021, 14, 7533. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Głąb, M.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Krzan, M.; Uthayakumar, M.; Kędzierska, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Containing Albumin Particles and Aloe vera Juice as Transdermal Systems Functionalized in the Viewpoint of Potential Biomedical Applications. Materials 2021, 14, 5832. [Google Scholar] [CrossRef]
- Kim, S.J.; Spinks, G.M.; Prosser, S.; Whitten, P.G.; Wallace, G.G.; Kim, S.I. Surprising shrinkage of expanding gels under an external load. Nat. Mater. 2006, 5, 48–51. [Google Scholar] [CrossRef]
- Kranz, H.; Bodmeier, R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. Eur. J. Pharm. Sci. 2008, 34, 164–172. [Google Scholar] [CrossRef]
- Sadeghi, M.; Heidari, B. Preparation and characterization of metronidazole loaded carboxymethyl cellulose-base superabsorbent for drug delivery application. Orient. J. Chem. 2011, 27, 417–427. [Google Scholar]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliver. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Wang, K.; Fu, Q.; Chen, X.; Gao, Y.; Dong, K. Preparation and characterization of pH-sensitive hydrogel for drug delivery system. RSC Adv. 2012, 2, 7772–7780. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Tan, T.W. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly(acrylic acid). Polymer 2006, 47, 7702–7710. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Composite poly-L-lactic acid/poly-(alpha,beta)-DL-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1443–1451. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.J.; Fang, L.; Tan, T.W. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Polyaspartic acid based superabsorbent polymers. Eur. Polym. J. 2014, 59, 363–376. [Google Scholar] [CrossRef]
- Yang, J.; Fang, L.; Tan, T.W. Synthesis and characterization of superabsorbent hydrogels composites based on polysuccinimide. J. Appl. Polym. Sci. 2006, 102, 550–557. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin and poly (acrylic acid)-based biodegradable, non-cytotoxic, chemically cross-linked hydrogel for sustained release of ornidazole and ciprofloxacin. ACS Appl. Mater. Interfaces 2015, 7, 4791–4803. [Google Scholar] [CrossRef]
- Ajekwene, K.K. Properties and applications of acrylates. In Acrylate Polymers for Advanced Applications; IntechOpen: London, UK, 2020; p. 35. [Google Scholar]
- Naeem, M.A.; Mahmood, A.; Khan, S.A.; Shahiq, Z. Development and Evaluation of Controlled-Release Bilayer Tablets Containing Microencapsulated Tramadol and Acetaminophen. Trop. J. Pharm. Res. 2010, 9, 347–354. [Google Scholar] [CrossRef]
- Gazi, A.S.; Sailaja, A.K. Preparation and characterization of paracetamol loaded eudragit s100 nanoparticles by salting uut technique. J. Develop. Drugs 2018, 7, 1000183. [Google Scholar]
- Schiodt, F.V.; Ott, P.; Christensen, E.; Bondesen, S. The value of plasma acetaminophen half-life in antidote-treated acetaminophen overdosage. Clin. Pharmacol. Ther. 2002, 71, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef]
- Breivik, E.K.; Barkvoll, P.; Skovlund, E. Combining diclofenac with acetaminophen or acetaminophen-codeine after oral surgery: A randomized, double-blind single-dose study. Clin. Pharmacol. Ther. 1999, 66, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Scolnik, D.; Kozer, E.; Jacobson, S.; Diamond, S.; Young, N.L. Comparison of oral versus normal and high-dose rectal acetaminophen in the treatment of febrile children. Pediatrics 2002, 110, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Preparation and characterization of drug-loaded chitosan–tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Su, H.; Cao, H.; Tan, T. pH-sensitive IPN hydrogel based on poly (aspartic acid) and poly (vinyl alcohol) for controlled release. Polym. Bull. 2013, 70, 2815–2827. [Google Scholar] [CrossRef]
