Research on the Mechanical Properties and Microstructure of Modified Silt Sediment Geopolymer Materials
Abstract
1. Introduction
2. Results and Discussion
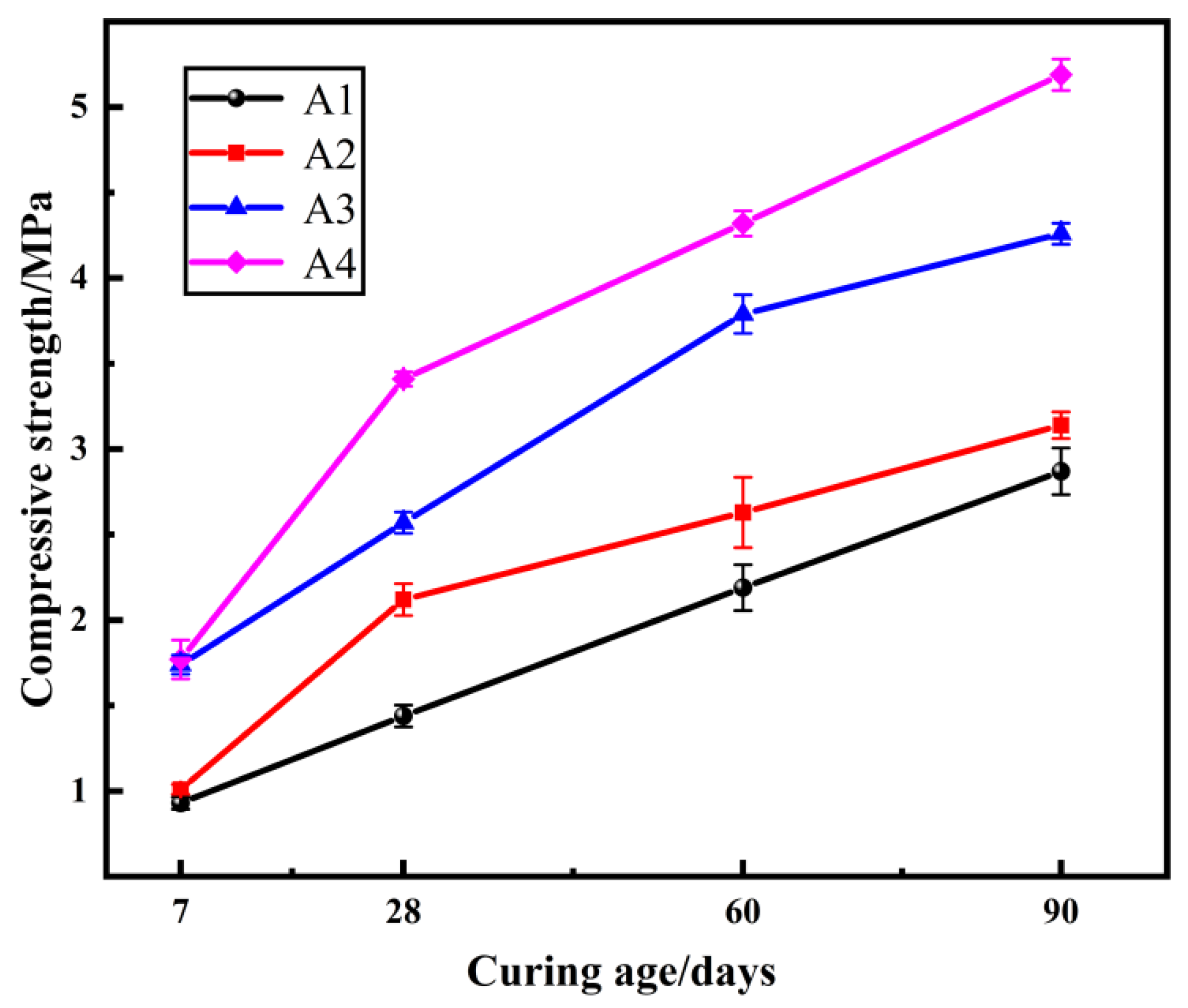
2.1. The Effect of the Mole Ratio of SiO2 to Na2O of the Sodium Silicate Solution on the Compressive Strength
2.2. The Effect of Quicklime Dosage on Compressive Strength
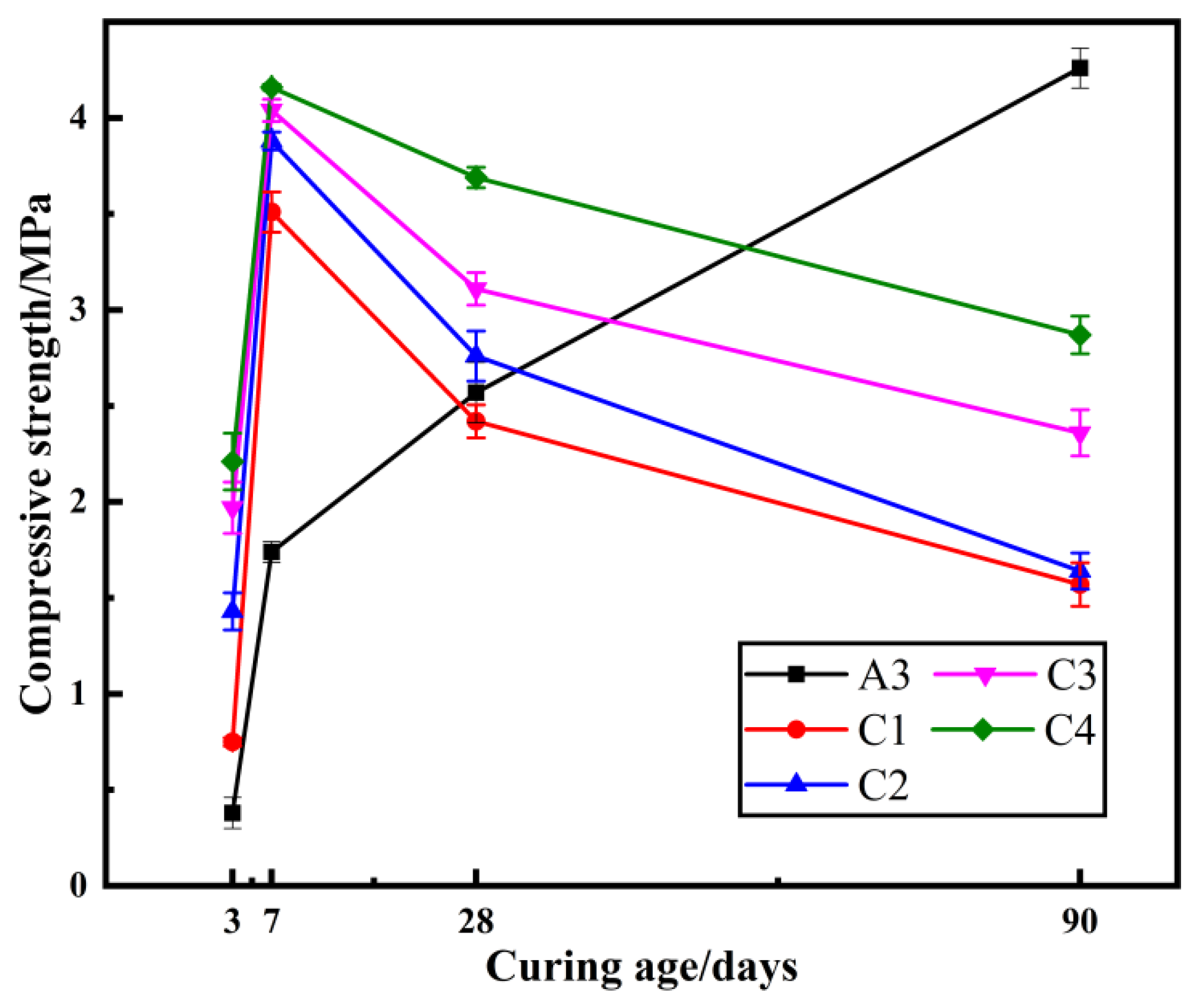
2.3. The Effect of Na2SO4 Dosage on Compressive Strength
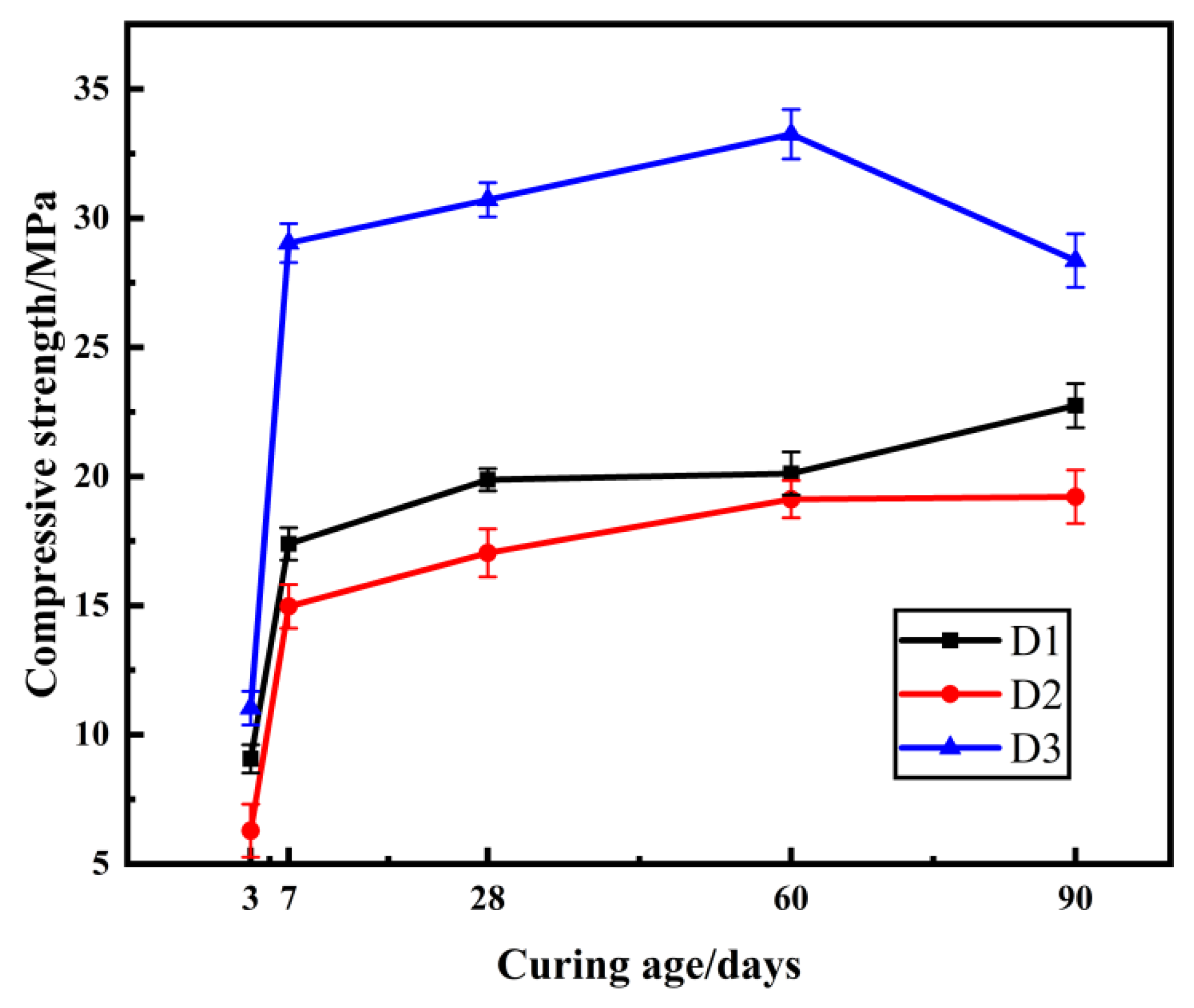
2.4. The Effect of Mineral Admixtures on Compressive Strength
2.5. The Effect of Curing Conditions on Compressive Strength
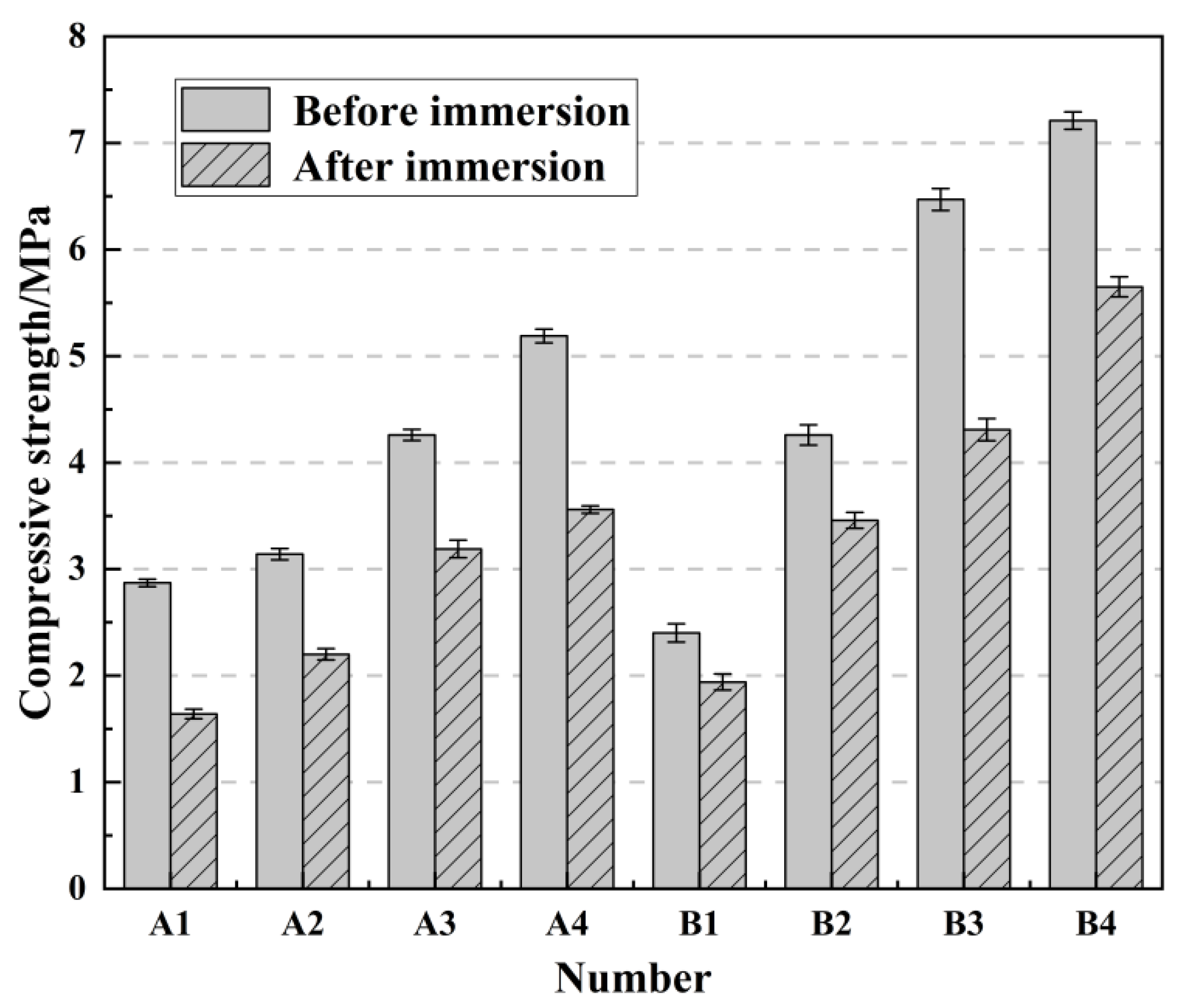
2.6. The Effect of Immersion Curing on Compressive Strength
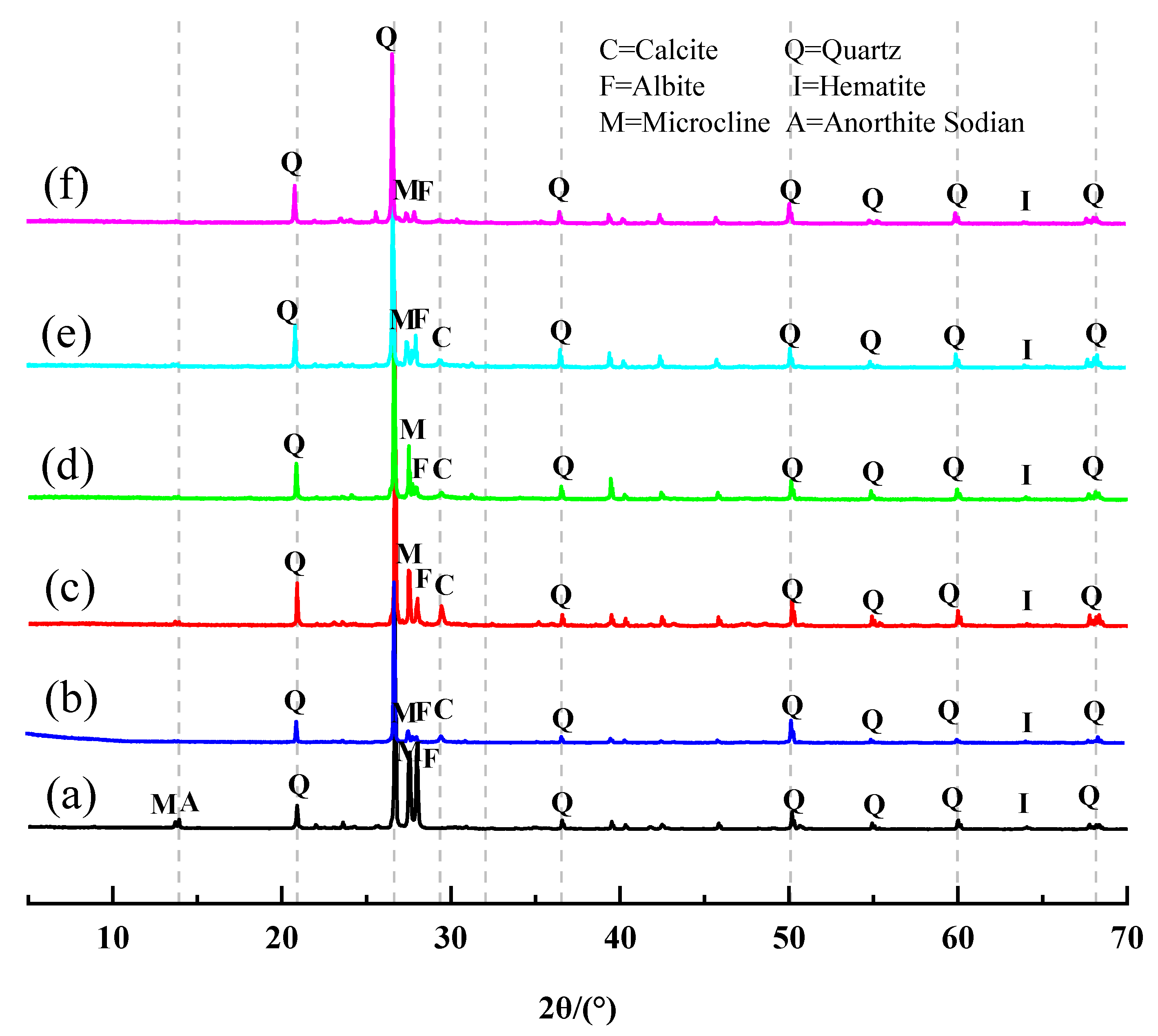
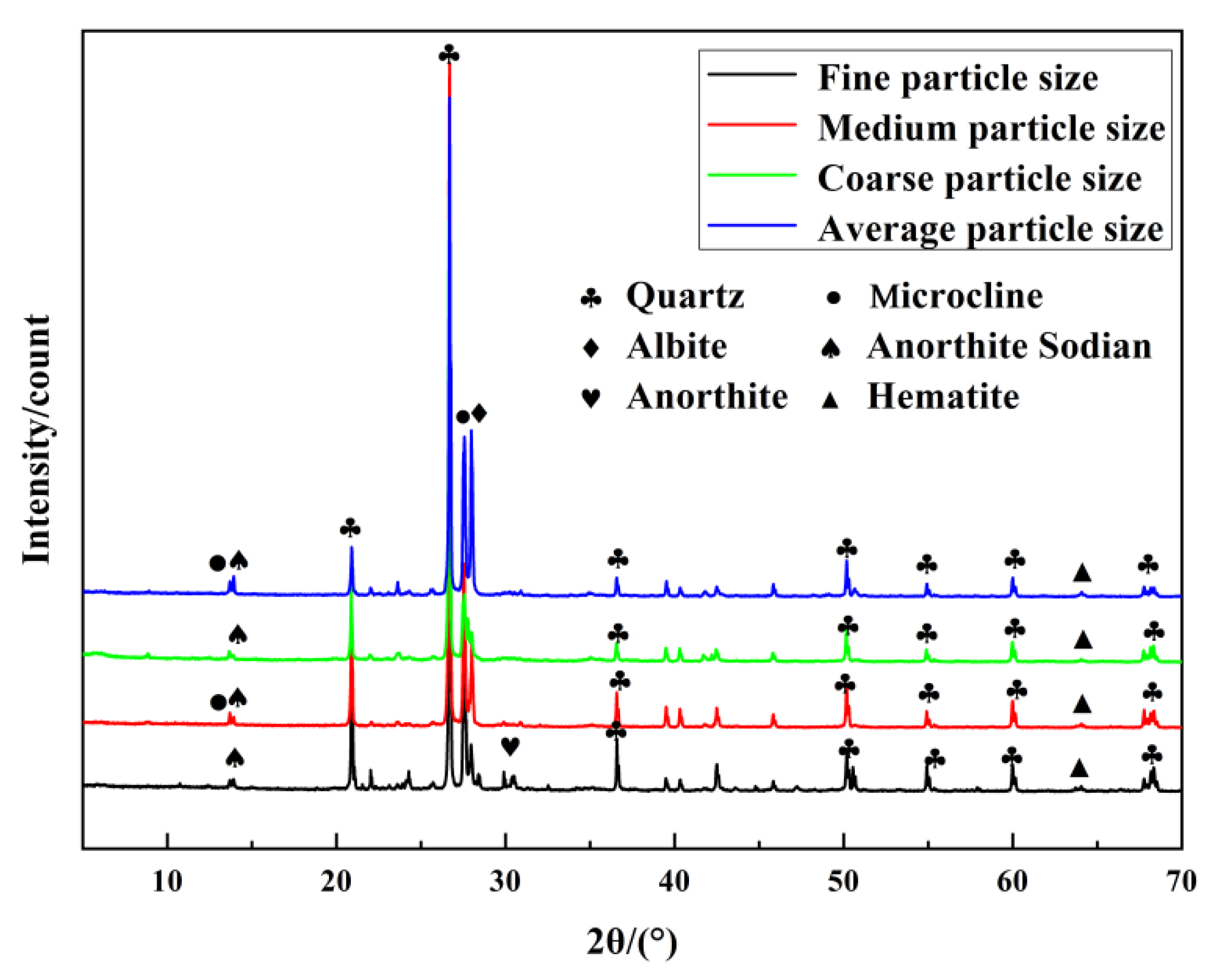
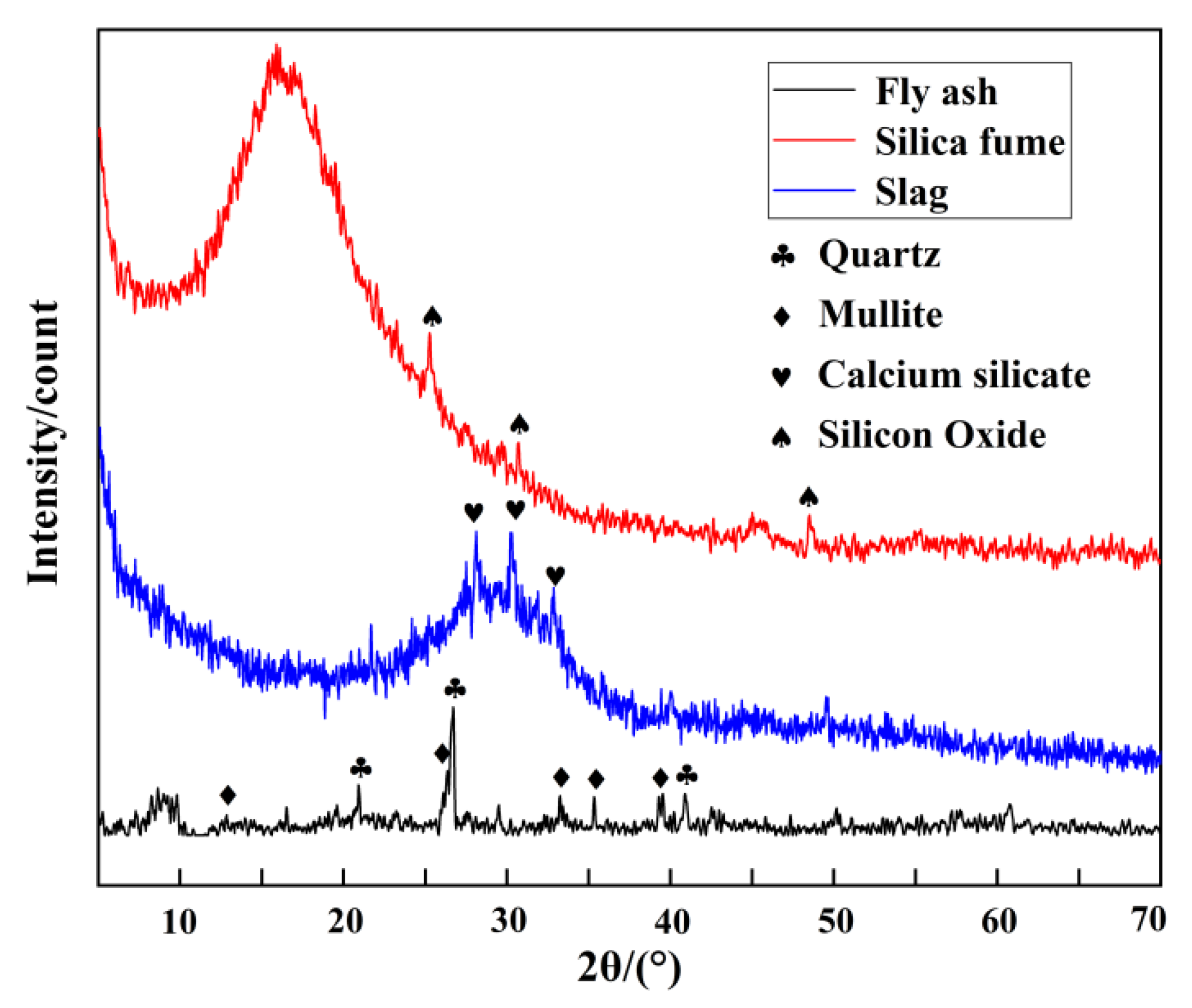
2.7. XRD Analysis
2.8. Microstructural Analysis
3. Conclusions
- (1)
- River and lake sediment can be made into new geopolymer materials using the alkali-activated gel modification method. The sediment geopolymer materials produced have a maximum strength of 33.25 MPa, and the sediment geopolymer materials made according to this method have the potential to be used in water conservation projects such as rock preparation along rivers and lakes.
- (2)
- Alkali dosage, mineral admixture type, and dosage, as well as curing condition and curing age, are significant factors affecting the mechanical strength of the composites. The strength of the samples will increase with increasing alkali dosage, but an excessive amount of alkali will have a negative impact on the compressive strength. Sulfate is not suitable as an alkali activator for geopolymer modification, and it will greatly reduce the durability of the modified material. Mineral dosage can significantly improve the early strength of the composites.
