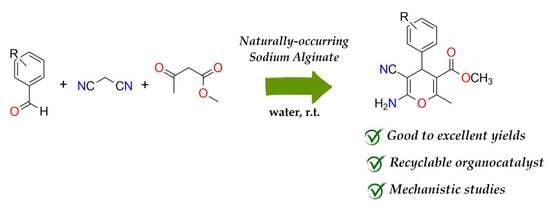
Mechanistic Insights into the Selective Synthesis of 4H-Pyran Derivatives On-Water Using Naturally Occurring Alginate from Sargassum muticum: Experimental and DFT Study
Abstract
1. Introduction
2. Results and Discussion
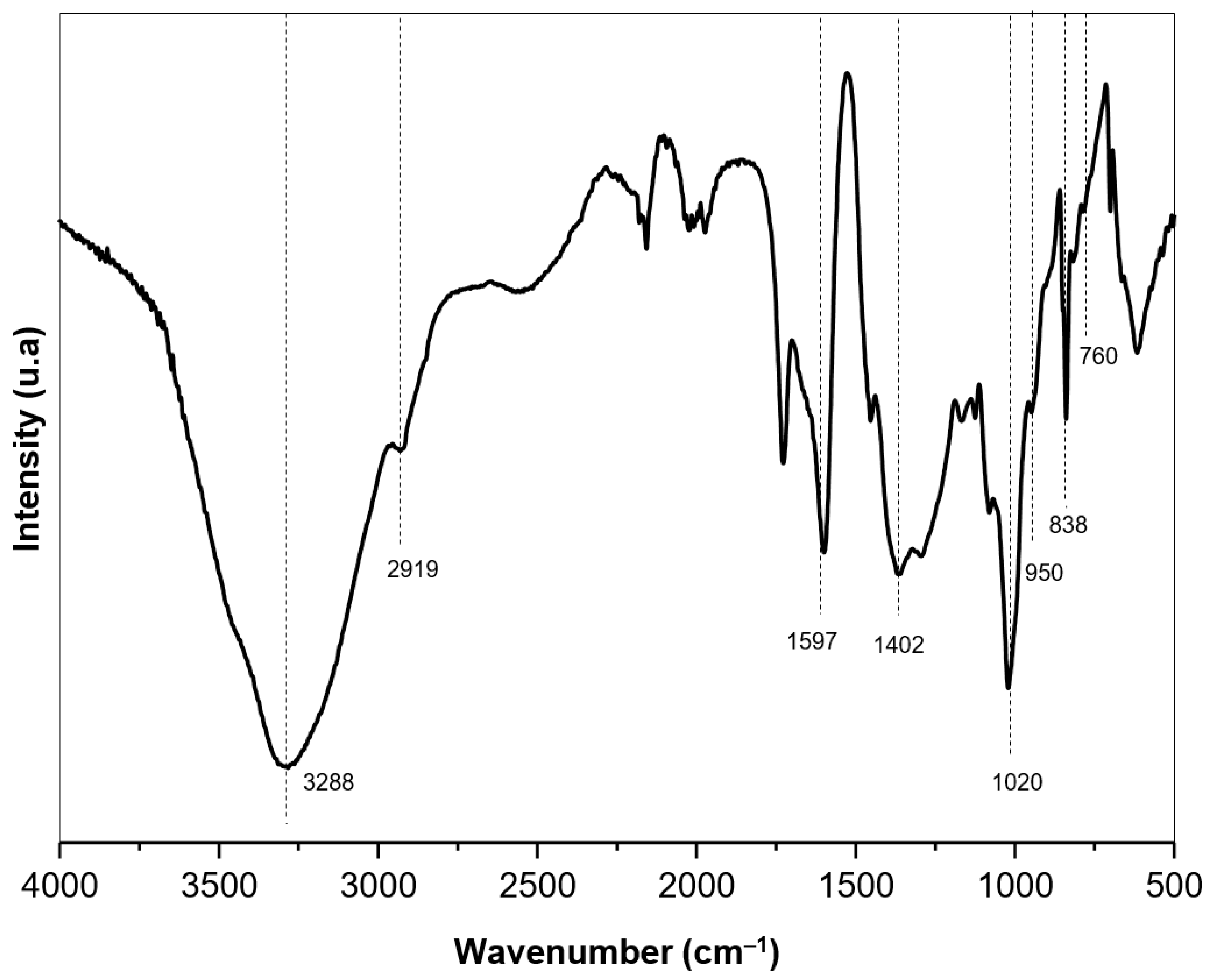
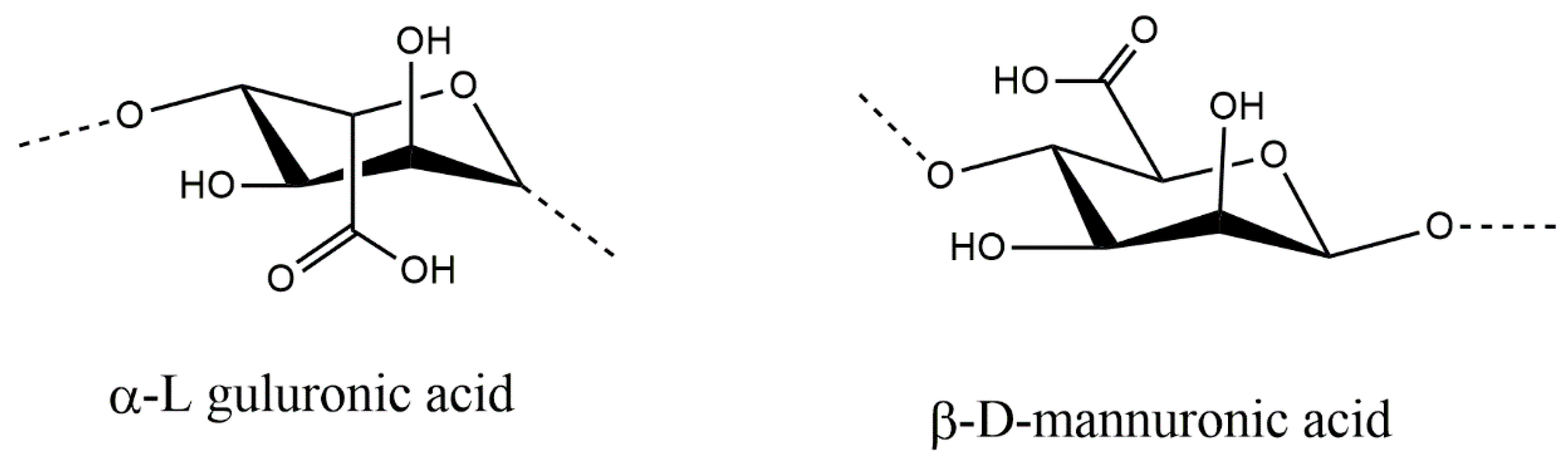
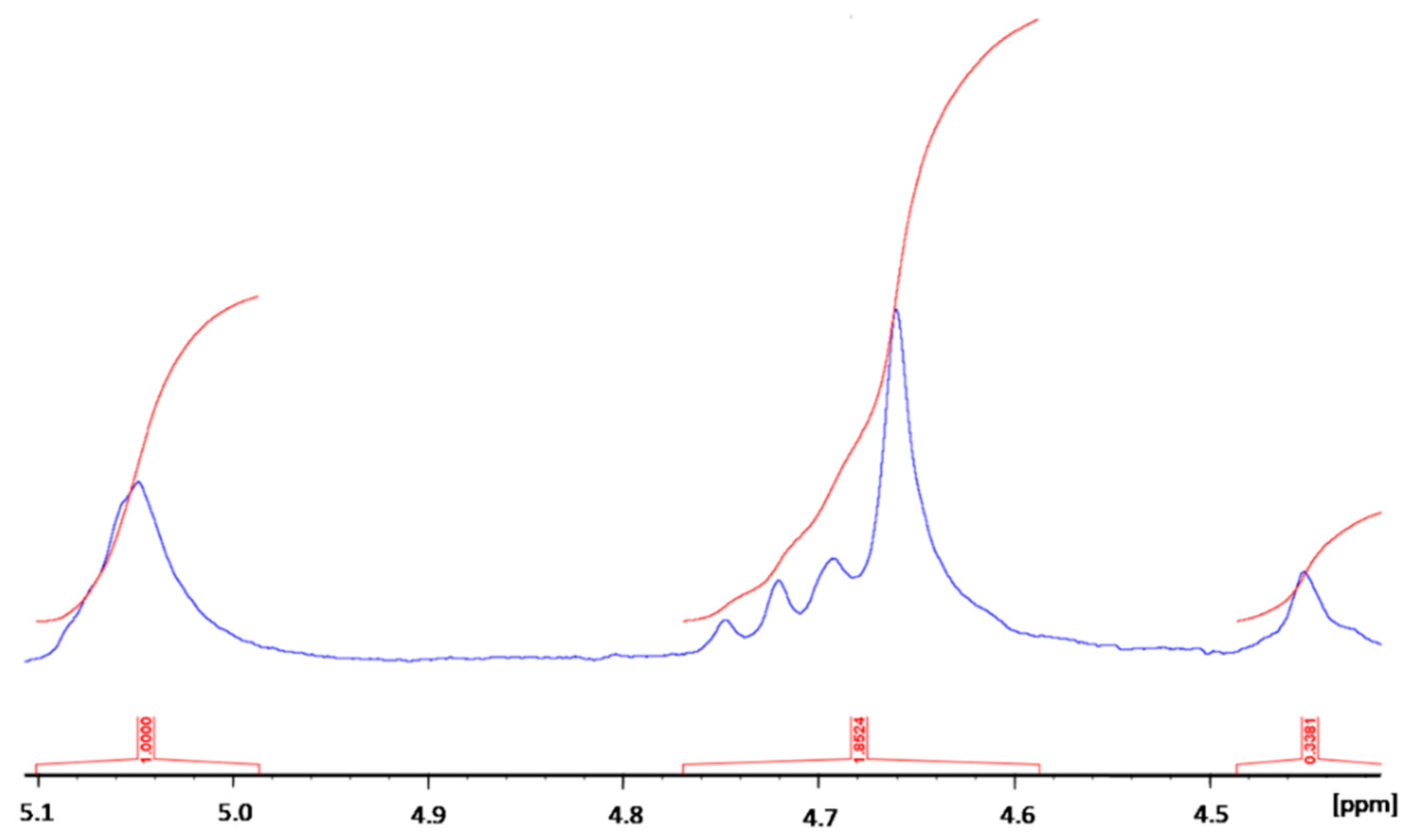
2.1. Sodium Alginate (SA) Biopolymer Characterization
2.2. Catalytic Activity
2.3. Mechanistic Studies
2.4. Reusability Test
2.5. Comparison of the Catalytic Activity of Our Organocatalyst with Other Reported Protocols
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Naturally-Occurring Sodium Alginate
4.3. Characterization
4.4. General Experimental Procedure for the Synthesis of 2-Amino-3-Cyano-4H-Pyran Derivatives
4.5. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A Critical Review on Production of Biopolymers from Algae Biomass and Their Applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for Surgical Applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Varma, K.; Gopi, S. Chapter 7—Biopolymers and Their Role in Medicinal and Pharmaceutical Applications. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–191. ISBN 978-0-12-819240-5. [Google Scholar]
- KloaregG, B.; Quatrano, R.S. Structure of the Cell Walls of Marine Algae and Ecophysiological Functions of the Matrix Polysaccharides. Oceanogr. Mar. Biol. 1988, 26, 259–315. [Google Scholar]
- Bahsis, L.; Ablouh, E.-H.; Anane, H.; Taourirte, M.; Julve, M.; Stiriba, S.-E. Cu( Ii )-Alginate-Based Superporous Hydrogel Catalyst for Click Chemistry Azide–Alkyne Cycloaddition Type Reactions in Water. RSC Adv. 2020, 10, 32821–32832. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Marine Algae: A Source of Biomass for Biotechnological Applications. In Natural Products From Marine Algae; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1308, pp. 1–37. