Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins
Abstract
:1. Introduction
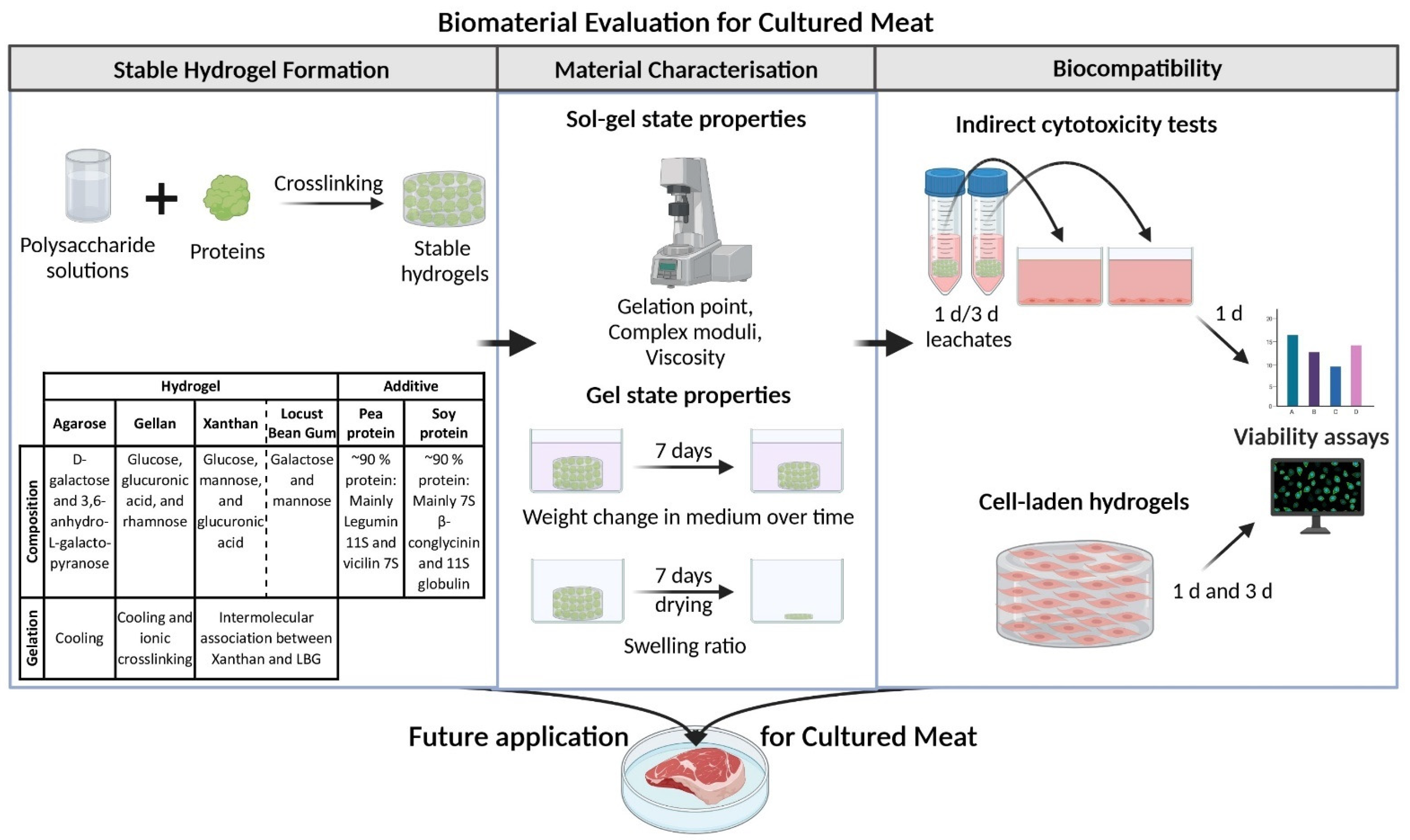
2. Results and Discussion
2.1. Characterization of Gel Properties
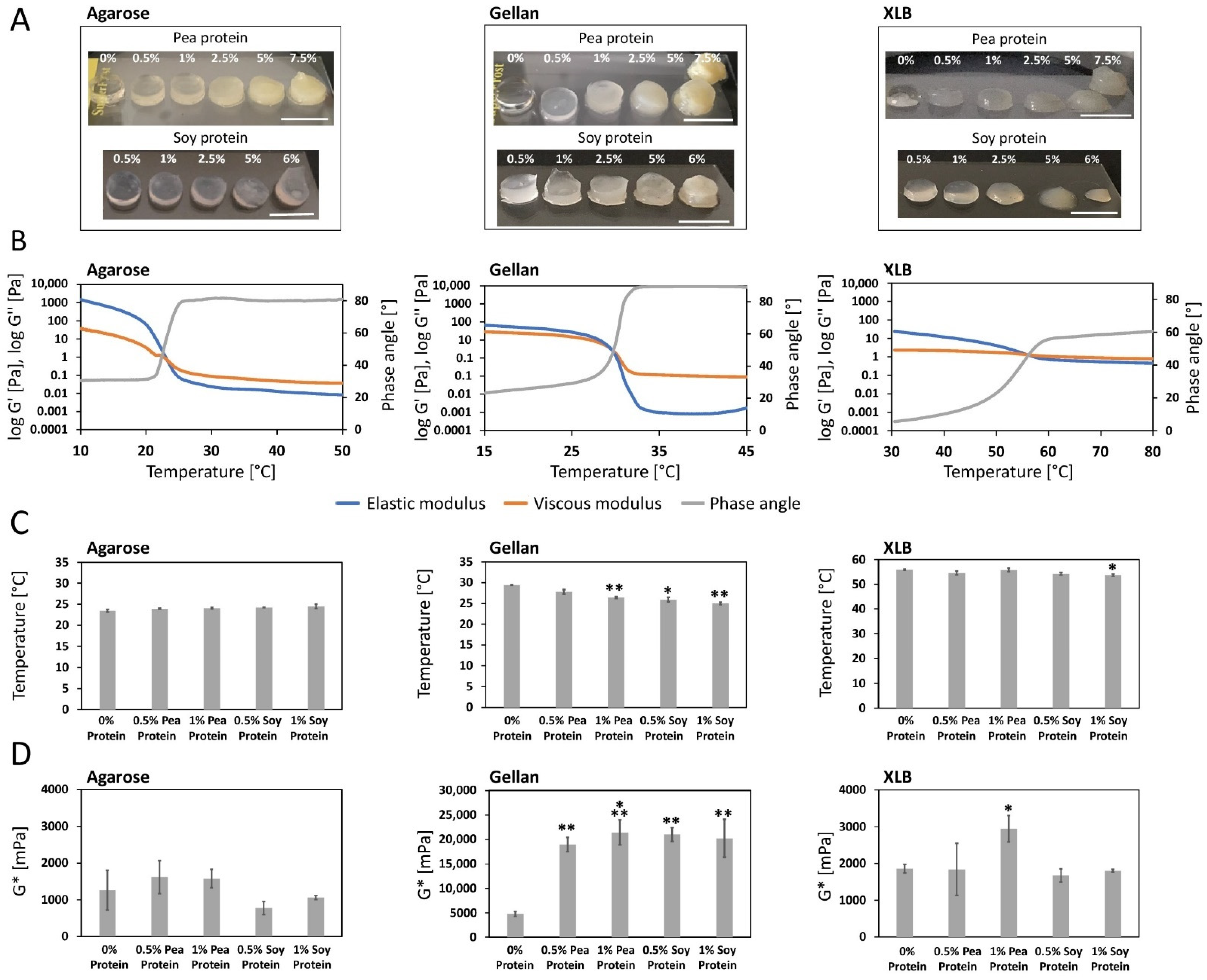
2.1.1. Impact of Protein Supplementation on Sol-Gel Transition
2.1.2. Impact of Protein Supplementation on the Complex Shear Modulus
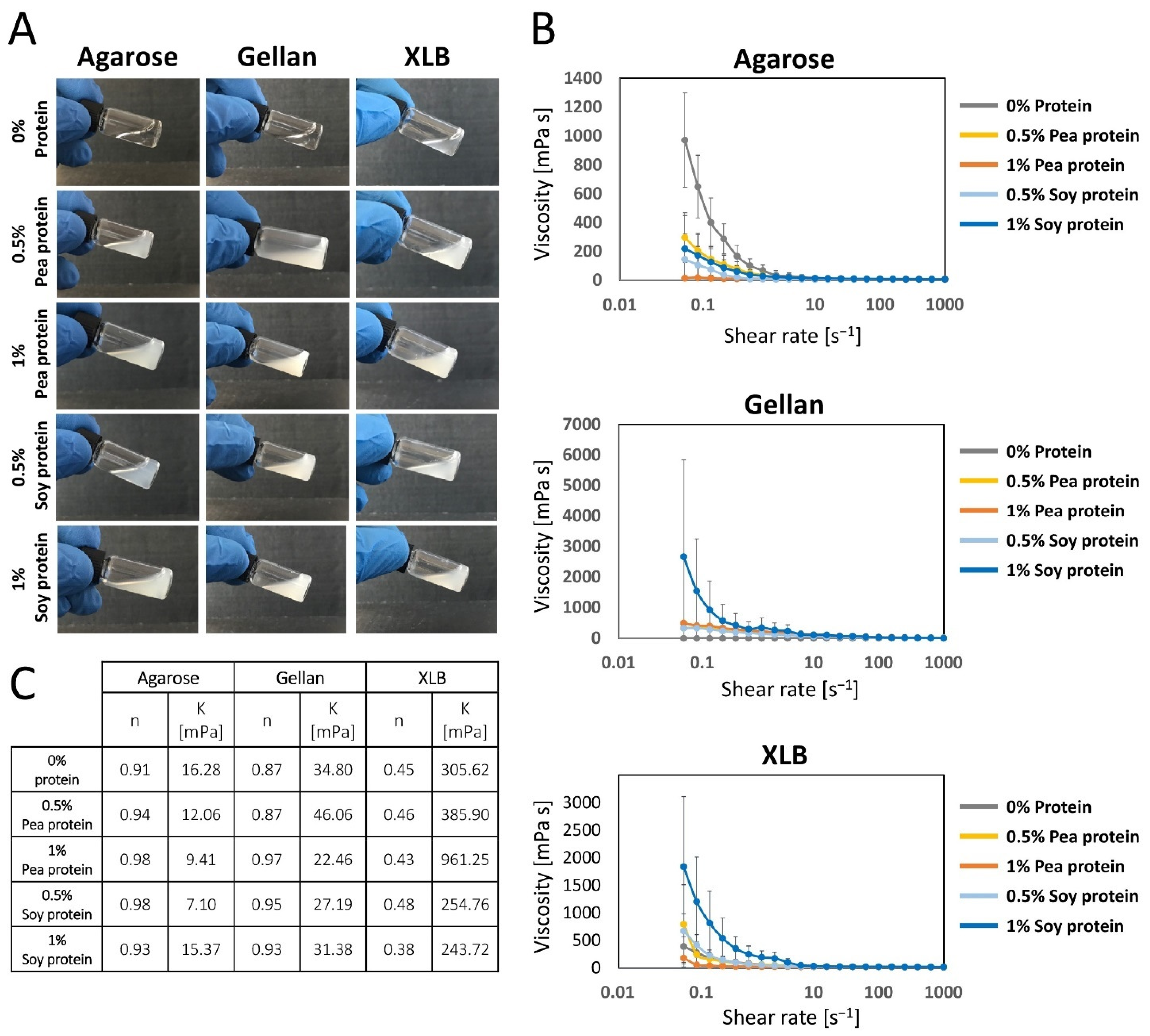
2.1.3. Impact of Protein Supplementation on the Homogeneity of the Polysaccharide Solution
2.1.4. Impact of Protein Supplementation on the Complex Shear Viscosity
2.1.5. Impact of Protein Supplementation on Swelling Properties of the Hydrogels
2.2. Biocompatibility of the Biomaterials
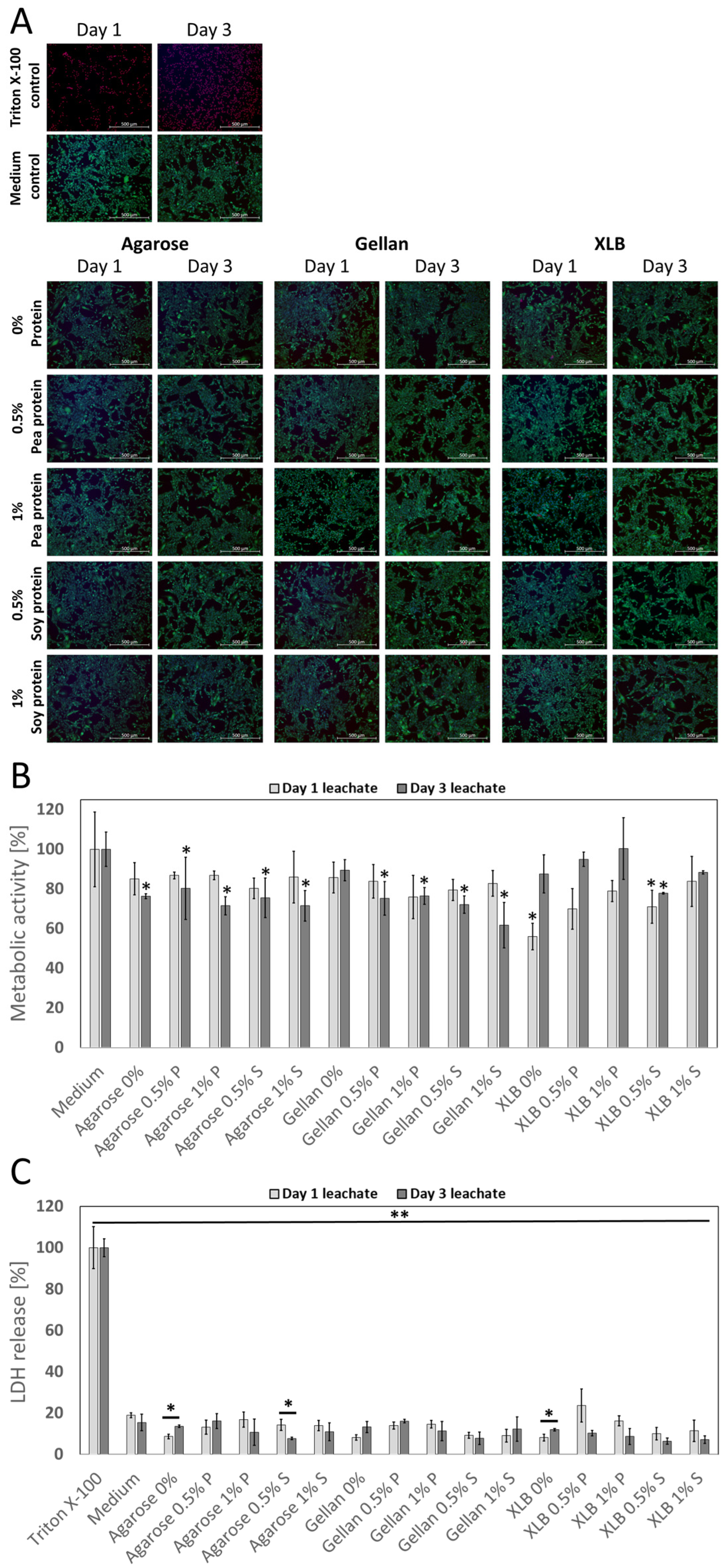
2.2.1. Impact of Biomaterial Leachates on Cell Viability and Metabolic Activity
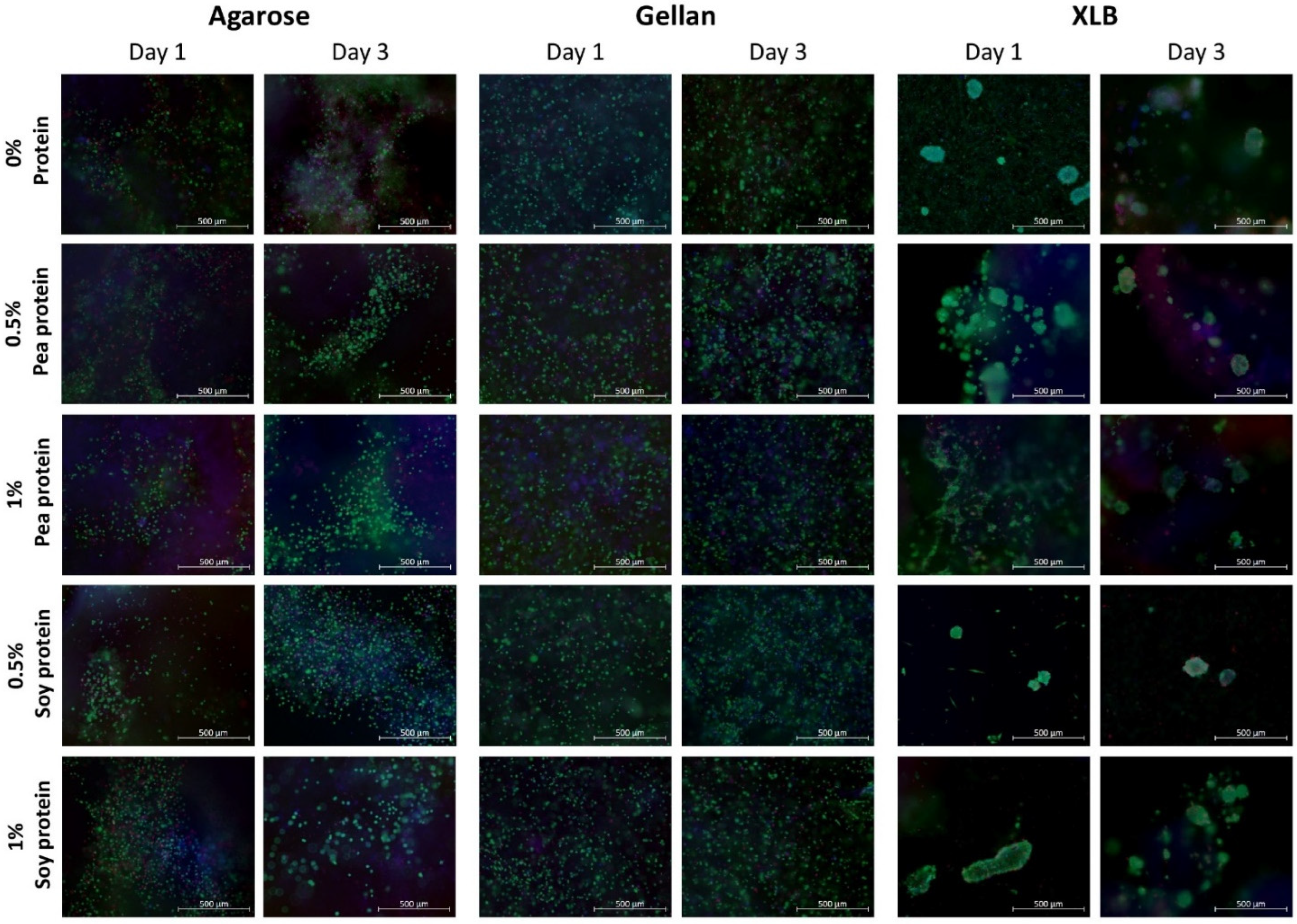
2.2.2. Impact of Cell Encapsulation on Cell Viability
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Preparation and Characterizations
4.1.1. Preparation of Hydrogels
4.1.2. Rheological Properties of Hydrogels
4.1.3. Swelling Characteristics of Hydrogels
4.2. Cell Culture
4.3. Biocompatibility Analysis
4.3.1. Indirect Cell Toxicity Test
4.3.2. Formation of Cell-Laden Gels
4.3.3. LDH Assay
4.3.4. Resazurin Assay
4.3.5. Live/Dead-Staining
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melzener, L.; Verzijden, K.E.; Buijs, A.J.; Post, M.J.; Flack, J.E. Cultured beef: From small biopsy to substantial quantity. J. Sci. Food Agric. 2021, 101, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiebers, D.O.; Feigin, V.L. What the COVID-19 Crisis Is Telling Humanity. Neuroepidemiology 2020, 54, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow; FAO of the UN: Rome, Italy, 2006. [Google Scholar]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials 2021, 280, 121274. [Google Scholar] [CrossRef]
- Bonny, S.P.F.; Gardner, G.E.; Pethick, D.W.; Hocquette, J.-F. What is artificial meat and what does it mean for the future of the meat industry? J. Integr. Agric. 2015, 14, 255–263. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2021, 42(2), 311–323. [Google Scholar] [CrossRef]
- Furuhashi, M.; Morimoto, Y.; Shima, A.; Nakamura, F.; Ishikawa, H.; Takeuchi, S. Formation of contractile 3D bovine muscle tissue for construction of millimetre-thick cultured steak. NPJ Sci. Food 2021, 5, 6. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2021, 9, e2102908. [Google Scholar] [CrossRef]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef]