- (3)
- High-temperature curing can accelerate the hydration reaction process and improve the reaction efficiency. Immersion curing can promote the growth of compressive strength of modified specimens in the early stage, while for long-age specimens immersion curing will weaken their compressive strength.
- (4)
- The compressive strength of the samples was significantly enhanced after mixing with slag, fly ash, and silica fume. The XRD and SEM-EDS test results showed that the alkali-activated products were mainly flocculent or honeycomb geopolymer gels. The geopolymer gels were mainly amorphous hydrated calcium silicate (C-S-H) gels and hydrated calcium aluminate (C-A-S-H) gels, which then reacted with carbon dioxide to produce calcium carbonate, and the crystalline phase of the calcium carbonate in the composites was determined by XRD.
4. Materials and Methods
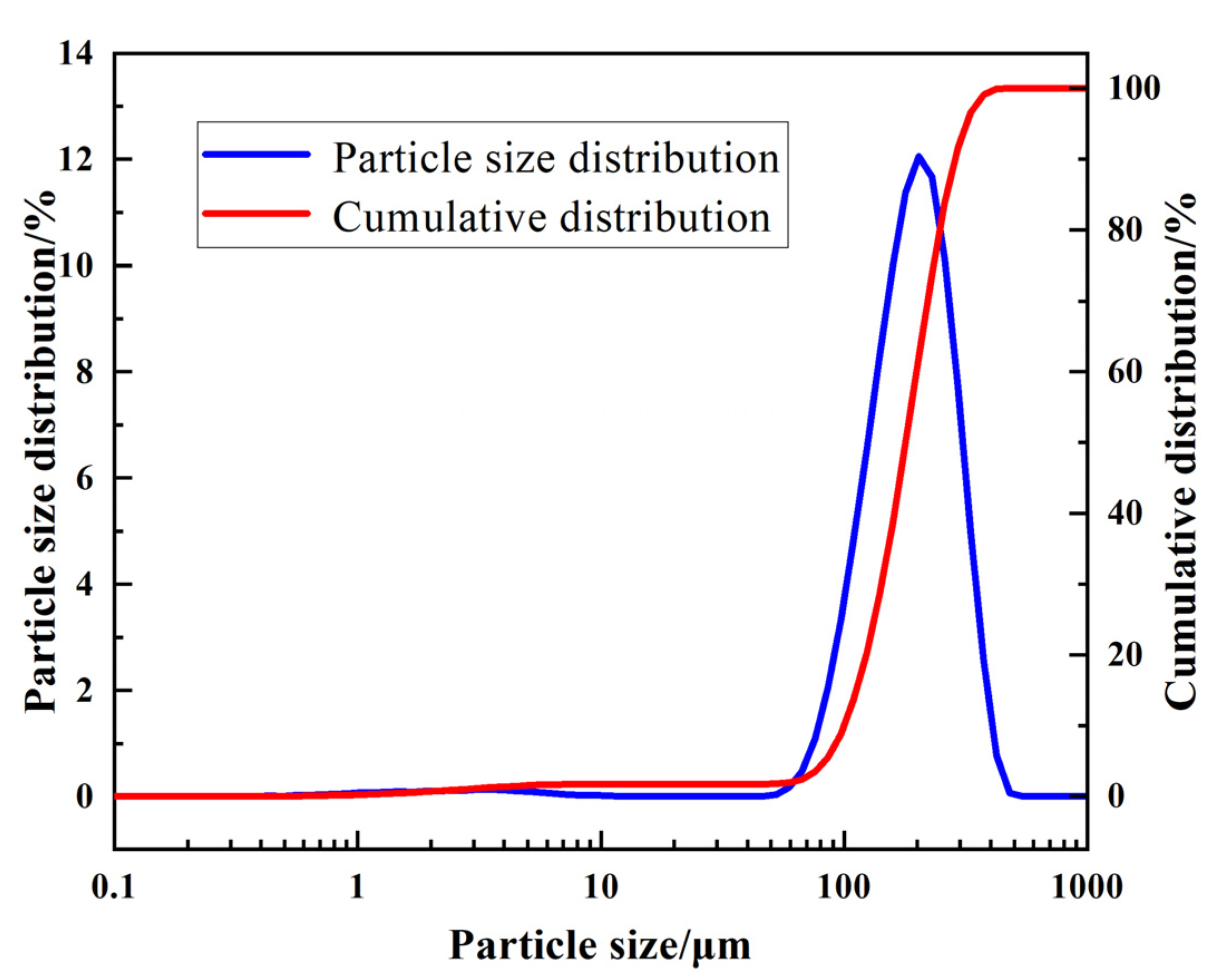
4.1. Raw Material
4.2. Admixture
4.3. Casting, Curing and Testing
4.4. Mix Proportion Design
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedraza, J.; Zimmermann, A.; Tobon, J.; Schomäcker, R.; Rojas, N. On the road to net zero-emission cement: Integrated assessment of mineral carbonation of cement kiln dust. Chem. Eng. J. 2021, 408, 127346. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Z.C.; Ma, L.; Liu, C.; Chen, Y.N.; Wang, Z.B.; Jia, Z.J.; Wang, X.G.; Jia, L.T.; Chen, C.; et al. Research progress and application of 3D printing concrete. Bull. Chin. Ceram. Soc. 2021, 40, 1769–1795. (In Chinese) [Google Scholar]
- Mao, Y.; Biasetto, L.; Colombo, P. Metakaolin-based geopolymer coatings on metals by airbrush spray deposition. J. Coat. Technol. Res. 2020, 17, 991–1002. [Google Scholar] [CrossRef]
- Ranjbar, N.; Zhang, M. Fiber-reinforced geopolymer composites: A review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Application, 4th ed.; Institut Geopolymere: Saint-Quentin, France, 2015. [Google Scholar]