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological Applications of Microalgal Oleaginous Compounds: Current Trends on Microalgal Bioprocessing of Products. Front. Energy Res. 2020, 8, 598803. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Huang, Y. Role of Alginate in Antibacterial Finishing of Textiles. Int. J. Biol. Macromol. 2017, 94, 466–473. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs 2018, 17, 18. [Google Scholar] [CrossRef]
- Ablouh, E.-H.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan Microspheres/Sodium Alginate Hybrid Beads: An Efficient Green Adsorbent for Heavy Metals Removal from Aqueous Solutions. Sustain. Environ. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Ablouh, E.-H.; Essaghraoui, A.; Eladlani, N.; Rhazi, M.; Taourirte, M. Uptake of Pb(II) onto Nanochitosan/Sodium Alginate Hybrid Beads: Mechanism and Kinetics Study. Water Environ. Res. 2019, 91, 239–249. [Google Scholar] [CrossRef]
- Boussetta, A.; Ablouh, E.-H.; Benhamou, A.A.; Taourirte, M.; Moubarik, A. Valorization of Moroccan Brown Seaweeds: Elaboration of Formaldehyde-Free Particleboards Based on Sodium Alginate–Corn-Starch—Mimosa Tannin Wood Adhesives. Int. J. Adhes. Adhes. 2021, 108, 102894. [Google Scholar] [CrossRef]
- Gopidas, S.K.; Subramani, N. Recent Advances and Research Challenges in Bioprospecting Of Brown Seaweeds. In Seaweed Biotechnology; Apple Academic Press: New York, NY, USA, 2022; ISBN 978-1-00-330085-4. [Google Scholar]
- Sangeetha, J.; Thangadurai, D. (Eds.) Seaweed Biotechnology: Biodiversity and Biotechnology of Seaweeds and Their Applications; Apple Academic Press: New York, NY, USA, 2022; ISBN 978-1-00-330085-4. [Google Scholar]
- Rioux, L.-E.; Turgeon, S.L. Chapter 7—Seaweed Carbohydrates. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 141–192. ISBN 978-0-12-418697-2. [Google Scholar]