- Liu, C.; Gan, X.; Chen, Y. A novel pH-sensitive hydrogels for potential colon-specific drug delivery: Characterization and in vitro release studies. Starch-Stärke 2011, 63, 503–511. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Nam, I.K.; Mun, G.A.; Nurkeeva, Z.S.; Shaikhutdinov, E.M. Radiation synthesis and characterization of stimuli-sensitive chitosan–polyvinyl pyrrolidone hydrogels. Radiat. Phys. Chem. 2005, 75, 619–623. [Google Scholar] [CrossRef]
- Harish, N.M.; Prabhu, P.; Charyulu, R.N.; Gulzar, M.A.; Subrahmanyam, E.V. Formulation and Evaluation of in situ Gels Containing Clotrimazole for Oral Candidiasis. Indian J. Pharm. Sci. 2009, 71, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Ranjha, N.M.; Shahzad, Y. Swelling and Controlled Release of Tramadol Hydrochloride from a pH-Sensitive Hydrogel. Des. Monomers Polym. 2011, 14, 233–249. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Moharram, M.A.; Khafagi, M.G. Application of FTIR spectroscopy for structural characterization of ternary poly(acrylic acid)-metal-poly(vinyl pyrrolidone) complexes. J. Appl. Polym. Sci. 2007, 105, 1888–1893. [Google Scholar] [CrossRef]
- Bhise, K.S.; Dhumal, R.S.; Chauhan, B.; Paradkar, A.; Kadam, S.S. Effect of oppositely charged polymer and dissolution medium on swelling, erosion, and drug release from chitosan matrices. AAPS PharmSciTech 2007, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Khalid, I.; Ahmad, M.; Minhad, M.U.; Barka, K.; Sohail, M. Cross-linked sodium alginate-g-poly (acrylic acid) structure: A potential hydrogel network for controlled delivery of loxoprofen sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and characterization of chemically cross-linked acrylic acid/gelatin hydrogels: Effect of pH and composition on swelling and drug release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Lim, S.L.; Tang, W.N.H.; Ooi, C.W.; Chan, E.S.; Tey, B.T. Rapid swelling and deswelling of semi-interpenetrating network poly (acrylic acid)/poly (aspartic acid) hydrogels prepared by freezing polymerization. J. Appl. Polym. Sci. 2016, 133, 43515. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals 2021, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH-Sensitive Hydrogels Prepared from the Blends of Poly(vinyl alcohol) with Acrylic Acid-graft-Guar Gum Matrixes for Isoniazid Delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- Caykara, T.; Turan, E. Effect of the amount and type of the crosslinker on the swelling behavior of temperature-sensitive poly(N-tert-butylacrylamide-co-acrylamide) hydrogels. Colloid Polym. Sci. 2006, 284, 1038–1048. [Google Scholar] [CrossRef]
- Teijon, C.; Olmo, R.; Blanco, M.D.; Teijon, J.M.; Romero, A. Effect of the crosslinking degree and the nickel salt load on the thermal decomposition of poly (2-hydroxyethyl methacrylate) hydrogels and on the metal release from them. J. Colloid Interf. Sci. 2006, 295, 393–400. [Google Scholar] [CrossRef]
- Teijon, J.M.; Trigo, R.M.; Garcia, O.; Blanco, M.D. Cytarabine trapping in poly(2-hydroxyethyl methacrylate) hydrogels: Drug delivery studies. Biomaterials 1997, 18, 383–388. [Google Scholar] [CrossRef]
- Vazquez, B.; Gurruchaga, M.; Goni, I. Hydrogels Based on Graft-Copolymerization of Hema Bma Mixtures onto Soluble Gelatin-Swelling Behavior. Polymer 1995, 36, 2311–2314. [Google Scholar] [CrossRef]
- Shen, Y.W.; Lin, H.T.; Gao, W.S.; Li, M.L. The effects of humic acid urea and polyaspartic acid urea on reducing nitrogen loss compared with urea. J. Sci. Food. Agric. 2020, 100, 4425–4432. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A. Functionalization of carbopol with NVP for designing antibiotic drug loaded hydrogel dressings for better wound management. J. Pharm. Biopharm. Res. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Azam, W.; Ashames, A.; Fahelelbom, K.M.; Ullah, K.; Mannan, A.; Murtaza, G. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, in-Vitro and in-Vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 101970. [Google Scholar] [CrossRef]