- Łach, M.; Korniejenko, K.; Mikuła, J. Thermal Insulation and Thermally Resistant Materials Made of Geopolymer Foams. Procedia Eng. 2016, 151, 410–416. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.-Y.; Li, L.-Y.; Jaya, N.A.; Abdullah, M.M.A.B.; Tan, S.J.; Hussin, K. Formation ofone-part-mixing geopolymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilize toxic metals: Part I. Theory and Applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Ghosh, S.; Mohanty, S.; Akcil, A.; Sukla, L.B.; Das, A. A greener approach for resource recycling: Manganese bioleaching. Chemosphere 2016, 154, 628–639. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer, Green chemistry and sustainable development solutions. In Proceedings of the World Congress Geopolymer 2005, Saint-Quentin, France, 29 June–1 July 2005; Geopolymer Institute: Saint-Quentin, France, 2005. [Google Scholar]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energ. Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of elevated temperatures on mechanical properties of lightweight geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- Arenas, C.; Luna-Galiano, Y.; Leiva, C.; Vilches, L.F.; Arroyo, F.; Villegas, R.; Fernández-Pereira, C. Development of a fly ash-based geopolymeric concrete with construction and demolition wastes as aggregates in acoustic barriers. Constr. Build. Mater. 2017, 134, 433–442. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement a Review; Geopolymer Institute: Saint-Quentin, France, 2013; pp. 1–11. [Google Scholar]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Sanni, S.H.; Khadiranaikar, R.B. Performance of geopolymer concrete under severe environmental conditions. Int. J. Civ. Eng. Struct. Eng. 2012, 3, 396–407. [Google Scholar]
