A Novel Poly(vinyl alcohol)–tetraethylorthosilicate Hybrid Gel Electrolyte for Lead Storage Battery
Abstract
1. Introduction
2. Results and Discussion
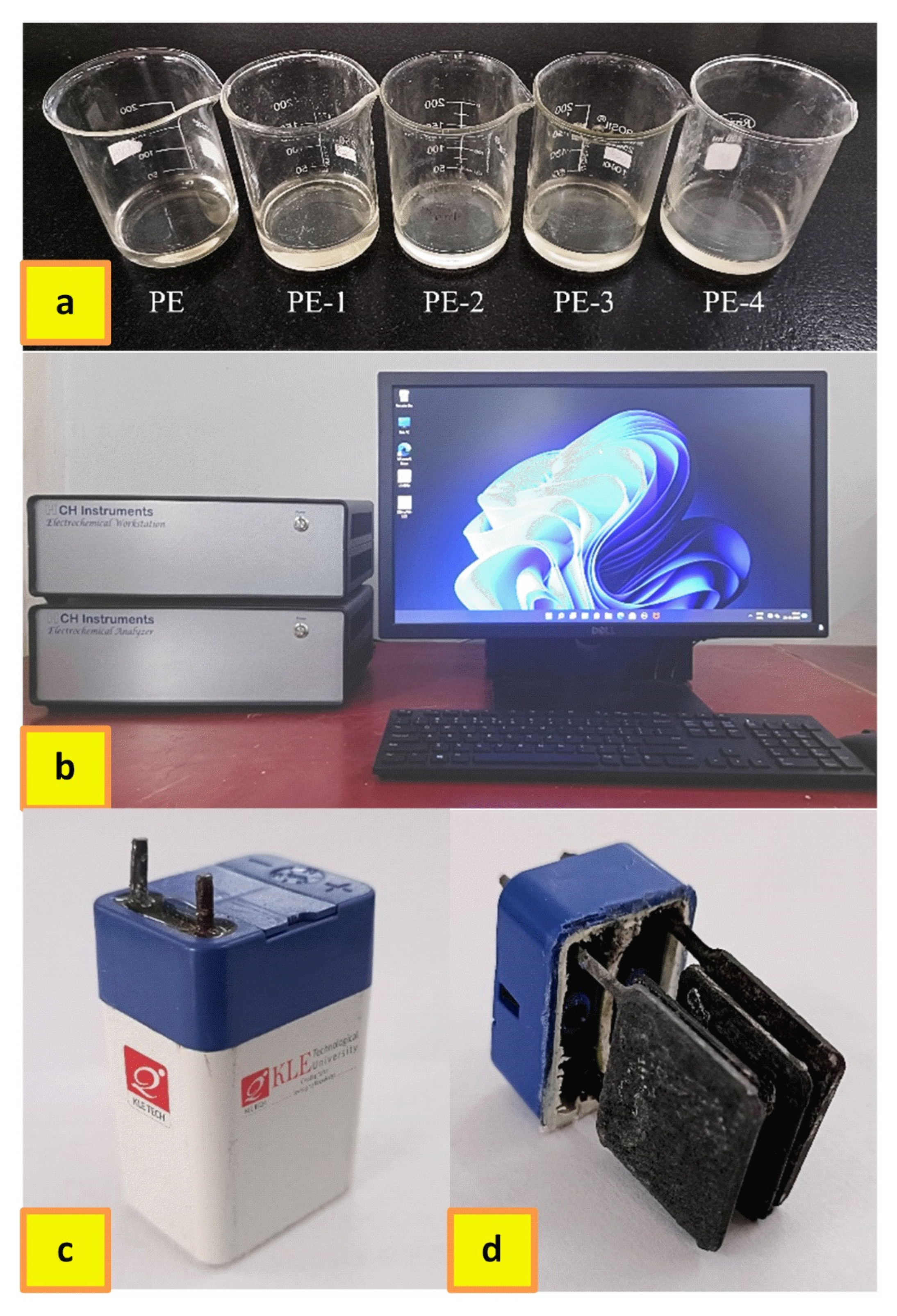
2.1. Physico-Chemical Characterization of Developed Polymer Gel Electrolytes
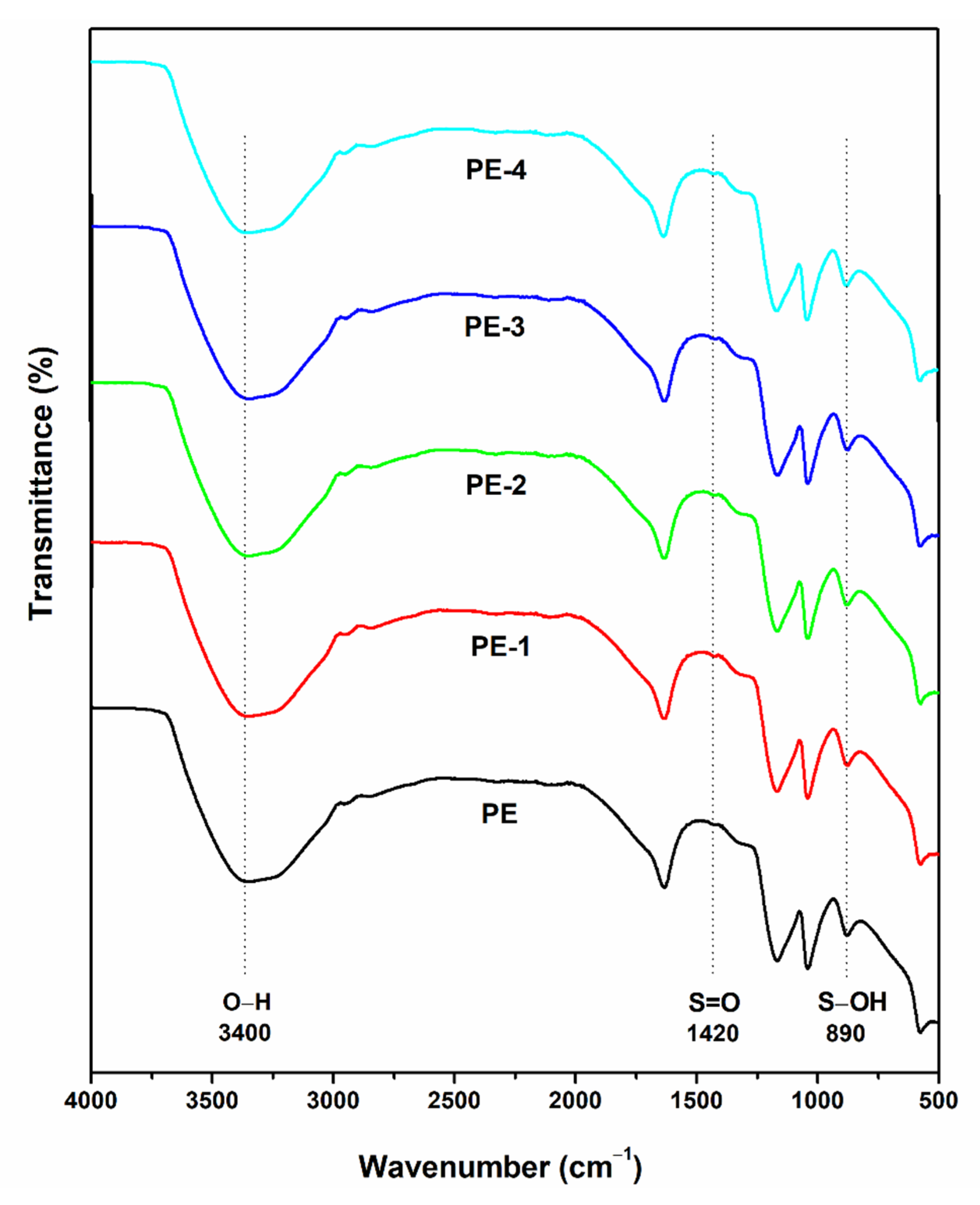
Fourier Transform Infrared Spectroscopy (FTIR)
2.2. Electrochemical Performance of Developed Polymer Gel Electrolytes
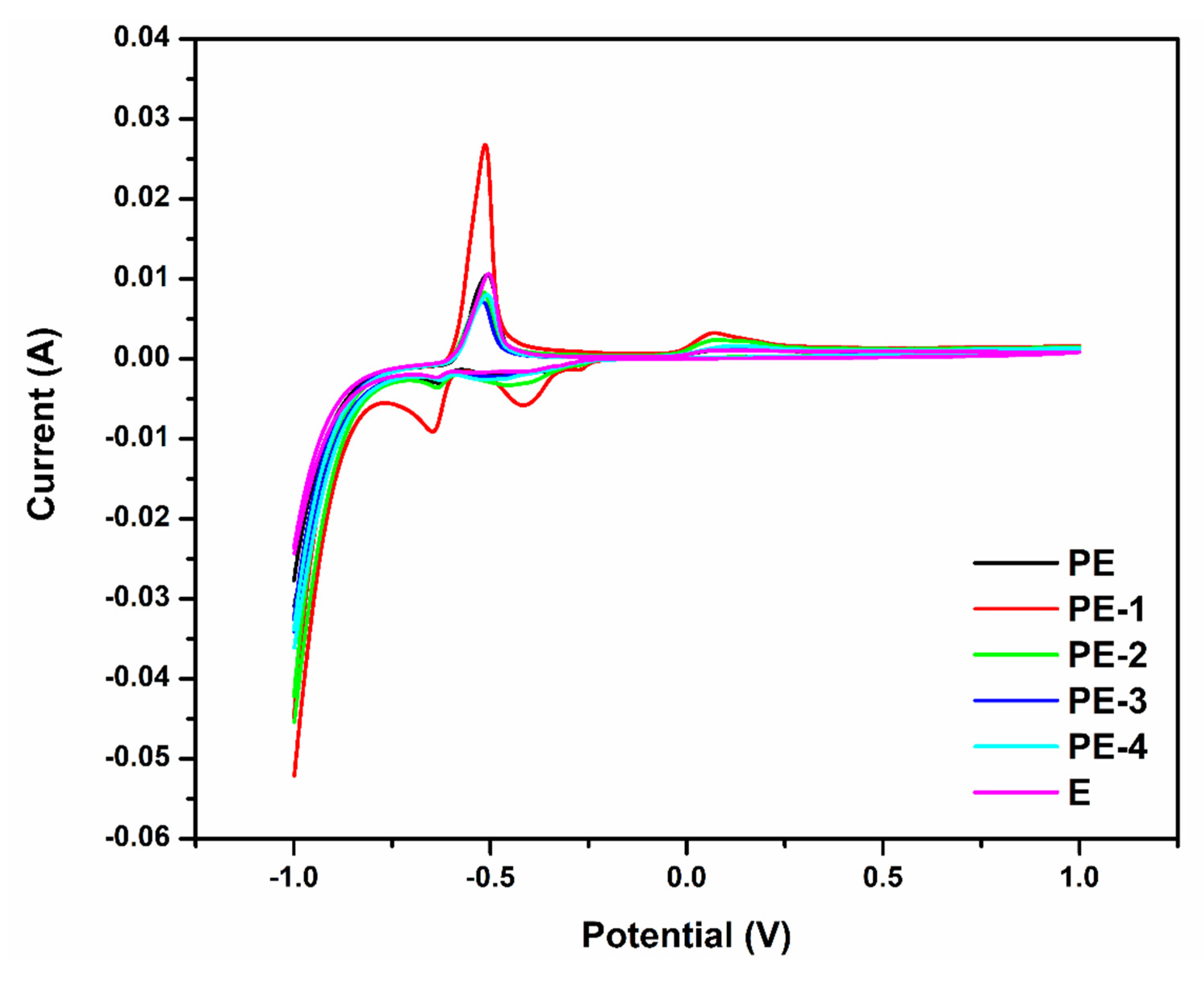
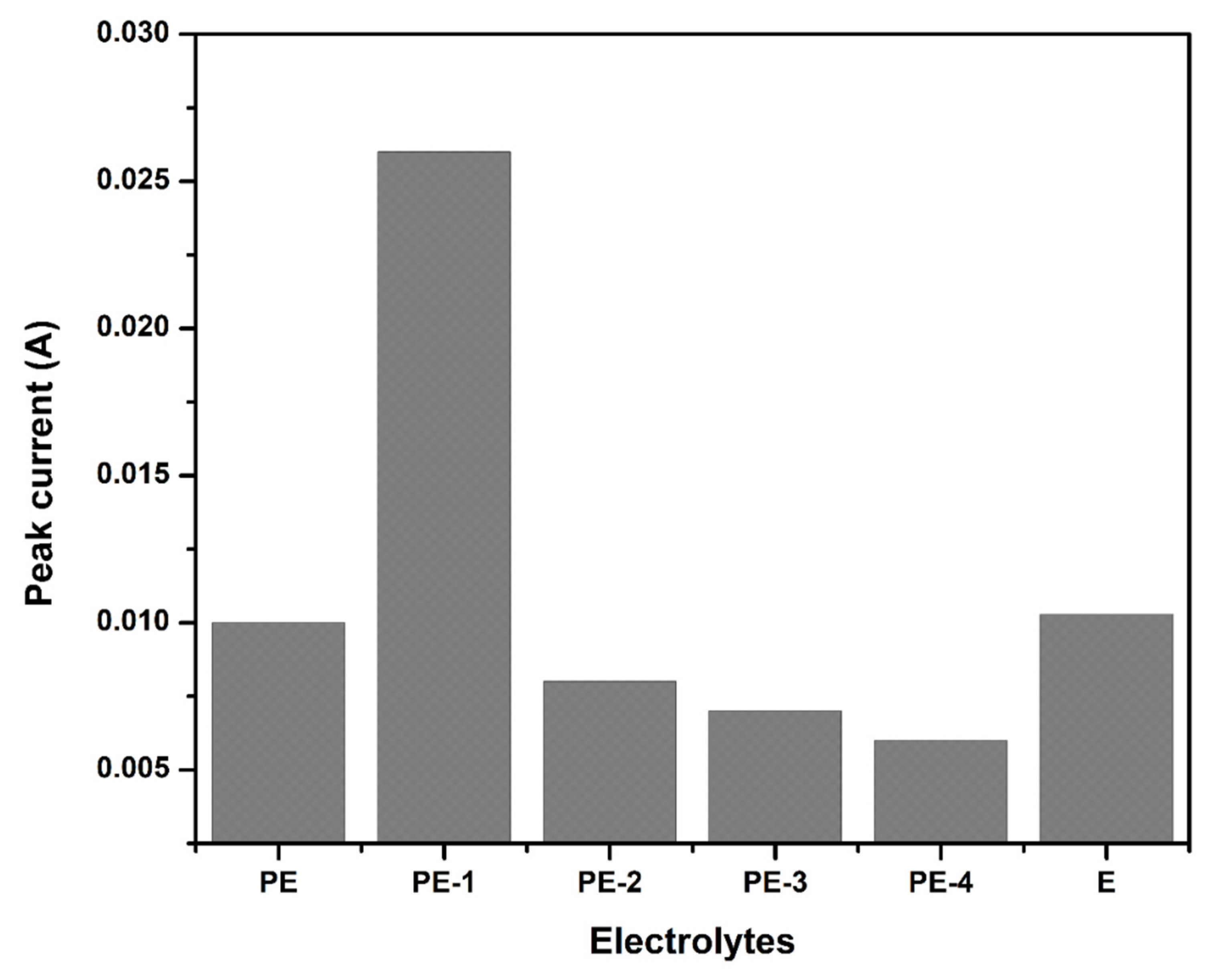
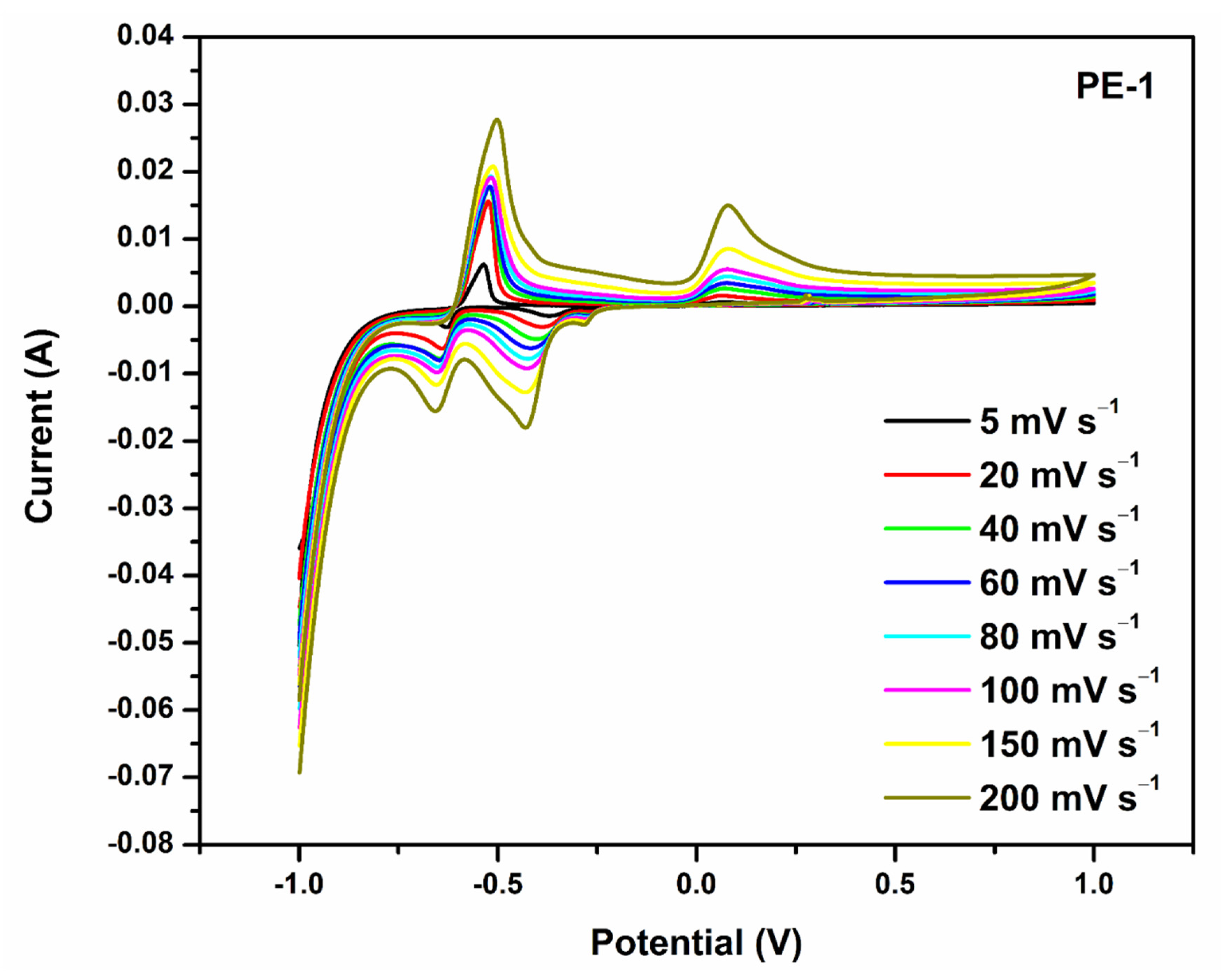
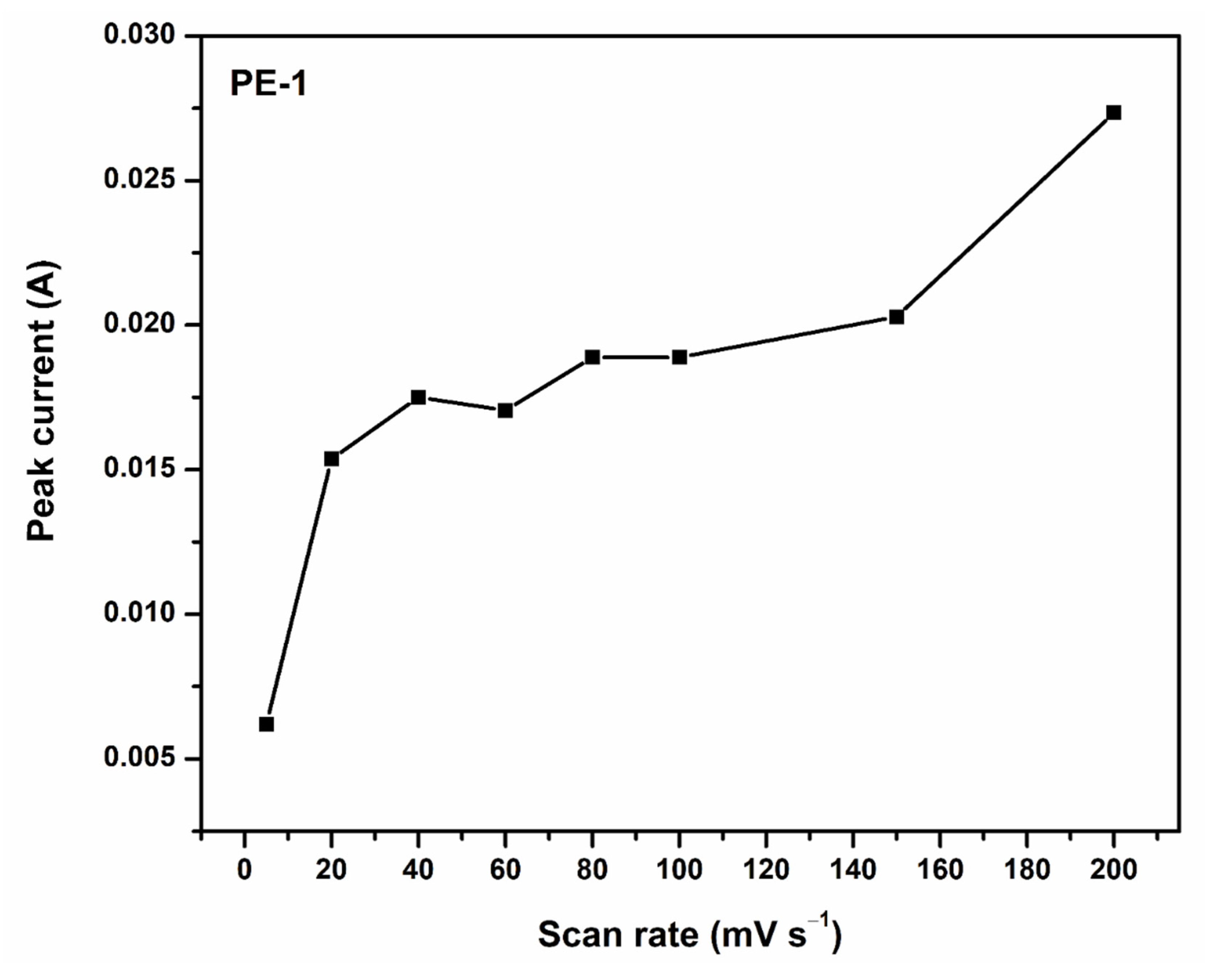
2.2.1. Cyclic Voltammetry (CV) Analysis
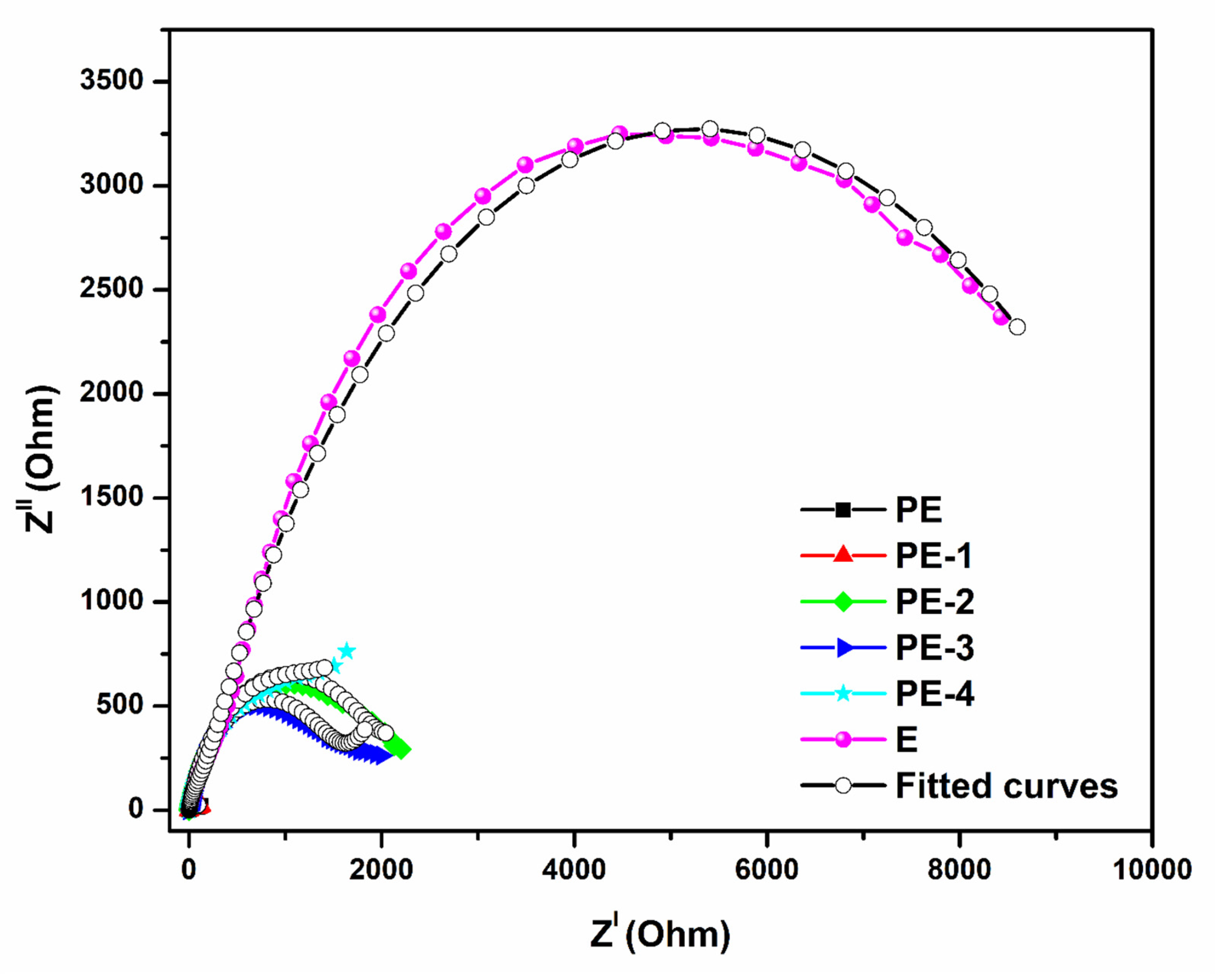
2.2.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
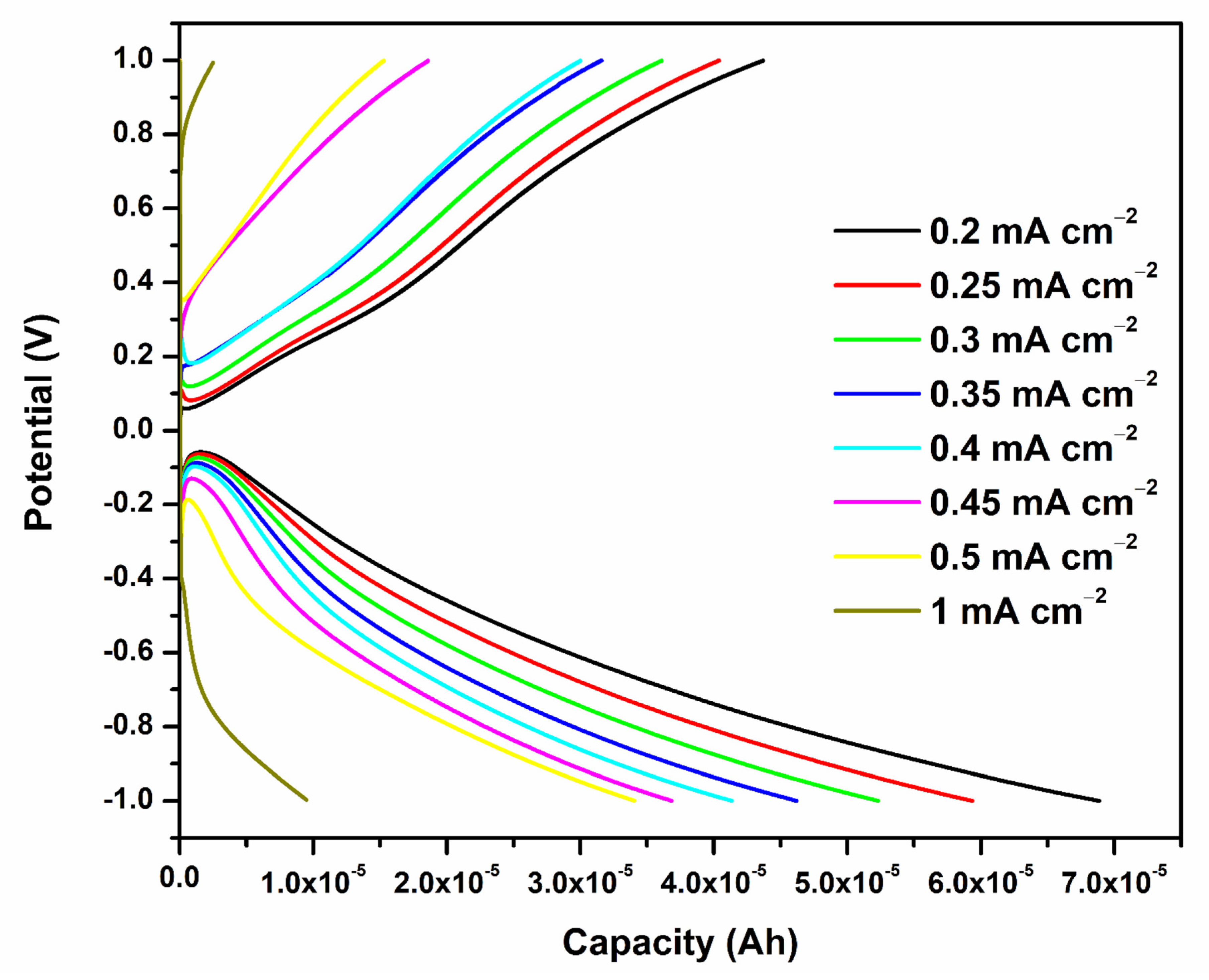
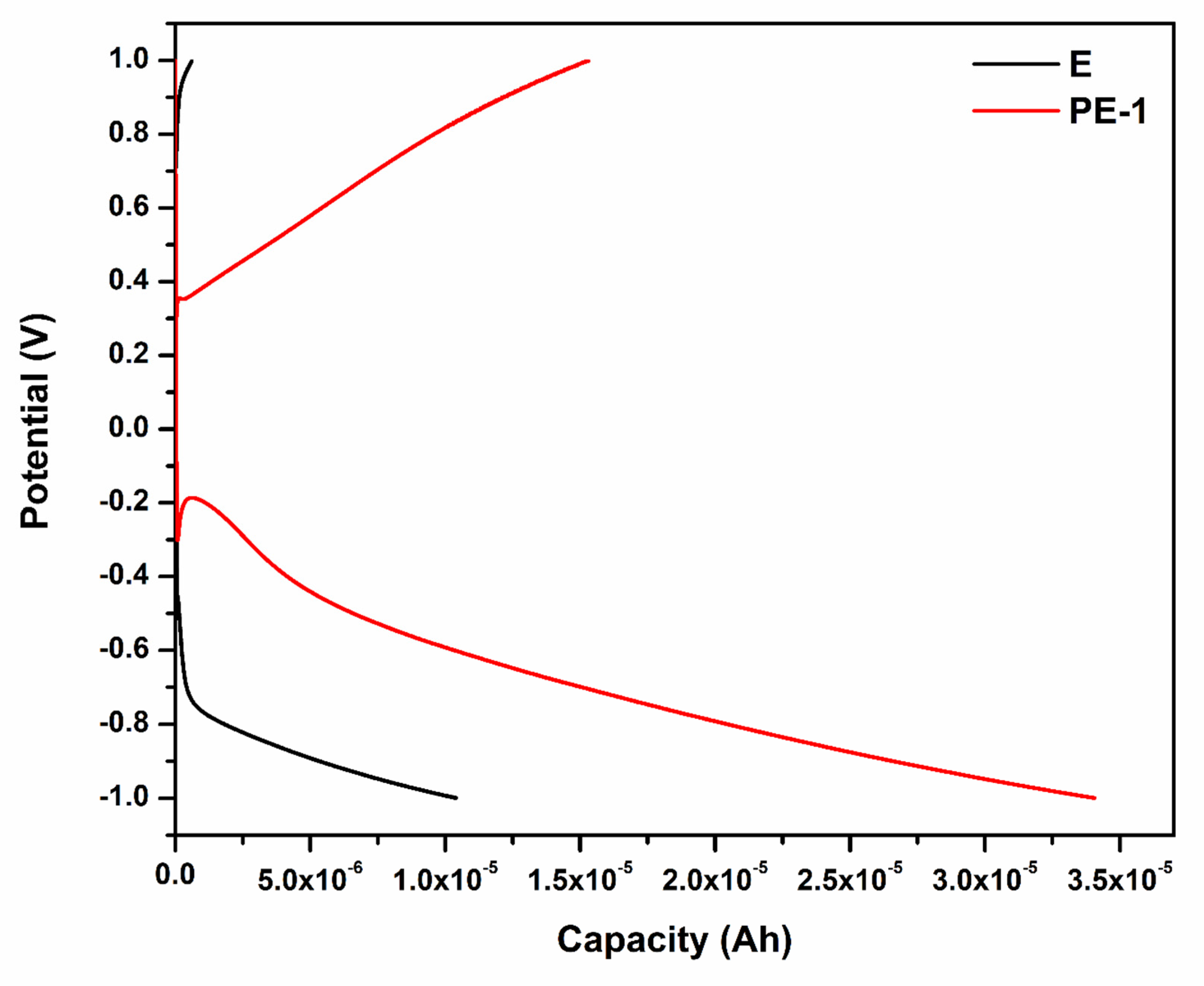
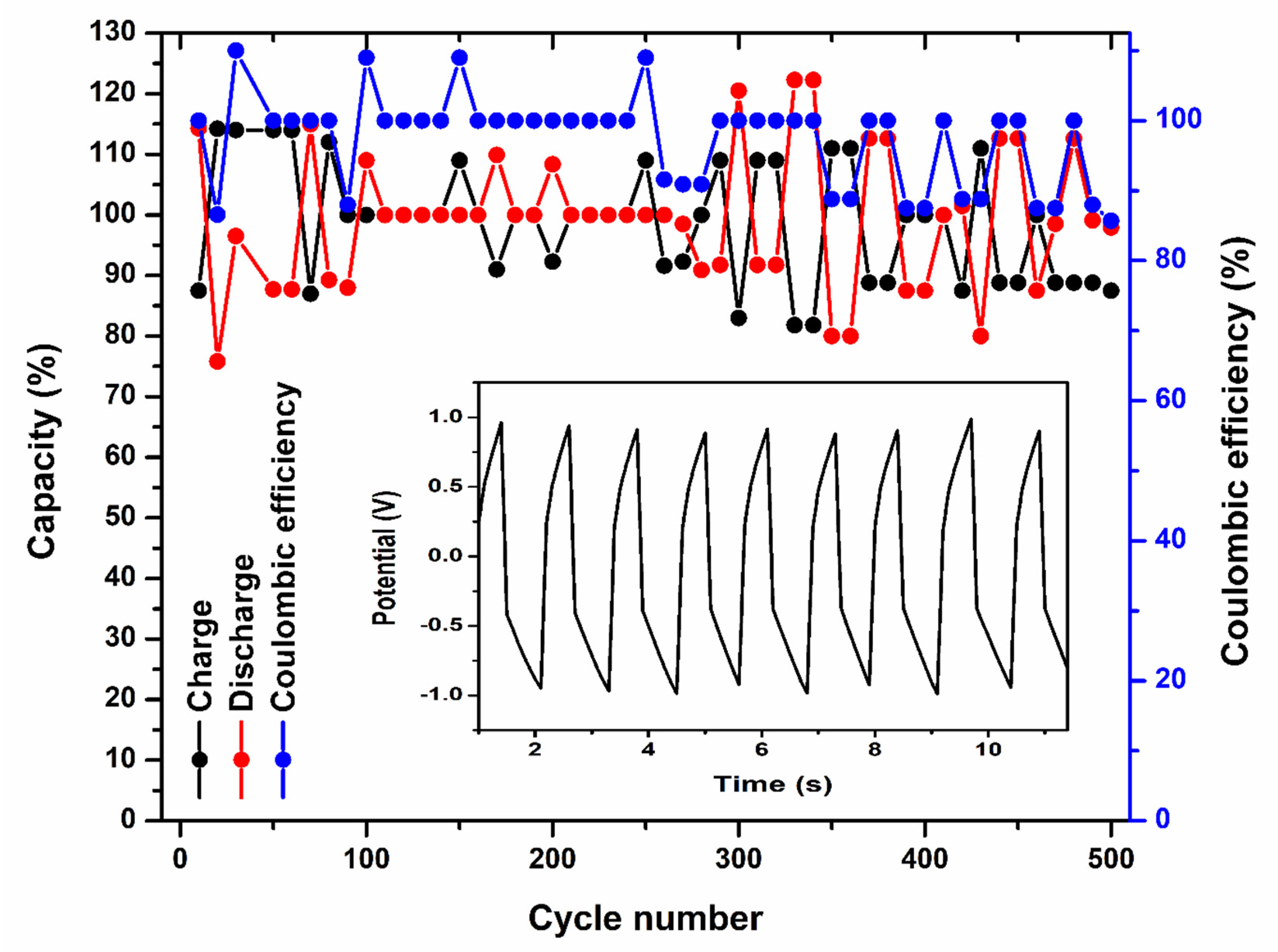
2.2.3. Galvanostatic Charge–Discharge (GCD) Analysis
3. Conclusions
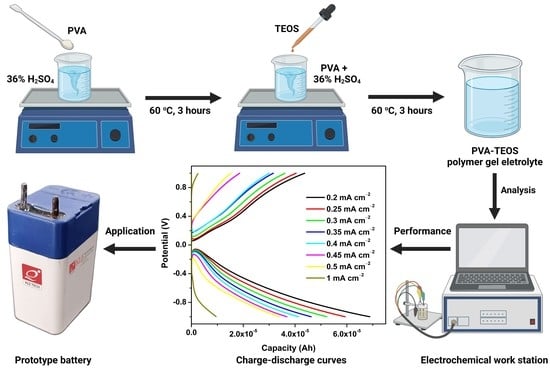
4. Materials and Methods
4.1. Materials
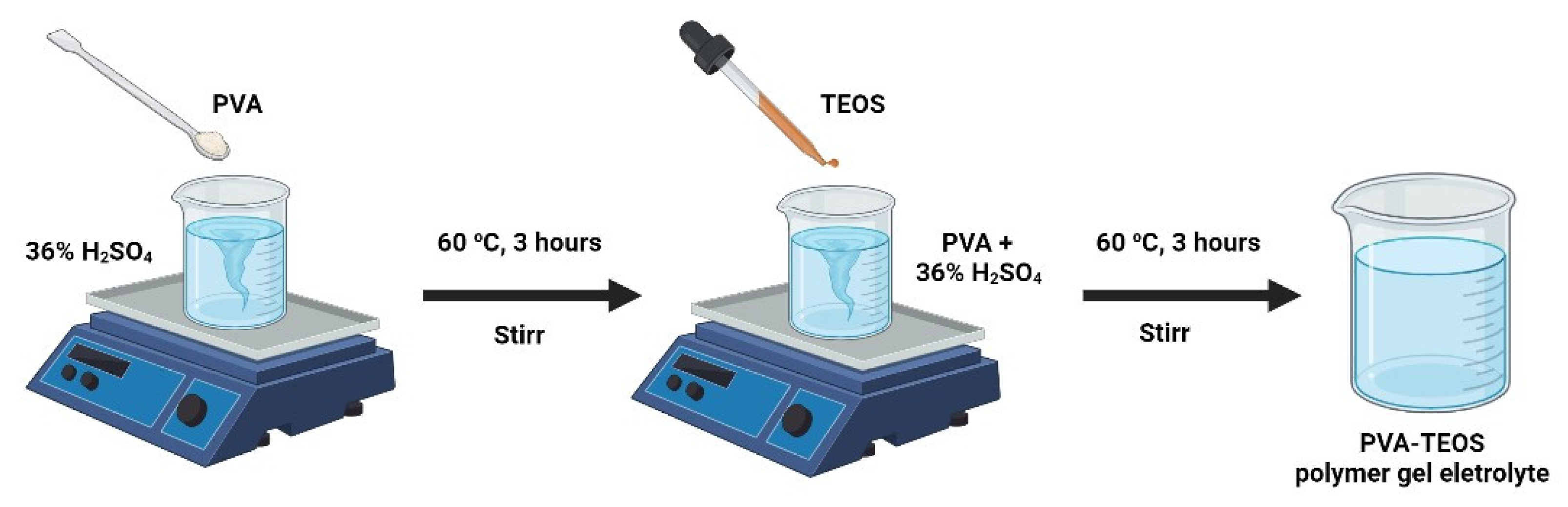
4.2. Development of Polymer Gel Electrolytes
4.3. Physico-Chemical Characterization
4.4. Electrochemical Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mansuroglu, A.; Gencten, M.; Arvas, M.B.; Sahin, M.; Bozdoğan, A.E.; Sahin, Y. A Novel Electrolyte Additive for Gel Type Valve Regulated Lead Acid Batteries: Sulfur Doped Graphene Oxide. Int. J. Energy Res. 2021, 45, 21390–21402. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A Review of Fractional-Order Techniques Applied to Lithium-Ion Batteries, Lead-Acid Batteries, and Supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef]
