Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids
Abstract
:1. Introduction
2. Results and Discussion
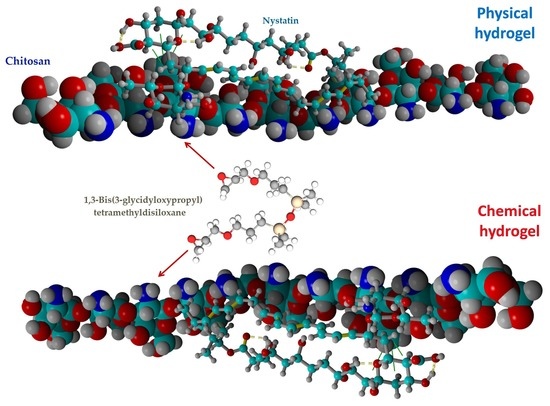
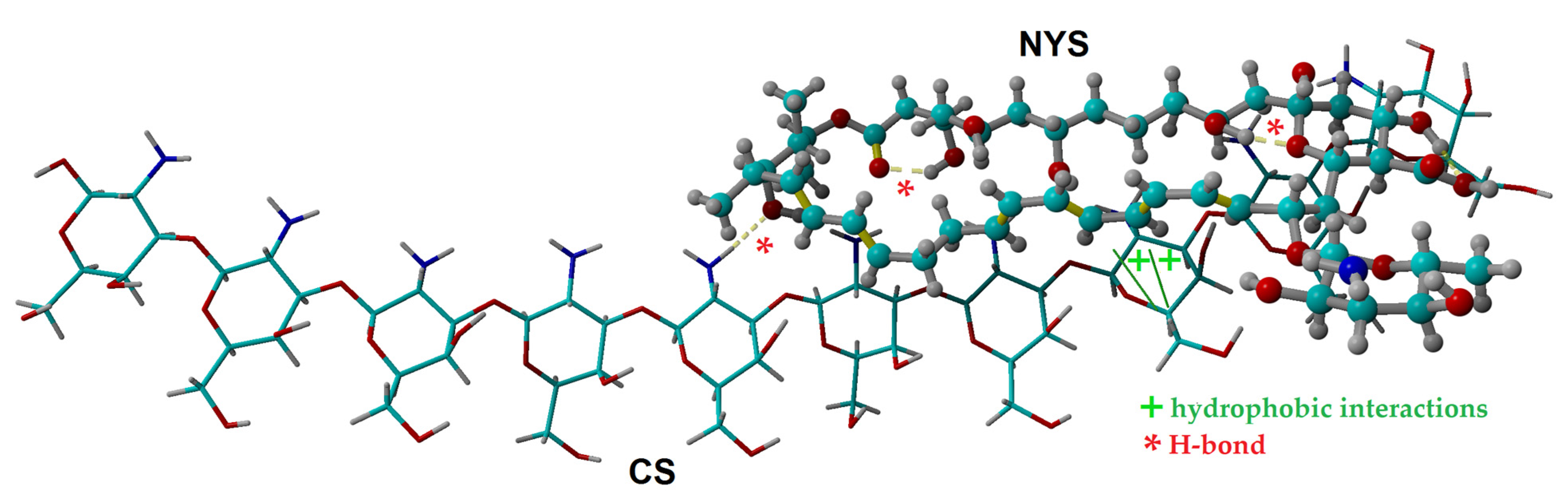
2.1. Molecular Docking Simulation of Physical Interactions between Chitosan and Nystatin
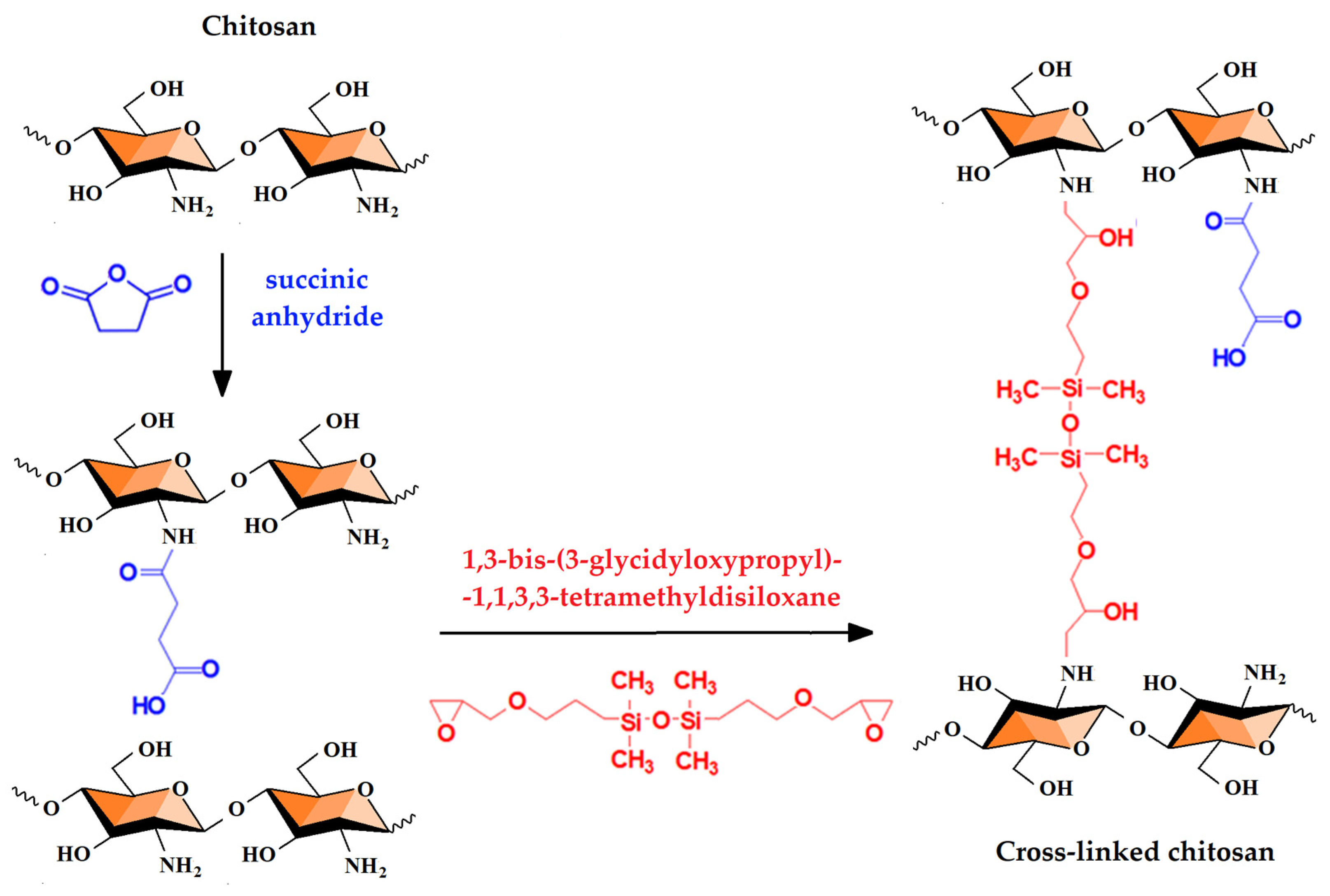
2.2. Chemical and Physical Hydrogels by Chitosan Modification
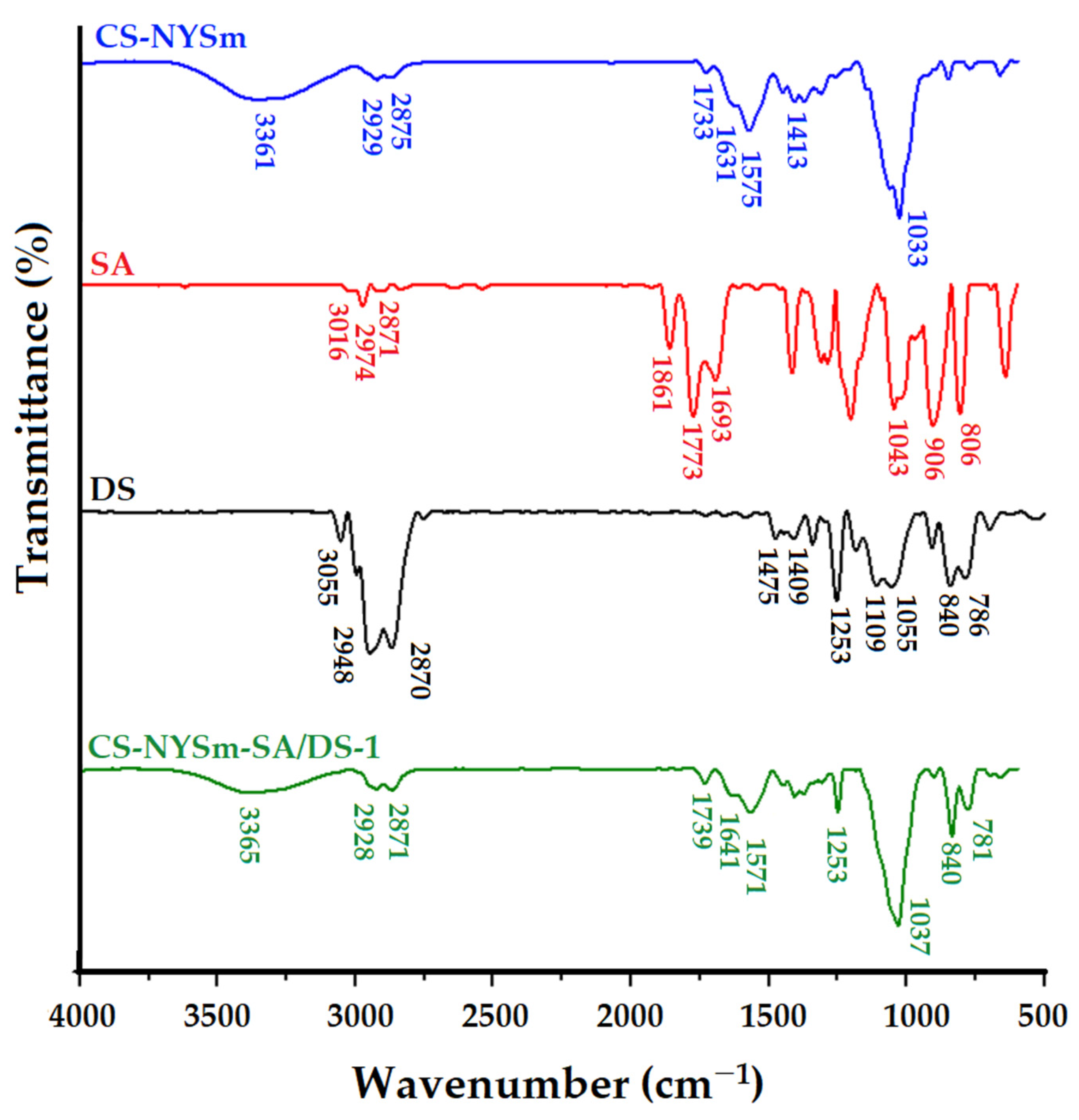
2.3. Structural Characterization by FTIR Spectroscopy
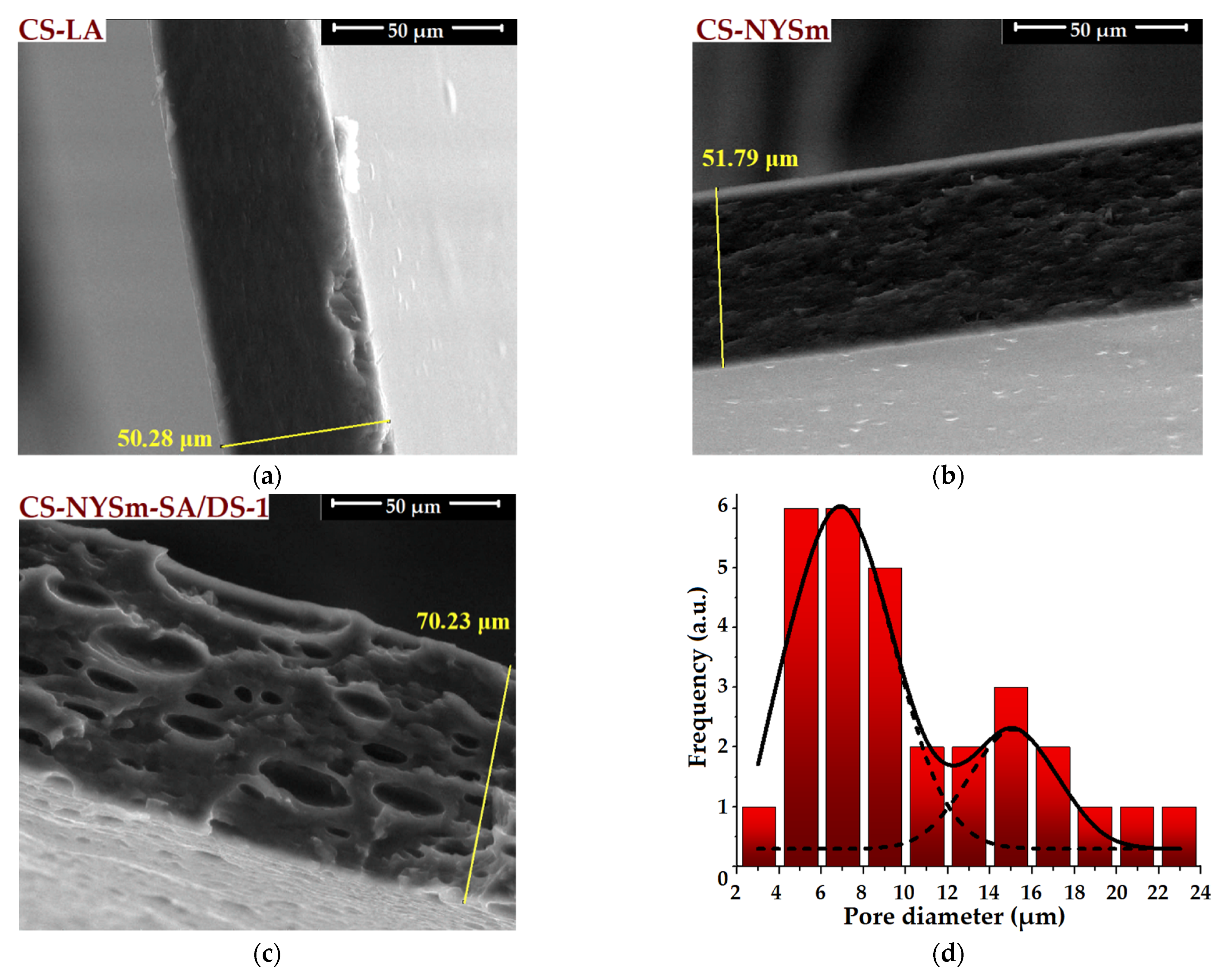
2.4. Morphological Evaluation of CS Films by SEM and EDX
2.5. Mechanical Properties of Dried Hy Drogels
2.6. Swelling Behavior of NYSm-Loaded Dried Hydrogels
2.7. Micronized Nystatin In Vitro Release from the Antifungal Film Formulations
2.8. Antifungal Activity of the Hydrogels against Candida spp.
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chemically and/or Physically Modified Chitosan Antifungal Formulation
4.3. Methods of Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinawati, W.; Kumalawati, J.; Bardosono, S.; Immanuel, S.; Sukartini, N.; Indrasari, N.D. Invasive candidiasis among high prevalence neurological patients. J. Infect. Dev. Ctries. 2022, 16, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef] [PubMed]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Singleton, A.L.; Jackson, B.R.; Molinari, N.A.M. Survey of incidence, lifetime prevalence, and treatment of self-reported vulvovaginal candidiasis, United States, 2020. BMC Women’s Health 2022, 22, 147. [Google Scholar] [CrossRef]
- Mareković, I.; Pleško, S.; Rezo Vranješ, V.; Herljević, Z.; Kuliš, T.; Jandrlić, M. Epidemiology of Candidemia: Three-Year Results from a Croatian Tertiary Care Hospital. J. Fungi 2021, 7, 267. [Google Scholar] [CrossRef]