- Reddy, D.V.; Edouard, J.B.; Sobhan, K. Durability of Fly Ash–Based Geopolymer Structural Concrete in the Marine Environment. J. Mater. Civ. Eng. 2012, 25, 781–787. [Google Scholar] [CrossRef]
- Sharif, H.H. Fresh and Mechanical Characteristics of Eco-efficient Geopolymer Concrete Incorporating Nano-silica: An Overview. Kurd. J. App. Res. 2021, 6, 64–74. [Google Scholar] [CrossRef]
- Solouki, A.; Viscomi, G.; Lamperti, R.; Tataranni, P. Quarry Waste as Precursors in Geopolymers for Civil Engineering Applications: A Decade in Review. Materials 2020, 13, 3146. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, C.; Liu, F.; Fan, F. Feasibility Study of Loess Stabilization with Fly Ash–Based Geopolymer. J. Mater. Civ. Eng. 2016, 28, 04016003. [Google Scholar] [CrossRef]
- Simoes, D.; Pereira, D.T.; Jose, F.; Beatriz, A.; Porto, R.; Scarpini, V.; Clay, A.; Da, F.; Garcia, C.; Neves, S. Comparative analysis between properties and microstructures of geopolymeric concrete and Portland concrete. Integr. Med. Res. 2018, 7, 606–611. [Google Scholar]
- Wang, X.L.; Ma, X.; Mei, R.F.; Yang, L.Y.; Li, C.Q.; Sun, Z.M. Research progress on the technologies for sediment utilization. Ind. Miner. Processing. 2021, 50, 36–44. (In Chinese) [Google Scholar]
- Ma, Y.X. Research on the countermeasures of sediment reduction and siltation control in the upper reaches of the Yellow River. Gansu Sci. Technol. 2019, 35, 56–57+27. (In Chinese) [Google Scholar]
- Yue, Y.S.; Wang, Z.L.; Xie, Z.G. Industrialization policies for utilization of sediment resources of Yellow River. China Water Resour. 2016, 49–50. (In Chinese) [Google Scholar]
- Yao, S.M.; Wang, J.; Guo, C. Utilization and Management of Sediment Resources in the Yangtze River Basin under the New Situation. Technol. Econ. Chang. 2020, 4, 21–28. (In Chinese) [Google Scholar]