- Gencten, M.; Sahin, Y. A Critical Review on Progress of the Electrode Materials of Vanadium Redox Flow Battery. Int. J. Energy Res. 2020, 44, 7903–7923. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, Z.; Wu, X.; Wang, X.; Wu, Y.; Holze, R. An Acid-Free Rechargeable Battery Based on PbSO4 and Spinel LiMn2O4. Chem. Commun. 2014, 50, 13714–13717. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J. The Valve-Regulated Battery—A Paradigm Shift in Lead-Acid Technology; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Lambert, D.W.H.; Greenwood, P.H.J.; Reed, M.C. Advances in Gelled-Electrolyte Technology for Valve-Regulated Lead-Acid Batteries. J. Power Sources 2002, 107, 173–179. [Google Scholar] [CrossRef]
- Pgsch, G. High Gelled-Electrolyte Quality with Polyacrylamide Polymer: Limitation of Cycle-Life through Water Loss. J. Power Sources 1991, 33, 127–133. [Google Scholar]
- Martha, S.K.; Hariprakash, B.; Gaffoor, S.A.; Ambalavanan, S.; Shukla, A.K. Assembly and Performance of Hybrid-VRLA Cells and Batteries. J. Power Sources 2005, 144, 560–567. [Google Scholar] [CrossRef]
- Hernández, J.C.; Soria, M.L.; González, M.; García-Quismondo, E.; Muñoz, A.; Trinidad, F. Studies on Electrolyte Formulations to Improve Life of Lead Acid Batteries Working under Partial State of Charge Conditions. J. Power Sources 2006, 162, 851–863. [Google Scholar] [CrossRef]
- Gençten, M.; Dönmez, K.; Şahin, Y. Investigation of the Temperature Effect on Electrochemical Behaviors of TiO2 for Gel Type Valve Regulated Lead-Acid Batteries. Anadolu Univ. J. Sci. Technol. A Appl. Sci. Eng. 2016, 17, 882. [Google Scholar] [CrossRef][Green Version]
