Advances of Hydrogel Therapy in Periodontal Regeneration—A Materials Perspective Review
Abstract
1. Introduction
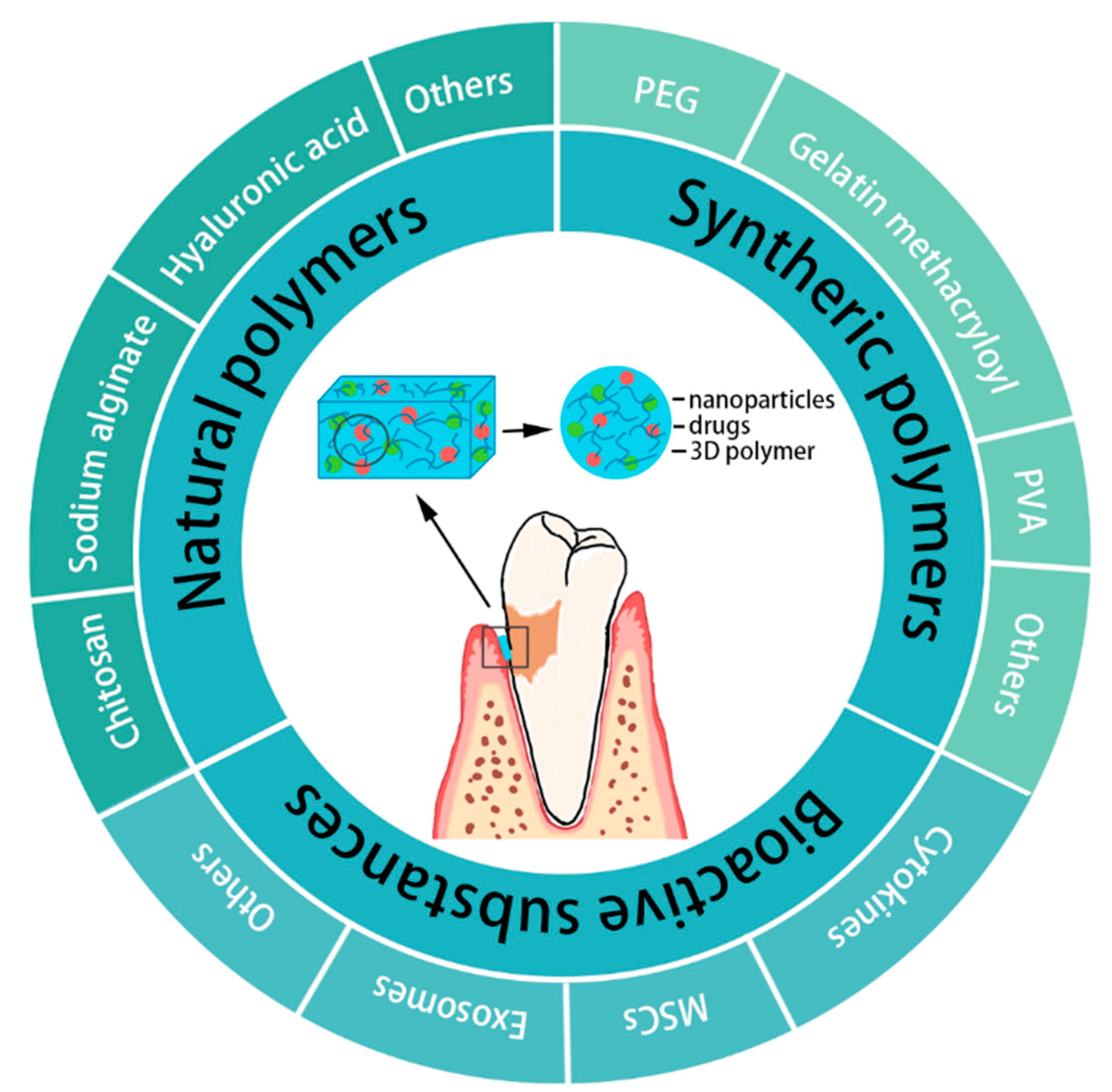
2. Components of Hydrogel in Periodontal Tissue Regeneration
2.1. The Fundamental Components of Hydrogels in Periodontal Tissue Regeneration
2.1.1. Natural Polymers
- Chitosan
- Sodium alginate
- Hyaluronic acid
- Collagen
- Others
2.1.2. Synthetic Polymers
- PEG
- Gelatin methacryloyl (GelMA)
- Others
2.2. The Multiple Components of Hydrogels in Periodontal Tissue Regeneration
- Antibacterial agents
- Cytokines
- Mesenchymal stem cells and exosomes
- Inorganic nanoparticles
- Natural compounds
3. Strategies of Hydrogels in Periodontal Tissue Regeneration
3.1. Biomimetic Hydrogel
3.2. Intelligent Hydrogels
3.3. Self-Healing Hydrogels
4. Summary and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontol 2000 2021, 87, 143–156. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef]
- Aljateeli, M.; Koticha, T.; Bashutski, J.; Sugai, J.V.; Braun, T.M.; Giannobile, W.V.; Wang, H.L. Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2014, 41, 693–700. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef]
- Jepsen, S.; Gennai, S.; Hirschfeld, J.; Kalemaj, Z.; Buti, J.; Graziani, F. Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2020, 47, 352–374. [Google Scholar] [CrossRef]
- Chen, F.M.; Jin, Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Feng, Z.; Dong, Y.; Zhou, W.; Li, B.; Bai, S.; Zhao, Y. Advanced biomaterials and their potential applications in the treatment of periodontal disease. Crit. Rev. Biotechnol. 2016, 36, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Löwik, D.W. A hybrid peptide amphiphile fiber PEG hydrogel matrix for 3D cell culture. Adv. Funct. Mater. 2019, 29, 1808505. [Google Scholar] [CrossRef]