- Bekker, G.A.; Tobi, H.; Fischer, A.R.H. Meet meat: An explorative study on meat and cultured meat as seen by Chinese, Ethiopians and Dutch. Appetite 2017, 114, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.; Luining, D.; van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2018, 70, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-Y.; Stoecklein, D.; Kommajosula, A.; Lin, J.; Owsley, K.; Ganapathysubramanian, B.; Di Carlo, D. Shaped 3D microcarriers for adherent cell culture and analysis. Microsyst. Nanoeng. 2018, 4, 21. [Google Scholar] [CrossRef]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109782. [Google Scholar] [CrossRef]
- Nie, M.; Shima, A.; Fukushima, K.; Morimoto, Y.; Takeuchi, S. A Cylindrical Molding Method for the Biofabrication of Plane-Shaped Skeletal Muscle Tissue. Micromachines 2021, 12, 1411. [Google Scholar] [CrossRef]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, I.; Seol, Y.-J.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Hume, S.L.; Hoyt, S.M.; Walker, J.S.; Sridhar, B.V.; Ashley, J.F.; Bowman, C.N.; Bryant, S.J. Alignment of multi-layered muscle cells within three-dimensional hydrogel macrochannels. Acta Biomater. 2012, 8, 2193–2202. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Bačáková, L.; Novotná, K.; Pařízek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29–S47. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Cidonio, G.; Cooke, M.; Glinka, M.; Dawson, J.I.; Grover, L.; Oreffo, R.O.C. Printing bone in a gel: Using nanocomposite bioink to print functionalised bone scaffolds. Mater. Today Bio. 2019, 4, 100028. [Google Scholar] [CrossRef]
- Tanaka, N.; Moriguchi, H.; Sato, A.; Kawai, T.; Shimba, K.; Jimbo, Y.; Tanaka, Y. Microcasting with agarose gel via degassed polydimethylsiloxane molds for repellency-guided cell patterning. RSC Adv. 2016, 6, 54754–54762. [Google Scholar] [CrossRef] [Green Version]
- Del Garcia Cruz, M.R.; Postma, A.; Frith, J.E.; Meagher, L. Printability and bio-functionality of a shear thinning methacrylated xanthan—Gelatin composite bioink. Biofabrication 2021, 13, 035023. [Google Scholar] [CrossRef]
- Jan, A.; Delcour, K.P. Fibre-Rich and Wholegrain Foods: Improving Quality; Woodhead: Sawston, UK, 2013; ISBN 9780857090386. [Google Scholar]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Czaja-Bulsa, G.; Bulsa, M. What Do We Know Now about IgE-Mediated Wheat Allergy in Children? Nutrients 2017, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Vadala’, M.; Laurino, C. Gluten-free diet in non-celiac patients: Beliefs, truths, advantages and disadvantages. Minerva Gastroenterol. Dietol. 2019, 65, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Armisén, R.; Galatas, F.; Hispanagar, S.A. Handbook of Hydrocolloids, 2nd ed.; CRC: Boca Raton, FL, USA; Woodhead: Oxford, UK, 2009; ISBN 978-1-84569-414-2. [Google Scholar]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.-C.; et al. Re-evaluation of agar (E 406) as a food additive. EFS2 2016, 14, e04645. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.; Melo, R.A. Global shortage of technical agars: Back to basics (resource management). J. Appl. Phycol. 2018, 30, 2463–2473. [Google Scholar] [CrossRef] [Green Version]
