Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins
Abstract
:1. Introduction
2. Results and Discussion
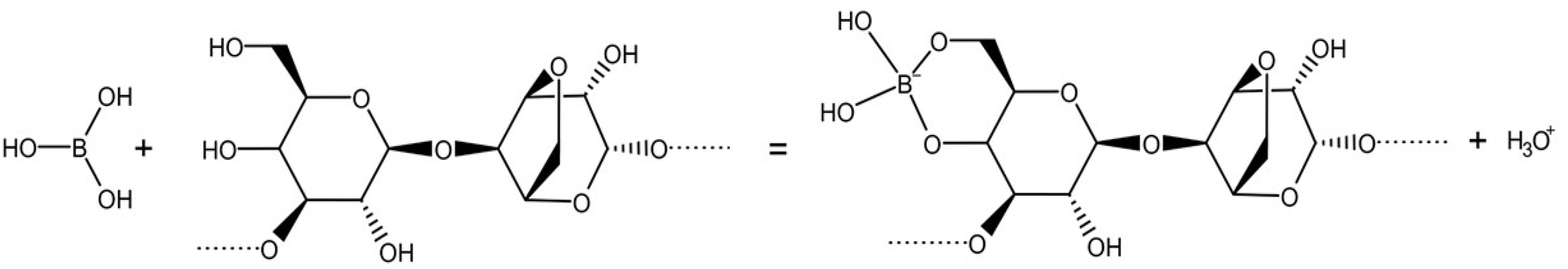
2.1. The Background Electrolyte
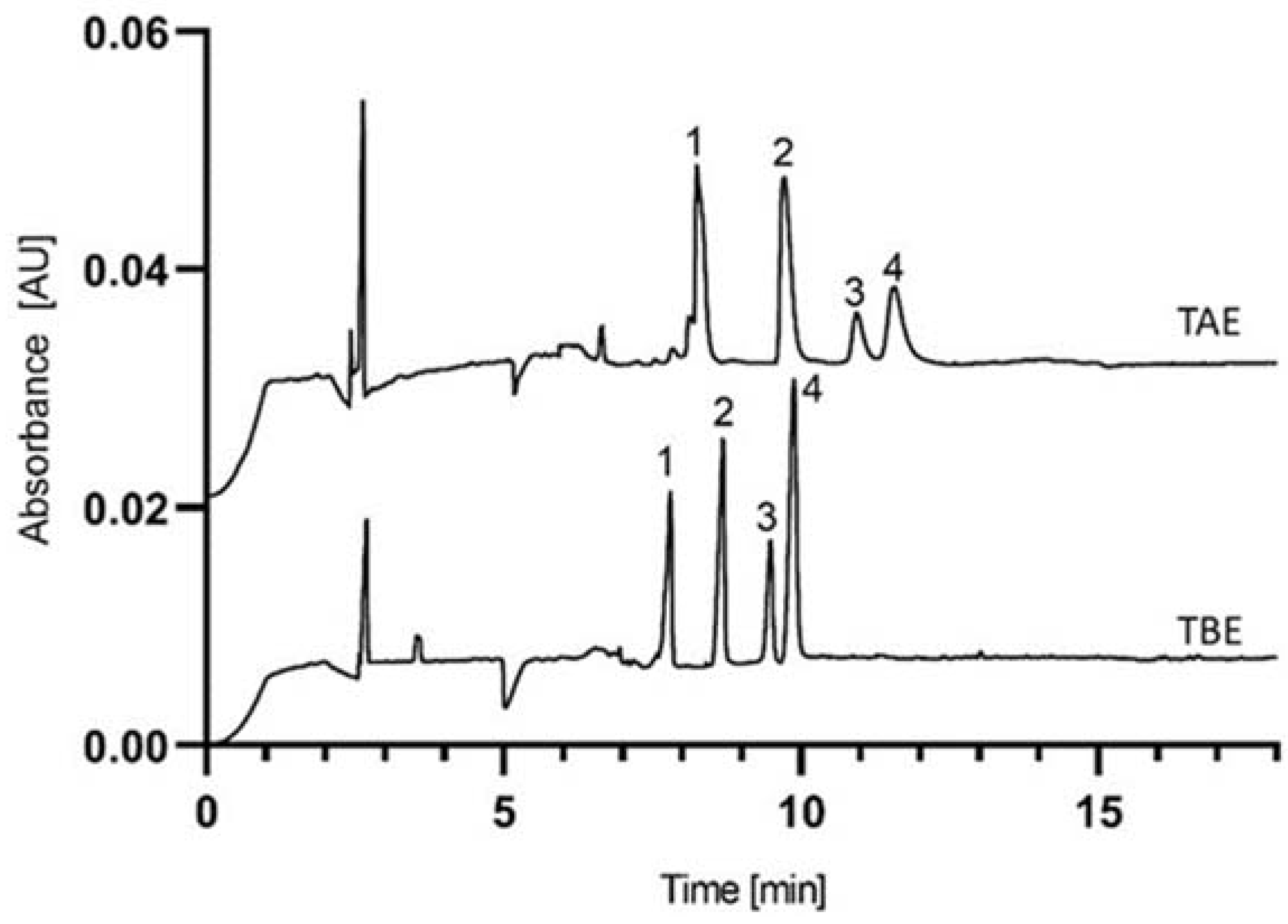
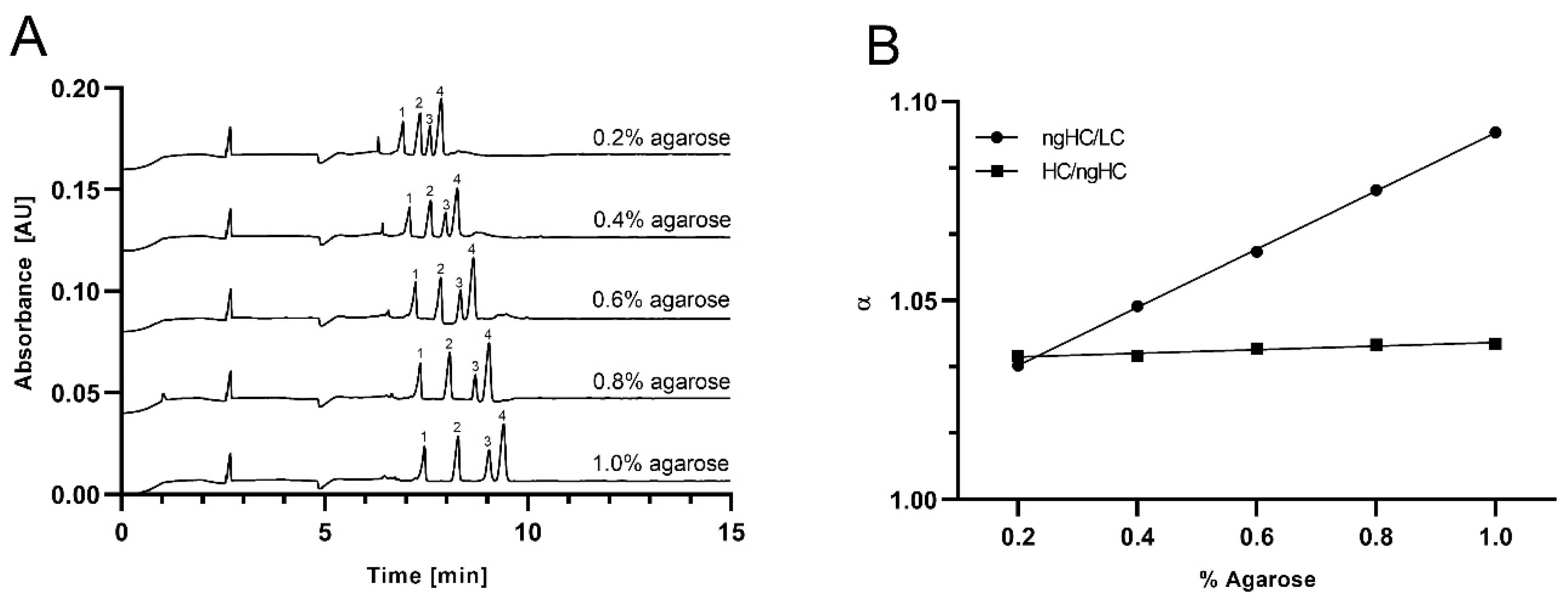
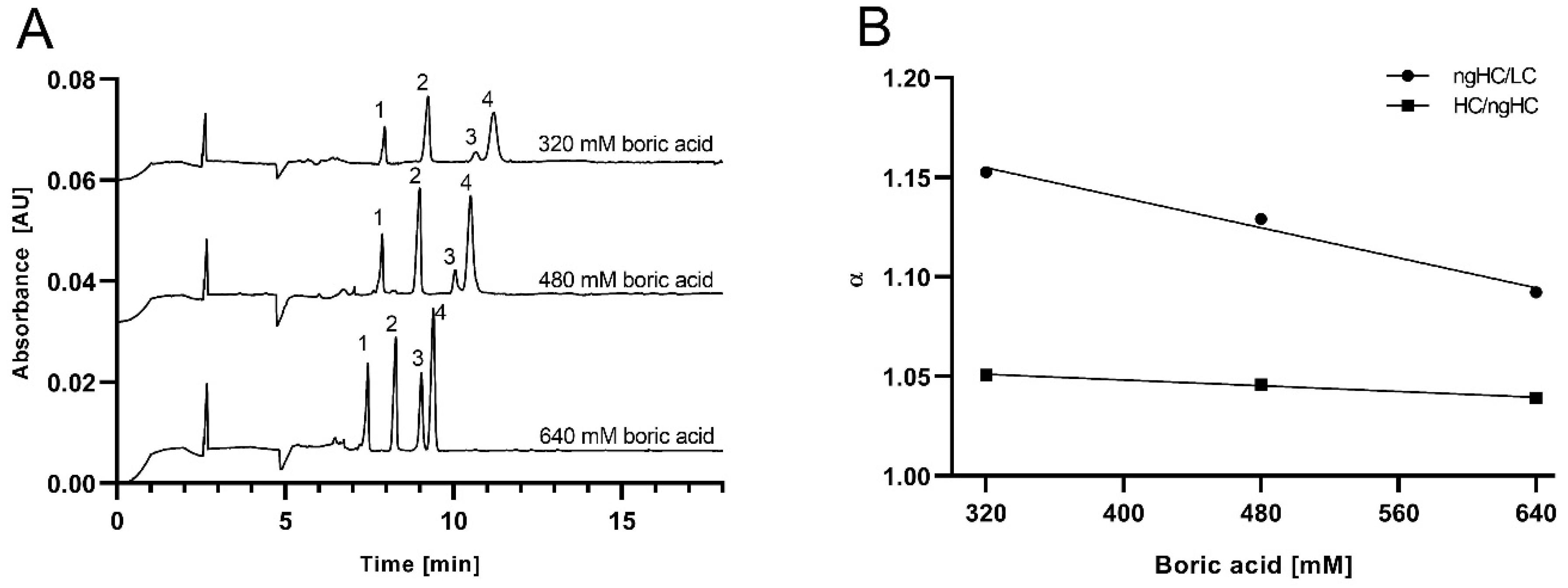
2.2. Separation of the SDS-Protein Complexes
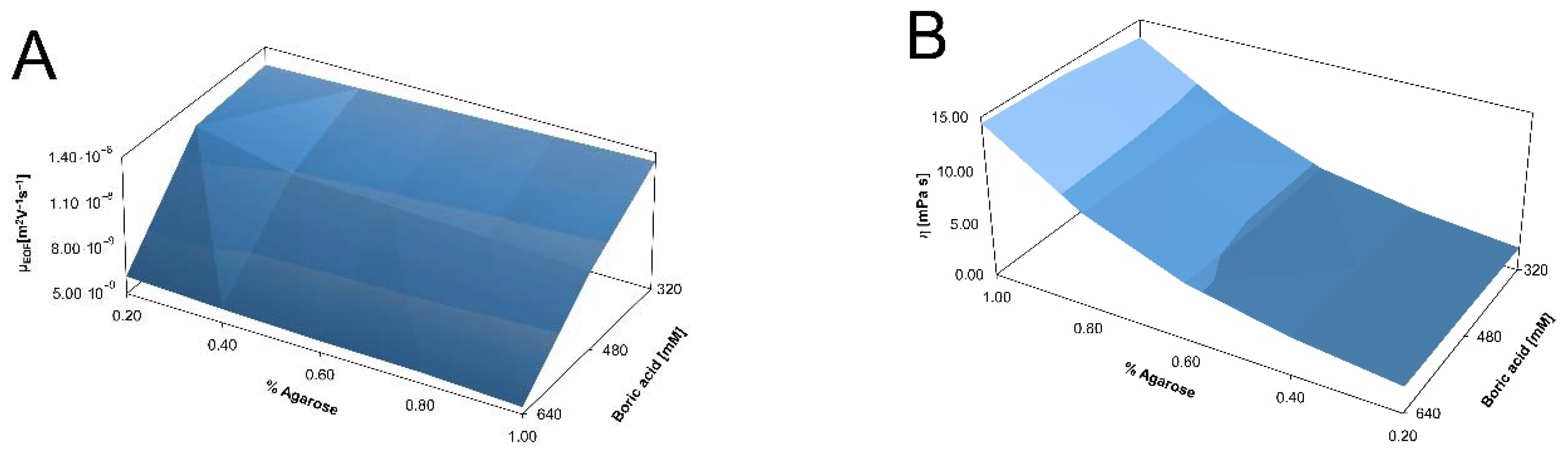
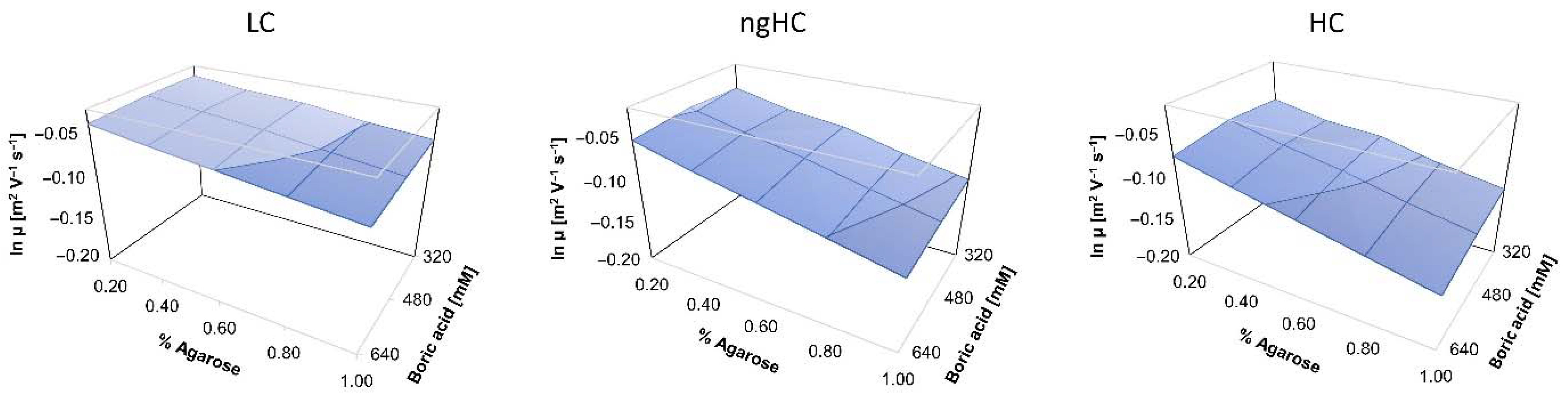
2.3. Ferguson Plots of Capillary SDS Agarose Gel Electrophoresis of Proteins
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Agarose Gel and Sample Preparation
4.3. Capillary Agarose Gel Electrophoresis
4.4. Electroosmotic Flow and Viscosity Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three dimensional |
| CGE | capillary gel electrophoresis |
| EOF | electroosmotic flow |
| HC | heavy chain |
| i.d. | internal diameter |
| LC | light chain |
| N | theoretical plate number |
| ngHC | non-glycosylated heavy chain |
| Rs | resolution |
| SDS | sodium dodecyl sulfate |
| TAE | Tris |
| acetate | EDTA |
| TBE | Tris |
| borate | EDTA |
| α | selectivity |
References
- Duckworth, M.; Yaphe, W. Preparation of agarose by fractionation from the spectrum of polysaccharides in agar. Anal. Biochem. 1971, 44, 636–641. [Google Scholar] [CrossRef]
