Comparative Study of Donepezil-Loaded Formulations for the Treatment of Alzheimer’s Disease by Nasal Administration
Abstract
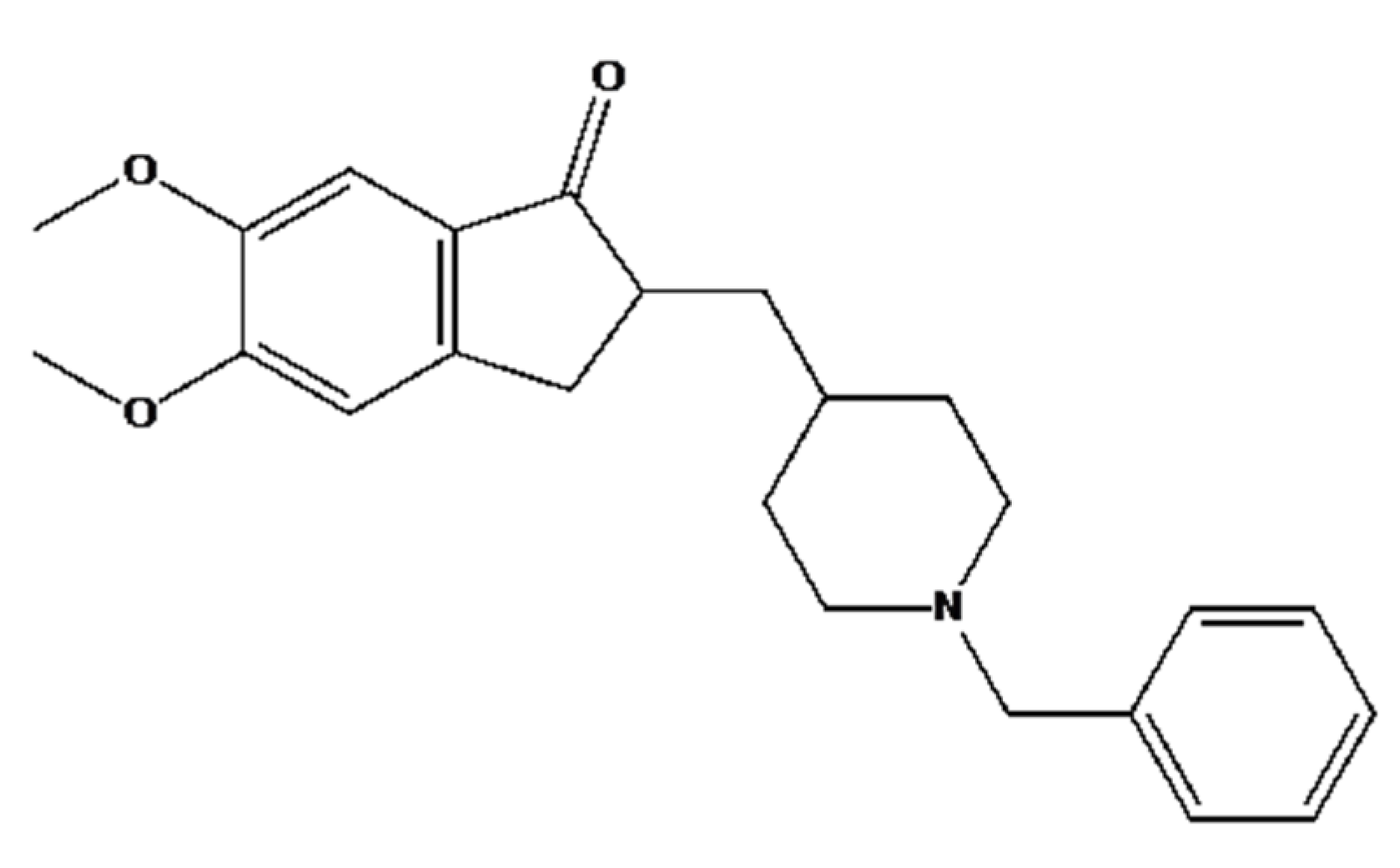
1. Introduction
2. Results and Discussion
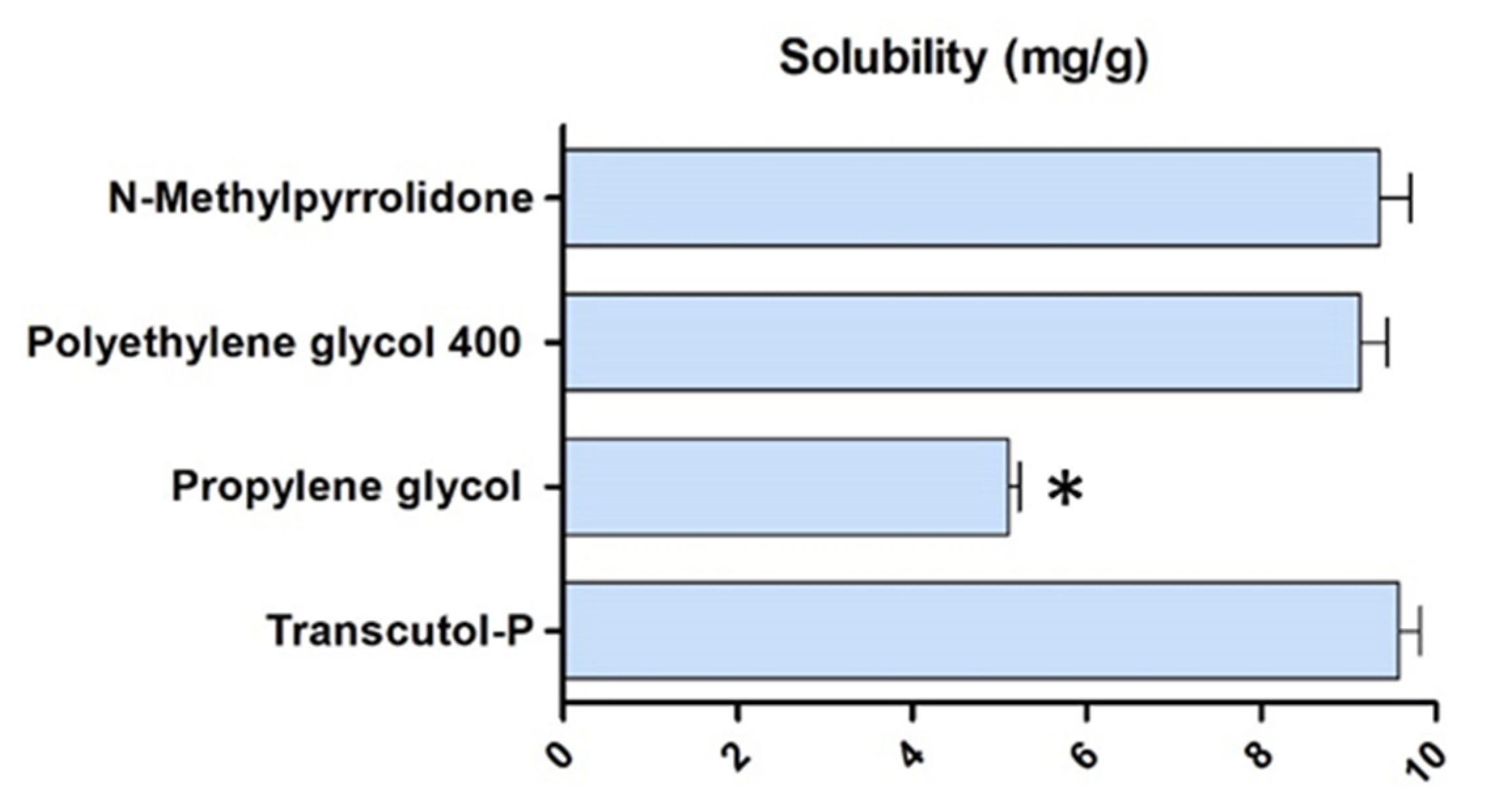
2.1. Solubility and Preparation of DPZ Gels
2.2. Physicochemical Characterization of DPZ-CGel, DPZ-PGel1 and DPZ-PGel2
2.3. Stability Studies
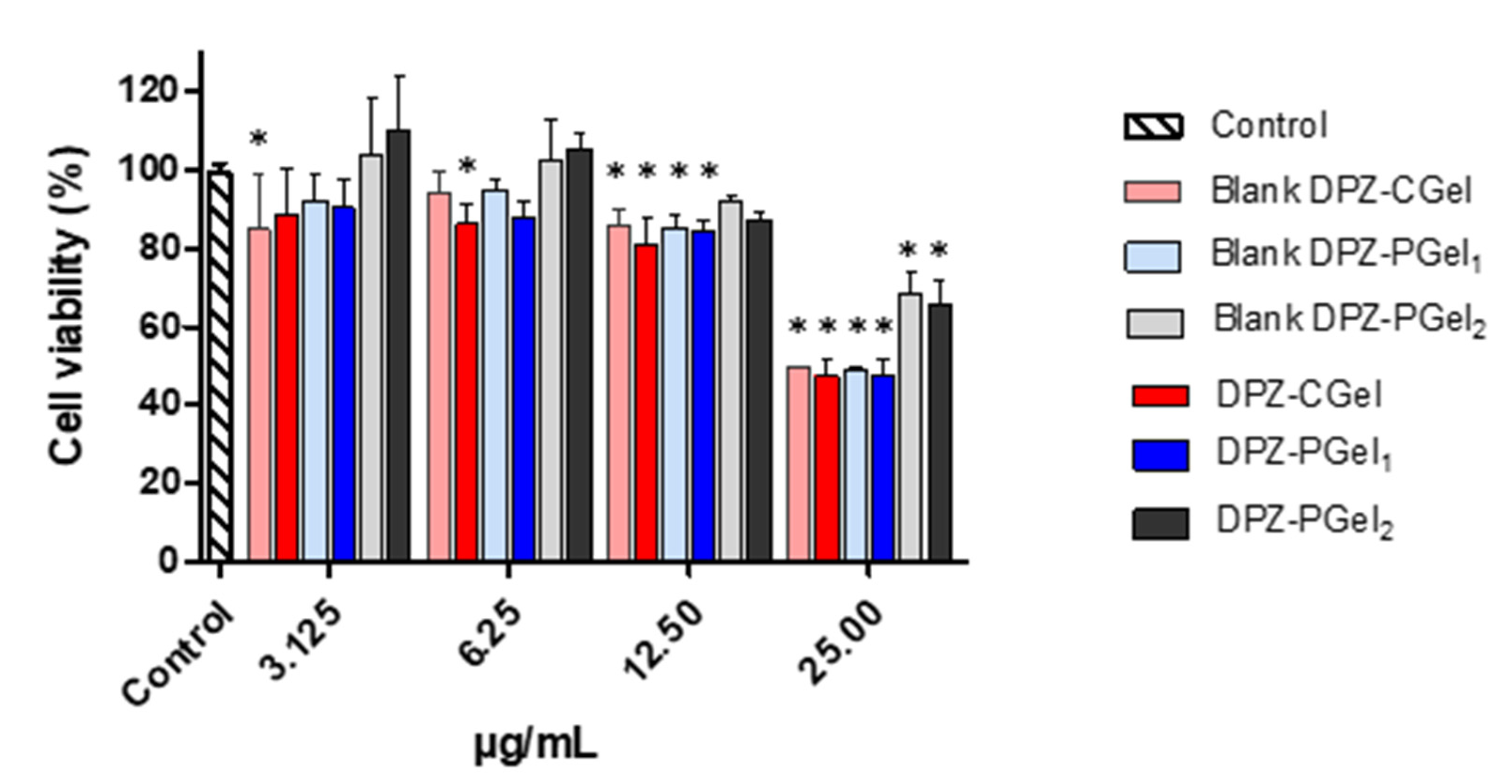
2.4. Cytotoxicity Results
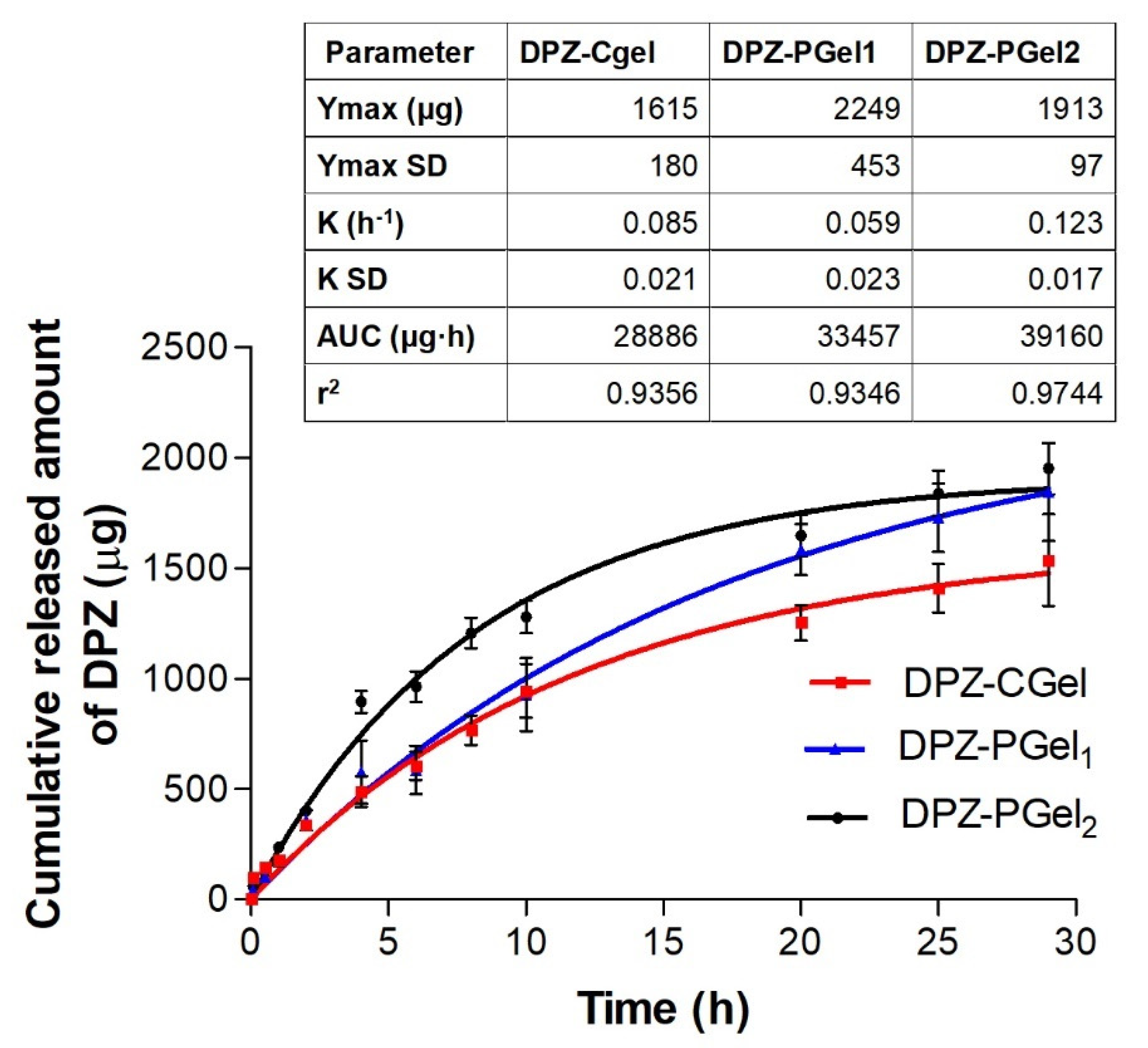
2.5. In Vitro Release Studies
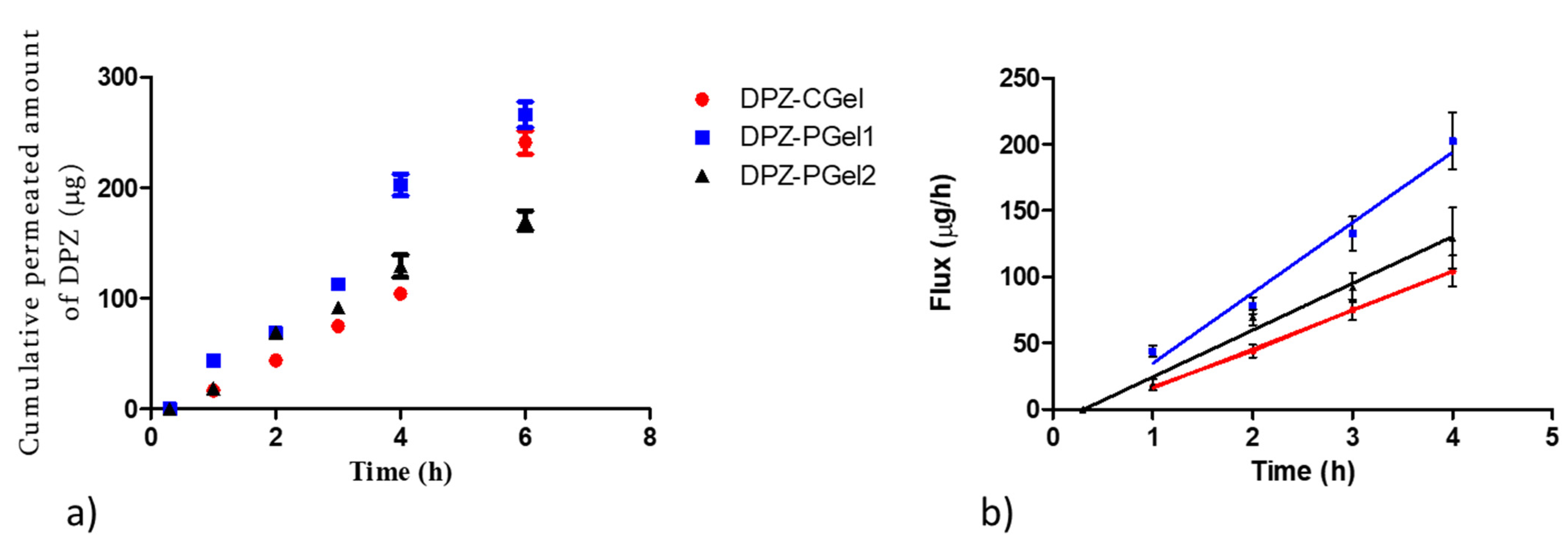
2.6. Ex vivo Permeation Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Solubility Studies
4.3. Preparation of Formulations
4.4. Physicochemical Characterization of DPZ-CGel, DPZ-PGel1 and DPZ-PGel2
4.4.1. pH Determination
4.4.2. Morphological Analysis
4.4.3. Gelation Temperature
4.4.4. Viscosity and Rheological Behavior
4.4.5. Swelling Test
4.5. Stability Assays
4.6. Cytotoxicity Assay
4.7. In Vitro Drug Release Study
4.8. Permeation Studies through Ex Vivo Nasal Mucosa
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, C.; Ceña, V. The Delivery Challenge in Neurodegenerative Disorders: The Nanoparticles Role in Alzheimer’s Disease Therapeutics and Diagnostics. Pharmaceutics 2018, 10, 190. [Google Scholar] [CrossRef]