- Vijay Anand, K.G.; Eswaran, K.; Ghosh, A. Life cycle impact assessment of a seaweed product obtained from Gracilaria edulis—A potent plant biostimulant. J. Clean. Prod. 2018, 170, 1621–1627. [Google Scholar] [CrossRef]
- Jenzer, H.; Müller, S.; Rotunno, F.; Maurer, N.D.; Rufener, A.; Marty, I.; Martins, S.; Sadeghi, L. PP-007 Evaluation of amylase-resistant gellan GUM (E418) as a rheology and texture modifier for oral preparations. Eur. J. Hosp. Pharm. 2016, 23, A197. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of gellan gum (E 418) as food additive. EFSA J. 2018, 16, e05296. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Li, A.; Hu, T.; Luo, H.; Alam, N.-U.; Xin, J.; Li, H.; Lin, Y.; Huang, J.; Huang, K.; Meng, Y.; et al. A Carotenoid- and Poly-β-Hydroxybutyrate-Free Mutant Strain of Sphingomonas elodea ATCC 31461 for the Commercial Production of Gellan. mSphere 2019, 4, e00668-19. [Google Scholar] [CrossRef] [Green Version]
- Raghunandan, K.; Kumar, A.; Kumar, S.; Permaul, K.; Singh, S. Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 2018, 8, 71. [Google Scholar] [CrossRef]
- West, T.P. Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts. Polysaccharides 2021, 2, 234–244. [Google Scholar] [CrossRef]
- Directorate General for Agriculture and Rural Development. Expert Group for Technical Advice on Organic Production: Final Report On Food (III); European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Industrial Microbiology; Wilson, D.B.; Sahm, H.; Stahmann, K.-P.; Koffas, M. (Eds.) Wiley-VCH: Weinheim, Germany, 2020; ISBN 978-3-527-34035-4. [Google Scholar]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.-C.; et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 2017, 15, e04909. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Zeng, Y.; Li, X.; Jiang, X.; Wang, Y.; Xie, T.; Zhang, Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr. Polym. 2016, 151, 684–691. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.-C. Introduction of Microbial Biopolymers in Soil Treatment for Future Environmentally-Friendly and Sustainable Geotechnical Engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Majid, M.A.; Rashid, A.S.A. Xanthan gum biopolymer: An eco-friendly additive for stabilization of tropical organic peat. Environ. Earth Sci. 2016, 75, 825. [Google Scholar] [CrossRef]
- Maiti, S.; Dey, P.; Banik, A.; Sa, B.; Ray, S.; Kaity, S. Tailoring of locust bean gum and development of hydrogel beads for controlled oral delivery of glipizide. Drug Deliv. 2010, 17, 288–300. [Google Scholar] [CrossRef]