- Grabar, P. Immunoelectrophoretic Analysis. In Methods of Biochemical Analysis 7; Interscience Publishers, Inc.: New York, NY, USA, 1959; pp. 1–38. [Google Scholar]
- Gordon, H.A.; Keil, B.; Sebesta, K. Electrophoresis of proteins in agar jelly. Nature 1949, 164, 498. [Google Scholar] [CrossRef] [PubMed]
- Wieme, R.J. Agar Gel Electrophoresis; Elsevier Publishing Company: New York, NY, USA, 1965; Volume xiii, 425p. [Google Scholar]
- Guiseley, K.B.; Renn, D.W. Agarose-Purification, Properties and Biomedical Applications; Marine Colloids, Inc.: Rockland, ME, USA, 1975. [Google Scholar]
- Hjerten, S. Agarose as an anticonvection agent in zone electrophoresis. Biochim. Biophys. Acta 1961, 53, 514–517. [Google Scholar] [CrossRef]
- Wu, M.; Kusukawa, N. SDS agarose gels for analysis of proteins. Biotechniques 1998, 24, 676–678. [Google Scholar] [CrossRef]
- Serwer, P. Agarose gels: Properties and use for electrophoresis. Electrophoresis 1983, 4, 375–382. [Google Scholar] [CrossRef]
- Righetti, P.G. Of matrices and men. J. Biochem. Biophys. Methods 1989, 19, 1–20. [Google Scholar] [CrossRef]