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR Characterization of Alginates from the Main Alginophyte Species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chowdhury, I.R.; Kabir, F.; Mazumder, M.A.J.; Zahir, M.H.; Alhooshani, K. Alginate-Based Biotechnology: A Review on the Arsenic Removal Technologies and Future Possibilities. J. Water Supply: Res. Technol.-Aqua 2019, 68, 369–389. [Google Scholar] [CrossRef]
- González, R.; Martín, N.; Seoane, C.; Marco, J.; Albert, A.; Cano, F.H. The First Asymmetric Synthesis of Polyfunctionalized 4H-Pyrans via Michael Addition of Malononitrile to 2-Acyl Acrylates. Tetrahedron Lett. 1992, 33, 3809–3812. [Google Scholar] [CrossRef]
- Shehab, W.S.; Ghoneim, A.A. Synthesis and Biological Activities of Some Fused Pyran Derivatives. Arab. J. Chem. 2016, 9, S966–S970. [Google Scholar] [CrossRef]
- Saranya, J.; Benhiba, F.; Anusuya, N.; Subbiah, R.; Zarrouk, A.; Chitra, S. Experimental and Computational Approaches on the Pyran Derivatives for Acid Corrosion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125231. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Z.-F.; Zhang, J.-F.; Zhang, B. Pyran Derivatives: Anti-Breast Cancer Activity and Docking Study. Russ. J. Gen. Chem. 2018, 88, 2664–2668. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, W.; Tian, H. Dicyanomethylene-4H-Pyran Chromophores for OLED Emitters, Logic Gates and Optical Chemosensors. Chem. Commun. 2012, 48, 6073–6084. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Wang, D.; Zhou, Y.; Lei, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Reversible Photochromic Properties of 4,5,6-Triaryl-4H-Pyran Derivatives in a Solid State. Mater. Chem. Front. 2021, 5, 3413–3421. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Hamedifar, H.; Larijani, B.; Ansari, S.; Mahdavi, M. Synthesis and Pharmacological Properties of Polysubstituted 2-Amino-4H-Pyran-3-Carbonitrile Derivatives. Mol. Divers. 2020, 24, 1385–1431. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Peyman, S.Z.; Karimi, Z.; Javanshir, S.; Naimi-Jamal, M.R.; Barikani, M. Sodium Alginate: An Efficient Biopolymeric Catalyst for Green Synthesis of 2-Amino-4H-Pyran Derivatives. Int. J. Biol. Macromol. 2016, 87, 172–179. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Gorji, S.; Ghorbani-Vaghei, R.; Alavinia, S. Sodium Alginate: Biopolymeric Catalyst for the Synthesis of 2-Amino-4-Arylthiazole Derivatives in Aqueous Medium. J. Mol. Struct. 2021, 1231, 129900. [Google Scholar] [CrossRef]
- Pettignano, A.; Aguilera, D.A.; Tanchoux, N.; Bernardi, L.; Quignard, F. Chapter 17—Alginate: A Versatile Biopolymer for Functional Advanced Materials for Catalysis. In Studies in Surface Science and Catalysis; Albonetti, S., Perathoner, S., Quadrelli, E.A., Eds.; Horizons in Sustainable Industrial Chemistry and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; Volume 178, pp. 357–375. [Google Scholar]
- Kühbeck, D.; Mayr, J.; Häring, M.; Hofmann, M.; Quignard, F.; Díaz, D.D. Evaluation of the Nitroaldol Reaction in the Presence of Metal Ion-Crosslinked Alginates. N. J. Chem. 2015, 39, 2306–2315. [Google Scholar] [CrossRef]
- Zafari, S.; Ghorbani-Vaghei, R.; Alavinia, S. Sodium Alginate: An Efficient Biopolymeric Catalyst for Green Synthesis of Phenylimidazo [1,2-a]Pyridine Derivatives. Polycycl. Aromat. Compd. 2022, 1–14. [Google Scholar] [CrossRef]
- Pathak, T.S.; Kim, J.S.; Lee, S.-J.; Baek, D.-J.; Paeng, K.-J. Preparation of Alginic Acid and Metal Alginate from Algae and Their Comparative Study. J. Polym. Environ. 2008, 16, 198–204. [Google Scholar] [CrossRef]
- Kaidi, S.; Belattmania, Z.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B. Synthesis and Characterization of Silver Nanoparticles Using Alginate from the Brown Seaweed Laminaria Ochroleuca: Structural Features and Antibacterial Activity. Biointerface Res. Appl. Chem. 2021, 12, 6046–6057. [Google Scholar] [CrossRef]