- Liu, Q.; Xu, Y. Status of Sewage Sludge Treatment and Utilization Research. Guizhou Chem. Ind. 2010, 35, 42–44. (In Chinese) [Google Scholar]
- Hui, Z.L.; Liu, W.K. Research on the characteristics of sediment distribution in the Yellow River and its rational management. Technol. Inno. App. 2018, 2018, 37–39. (In Chinese) [Google Scholar]
- Hao, H.S.; Xu, L.H.; Zhang, X.M.; Zhang, Z.S.; Zhai, W.; Xie, Z.P. Progress and Prospect for Transformation Utilization of Sediment. Mater. Rep. 2010, 24, 125–128. (In Chinese) [Google Scholar]
- Hu, L.J.; Cao, Y.J.; Chen, Z. Research Progress on Sediment of Dredged River in Utilization of Resources. Guangzhou Chem. Indu. 2019, 47, 36–38. (In Chinese) [Google Scholar]
- Shi, Y.C.; Ye, H.; Hou, H.B.; Bi, Z.W. The Internal Cause of the Erosion in “Pisha” Sandstone Area, Southern Inner Mongolia. Acta Geosci. Sin. 2004, 25, 659–664. (In Chinese) [Google Scholar]
- Li, G.N.; Wang, B.M.; Liu, H.; Song, W.Z.; Han, J.N. Mechanical Property and Microstructure of Alkali-activated Yellow River Sediment-Coal Slime Ash Composites. J. Wuhan Univ. Technol. 2017, 32, 1080–1086. (In Chinese) [Google Scholar] [CrossRef]
- Li, G.N.; Wang, B.M.; Liu, H. Properties of Alkali-activated Yellow River Sediment-slag Composite Material. J. Wuhan Univ. Technol. 2019, 34, 114–121. (In Chinese) [Google Scholar] [CrossRef]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Mechanical properties and microstructure of alkali activated Pisha sandstone geopolymer composites. Constr. Build. Mater. 2014, 68, 233–239. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, L.J.; Zhang, T.T.; Dong, J.L. Development of building material utilizing a low pozzolanic activity mineral. Constr. Build. Mater. 2016, 121, 300–309. [Google Scholar] [CrossRef]