- Sänger-van de Griend, C.E. CE-SDS method development, validation, and best practice-An overview. Electrophoresis 2019, 40, 2361–2374. [Google Scholar] [CrossRef]
- Guttman, A. Capillary sodium dodecyl sulfate-gel electrophoresis of proteins. Electrophoresis 1996, 17, 1333–1341. [Google Scholar] [CrossRef]
- Olivera, B.M.; Baine, P.; Davidson, N. Electrophoresis of the nucleic acids. Electrophoresis 1964, 2, 245–257. [Google Scholar] [CrossRef]
- Lacher, N.A.; Roberts, R.K.; He, Y.; Cargill, H.; Kearns, K.M.; Holovics, H.; Ruesch, M.N. Development, validation, and implementation of capillary gel electrophoresis as a replacement for SDS-PAGE for purity analysis of IgG2 mAbs. J. Sep. Sci. 2010, 33, 218–227. [Google Scholar] [CrossRef]
- Guttman, A.; Filep, C.; Karger, B.L. Fundamentals of Capillary Electrophoretic Migration and Separation of SDS Proteins in Borate Cross-Linked Dextran Gels. Anal Chem. 2021, 93, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Werner, W.E.; Demorest, D.M.; Stevens, J.; Wiktorowicz, J.E. Size-dependent separation of proteins denatured in SDS by capillary electrophoresis using a replaceable sieving matrix. Anal Biochem. 1993, 212, 253–258. [Google Scholar] [CrossRef]