- Liu, F.; McConnell, E.L.; Pygall, S. Update on Polymers for Oral Drug Delivery; iSmithers: Shawsbury, UK, 2011; ISBN 978-1847355379. [Google Scholar]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.-C.; et al. Re-evaluation of locust bean gum (E 410) as a food additive. EFS2 2017, 15, e04646. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J. Locust bean gum: A versatile biopolymer. Carbohydr. Polym. 2013, 94, 814–821. [Google Scholar] [CrossRef]
- Cerqueira, M.A.P.R.; Pereira, R.N.C.; Da Ramos, O.L.S.; Teixeira, J.A.C.; Vicente, A.A. (Eds.) Edible Food Packaging: Materials and Processing Technologies; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482234169. [Google Scholar]
- Boublenza, I.; Boublenza, I.; Boublenza, A.; Madji, S.; Fabiano-Tixier, A.-S.; Chemat, F. Carob as Source for Sustainable Ingredients and Products. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019; pp. 257–275. ISBN 978-981-13-3809-0. [Google Scholar]
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability Opportunities for Mediterranean Food Products through New Formulations Based on Carob Flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. J. 2017, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Gläser, P.; Dawid, C.; Meister, S.; Bader-Mittermaier, S.; Schott, M.; Eisner, P.; Hofmann, T. Molecularization of Bitter Off-Taste Compounds in Pea-Protein Isolates (Pisum sativum L.). J. Agric. Food Chem. 2020, 68, 10374–10387. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notice 788 for Pea Protein Concentrate; Recently Published GRAS Notices and FDA Letters; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A perspective on pea allergy and pea allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Barac, M.; Pesic, M.; Stanojevic, S.; Kostic, A.; Cabrilo, S. Techno-functional properties of pea (Pisum sativum) protein isolates: A review. Acta Period. Techol. 2015, 46, 1–18. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. GRAS Notice 851 Pea Protein; No. 851; Recently Published GRAS Notices and FDA Letters; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Markets and Markets. Pea Protein Market by Type (Isolates, Concentrates, and Textured), Form (Dry and Wet): Source (Yellow Split Peas, Lentils, and Chickpeas), Application, and Region (North America, Europe, Asia Pacific, South America, and Rest of the World)—Global Forecast to 2025; No. 5003970; Research and Markets: Rockville, MD, USA, 2020. [Google Scholar]
- Berardy, A.; Johnston, C.S.; Plukis, A.; Vizcaino, M.; Wharton, C. Integrating Protein Quality and Quantity with Environmental Impacts in Life Cycle Assessment. Sustainability 2019, 11, 2747. [Google Scholar] [CrossRef] [Green Version]
- Final Rule Department of Health and Human Services Food and Drug Administration. Federal Register Volume 64, Issue 206 (October 26, 1999): Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease; Regulatory Information 64 FR 57700, Docket No. 98P–0683; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1999.