- Suhail, A.; Wadha, A. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms 2021, 9, 807. [Google Scholar] [CrossRef]
- Rundjan, L.; Wahyuningsih, R.; Oeswadi, C.A.; Marsogi, M.; Purnamasari, A. Oral nystatin prophylaxis to prevent systemic fungal infection in very low birth weight preterm infants: A randomized controlled trial. BMC Pediatr. 2020, 20, 170. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, H.-J.; Han, C.-Y.; Nah, H.-J.; Choi, S.-S.; Kim, E.-S. Stimulated Biosynthesis of an C10-Deoxy Heptaene NPP B2 via Regulatory Genes Overexpression in Pseudonocardia autotrophica. Front. Microbiol. 2020, 11, 19. [Google Scholar] [CrossRef]
- La Clair, J.J. Accessing Nystatin through Mariculture. Molecules 2021, 26, 7649. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Antibiotice, S.A. Antibiotice Annual Report 2019. Available online: https://www.antibiotice.ro (accessed on 6 July 2020).
- Skopinska-Wisniewska, J.; De la Flor, S.; Kozlowska, J. From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances. Int. J. Mol. Sci. 2021, 22, 7402. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Menghani, S.S.; Kerzare, D.R.; Khedekar, P.B.; Bharne, A.P.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Sreeharsha, N.; Asdaq, S.M.B. Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study. Pharmaceutics 2022, 14, 1393. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual cross-linked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Demirci, T.; Hasköylü, M.E.; Eroğlu, M.S.; Hemberger, J.; Öner, E.T. Levan-based hydrogels for controlled release of Amphotericin B for dermal local antifungal therapy of Candidiasis. Eur. J. Pharm. Sci. 2020, 145, 105255. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides: Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Gegel, N.O.; Shipovskaya, A.B.; Khaptsev, Z.Y.; Radionov, R.V.; Belyaeva, A.A.; Kharlamov, V.N. Thermosensitive ChitosanContaining Hydrogels: Their Formation, Properties, Antibacterial Activity, and Veterinary Usage. Gels 2022, 8, 93. [Google Scholar] [CrossRef]
- Oliveira Lima, K.; Barreto Pinilla, C.M.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model. Polymers 2021, 13, 3331. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Condrò, G.; Perugini, P. Evaluation of the Mucoadhesive Properties of Chitosan-Based Microstructured Lipid Carrier (CH-MLC). Pharmaceutics 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Humelnicu, A.-C.; Samoila, P.; Cojocaru, C.; Dumitriu, R.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V.; Simionescu, B.C. Chitosan-Based Therapeutic Systems for Superficial Candidiasis Treatment. Synergetic Activity of Nystatin and Propolis. Polymers 2022, 14, 689. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—A self-parameterizing force field. Proteins 2002, 47, 393–402. [Google Scholar] [CrossRef]
- Perchyonok, V.T.; Zhang, S.; Basson, N.; Grobler, S.; Oberholzer, T.; Massey, W. Insights into chitosan based gels as functional restorative biomaterials prototypes: In vitro approach. Open J. Stomatol. 2013, 3, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T. Glycerol-plasticized chitosan film for the preservation of orange. J. Food Saf. 2021, 42, E12943. [Google Scholar] [CrossRef]