- Sekhon, S.S. Conductivity Behaviour of Polymer Gel Electrolytes: Role of Polymer. Bull. Mater. Sci. 2003, 26, 321–328. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-Conductive Macromolecular Gels and Membranes for Solid Lithium Cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Tantichanakul, T.; Chailapakul, O.; Tantavichet, N. Influence of Fumed Silica and Additives on the Gel Formation and Performance of Gel Valve-Regulated Lead-Acid Batteries. J. Ind. Eng. Chem. 2013, 19, 2085–2091. [Google Scholar] [CrossRef]
- Bhad, S.N.V.S. SANGAWAR Synthesis and Study of PVA Based Gel Electrolyte. Chem. Sci. Trans. 2012, 1, 653–657. [Google Scholar] [CrossRef][Green Version]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-Based Gel Polymer Electrolytes in Flexible Solid-State Supercapacitors: Opportunities and Challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Zhang, S.S. A Concept for Making Poly(Ethylene Oxide) Based Composite Gel Polymer Electrolyte Lithium/Sulfur Battery. J. Electrochem. Soc. 2013, 160, A1421–A1424. [Google Scholar] [CrossRef]
- Sharma, R.; Sil, A.; Ray, S. Poly(Methyl Methacrylate) Based Nanocomposite Gel Polymer Electrolytes with Enhanced Safety and Performance. J. Polym. Res. 2016, 23, 194. [Google Scholar] [CrossRef]
- Gao, S.; Wang, K.; Wang, R.; Jiang, M.; Han, J.; Gu, T.; Cheng, S.; Jiang, K. Poly(Vinylidene Fluoride)-Based Hybrid Gel Polymer Electrolytes for Additive-Free Lithium Sulfur Batteries. J. Mater. Chem. A Mater 2017, 5, 17889–17895. [Google Scholar] [CrossRef]