- Cordle, C.T. Soy protein allergy: Incidence and relative severity. J. Nutr. 2004, 134, 1213S–1219S. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and Edible Uses of Soy Protein Products. Comp. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of a health claim related to soy protein and reduction of blood cholesterol concentrations pursuant to Article 14 of the Regulation (EC) No 1924/2006. EFS2 2010, 8, 1688. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, T.; Jiang, L. Soy Protein: Molecular Structure Revisited and Recent Advances in Processing Technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef]
- Andrew, B.; Christine, C.; Thomas, S. Life Cycle Assessment of Soy Protein Isolate. In Proceedings of the International Symposium on Sustainable Systems and Technologies, Phoenix, AZ, USA, 16–18 May 2015. [Google Scholar]
- Reijnders, L.; Soret, S. Quantification of the environmental impact of different dietary protein choices. Am. J. Clin. Nutr. 2003, 78, 664S–668S. [Google Scholar] [CrossRef]
- Casas, J.A.; Garca-Ochoa, F. Viscosity of solutions of xanthan/locust bean gum mixtures. J. Sci. Food Agric. 1999, 79, 25–31. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Renou, F.; Petibon, O.; Malhiac, C.; Grisel, M. Effect of xanthan structure on its interaction with locust bean gum: Toward prediction of rheological properties. Food Hydrocoll. 2013, 32, 331–340. [Google Scholar] [CrossRef]
- Higiro, J.; Herald, T.J.; Alavi, S.; Bean, S. Rheological study of xanthan and locust bean gum interaction in dilute solution: Effect of salt. Food Res. Int. 2007, 40, 435–447. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Schreiber, C.; Zielbauer, B.; Dietz, N.; Vilgis, T.A. Interaction of xanthan gums with galacto- and glucomannans. Part II: Heat induced synergistic gelation mechanism and their interaction with salt. J. Phys. Mater. 2021, 3, 34014. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chien, K.B.; Chung, E.J.; Shah, R.N. Investigation of soy protein hydrogels for biomedical applications: Materials characterization, drug release, and biocompatibility. J. Biomater. Appl. 2014, 28, 1085–1096. [Google Scholar] [CrossRef]
- Tansaz, S.; Singh, R.; Cicha, I.; Boccaccini, A.R. Soy Protein-Based Composite Hydrogels: Physico-Chemical Characterization and In Vitro Cytocompatibility. Polymers 2018, 10, 1159. [Google Scholar] [CrossRef] [Green Version]
- Nordqvist, D.; Vilgis, T.A. Rheological Study of the Gelation Process of Agarose-Based Solutions. Food Biophys. 2011, 6, 450–460. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Hege, J.; Palberg, T.; Vilgis, T.A. Interactions of different hydrocolloids with milk proteins. J. Phys. Mater. 2020, 3, 44003. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Blaeser, A. 3D-Bioprinting. In Basic Concepts on 3D Cell Culture; Kasper, C., Egger, D., Lavrentieva, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 201–232. ISBN 978-3-030-66748-1. [Google Scholar]