- Vildanova, R.; Lobov, A.; Spirikhin, L.; Kolesov, S. Hydrogels on the Base of Modified Chitosan and Hyaluronic Acid Mix as Polymer Matrices for Cytostatics Delivery. Gels 2022, 8, 104. [Google Scholar] [CrossRef]
- Gupta, S.; Tyagi, R.; Parmar, V.S.; Sharma, S.K.; Haag, R. Polyether based amphiphiles for delivery of active components. Polymer 2012, 53, 3053–3078. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Ştefănoiu, F.; Candy, L.; Vaca-Garcia, C.; Borredon, M.-E. Kinetics and mechanism of the reaction between maleic anhydride and fatty acid esters and the structure of the products. Eur. J. Lipid Sci. Technol. 2008, 110, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Enescu, D.; Hamciuc, V.; Pricop, L.; Hamaide, T.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane-modified chitosan I. Synthesis and structural characterisation of graft and cross-linked copolymers. J. Polym. Res. 2008, 16, 73–80. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Physico-chemical characterization of pH-sensitive N-Succinyl chitosan-g-poly (acrylamide-co-acrylic acid) hydrogels and in vitro drug release studies. Polym. Degrad. Stab. 2017, 139, 38–54. [Google Scholar] [CrossRef]
- Tang, F.; Lv, L.; Lu, F.; Rong, B.; Li, Z.; Lu, B.; Yu, K.; Liu, J.; Dai, F.; Wu, D.; et al. Preparation and characterization of N -chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef]
- Llanos, J.H.R.; de Oliveira Vercik, L.C.; Vercik, A. Physical Properties of Chitosan Films Obtained after Neutralization of Polycation by Slow Drip Method. J. Biomater. Nanobiotechnol. 2015, 6, 276–291. [Google Scholar] [CrossRef] [Green Version]