- Cavanaugh, S.E.; Pippin, J.J.; Barnard, N.D. Animal Models of Alzheimer Disease: Historical Pitfalls and A Path Forward. Altex 2014, 31, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Madav, Y.; Wairkar, S.; Prabhakar, B. Recent Therapeutic Strategies Targeting Beta Amyloid and Tauopathies in Alzheimer’s Disease. Brain Res. Bull. 2019, 146, 171–184. [Google Scholar] [CrossRef]
- Li, Q.; He, S.; Chen, Y.; Feng, F.; Qu, W.; Sun, H. Donepezil-Based Multi-Functional Cholinesterase Inhibitors for Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2018, 158, 463–477. [Google Scholar] [CrossRef]
- Jacobson, S.A.; Sabbagh, M.N. Donepezil: Potential Neuroprotective and Disease-Modifying Effects. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1363–1369. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems In Nose-to-Brain Drug Delivery: A Review of Non-Clinical Brain Targeting Studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Md, S.; Bhattmisra, S.K.; Zeeshan, F.; Shahzad, N.; Mujtaba, M.A.; Srikanth Meka, V.; Radhakrishnan, A.; Kesharwani, P.; Baboota, S.; Ali, J. Nano-Carrier Enabled Drug Delivery Systems for Nose to Brain Targeting for the Treatment of Neurodegenerative Disorders. J. Drug Deliv. Sci. Technol. 2018, 43, 295–310. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Espinoza, L.C.; Halbaut, L.; Espina, M.; García, M.L.; Calpena, A.C. Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers 2018, 10, 316. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in Nasal Delivery Systems for Therapeutic Drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Vacacela, M.; Clares, B.; Garcia, M.L.; Fabrega, M.J.; Calpena, A.C. Development of a Nasal Donepezil-Loaded Microemulsion for Treatment of Alzheimer’s Disease: In Vitro and Ex Vivo Characterization. Cns Neurol. Disord.-Drug Targets 2018, 17, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A Review of the Nonclinical Safety of Transcutol®, a Highly Purified Form of Diethylene Glycol Monoethyl Ether (Degee) Used as a Pharmaceutical Excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Roche-Molina, M.; Hardwick, B.; Sanchez-Ramos, C.; Sanz-Rosa, D.; Gewert, D.; Cruz, F.M.; Gonzalez-Guerra, A.; Andres, V.; Palma, J.A.; Ibanez, B.; et al. The Pharmaceutical Solvent N-Methyl-2-Pyrollidone (Nmp) Attenuates Inflammation through Kruppel-Like Factor 2 Activation to Reduce Atherogenesis. Sci. Rep. 2020, 10, 11636. [Google Scholar] [CrossRef]
- Ciurlizza, C.; Fernandez, F.; Calpena, A.C.; Lazaro, R.; Parra, A.; Clares, B. Semisolid Formulations Containing Cetirizine: Human Skin Permeation and Topical Antihistaminic Evaluation in a Rabbit Model. Arch. Dermatol. Res. 2014, 306, 711–717. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Vera-Garcia, R.; Silva-Abreu, M.; Domenech, O.; Badia, J.; Rodriguez-Lagunas, M.J.; Clares, B.; Calpena, A.C. Topical Pioglitazone Nanoformulation for the Treatment of Atopic Dermatitis: Design, Characterization and Efficacy in Hairless Mouse Model. Pharmaceutics 2020, 12, 255. [Google Scholar] [CrossRef]