- Wang, S.H.; Kuo, P.L.; Hsieh, C.; Teng, H. Design of Poly(Acrylonitrile)-Based Gel Electrolytes for High-Performance Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 19360–19370. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.P. Anti-Freezing Flexible Aqueous Zn-MnO2 Batteries Working at −35 °C Enabled by a Borax-Crosslinked Polyvinyl Alcohol/Glycerol Gel Electrolyte. J. Mater. Chem. A Mater. 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Awadhia, A.; Agrawal, S.L. Structural, Thermal and Electrical Characterizations of PVA:DMSO:NH4SCN Gel Electrolytes. Solid State Ion 2007, 178, 951–958. [Google Scholar] [CrossRef]
- Didier, B.; Mercier, R.; Alberola, N.D.; Bas, C. Preparation of Polyimide/Silica Hybrid Material by Sol-Gel Process under Basic Catalysis: Comparison with Acid Conditions. J. Polym. Sci. B Polym. Phys. 2008, 46, 1891–1902. [Google Scholar] [CrossRef]
- Jamwal, H.S.; Kumari, S.; Chauhan, G.S.; Reddy, N.S.; Ahn, J.H. Silica-Polymer Hybrid Materials as Methylene Blue Adsorbents. J. Environ. Chem. Eng. 2017, 5, 103–113. [Google Scholar] [CrossRef]
- Li, R.; Pang, C.; Li, Z.; Yang, M.; Amekura, H.; Dong, N.; Wang, J.; Ren, F.; Wu, Q.; Chen, F. Fused Silica with Embedded 2D-Like Ag Nanoparticle Monolayer: Tunable Saturable Absorbers by Interparticle Spacing Manipulation. Laser Photon. Rev. 2020, 14, 1900302. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, S.; Wang, H.; Yan, D.; Wang, M.; Zang, Z. Room Temperature Synthesis of Stable Single Silica-Coated CsPbBr3 Quantum Dots Combining Tunable Red Emission of Ag–In–Zn–S for High-CRI White Light-Emitting Diodes. Nano Energy 2020, 67, 1900302. [Google Scholar] [CrossRef]
- Elsinger, L.; Petit, R.; van Acker, F.; Zawacka, N.K.; Tanghe, I.; Neyts, K.; Detavernier, C.; Geiregat, P.; Hens, Z.; van Thourhout, D. Waveguide-Coupled Colloidal Quantum Dot Light Emitting Diodes and Detectors on a Silicon Nitride Platform. Laser Photonics Rev. 2021, 15, 2000230. [Google Scholar] [CrossRef]