- Wang, B.M.; Wang, W.L.; Liang, X.X.; Liu, H.; Han, J.N.; Zhao, L.; Yang, X.X.; Yan, J.F. Development of Cementitious Materials Utilizing Alkali-activated Yellow River Silt. J. Wuhan Univ. Technol. 2021, 36, 364–373. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, L. Research on the consolidation and cementation technology of flood control stone by using the sediment of the Yellow River. Master’s Thesis, Dalian University of Technology, Dalian, China, 2016. (In Chinese). [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, X.; Long, F. Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Bakhareva, T.; Sanjayana, J.G.; Cheng, Y.B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Palomoa, A.; Grutzeckb, M.W.; Blancoa, M.T. Alkali-activated fly ashs a cement for future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Lee, Y.; Bang, J.J.; Kang, S. Study on a Nano-Microstructure and Properties of Geopolymer by Recycling Integrated Gasification Combined Cycle Coal Ash Slag. J. Nanosci. Nanotechnol. 2019, 19, 2193–2197. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yu, X.; Zhang, H.J.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry, 2nd ed; Thomas Telford: London, UK, 1997; pp. 114–115. [Google Scholar]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Naggar, K.A.M.; Tarek, D.; Ragab, A.; Sameh, H.; Zeyad, A.M.; Tayeh, B.A.; Maafa, I.M.; Yousef, A. Fabrication of thermal insulation geopolymer bricks using ferrosilicon slag and alumina waste. Case Stud. Constr. Mater. 2021, 15, e00737. [Google Scholar] [CrossRef]
- Sun, H.P. Value Analysis of Mixing Mineral Powder and Fly Ash in Concrete. China Plant Eng. 2022, 2022, 252–253. (In Chinese) [Google Scholar]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e007733. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Barnes, P.; Bensted, J. Structure and Performance of Cements; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Zhao, X.X. Experimental study on the properties of silica fume and its influence on the properties of porous cement stabilized crushed stone. Master’s Thesis, Hefei University of Technology, Hefei, China, 2021. (In Chinese). [Google Scholar]