- Benedek, K.; Thiede, S. High-performance capillary electrophoresis of proteins usingsodium dodecyl sulfate-poly(ethylene oxide). J. Chromatogr. A 1994, 676, 209–217. [Google Scholar] [CrossRef]
- Ganzler, K.; Greve, K.S.; Cohen, A.S.; Karger, B.L. High-performance capillary electrophoresis of SDS-protein complexes using UV-transparent polymer networks. Anal Chem. 1992, 64, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, K.R.; Cheng, J. Capillary electrophoresis with glycerol as an additive. Methods Mol. Biol. 2001, 162, 259–277. [Google Scholar] [PubMed]
- Stellwagen, N.C.; Gelfi, C.; Righetti, P.G. DNA and buffers: The hidden danger of complex formation. Biopolymers 2000, 54, 137–142. [Google Scholar] [CrossRef]
- Foster, A.B. Advances in Carbohydrate Chemistry; Wolfrom, M.L., Ed.; Academic: New York, NY, USA, 1957; pp. 81–115. [Google Scholar]
- Guttman, A.; Paulus, A.; Cohen, A.S.; Grinberg, N.; Karger, B.L. Use of complexing agents for selective separation in high-performance capillary electrophoresis: Chiral resolution via cyclodextrins incorporated within polyacrylamide gel columns. J. Chromatogr. A 1988, 448, 41–53. [Google Scholar] [CrossRef]