- Yue, Y.; Pang, S.; Li, N.; Tong, L.; Wang, L.; Fan, B.; Li, C.; Wang, F.; Liu, L. Interactions between Pea Protein Isolate and Carboxymethylcellulose in Neutral and Acid Aqueous Systems. Foods 2021, 10, 1560. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Braudo, E.E.; Plashchina, I.G.; Schwenke, K.D. Plant protein interactions with polysaccharides and their influence on legume protein functionality A Review. Nahrung 2001, 45, 382. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef]
- Roterman, I.; Banach, M.; Kalinowska, B.; Konieczny, L. Influence of the Aqueous Environment on Protein Structure—A Plausible Hypothesis Concerning the Mechanism of Amyloidogenesis. Entropy 2016, 18, 351. [Google Scholar] [CrossRef] [Green Version]
- Guzzo, A.V. The Influence of Amino Acid Sequence on Protein Structure. Biophys. J. 1965, 5, 809–822. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, J.; Luo, J.; Gao, Q.; Mao, A.; Li, J. Research progress on double-network hydrogels. Mater. Today Commun. 2021, 29, 102757. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Abchiche, H.; Mellal, M.; Sahraoui, N.; Bertouche, S.; Tebachi, L.; Mameri, A. The Study of The Effect of Concentration of The Agar-Agar Solution on The Rheological and Thermo Rheological Behavior. EJEST 2020, 3, 105–113. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- De Silva, D.A.; Poole-Warren, L.A.; Martens, P.J.; Panhuis, M.I.H. Mechanical characteristics of swollen gellan gum hydrogels. J. Appl. Polym. Sci. 2013, 130, 3374–3383. [Google Scholar] [CrossRef]
- Hayes, M. Measuring Protein Content in Food: An Overview of Methods. Foods 2020, 9, 1340. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Northoff, H.; Flegel, W.A. Fetal Calf Serum. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 896–897. ISBN 9780122267659. [Google Scholar]
- Kolkmann, A.M.; Post, M.J.; Rutjens, M.A.M.; van Essen, A.L.M.; Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020, 72(1), 111–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhao, X.; Li, X.; Du, G.; Zhou, J.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Messmer, T.; Klevernic, I.; Furquim, C.; Ovchinnikova, E.; Dogan, A.; Cruz, H.; Post, M.J.; Flack, J.E. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food 2022, 3, 74–85. [Google Scholar] [CrossRef]
- Volz, A.-C.; Kluger, P.J. Completely serum-free and chemically defined adipocyte development and maintenance. Cytotherapy 2018, 20, 576–588. [Google Scholar] [CrossRef]
- Leber, B.; Mayrhauser, U.; Leopold, B.; Koestenbauer, S.; Tscheliessnigg, K.; Stadlbauer, V.; Stiegler, P. Impact of temperature on cell death in a cell-culture model of hepatocellular carcinoma. Anticancer Res. 2012, 32, 915–921. [Google Scholar]