- Warnken, Z.N.; Smyth, H.D.C.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and Device Design to Increase Nose to Brain Drug Delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. [Google Scholar] [CrossRef]
- Lenaerts, V.; Triqueneaux, C.; Quartern, M.; Rieg-Falson, F.; Couvreur, P. Temperature-Dependent Rheological Behavior of Pluronic F-127 Aqueous Solutions. Int. J. Pharm. 1987, 39, 121–127. [Google Scholar] [CrossRef]
- Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guillen, C.; Boix-Montanes, A.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C.; Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernandez-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduno-Ramirez, M.L.; Andrade, B.; Del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincon, M.; Bozal, N.; Domenech, O.; Rodriguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the Treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Vildanova, R.; Lobov, A.; Spirikhin, L.; Kolesov, S. Hydrogels on the Base of Modified Chitosan and Hyaluronic Acid Mix As Polymer Matrices for Cytostatics Delivery. Gels 2022, 8, 104. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.L.; et al. The Nasal Delivery of Nanoencapsulated Statins–an Approach for Brain Delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [PubMed]
- Wengst, A.; Reichl, S. Rpmi 2650 Epithelial Model and Three-Dimensional Reconstructed Human Nasal Mucosa as In Vitro Models for Nasal Permeation Studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodriguez-Lagunas, M.J.; Halbaut, L.; Canas, M.A.; Calpena, A.C. Formulation Strategies to Improve Nose-to-Brain Delivery of Donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef]
- Rajput, A.; Butani, S. Donepezil Hcl Liposomes: Development, Characterization, Cytotoxicity, and Pharmacokinetic Study. AAPS PharmSciTech 2022, 23, 74. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Dandagi, P.M.; Sutar, K.P. Development and Evaluation of Thermoreversible Ethosomal Gel of Donepezil Hydrochloride for Intranasal Delivery. J. Pharm. Innov. 2022. [Google Scholar] [CrossRef]
- Gangane, P.; Kawtikwar, P. Development of Donepezil Hydrochloride Loaded Gellan Gum Based Nasal Mucoadhesive Microspheres by Spray Drying Method. Indian J. Pharm. Educ. Res. 2020, 54, 935–945. [Google Scholar] [CrossRef]
- Gangane, P.S.; Ghormare, N.V.; Mahapatra, D.K.; Mahajan, N.M. Gellan Gum Assisted Fabrication and Characterization of Donepezil Hydrochloride Mucoadhesive Intranasal Microspheres. Int. J. Curr. Res. Rev. 2020, 12, 105–115. [Google Scholar] [CrossRef]