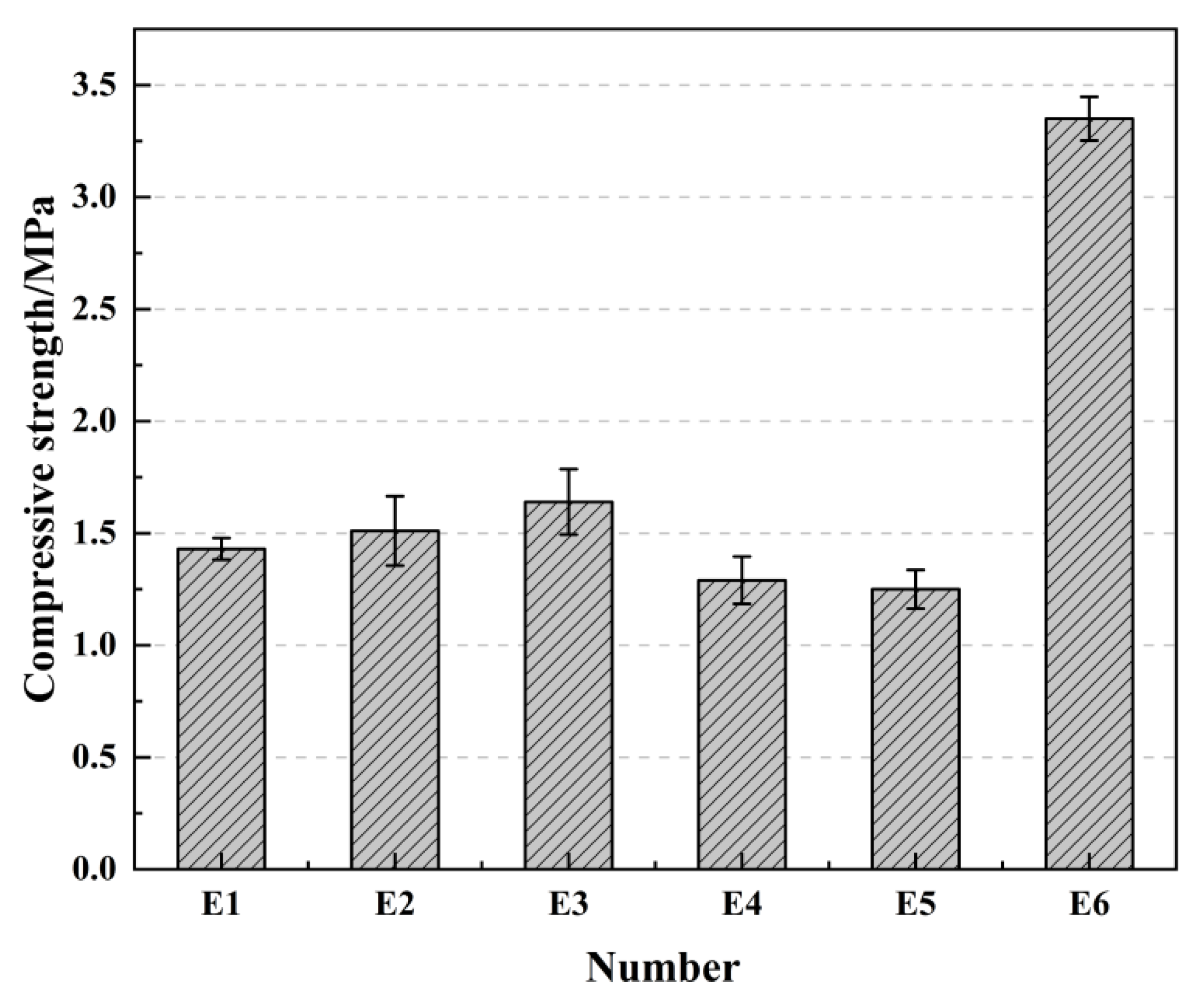




| Number | E1 | E2 | E3 | E4 | E5 | E6 |
|---|---|---|---|---|---|---|
| Curing conditions | Natural curing 28 days | Standard curing 28 days | Immersion 7 days after natural maintenance 28 days | Immersion curing 28 days | 90 °C high temperature curing 12 h | Natural curing for 28 days after 90 °C high temperature curing |
| Element | Sample A | Sample B | Sample C | |||
|---|---|---|---|---|---|---|
| Weight Percentage/wt.% | Atomic Percentage/at% | Weight Percentage/wt.% | Atomic Percentage/at% | Weight Percentage/wt.% | Atomic Percentage/at% | |
| C | 5.54 | 9.75 | 6.73 | 11.22 | 2.7 | 5.33 |
| O | 41.39 | 54.63 | 47.18 | 58.99 | 23.9 | 35.31 |
| Na | 2.1 | 1.93 | 5.54 | 4.82 | 2.26 | 2.32 |
| Al | 7.46 | 5.84 | 5.25 | 3.89 | 9.15 | 9.13 |
| Si | 20.96 | 15.81 | 15.02 | 10.73 | 47.23 | 40.13 |
| Ca | 19.27 | 10.17 | 17.75 | 8.88 | 11.9 | 7.03 |
| Fe | 2.03 | 0.77 | 1.39 | 0.52 | 1.32 | 0.6 |
| Mg | 1.25 | 1.1 | 1.14 | 0.95 | 1.54 | 0.15 |
| Total | 100 | 100 | 100 | |||
| Sample | Content of Each Component in the Experimental Material (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | K2O | Na2O | Fe2O3 | CaO | MgO | TiO2 | SO3 | LOI | |
| Xiaoluan River sediment | 79.05 | 9.22 | 5.33 | 1.90 | 1.79 | 1.29 | 0.45 | 0.43 | 0.08 | 0.54 |
| Sample | Content of Each Component in the Experimental Material (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | K2O | Na2O | Fe2O3 | CaO | MgO | TiO2 | SO3 | LOI | |
| Slag | 27.83 | 12.98 | 0.35 | 0.35 | 0.39 | 46.21 | 7.52 | 1.64 | 1.82 | 0.91 |
| Fly ash | 43.61 | 20.14 | 1.95 | 0.50 | 4.91 | 21.28 | 3.91 | 1.65 | 1.16 | 2.33 |