- Dani, S.; Ahlfeld, T.; Albrecht, F.; Duin, S.; Kluger, P.; Lode, A.; Gelinsky, M. Homogeneous and Reproducible Mixing of Highly Viscous Biomaterial Inks and Cell Suspensions to Create Bioinks. Gels 2021, 7, 227. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Chu, B.; Gao, Y.; Wu, C.; Zhang, L.; Chen, P.; Wang, X.; Tang, S. Modification of agarose with carboxylation and grafting dopamine for promotion of its cell-adhesiveness. Carbohydr. Polym. 2013, 92, 2245–2251. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [Green Version]
- Bektas, E.I.; Gurel Pekozer, G.; Kök, F.N.; Torun Kose, G. Evaluation of natural gum-based cryogels for soft tissue engineering. Carbohydr. Polym. 2021, 271, 118407. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, Y.; Wang, X.; Huang, J.; Chang, P.R.; Anderson, D.P. Improvement in physical properties and cytocompatibility of zein by incorporation of pea protein isolate. J. Mater. Sci. 2010, 45, 6775–6785. [Google Scholar] [CrossRef]
- Ferris, C.J.; Panhuis, M.I.H. Conducting bio-materials based on gellan gum hydrogels. Soft Matter. 2009, 5, 3430. [Google Scholar] [CrossRef]
- Hara, S.; Aoki, S.; Nagata, M.; Shirasuna, K.; Noguchi, T.; Iwata, H. Xanthan gum and locust bean gum substrate improves bovine embryo development. Reprod. Domest. Anim. 2020, 55, 1124–1131. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, J.; Wang, J.; Huang, R.; Qiao, X.; Wang, H.; Tan, Z. Enhanced growth and differentiation of myoblast cells grown on E-jet 3D printed platforms. Int. J. Nanomed. 2019, 14, 937–950. [Google Scholar] [CrossRef] [Green Version]

| Biomaterial | Taste | Maximum Daily Intake | Allergy Risks | Source/Origin | Regulations as Food | Usage in Food Industry | Annual Production | Environmental Impact | |
|---|---|---|---|---|---|---|---|---|---|
| Hydrogel | Agarose/Agar | Tasteless [31] | 64 mg/kg body weight per day [32] | Very low [32] | Sea weed, red algae [23] | Evaluated as food additive [32] | Thickener, stabiliser, gelling agent [31] | >20,000 t [33] | Low [34] |
| Gellan | Tasteless [35] | 200 mg/kg body weight per day [36] | Very low [36] | Pseudomonas bacteria [37] | Evaluated as food additive [36] | Thickener, gelling agent and stabiliser [31] | >10,000 t [38] | Low-medium [39,40,41] | |
| Xanthan | Tasteless [42] | 214 mg/kg body weight per day [43] | Very low [43] | Xanthomonas [44] | Evaluated as food additive [43] | Thickener, stabiliser, emulsifier, foaming agent [31] | >30,000 t [45] | Low-medium [46,47] | |
| Locust Bean gum | Tasteless, Risk of leguminous taste when heated [48,49] | 500 mg/kg body weight per day [50] | Very low [50] | Carob tree seeds [51] | Evaluated as food additive [50] | Thickener, gelling agent [31] | >10,000 t [52] | Low [53,54] | |