- Patil, R.; Pawara, D.; Gudewar, C.; Tekade, A. Nanostructured Cubosomes in an In Situ Nasal Gel System: An Alternative Approach for the Controlled Delivery of Donepezil Hcl to Brain. J. Liposome Res. 2019, 29, 264–273. [Google Scholar] [CrossRef] [PubMed]



| Ingredients (% w/w) | DPZ-CGel | DPZ-PGel1 | DPZ-PGel2 |
|---|---|---|---|
| DPZ | 6.25 mg/mL | 6.25 mg/mL | 6.25 mg/mL |
| Chitosan | 1 | - | - |
| Pluronic F-127 | - | 18 | 18 |
| Transcutol® P | 20 | 20 | - |
| N-Methylpyrrolidone | - | - | 5 |
| Water | 59 | 62 | 77 |
| Acid acetic solution | 20 | - | - |
| Formulas | 25 °C | 35 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Viscosity (mPa·s) at 50 s−1 | Apparent Thixotropy (Pa/s) | Rheological Behavior | Mathematical Model | Viscosity (mPa.s) at 50 s−1 | Apparent Thixotropy (Pa/s) | Rheological Behavior | Mathematical Model | |
| DPZ-CGEL | 487 ± 1.5 | 72.7 | Pseudoplastic | Cross (r = 1) | 335 ± 0.9 | 43.3 | Pseudoplastic | Cross (r = 1) |
| DPZ-PGel1 | 336 ± 3.3 | 39.5 | Pseudoplastic | Cross (r = 1) | 1345 ± 16.6 | 441.4 | Pseudoplastic | Cross (r = 1) |
| DPZ-PGel2 | 232 ± 0.9 | 15.3 | Pseudoplastic | Cross (r = 1) | 1112 ± 21.5 | 568.7 | Pseudoplastic | Cross (r = 1) |
| Parameters | DPZ-CGel (Days) | DPZ-PGel1 (Days) | DPZ-PGel2 (Days) | |||
|---|---|---|---|---|---|---|
| 1 | 30 | 1 | 30 | 1 | 30 | |
| Appearance | Homogeneous and slightly yellowish | Homogeneous and slightly yellowish | Homogeneous and translucent | Homogeneous and translucent | Homogeneous and translucent | Homogeneous and translucent |