- Drebezghova, V.; Gojzewski, H.; Allal, A.; Hempenius, M.A.; Nardin, C.; Vancso, G.J. Network Mesh Nanostructures in Cross-Linked Poly(Dimethylsiloxane) Visualized by AFM. Macromol. Chem. Phys. 2020, 221, 2000170. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, S.-Y.; Liu, D.-M. An amphiphilic silicone-modified polysaccharide molecular hybrid with in situ forming of hierarchical superporous architecture upon swelling. Soft Matter 2012, 8, 10868. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Ngamdee, P.; Phinyocheep, P. Preparation and properties of polydimethylsiloxane-modified chitosan. Carbohydr. Polym. 2006, 63, 229–237. [Google Scholar] [CrossRef]
- Gleadall, A.; Ruiz-Cantu, L. Transplantable scaffolds. In 3D Printing in Medicine and Surgery-Applications in Healthcare, 1st ed.; Thomas, D.J., Singh, D., Eds.; Woodhead Publishing: Kidlington, UK, 2020; pp. 199–222. [Google Scholar]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Khan, A.A. N-succinyl chitosan preparation, characterization, properties and biomedical applications: A state of the art review. Rev. Chem. Eng. 2015, 31, 563–597. [Google Scholar] [CrossRef]
- Mello, K.G.P.C.; Bernusso, L.C.; Pitombo, R.N.M.; Polakiewicz, B. Synthesis and physicochemical characterization of chemically modified chitosan by succinic anhydride. Braz. Arch. Biol. Technol. 2006, 49, 665–668. [Google Scholar] [CrossRef]
- Wang, D.; Liu, W.; Feng, Q.; Dong, C.; Liu, Q.; Duan, L.; Huang, J.; Zhu, W.; Li, Z.; Xiong, J.; et al. Effect of inorganic/organic ratio and chemical coupling on the performance of porous silica/chitosan hybrid scaffolds. Mater. Sci. Eng. C 2017, 70, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Budianto, E.; Amalia, A. Swelling behavior and mechanical properties of Chitosan-Poly(N-vinyl-pyrrolidone) hydrogels. J. Polym. Eng. 2020, 40, 551–560. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Ahmed, R.; Hashmi, S.; Awan, Z.U.H. Comparative performance analysis of different swelling kinetic models for the evaluation of shale swelling. J. Pet. Explor. Prod. Technol. 2022, 12, 1237–1249. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Rehman, Q.; Hamid Akash, M.S.; Rasool, M.F.; Rehman, K. Role of kinetic Models in Drug Stability. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 155–166. [Google Scholar]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Humelnicu, A.-C.; Samoila, P.; Asandulesa, M.; Cojocaru, C.; Bele, A.; Marinoiu, A.T.; Saccà, A.; Harabagiu, V. Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature. Polymers 2020, 12, 1125. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Concha, L.; Resende Pires, A.L.; Moraes, A.M.; Mas-Hernández, E.; Berres, S.; Hernandez Montelongo, J. Cost Function Analysis Applied to Different Kinetic Release Models of Arrabidaea chica Verlot Extract from Chitosan/Alginate Membranes. Polymers 2022, 14, 1109. [Google Scholar] [CrossRef]
- Chopra, L.; Chohan, J.S.; Sharma, S.; Pelc, M.; Kawala-Sterniuk, A. Multifunctional Modified Chitosan Biopolymers for Dual Applications in Biomedical and Industrial Field: Synthesis and Evaluation of Thermal, Chemical, Morphological, Structural, In Vitro Drug-Release Rate, Swelling and Metal Uptake Studies. Sensors 2022, 22, 3454. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.; Arendrup, M.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.; Donnelly, J.; Dromer, F.; et al. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]