- Sarika, P.; James, N.R.; Raj, D.K. Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde–gelatin nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar]
- Murthy, P.S.K.; Mohan, Y.M.; Sreeramulu, J.; Raju, K.M. Semi-IPNs of starch and poly(acrylamide-co-sodium methacrylate): Preparation, swelling and diffusion characteristics evaluation. React. Funct. Polym. 2006, 66, 1482–1493. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, C.P.; Lee, Y.D. Synthesis and characterizations of amphiphilic poly(L-lactide)-grafted chondroitin sulfate copolymer and its application as drug carrier. Biomol. Eng. 2007, 24, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Chen, J. Synthesis and characteristics of pH-sensitive semi-interpenetrating polymer network hydrogels based on konjac glucomannan and poly (aspartic acid) for in vitro drug delivery. Carbohydr. Polym. 2010, 79, 500–506. [Google Scholar] [CrossRef]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Sanli, O.; Ay, N.; Isiklan, N. Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur. J. Pharm. Biopharm. 2007, 65, 204–214. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ranjha, N.M.; Hanif, M. Effect of ethylene glycol dimethacrylate on swelling and on metformin hydrochloride release behavior of chemically crosslinked pH-sensitive acrylic acid-polyvinyl alcohol hydrogel. Daru 2015, 23, 41. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.H.; Tazeen, J.; Merchant, M.A.; Yousuf, R.I. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak. J. Pharm. Sci. 2006, 19, 119–124. [Google Scholar]
- Maziad, N.A.; EL-Hamouly, S.; Zied, E.; EL Kelani, T.A.; Nasef, N.R. Radiation preparation of smart hydrogel has antimicrobial properties for controlled release of ciprofloxacin in drug delivery systems. Drug Deliv. 2015, 14, 15. [Google Scholar]
- Lai, M.K.; Tsiang, R.C.C. Encapsulating acetaminophen into poly(L-lactide) microcapsules by solvent-evaporation technique in an O/W emulsion. J. Microencapsul. 2004, 21, 307–316. [Google Scholar] [CrossRef]
- Cao, Q.-R.; Choi, Y.-W.; Cui, J.-H.; Lee, B.-J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J. Control. Release 2005, 108, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Choi, H.-G. Acetaminophen and tramadol hydrochloride-loaded soft gelatin capsule: Preparation, dissolution and pharmacokinetics in beagle dogs. Pharm. Dev. Technol. 2021, 26, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and synthetic materials based cmch/PVA hydrogels for oxaliplatin delivery: Fabrication, characterization, in-vitro and in-vivo safety profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Azam, F.; Qureshi, J. Thiolation of arabinoxylan and its application in the fabrication of pH-sensitive thiolated arabinoxylan grafted acrylic acid copolymer. Drug Dev. Ind. Pharm. 2019, 45, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Buabeid, M.A.; Ullah, K.; Murtaza, G.; Mannan, A.; Khan, S.A. Synthesis, characterization and safety profiling of eudragit-based pH-responsive hydrogels: A promising platform for colonic delivery of losartan potassium. Curr. Drug Deliv. 2019, 16, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Buabeid, M.A.; Murtaza, G.; Ullah, A.; Rashid, H.; Khan, M.A.; Khan, S.A. Pectin-based (LA-co-MAA) semi-IPNS as a potential biomaterial for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 569, 118557. [Google Scholar] [CrossRef]
- Zia, M.A.; Sohail, M.; Minhas, M.U.; Sarfraz, R.M.; Khan, S.; de Matas, M.; Hussain, Z.; Abbasi, M.; Shah, S.A.; Kousar, M. HEMA based pH-sensitive semi IPN microgels for oral delivery; a rationale approach for ketoprofen. Drug Dev. Ind. Pharm. 2020, 46, 272–282. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Khan, H.U.; Mahmood, A.; Ahmad, M.; Maheen, S.; Sher, M. Formulation and evaluation of mouth disintegrating tablets of atenolol and atorvastatin. Indian J. Pharm. Sci. 2015, 77, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Mannan, A.; Rashid, H.; Shah, A.; Murtaza, G.; Khan, S.A. Facile synthesis of chitosan based-(AMPS-co-AA) semi-IPNs as a potential drug carrier: Enzymatic degradation, cytotoxicity, and preliminary safety evaluation. Curr. Drug Deliv. 2019, 16, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]