- Filep, C.; Guttman, A. Effect of the Monomer Cross-Linker Ratio on the Separation Selectivity of Monoclonal Antibody Subunits in Sodium Dodecyl Sulfate Capillary Gel Electrophoresis. Anal Chem. 2021, 93, 3535–3541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Mitchelson, K.R. Glycerol-enhanced separation of DNA fragments in entangled solution capillary electrophoresis. Anal. Chem. 1994, 66, 4210–4214. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.A. Starch-gel electrophoresis--application to the classification of pituitary proteins and polypeptides. Metabolism 1964, 13, 985–1002. [Google Scholar] [CrossRef]
- Filep, C.; Guttman, A. Capillary sodium dodecyl sulfate gel electrophoresis of proteins: Introducing the three dimensional Ferguson method. Anal Chim. Acta 2021, 1183, 338958. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, M.; Guttman, A. Sample Preparation Scale-Up for Deep N-glycomic Analysis of Human Serum by Capillary Electrophoresis and CE-ESI-MS. Mol. Cell Proteom. 2019, 18, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Dieu, L.-H.; Fischer, S.; Mueller, R.; Mahler, H.-C.; Huwyler, J. High-throughput viscosity measurement using capillary electrophoresis instrumentation and its application to protein formulation. J. Pharm. Biomed. Anal 2014, 99, 51–58. [Google Scholar] [CrossRef] [PubMed]