- Xie, Z.; Hoang, M.; Duong, T.; Ng, D.; Dao, B.; Gray, S. Sol-Gel Derived Poly(Vinyl Alcohol)/Maleic Acid/Silica Hybrid Membrane for Desalination by Pervaporation. J. Memb. Sci. 2011, 383, 96–103. [Google Scholar] [CrossRef]
- Haouas, M.; Petry, D.P.; Anderson, M.W.; Taulelle, F. Tetrapropylammonium Occlusion in Nanoaggregates of Precursor of Silicalite-1 Zeolite Studied by 1H and 13C NMR. Inorganics 2016, 4, 18. [Google Scholar] [CrossRef]
- Pirzada, T.; Shah, S.S. Water-Resistant Poly(Vinyl Alcohol)-Silica Hybrids through Sol-Gel Processing. Chem. Eng. Technol. 2014, 37, 620–626. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Propertles, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Sahin, A. The Development of Speek/Pva/Teos Blend Membrane for Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2018, 271, 127–136. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, S.H.; Lee, H.C.; Hong, M.Z.; Kim, K.; Lee, H.I. Organic-Inorganic Composite Membranes as Addition of SiO2 for High Temperature-Operation in Polymer Electrolyte Membrane Fuel Cells (PEMFCs). Electrochim. Acta 2004, 49, 4787–4796. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Stefanithis, D.; Davis, S.V.; Scheetz, R.W.; Pope, R.K.; Huanc, H. Microstructural Evolution of a Silicon Oxide Phase in a Perfluorosulfonic Acid Lonomer by an In Situ Sol-Gel Reaction. J. Appl. Polym. Sci. 1995, 55, 181–190. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Kulkarni, A.S.; Sajjan, A.M.; Yunus Khan, T.M.; Badruddin, I.A.; Kamangar, S.; Banapurmath, N.R.; Ayachit, N.H.; Naik, M.L.; Marakatti, V.S. Fabrication and Physicochemical Study of B2SA-Grafted Poly(Vinyl Alcohol)–Graphene Hybrid Membranes for Dehydration of Bioethanol by Pervaporation. Membranes 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Kittur, A.A.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Synthesis and Characterization of Hybrid Membranes Using Poly(Vinyl Alcohol) and Tetraethylorthosilicate for the Pervaporation Separation of Water-Isopropanol Mixtures. J. Appl. Polym. Sci. 2004, 94, 1304–1315. [Google Scholar] [CrossRef]
- Raza, M.A.; Islam, A.; Sabir, A.; Gull, N.; Ali, I.; Mehmood, R.; Bae, J.; Hassan, G.; Khan, M.U. PVA/TEOS Crosslinked Membranes Incorporating Zinc Oxide Nanoparticles and Sodium Alginate to Improve Reverse Osmosis Performance for Desalination. J. Appl. Polym. Sci. 2019, 136, 47559. [Google Scholar] [CrossRef]
- Buzzoni, R.; Bordiga, S.; Ricchiardi, G.; Spoto, G.; Zecchina, A. Interaction of HzO, CH30H, (CH3)20, CH&N, and Pyridine with the Superacid Perfluorosulfonic Membrane Nafion: An IR and Raman Study. Ph.D. Thesis, Northeastern University, Boston, MA, USA, 1995. Volume 99. [Google Scholar]
- Dönmez, K.B.; Gençten, M.; Şahin, Y. A Novel Polysiloxane-Based Polymer as a Gel Agent for Gel–VRLA Batteries. Ionics 2017, 23, 2077–2089. [Google Scholar] [CrossRef]
- Gençten, M. Investigation the Effects of Boehmite and Gibbsite on the Electrochemical Behaviours of Gel-VRLA Batteries. Int. J. Electrochem. Sci. 2018, 13, 11741–11751. [Google Scholar] [CrossRef]
- Gençten, M.; Dönmez, K.B.; Şahin, Y.; Pekmez, K.; Suvacı, E. Voltammetric and Electrochemical Impedimetric Behavior of Silica-Based Gel Electrolyte for Valve-Regulated Lead-Acid Battery. J. Solid State Electrochem. 2014, 18, 2469–2479. [Google Scholar] [CrossRef]