| Silica fume | 96.87 | 0.86 | — | — | 0.11 | 0.41 | 0.40 | — | — | 1.35 |
| Number | S/% | W/% | Sodium Silicate Solution/% | SiO2/Na2O | Q/% | Na2SO4/% | Mineral Admixture |
|---|---|---|---|---|---|---|---|
| A1 | 82 | 5 | 7 | 3.0 | 6 | 0 | 0 |
| A2 | 82 | 5 | 7 | 2.5 | 6 | 0 | 0 |
| A3 | 82 | 5 | 7 | 2.0 | 6 | 0 | 0 |
| A4 | 82 | 5 | 7 | 1.5 | 6 | 0 | 0 |
| B1 | 84 | 5 | 7 | 2.0 | 4 | 0 | 0 |
| B2 | 82 | 5 | 7 | 2.0 | 6 | 0 | 0 |
| B3 | 80 | 5 | 7 | 2.0 | 8 | 0 | 0 |
| B4 | 78 | 5 | 7 | 2.0 | 10 | 0 | 0 |
| C1 | 81 | 5 | 7 | 2.0 | 6 | 1 | 0 |
| C2 | 79 | 5 | 7 | 2.0 | 6 | 3 | 0 |
| C3 | 77 | 5 | 7 | 2.0 | 6 | 5 | 0 |
| C4 | 75 | 5 | 7 | 2.0 | 6 | 7 | 0 |
| D1 | 72 | 5 | 7 | 2.0 | 6 | 0 | Slag 10% |
| D2 | 72 | 5 | 7 | 2.0 | 6 | 0 | Fly ash10% |
| D3 | 72 | 5 | 7 | 2.0 | 6 | 0 | Silica fume10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chai, X.; Liu, H.; Cheng, H.; Jia, D.; Di, L.; Qin, S.; Jin, Y. Research on the Mechanical Properties and Microstructure of Modified Silt Sediment Geopolymer Materials. Gels 2022, 8, 792. https://doi.org/10.3390/gels8120792
Li C, Chai X, Liu H, Cheng H, Jia D, Di L, Qin S, Jin Y. Research on the Mechanical Properties and Microstructure of Modified Silt Sediment Geopolymer Materials. Gels. 2022; 8(12):792. https://doi.org/10.3390/gels8120792
Chicago/Turabian StyleLi, Changming, Xiaoxiong Chai, Hui Liu, Haifeng Cheng, Dongyang Jia, Longfei Di, Songlin Qin, and Yongbao Jin. 2022. "Research on the Mechanical Properties and Microstructure of Modified Silt Sediment Geopolymer Materials" Gels 8, no. 12: 792. https://doi.org/10.3390/gels8120792
APA StyleLi, C., Chai, X., Liu, H., Cheng, H., Jia, D., Di, L., Qin, S., & Jin, Y. (2022). Research on the Mechanical Properties and Microstructure of Modified Silt Sediment Geopolymer Materials. Gels, 8(12), 792. https://doi.org/10.3390/gels8120792