- Grasdalen, H.; Larsen, B.; Smidsrød, O. A p.m.r. Study of the Composition and Sequence of Uronate Residues in Alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and Differences between Alginic Acid Gels and Ionically Crosslinked Alginate Gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Kolvari, E.; Koukabi, N.; Ozmaei, Z.; Khoshkho, H.; Seidi, F. Synthesis of 2-Amino-4H-Pyran and 2-Benzylidene Malononitrile Derivatives Using a Basil Seed as a Cheap, Natural, and Biodegradable Catalyst. Curr. Res. Green Sustain. Chem. 2022, 5, 100327. [Google Scholar] [CrossRef]
- van der Helm, M.P.; Klemm, B.; Eelkema, R. Organocatalysis in Aqueous Media. Nat. Rev. Chem. 2019, 3, 491–508. [Google Scholar] [CrossRef]
- Banik, B.K.; Banerjee, B. Organocatalysis: A Green Tool for Sustainable Developments; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022; ISBN 978-3-11-073254-2. [Google Scholar]
- Saini, S.; Kaur, N.; Singh, N. A Cytochrome C-Urea Functionalized Dipeptide Conjugate: An Efficient HBD Framework to Synthesize 4H-Pyrans via One-Pot Multicomponent Reaction. Green Chem. 2020, 22, 956–968. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Dogari, H.; Esmailzadeh, F.; Maleki, A. Magnetized Melamine-Modified Polyacrylonitrile (PAN@melamine/Fe3O4) Organometallic Nanomaterial: Preparation, Characterization, and Application as a Multifunctional Catalyst in the Synthesis of Bioactive Dihydropyrano [2,3-c]Pyrazole and 2-Amino-3-Cyano 4H-Pyran Derivatives. Appl. Organomet. Chem. 2021, 35, e6363. [Google Scholar] [CrossRef]
- Khazaei, A.; Abbasi, F.; Moosavi-Zare, A.R. Catalytic Application of N,2-Dibromo-6-Chloro-3,4-Dihydro-2H-Benzo[e][1,2,4]Thiadiazine-7-Sulfonamide 1,1-Dioxide on the Synthesis of 1-Carbamato-Alkyl-2-Naphthols and 1-Thioamido-Alkyl-2-Naphthols. J. Sulfur Chem. 2015, 36, 364–372. [Google Scholar] [CrossRef]