| F. Code | Dynamic Swelling up to 72 h | Drug Loading (mg)/400 mg of Dry Gels | Polymer Volume Fraction | |||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 7.4 | Weight method | Extraction method | pH 1.2 | pH 7.4 | |
| APAF-1 | 3.63 ± 0.21 | 09.89 ± 0.18 | 93.3 ± 1.5 | 91.8 ± 0.9 | 0.275 | 0.101 |
| APAF-2 | 3.92 ± 0.19 | 10.12 ± 0.12 | 98.2 ± 1.2 | 95.7 ± 1.6 | 0.255 | 0.098 |
| APAF-3 | 4.53 ± 0.18 | 10.29 ± 0.24 | 105.4 ± 1.2 | 103.1 ± 1.8 | 0.220 | 0.097 |
| APAF-4 | 3.77 ± 0.24 | 9.56 ± 0.27 | 81.2 ± 1.1 | 78.3 ± 1.1 | 0.265 | 0.104 |
| APAF-5 | 4.53 ± 0.18 | 10.29 ± 0.24 | 105.4 ± 1.2 | 103.1 ± 1.8 | 0.220 | 0.097 |
| APAF-6 | 4.70 ± 0.20 | 10.80 ± 0.26 | 112.8 ± 0.8 | 110.2 ± 1.1 | 0.212 | 0.092 |
| APAF-7 | 4.53 ± 0.18 | 10.29 ± 0.24 | 105.4 ± 1.2 | 103.1 ± 1.8 | 0.220 | 0.097 |
| APAF-8 | 3.67 ± 0.26 | 6.76 ± 0.21 | 96.3 ± 1.0 | 94.5 ± 1.3 | 0.272 | 0.147 |
| APAF-9 | 3.41 ± 0.14 | 6.10 ± 0.13 | 86.5 ± 0.1 | 84.9 ± 0.9 | 0.293 | 0.163 |
| F. Code | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | |
| APAF-1 | 0.8587 | 0.9071 | 0.7013 | 0.8880 | 0.9417 |
| APAF-2 | 0.9493 | 0.9631 | 0.9545 | 0.9697 | 0.8448 |
| APAF-3 | 0.9635 | 0.9856 | 0.9760 | 0.9716 | 0.8260 |
| APAF-4 | 0.9469 | 0.9491 | 0.9063 | 0.9094 | 0.6506 |
| APAF-5 | 0.9635 | 0.9856 | 0.9760 | 0.9716 | 0.8260 |
| APAF-6 | 0.9372 | 0.9757 | 0.9823 | 0.9726 | 0.7507 |
| APAF-7 | 0.9635 | 0.9856 | 0.9760 | 0.9716 | 0.8260 |
| APAF-8 | 0.9761 | 0.9893 | 0.9812 | 0.9858 | 0.7076 |
| APAF-9 | 0.9912 | 0.9954 | 0.9422 | 0.9700 | 0.6864 |
| S. No. | Formulation | Intended Quantity of Loaded Formulation for Drug Release (mg) | Maximum % of Drug Release | Time for Maximum % of Drug Release | Reference |
|---|---|---|---|---|---|
| 1 | Eudragit S100-based nanoparticles | 50 | 28.31 | 12 h | [22] |
| 2 | Tramadol HCl and acetaminophen microparticles | 531 | 99.5 | 12 h | [21] |
| 3 | Acetaminophen-loaded poly(L-lactide) microcapsules | 80 | 83.50 | 24 h | [61] |
| 4 | Hydroxypropylmethylcellulose matrix tablets containing acetaminophen | - | 100 | 8 h | [62] |
| 5 | Acetaminophen- and tramadol hydrochloride-loaded soft gelatin capsule | 325 | 100 | 0.5 h | [63] |
| 6 | APA-g-PACA hydrogels | 400 | 84.62 | 24 h | Current study |
| Formulation Code | Polymer (APA) g/100 g | Monomer (ACA) g/100 g | Initiator (APS) g/100 g | Crosslinker (EGDMA) g/100 g |
|---|---|---|---|---|
| APAF-1 | 0.30 | 25 | 0.5 | 0.50 |
| APAF-2 | 0.65 | 25 | 0.5 | 0.50 |
| APAF-3 | 1.00 | 25 | 0.5 | 0.50 |
| APAF-4 | 1.00 | 20 | 0.5 | 0.50 |
| APAF-5 | 1.00 | 25 | 0.5 | 0.50 |
| APAF-6 | 1.00 | 30 | 0.5 | 0.50 |
| APAF-7 | 1.00 | 25 | 0.5 | 0.50 |
| APAF-8 | 1.00 | 25 | 0.5 | 0.75 |
| APAF-9 | 1.00 | 25 | 0.5 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhail, M.; Fang, C.-W.; Chiu, I.-H.; Hung, M.-C.; Vu, Q.L.; Lin, I.-L.; Wu, P.-C. Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers. Gels 2022, 8, 521. https://doi.org/10.3390/gels8080521
Suhail M, Fang C-W, Chiu I-H, Hung M-C, Vu QL, Lin I-L, Wu P-C. Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers. Gels. 2022; 8(8):521. https://doi.org/10.3390/gels8080521
Chicago/Turabian StyleSuhail, Muhammad, Chih-Wun Fang, I-Hui Chiu, Ming-Chia Hung, Quoc Lam Vu, I-Ling Lin, and Pao-Chu Wu. 2022. "Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers" Gels 8, no. 8: 521. https://doi.org/10.3390/gels8080521
APA StyleSuhail, M., Fang, C.-W., Chiu, I.-H., Hung, M.-C., Vu, Q. L., Lin, I.-L., & Wu, P.-C. (2022). Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers. Gels, 8(8), 521. https://doi.org/10.3390/gels8080521