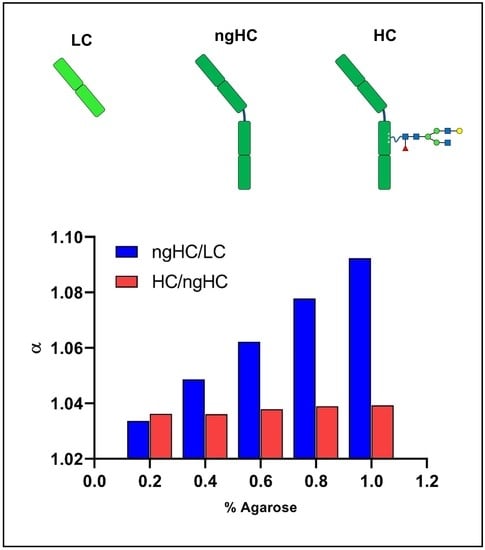
| Peak | α | N | Rs | ||||
|---|---|---|---|---|---|---|---|
| ngHC/LC | HC/ngHC | LC | ngHC | HC | ngHC/LC | HC/ngHC | |
| Agarose-TAE | 1.140 | 1.055 | 15967 | 10579 | 6455 | 3.000 | 0.962 |
| Agarose-TBE | 1.092 | 1.039 | 39316 | 45012 | 32201 | 3.435 | 1.505 |
| %Agarose | 320 mM Boric acid | ||||||
|---|---|---|---|---|---|---|---|
| α | N | Rs | |||||
| ngHC/LC | HC/ngHC | LC | ngHC | HC | ngHC/LC | HC/ngHC | |
| 0.2 | 1.048 | 1.049 | 22321 | 31125 | 16204 | 1.504 | 1.450 |
| 0.4 | 1.073 | 1.051 | 27609 | 29863 | 22158 | 2.476 | 1.593 |
| 0.6 | 1.088 | 1.045 | 29155 | 30386 | 19964 | 3.799 | 1.545 |
| 0.8 | 1.124 | 1.051 | 29528 | 24799 | 20088 | 4.051 | 1.580 |
| 1.0 | 1.153 | 1.051 | 22114 | 18238 | 12858 | 4.319 | 1.254 |
| %Agarose | 480 mM Boric acid | ||||||
| α | N | Rs | |||||
| ngHC/LC | HC/ngHC | LC | ngHC | HC | ngHC/LC | HC/ngHC | |
| 0.2 | 1.043 | 1.050 | 22755 | 38553 | 21541 | 1.374 | 1.440 |
| 0.4 | 1.063 | 1.045 | 27691 | 31503 | 22210 | 1.718 | 1.318 |
| 0.6 | 1.082 | 1.044 | 29063 | 30346 | 25263 | 2.789 | 1.581 |