| Hydrogel/Film 1 Code | CS (g) | NYSm (mg) | Glycerin (g) | SA 2 (mmol) | DS 2 (mmol) |
|---|---|---|---|---|---|
| CS-LA | 0.3 | - | - | - | - |
| CS-NYSm | 0.3 | 15 | 4 | - | - |
| CS-NYSm-SA/DS-1 | 0.3 | 15 | 4 | 0.15 | 0.53 |
| CS-NYSm-SA/DS-2 | 0.3 | 15 | 4 | 0.45 | 0.38 |
| Film Formulation | σ 1 (MPa) | ε 1 (%) | Y 2 (MPa) |
|---|---|---|---|
| CS-LA | 101.63 ± 2.24 | 4.31 ± 0.13 | 43.23 |
| CS-NYSm | 2.89 ± 0.07 | 74.40 ± 1.52 | 0.12 |
| CS-NYSm-SA/DS-1 | 1.60 ± 0.04 | 34.43 ± 0.99 | 0.29 |
| CS-NYSm-SA/DS-2 | 1.04 ± 0.03 | 4.41 ± 0.16 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, A.-C.; Cojocaru, C.; Samoila, P.; Bele, A.; Bostanaru, A.-C.; Mares, M.; Harabagiu, V. Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids. Gels 2022, 8, 495. https://doi.org/10.3390/gels8080495
Enache A-C, Cojocaru C, Samoila P, Bele A, Bostanaru A-C, Mares M, Harabagiu V. Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids. Gels. 2022; 8(8):495. https://doi.org/10.3390/gels8080495
Chicago/Turabian StyleEnache, Andra-Cristina, Corneliu Cojocaru, Petrisor Samoila, Adrian Bele, Andra-Cristina Bostanaru, Mihai Mares, and Valeria Harabagiu. 2022. "Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids" Gels 8, no. 8: 495. https://doi.org/10.3390/gels8080495
APA StyleEnache, A.-C., Cojocaru, C., Samoila, P., Bele, A., Bostanaru, A.-C., Mares, M., & Harabagiu, V. (2022). Evaluation of Physically and/or Chemically Modified Chitosan Hydrogels for Proficient Release of Insoluble Nystatin in Simulated Fluids. Gels, 8(8), 495. https://doi.org/10.3390/gels8080495