- Li, J.P.H.; Kennedy, E.M.; Adesina, A.A.; Stockenhuber, M. Mechanistic Insights into the Knoevenagel Condensation Reaction over ZnO Catalysts: Direct Observation of Surface Intermediates Using in Situ FTIR. J. Catal. 2019, 369, 157–167. [Google Scholar] [CrossRef]
- El Jemli, Y.; Khallouk, K.; Lanaya, S.; Brulé, M.; Barakat, A.; Abdelouahdi, K.; Solhy, A. Hybrid Alginate–Brushite Beads Easily Catalyze the Knoevenagel Condensation On-Water. ACS Omega 2022, 7, 27831–27838. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Jonnalagadda, S.B. An Efficient, Multicomponent, One-pot Synthesis of Tetra Substituted Pyrans in Water. J. Heterocycl. Chem. 2015, 52, 1226–1229. [Google Scholar] [CrossRef]
- Ardiles, C.S.; Rodríguez, C.C. Theoretical Study for Determining the Type of Interactions between a GG Block of an Alginate Chain with Metals Cu2+, Mn2+, Ca2+ and Mg2+. Arab. J. Chem. 2021, 14, 103325. [Google Scholar] [CrossRef]
- Costa, M.P.M.; Prates, L.M.; Baptista, L.; Cruz, M.T.M.; Ferreira, I.L.M. Interaction of Polyelectrolyte Complex between Sodium Alginate and Chitosan Dimers with a Single Glyphosate Molecule: A DFT and NBO Study. Carbohydr. Polym. 2018, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Plazinska, A. Molecular Dynamics Study of the Interactions between Phenolic Compounds and Alginate/Alginic Acid Chains. N. J. Chem. 2011, 35, 1607–1614. [Google Scholar] [CrossRef]
- Knani, D.; Barkay-Olami, H.; Alperstein, D.; Zilberman, M. Simulation of Novel Soy Protein-Based Systems for Tissue Regeneration Applications. Polym. Adv. Technol. 2017, 28, 496–505. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Kim, M.R.; Kim, I. Dibutylamine-Catalysed Efficient One-Pot Synthesis of Biologically Potent Pyrans. Tetrahedron Lett. 2015, 56, 717–720. [Google Scholar] [CrossRef]
- Amirnejat, S.; Nosrati, A.; Peymanfar, R.; Javanshir, S. Synthesis and Antibacterial Study of 2-Amino-4H-Pyrans and Pyrans Annulated Heterocycles Catalyzed by Sulfated Polysaccharide-Coated BaFe12O19 Nanoparticles. Res. Chem. Intermed. 2020, 46, 3683–3701. [Google Scholar] [CrossRef]