| 0.8 | 1.108 | 1.048 | 30118 | 34139 | 28627 | 3.931 | 1.602 |
| 1.0 | 1.129 | 1.046 | 27624 | 39007 | 19911 | 3.939 | 1.536 |
| %Agarose | 640 mM Boric acid | ||||||
| α | N | Rs | |||||
| ngHC/LC | HC/ngHC | LC | ngHC | HC | ngHC/LC | HC/ngHC | |
| 0.2 | 1.034 | 1.036 | 32775 | 52624 | 29885 | 1.170 | 1.342 |
| 0.4 | 1.049 | 1.036 | 33757 | 50221 | 28982 | 1.811 | 1.292 |
| 0.6 | 1.062 | 1.038 | 34210 | 48996 | 29605 | 2.155 | 1.389 |
| 0.8 | 1.078 | 1.039 | 32818 | 66250 | 34239 | 3.032 | 1.653 |
| 1.0 | 1.092 | 1.039 | 39316 | 45012 | 32201 | 3.435 | 1.505 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkozy, D.; Guttman, A. Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins. Gels 2022, 8, 67. https://doi.org/10.3390/gels8020067
Sarkozy D, Guttman A. Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins. Gels. 2022; 8(2):67. https://doi.org/10.3390/gels8020067
Chicago/Turabian StyleSarkozy, Daniel, and Andras Guttman. 2022. "Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins" Gels 8, no. 2: 67. https://doi.org/10.3390/gels8020067
APA StyleSarkozy, D., & Guttman, A. (2022). Capillary Sodium Dodecyl Sulfate Agarose Gel Electrophoresis of Proteins. Gels, 8(2), 67. https://doi.org/10.3390/gels8020067