| pH | 6.30 ± 0.09 | 6.18 ± 0.05 | 6.30 ± 0.09 | 6.22 ± 0.03 | 6.30 ± 0.09 | 6.39 ± 0.05 |
| Drug content (%) | 99.70 ± 0.06 | 99.70 ± 0.09 | 99.60 ± 0.03 | 99.30 ± 0.08 | 101.30 ± 0.05 | 99.7 ± 0.10 |
| Parameters | DPZ-CGel (Days) | DPZ-PGel1 (Days) | DPZ-PGel2 (Days) | |||
|---|---|---|---|---|---|---|
| 1 | 30 | 1 | 30 | 1 | 30 | |
| Appearance | Homogeneous and slightly yellowish | Homogeneous and slightly yellowish | Homogeneous and translucent | Homogeneous and translucent | Homogeneous and translucent | Homogeneous and translucent |
| pH | 6.30 ± 0.09 | 6.33 ± 0.03 | 6.30 ± 0.09 | 6.26 ± 0.08 | 6.30 ± 0.09 | 6.39 ± 0.07 |
| Drug content (%) | 99.70 ± 0.06 | 98.67 ± 0.05 | 99.60 ± 0.03 | 99.50 ± 0.06 | 101.30 ± 0.05 | 100.50 ± 0.15 |
| Permeation Parameters | DPZ-CGel | DPZ-PGel1 | DPZ-PGel2 |
|---|---|---|---|
| Jss (µg/(h/cm2)) | 0.83 ± 0.07 b,c | 8.76 ± 0.92 a,c | 3.54 ± 0.29 |
| Tl (h) | 0.45 ± 0.05 b,c | 0.34 ± 0.02 | 0.31 ± 0.03 |
| P2 (1/h) | 0.37 ± 0.04 b,c | 0.48 ± 0.05 | 0.54 ± 0.05 |
| P1 (cm) x 103 | 0.36 ± 0.04 b,c | 2.90 ± 0.35 a,c | 1.05 ± 0.18 |
| Kp (cm/h) x 103 | 0.13 ± 0.01 b,c | 1.40 ± 0.20 a,c | 0.57 ± 0.07 |
| Css (µg/mL) | 0.01 ± 0.00 b,c | 0.13 ± 0.02 a,c | 0.05 ± 0.00 |
| Qret (µg drug/g tissue/cm2) | 546.96 ± 53.81 b,c | 620.23 ± 59.34 | 518.74 ± 57.21 |
| Model | Equation |
|---|---|
| Newton | |
| Bingham | |
| Ostwald-de-Waele | |