- Hakiminasab, S.; Habibi, A.; Shahcheragh, S.M.; Farahani, Y.; Sardari, S.; Dolati, H.; Mahdavi, S.M.; Habibi, M. Efficient Pyran Derivatives Synthesis in DES Medium and Their Antimicrobial Evaluation as Inhibitors of Mycobacterium Bovis (BCG). J. Iran. Chem. Soc. 2021, 18, 2575–2582. [Google Scholar] [CrossRef]
- Kharbangar, I.; Rohman, M.R.; Mecadon, H.; Myrboh, B. KF-Al2O3 as an Efficient and Recyclable Basic Catalyst for the Synthesis of 4H-Pyran-3-Carboxylates and 5-Acetyl-4H-Pyrans. Int. J. Org. Chem. 2012, 2, 282–286. [Google Scholar] [CrossRef]
- Ahmad, I.; Jasim, S.A.; Yasin, G.; Al-Qargholi, B.; Hammid, A.T. Synthesis and Characterization of New 1,4-Dihydropyran Derivatives by Novel Ta-MOF Nanostructures as Reusable Nanocatalyst with Antimicrobial Activity. Front. Chem. 2022, 10, 967111. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]

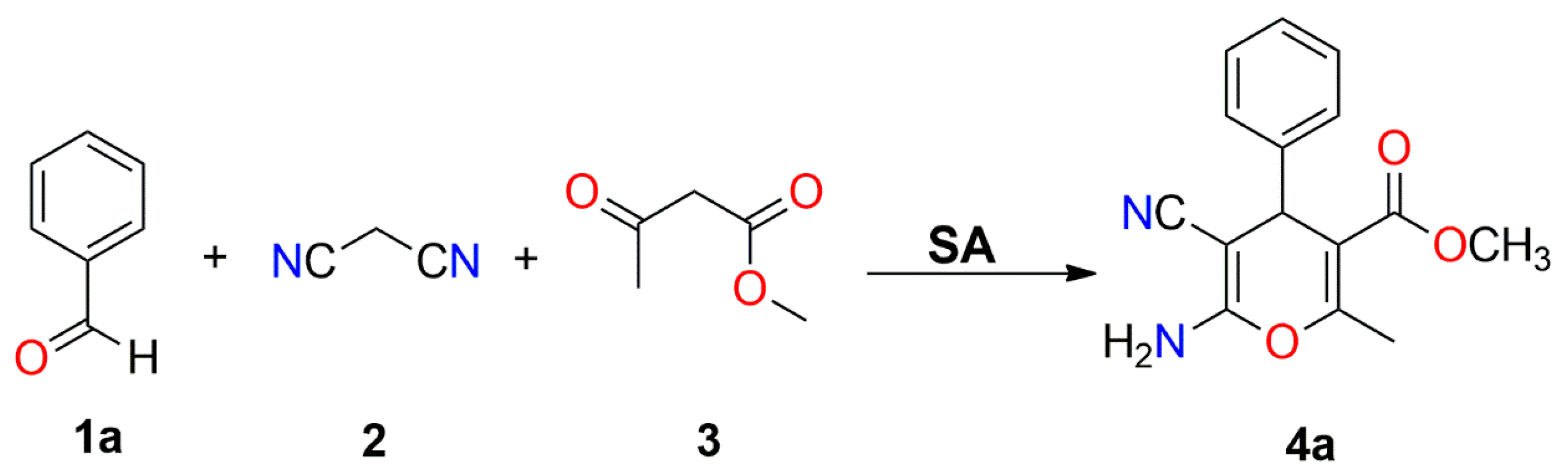
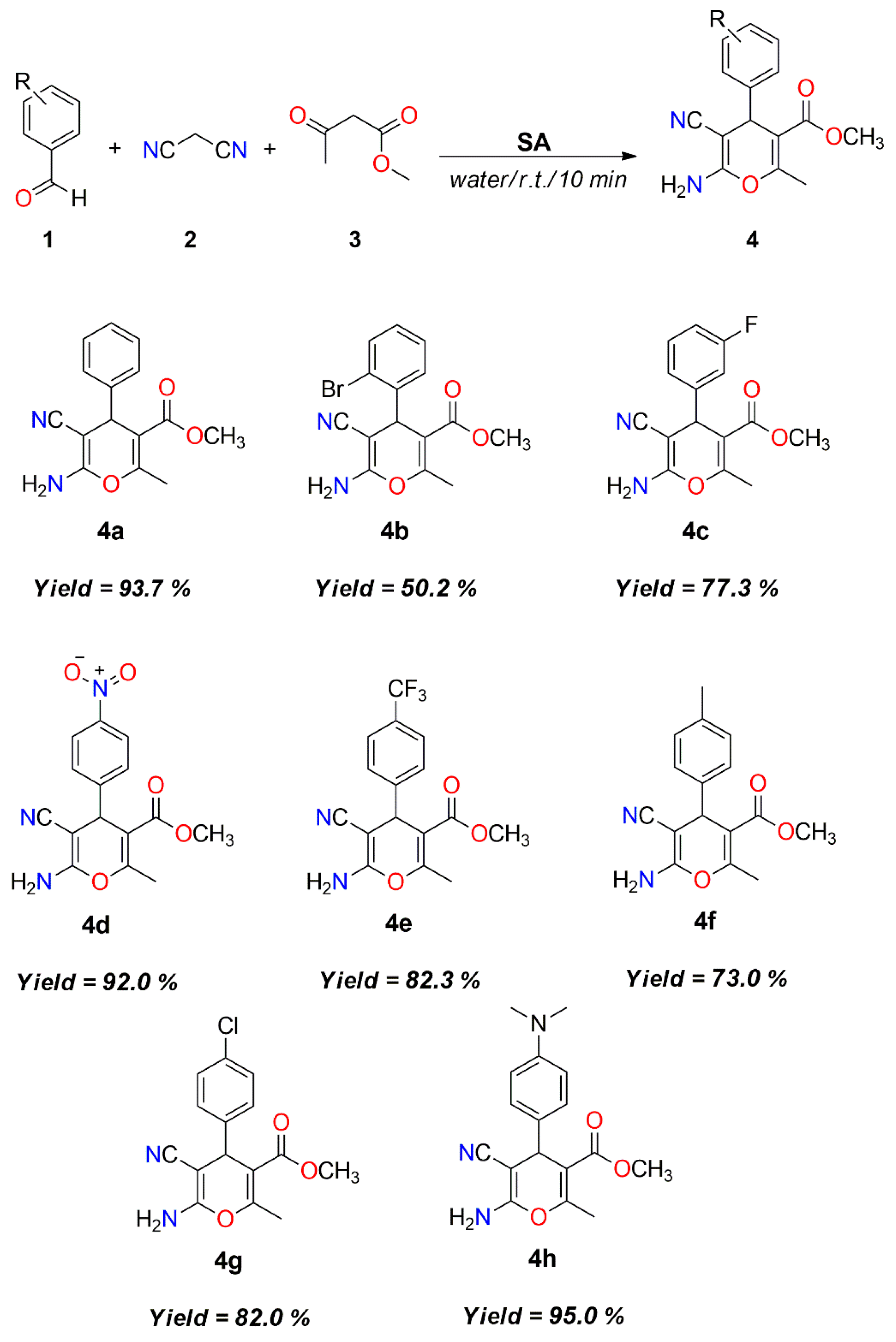
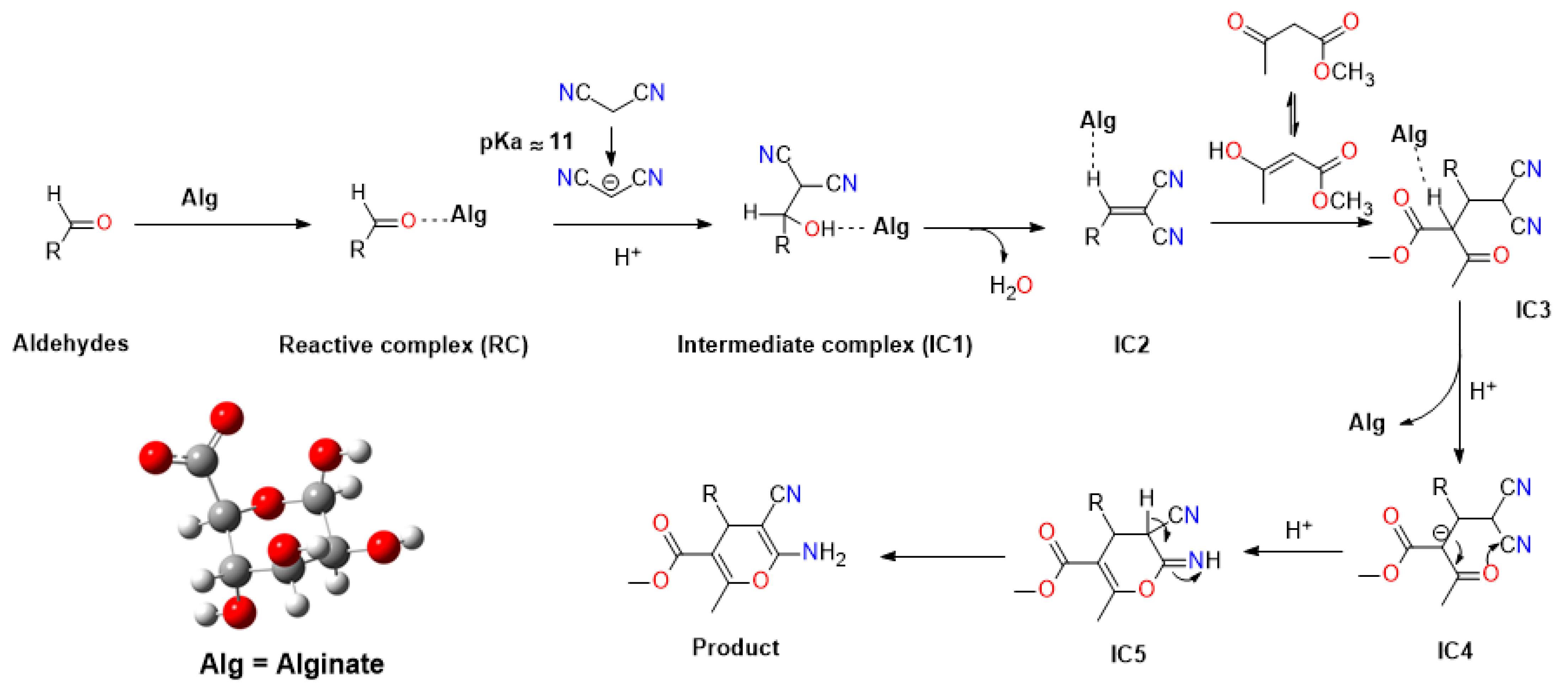
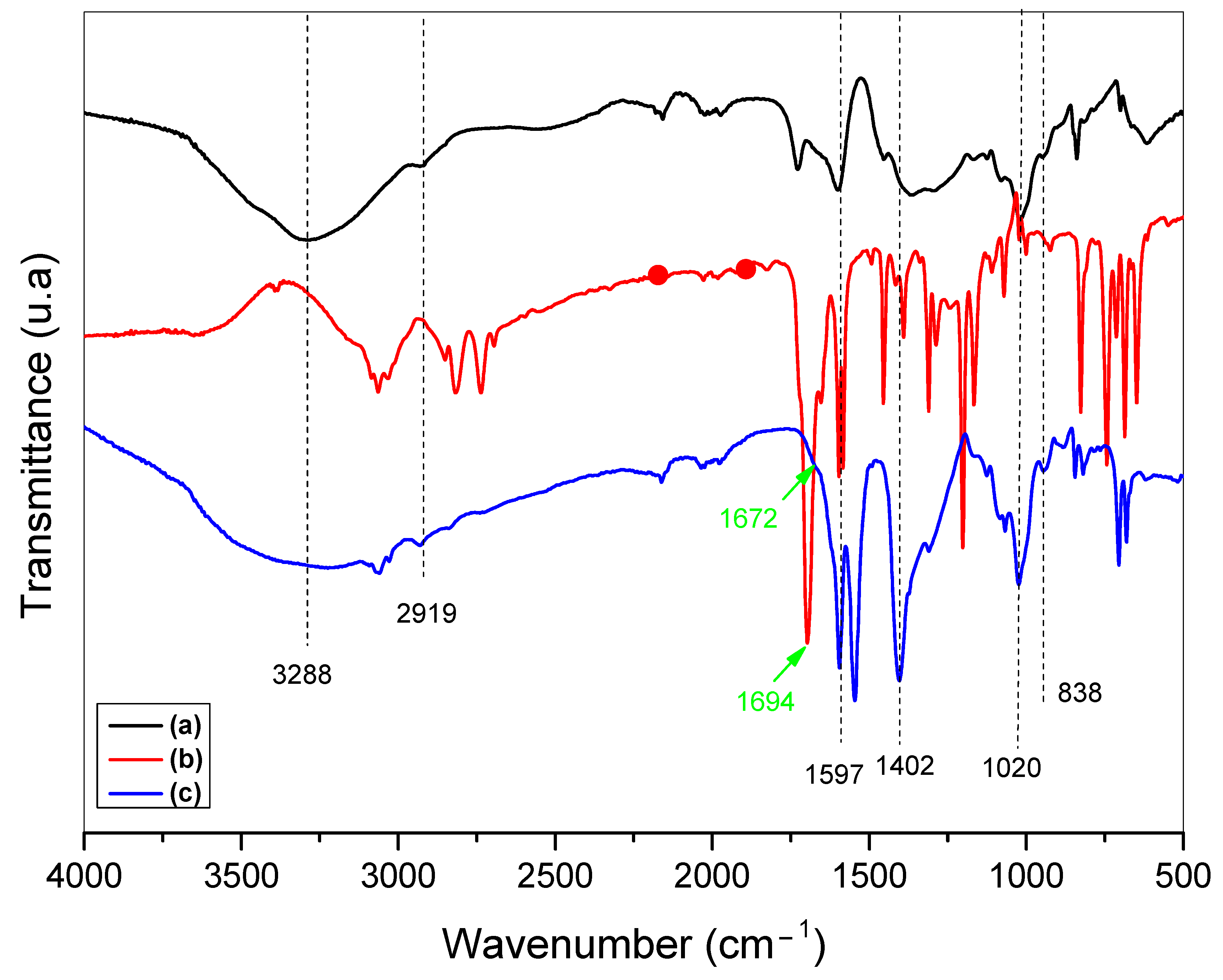

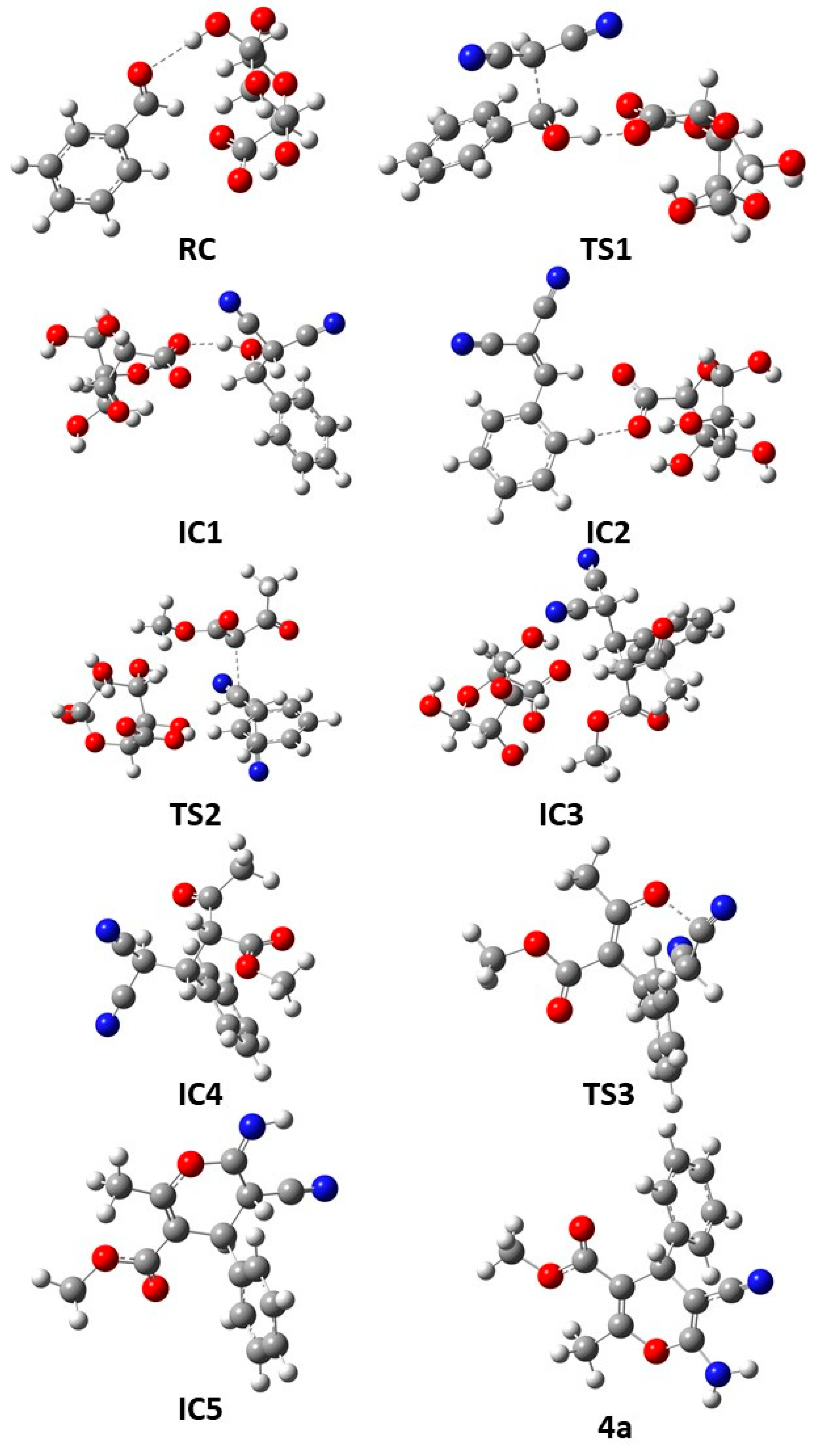
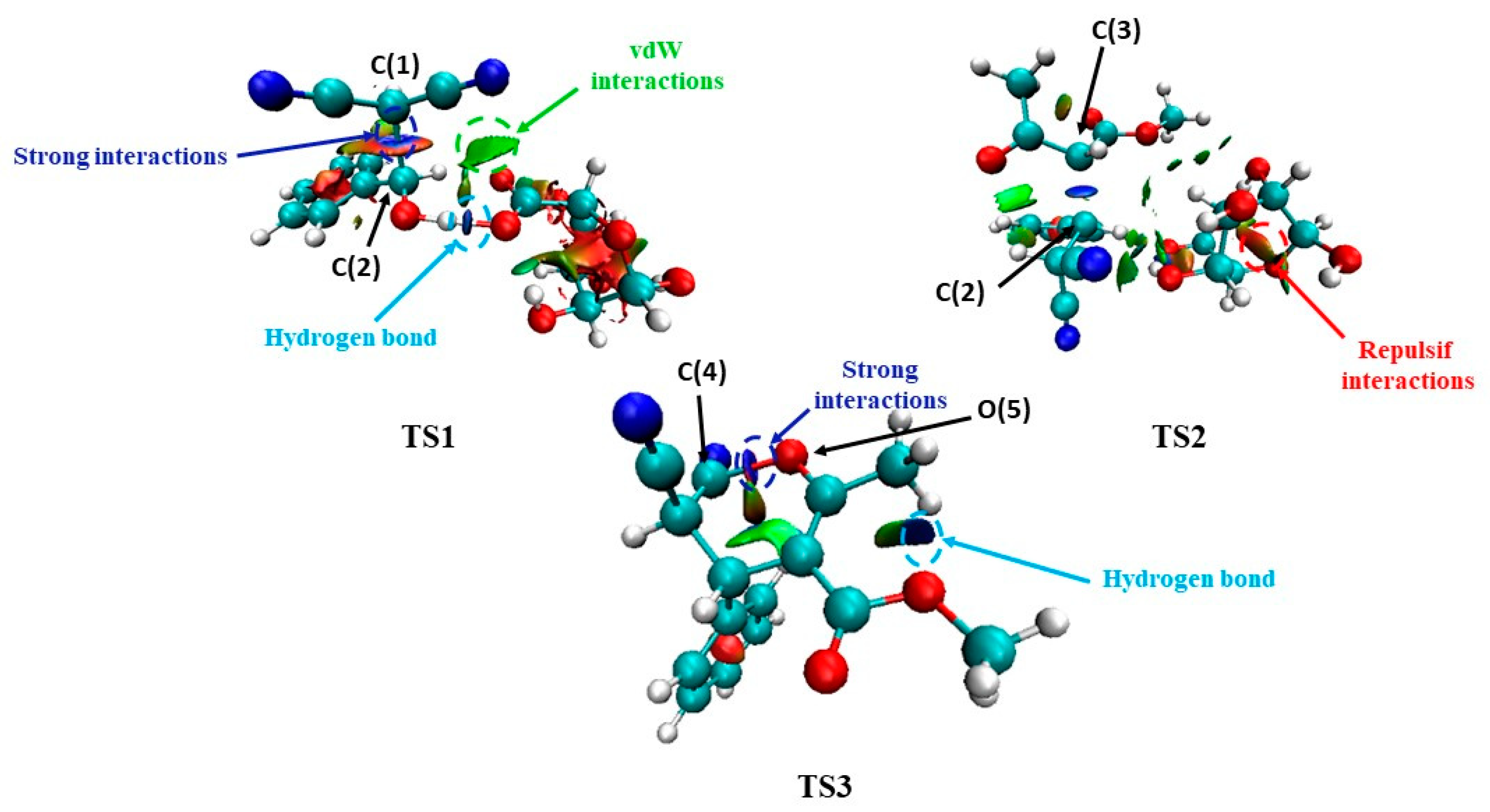
| Entry | Catalyst | Loading (mg) | Solvent | Time (h) | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | Neat | - | H2O | 24 | r.t. | 0 |
| 2 | SA | 40 | H2O | 24 | r.t. | 97.3 |
| 3 | SA | 40 | Ethanol | 24 | r.t. | 57.3 |
| 4 | SA | 40 | Acetonitrile | 24 | r.t. | 80.3 |
| 5 | SA | 40 | Hexane | 24 | r.t. | 88.3 |
| 6 | SA | 40 | Acetone | 24 | r.t. | 39.3 |
| 7 | SA | 5 | H2O | 0.2 | r.t. | 16.8 |
| 8 | SA | 20 | H2O | 0.2 | r.t. | 35.8 |
| 9 | SA | 40 | H2O | 0.2 | r.t. | 73.2 |
| 10 | SA | 100 | H2O | 0.2 | r.t. | 93.7 |
| 11 | SA | 100 | Hexane | 2 | r.t. | 64.0 |
| 12 | SA | 40 | H2O | 3 | 40 | 56.4 |
| 13 | SA | 40 | H2O | 3 | 60 | 72.4 |