- Seidel, M.; Kugaraj, M.; Nikolowski, K.; Wolter, M.; Kinski, I.; Jähnert, T.; Michaelis, A. Comparison of Electrochemical Degradation for Spray Dried and Pulse Gas Dried LiNi0.5Mn1.5O4. J. Electrochem. Soc. 2019, 166, A2860–A2869. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Xu, S.; Huang, S.; Zhu, J. Synthesis and Electrochemical Properties of LiNi0.5Mn1.5O4 Cathode Materials with Cr3+ and F− Composite Doping for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 414. [Google Scholar] [CrossRef][Green Version]
- Ates, M.; Yilmaz, K.; Shahryari, A.; Omanovic, S.; Sezai Sarac, A. A Study of the Electrochemical Behavior of Poly [N-Vinyl Carbazole] Formed on Carbon-Fiber Microelectrodes and Its Response to Dopamine. IEEE Sens. J. 2008, 8, 1628–1639. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Ni, Z.; Gu, Y.; Wang, B.; Guo, Z.; Wang, Z.; Bin, D.; Ma, J.; Wang, Y. Binding Zinc Ions by Carboxyl Groups from Adjacent Molecules toward Long-Life Aqueous Zinc–Organic Batteries. Adv. Mater. 2020, 32, 2000338. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Pan, L.; Lu, T.; Yao, Y.; Sun, Z.; Chua, D.H.C.; Chen, Q. Electrospun Carbon Nanofibers as Anode Materials for Sodium Ion Batteries with Excellent Cycle Performance. J. Mater. Chem. A Mater. 2014, 2, 4117–4121. [Google Scholar] [CrossRef]
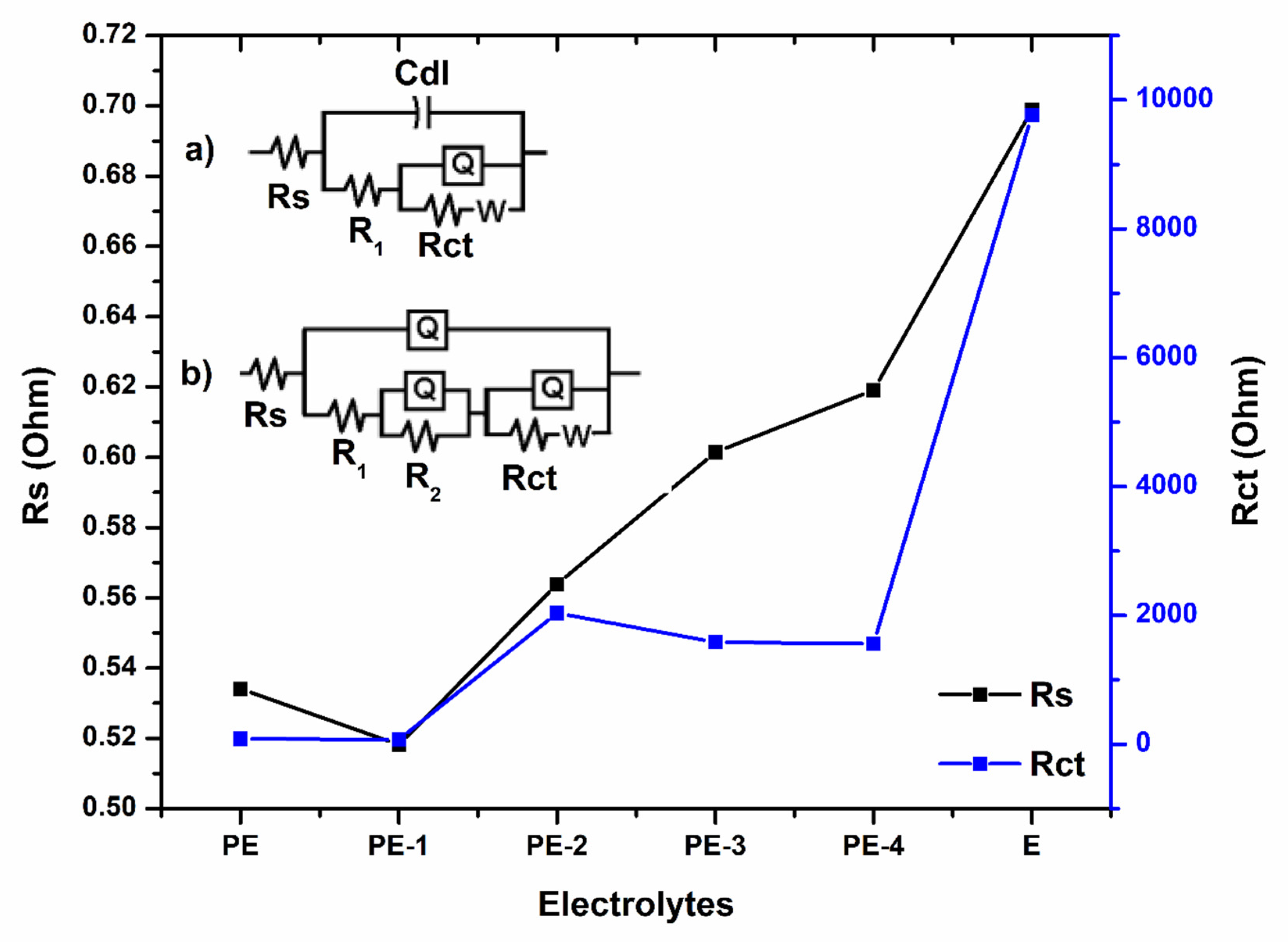

| Polymer Gel Electrolytes | Rs (Ohm) | Cdl (F) | R1 (Ohm) | Q (S-sec^n) | n | Rct (Ohm) | W (S-sec^5) |
| PE | 0.5341 | 0.0002509 | 1.97 | 0.005817 | 0.5971 | 82.59 | 0.1447 |
| PE-1 | 0.5182 | 0.0002258 | 1.859 | 0.007118 | 0.5288 | 72.33 | 0.22 |