| Herschel-Bulkley | |
| Casson | |
| Cross |
| Kinetic Model | Equation |
|---|---|
| Zero Order | |
| Higuchi | |
| First Order | |
| Korsmeyer-Peppas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, L.C.; Guaya, D.; Calpena, A.C.; Perotti, R.M.; Halbaut, L.; Sosa, L.; Brito-Llera, A.; Mallandrich, M. Comparative Study of Donepezil-Loaded Formulations for the Treatment of Alzheimer’s Disease by Nasal Administration. Gels 2022, 8, 715. https://doi.org/10.3390/gels8110715
Espinoza LC, Guaya D, Calpena AC, Perotti RM, Halbaut L, Sosa L, Brito-Llera A, Mallandrich M. Comparative Study of Donepezil-Loaded Formulations for the Treatment of Alzheimer’s Disease by Nasal Administration. Gels. 2022; 8(11):715. https://doi.org/10.3390/gels8110715
Chicago/Turabian StyleEspinoza, Lupe Carolina, Diana Guaya, Ana Cristina Calpena, Rodolfo Miguel Perotti, Lyda Halbaut, Lilian Sosa, Adriel Brito-Llera, and Mireia Mallandrich. 2022. "Comparative Study of Donepezil-Loaded Formulations for the Treatment of Alzheimer’s Disease by Nasal Administration" Gels 8, no. 11: 715. https://doi.org/10.3390/gels8110715
APA StyleEspinoza, L. C., Guaya, D., Calpena, A. C., Perotti, R. M., Halbaut, L., Sosa, L., Brito-Llera, A., & Mallandrich, M. (2022). Comparative Study of Donepezil-Loaded Formulations for the Treatment of Alzheimer’s Disease by Nasal Administration. Gels, 8(11), 715. https://doi.org/10.3390/gels8110715