| 14 | SA | 40 | H2O | 3 | 80 | 83 |
 | |||||
|---|---|---|---|---|---|
| Catalyst | Loading | Conditions | Time (h) | Yield (%) | Ref. |
| Dibutylamine | 2.5 a | Neat; r.t | 0.14 | 98 | [53] |
| BaFe12O19@ IM | 3.12 b | Refux; EtOH | 0.25 | 96 | [54] |
| DES (Urea-ChCl) | 30 a | EtOH; 80 °C | 0.5 | 88 | [55] |
| KF-Al2O3 | 5 a | EtOH; r.t | 5 | 90 | [56] |
| Ta-MOF nanostructures | 0.003 b | EtOH; r.t | 0.5 | 71 | [57] |
| Sodium Alginate (SA) | 0.100 b | water; r.t | 0.17 | 97.3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudghiri, K.; Belattmania, Z.; Elmouli, H.; Guesmi, S.; Bentiss, F.; Sabour, B.; Bahsis, L.; Taourirte, M. Mechanistic Insights into the Selective Synthesis of 4H-Pyran Derivatives On-Water Using Naturally Occurring Alginate from Sargassum muticum: Experimental and DFT Study. Gels 2022, 8, 713. https://doi.org/10.3390/gels8110713
Oudghiri K, Belattmania Z, Elmouli H, Guesmi S, Bentiss F, Sabour B, Bahsis L, Taourirte M. Mechanistic Insights into the Selective Synthesis of 4H-Pyran Derivatives On-Water Using Naturally Occurring Alginate from Sargassum muticum: Experimental and DFT Study. Gels. 2022; 8(11):713. https://doi.org/10.3390/gels8110713
Chicago/Turabian StyleOudghiri, Khaoula, Zahira Belattmania, Hamid Elmouli, Salaheddine Guesmi, Fouad Bentiss, Brahim Sabour, Lahoucine Bahsis, and Moha Taourirte. 2022. "Mechanistic Insights into the Selective Synthesis of 4H-Pyran Derivatives On-Water Using Naturally Occurring Alginate from Sargassum muticum: Experimental and DFT Study" Gels 8, no. 11: 713. https://doi.org/10.3390/gels8110713
APA StyleOudghiri, K., Belattmania, Z., Elmouli, H., Guesmi, S., Bentiss, F., Sabour, B., Bahsis, L., & Taourirte, M. (2022). Mechanistic Insights into the Selective Synthesis of 4H-Pyran Derivatives On-Water Using Naturally Occurring Alginate from Sargassum muticum: Experimental and DFT Study. Gels, 8(11), 713. https://doi.org/10.3390/gels8110713