| PE-2 | 0.5639 | 0.00004742 | 8.848 | 0.000348 | 0.6661 | 2035 | 0.01576 |
| PE-3 | 0.6014 | 0.00003007 | 13.27 | 0.000189 | 0.6616 | 1585 | 0.008965 |
| PE-4 | 0.6191 | 0.0001082 | 1.831 | 0.001566 | 0.7558 | 1561 | 0.007856 |
| Rs (Ohm) | Q (S-sec^n) | n | R1 | Q (S-sec^n) | n | R2 | Q (S-sec^n) | n | Rct | W (S-sec^5) |
| 0.6988 | 0.00006129 | 0.8374 | 0.02246 | 2.502×10−18 | 0.7992 | 350.5 | 0.0001736 | 0.6981 | 9763 | 0.009552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikkatti, B.S.; Sajjan, A.M.; Kalahal, P.B.; Banapurmath, N.R.; Khan, T.M.Y.; Khadar, S.D.A.; Shamsudeen, S.M.; Raju, A.B. A Novel Poly(vinyl alcohol)–tetraethylorthosilicate Hybrid Gel Electrolyte for Lead Storage Battery. Gels 2022, 8, 791. https://doi.org/10.3390/gels8120791
Chikkatti BS, Sajjan AM, Kalahal PB, Banapurmath NR, Khan TMY, Khadar SDA, Shamsudeen SM, Raju AB. A Novel Poly(vinyl alcohol)–tetraethylorthosilicate Hybrid Gel Electrolyte for Lead Storage Battery. Gels. 2022; 8(12):791. https://doi.org/10.3390/gels8120791
Chicago/Turabian StyleChikkatti, Bipin S., Ashok M. Sajjan, Prakash B. Kalahal, Nagaraj R. Banapurmath, T. M. Yunus Khan, Shaik Dawood Abdul Khadar, Shaik Mohamed Shamsudeen, and A. B. Raju. 2022. "A Novel Poly(vinyl alcohol)–tetraethylorthosilicate Hybrid Gel Electrolyte for Lead Storage Battery" Gels 8, no. 12: 791. https://doi.org/10.3390/gels8120791
APA StyleChikkatti, B. S., Sajjan, A. M., Kalahal, P. B., Banapurmath, N. R., Khan, T. M. Y., Khadar, S. D. A., Shamsudeen, S. M., & Raju, A. B. (2022). A Novel Poly(vinyl alcohol)–tetraethylorthosilicate Hybrid Gel Electrolyte for Lead Storage Battery. Gels, 8(12), 791. https://doi.org/10.3390/gels8120791