| Additive | Pea protein | Untreated: Bitter, beany, green, grassy, and leafy [55,56] | 30 g per day [57] | Low [58] | Pea, Pisum sativum [59] | Evaluated as food additive [60] | Emulsifier, foaming agent, gelling agent [56] | >200,000 t [61] | Medium [62] |
| Soy protein | Untreated: Bitter, beany, fatty, green [55] | 25–100 g per day [63] | Low [64] | Soybean, Glycine max [65] | Evaluated as food additive [66] | Emulsifier, foaming agent, gelling agent, fat and water absorption [67] | >1,000,000 t [65] | Low-medium [62,68,69] |
| Polysaccharide | Gel Formation with Protein | Form Stability over Time | Gelation Temperature | Biocompatibility | Encapsulation of Cells |
|---|---|---|---|---|---|
| Agarose | uniform gels > 2.5% protein less uniform < 7.5% protein | no weight change | 23–24 °C with and without protein | non toxic | possible, but with inhomogeneous cell distribution |
| (+) | (+) | (+) | (+) | (+/−) | |
| Gellan | uniform gels > 1% protein less uniform gels < 7.5% protein | slight weight change | 29.4 °C without protein Less with protein | non toxic | possible with homogeneous cell distribution |
| (+/−) | (+/o) | (+) | (+) | (+) | |
| XLB | uniform gels > 1% protein | significant weight change | 56 °C without protein Less with soy protein | non toxic | not possible |
| (+/−) | (−) | (−) | (+) | (−) |
| Protein Concentration [%] | 2% Agarose, 2% Gellan or 1% XLB [µL] | 12% SPI [µL] | Distilled H2O [µL] | 15% PPI [µL] | Distilled H2O [µL] | 2% SPI or PPI [µL] | Distilled H2O [µL] |
|---|---|---|---|---|---|---|---|
| 0 | 750 | 0 | 750 | 0 | 750 | 0 | 750 |
| 0.5 | 750 | 62.5 | 687.5 | 50 | 700 | 375 | 375 |
| 1 | 750 | 125 | 625 | 100 | 650 | 750 | 750 |
| 2.5 | 750 | 312 | 438 | 250 | 500 | - | - |
| 5 | 750 | 625 | 125 | 500 | 250 | - | - |
| 6 | 750 | 750 | 0 | - | - | - | - |
| 7.5 | 750 | - | - | 750 | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollschlaeger, J.O.; Maatz, R.; Albrecht, F.B.; Klatt, A.; Heine, S.; Blaeser, A.; Kluger, P.J. Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels 2022, 8, 94. https://doi.org/10.3390/gels8020094
Wollschlaeger JO, Maatz R, Albrecht FB, Klatt A, Heine S, Blaeser A, Kluger PJ. Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels. 2022; 8(2):94. https://doi.org/10.3390/gels8020094
Chicago/Turabian StyleWollschlaeger, Jannis O., Robin Maatz, Franziska B. Albrecht, Annemarie Klatt, Simon Heine, Andreas Blaeser, and Petra J. Kluger. 2022. "Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins" Gels 8, no. 2: 94. https://doi.org/10.3390/gels8020094
APA StyleWollschlaeger, J. O., Maatz, R., Albrecht, F. B., Klatt, A., Heine, S., Blaeser, A., & Kluger, P. J. (2022). Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels, 8(2), 94. https://doi.org/10.3390/gels8020094