- Abboud, A.R.; Ali, A.M.; Youssef, T. Preparation and characterization of insulin-loaded injectable hydrogels as potential adjunctive periodontal treatment. Dent. Med. Probl. 2020, 57, 377–384. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, H.; Fang, C.; Yang, Y.; Xia, X.; Yang, B.; Lin, Y.; Li, G.; Bian, L. Microscopic local stiffening in a supramolecular hydrogel network expedites stem cell mechanosensing in 3D and bone regeneration. Mater. Horiz. 2021, 8, 1722–1734. [Google Scholar] [CrossRef]
- Zang, S.; Mu, R.; Chen, F.; Wei, X.; Zhu, L.; Han, B.; Yu, H.; Bi, B.; Chen, B.; Wang, Q.; et al. Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 919–928. [Google Scholar] [CrossRef]
- Pan, J.; Deng, J.; Yu, L.; Wang, Y.; Zhang, W.; Han, X.; Camargo, P.H.C.; Wang, J.; Liu, Y. Investigating the repair of alveolar bone defects by gelatin methacrylate hydrogels-encapsulated human periodontal ligament stem cells. J. Mater. Sci. Mater. Med. 2019, 31, 3. [Google Scholar] [CrossRef]
- Momose, T.; Miyaji, H.; Kato, A.; Ogawa, K.; Yoshida, T.; Nishida, E.; Murakami, S.; Kosen, Y.; Sugaya, T.; Kawanami, M. Collagen Hydrogel Scaffold and Fibroblast Growth Factor-2 Accelerate Periodontal Healing of Class II Furcation Defects in Dog. Open Dent. J. 2016, 10, 347–359. [Google Scholar] [CrossRef]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A.R. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Li, B.; Feng, B.; He, X.; Fu, W.; Yuan, H.; Xu, Z. Enhanced chondrogenic differentiation of human mesenchymal stems cells on citric acid-modified chitosan hydrogel for tracheal cartilage regeneration applications. RSC Adv. 2018, 8, 16910–16917. [Google Scholar] [CrossRef]
- Meena, L.K.; Raval, P.; Kedaria, D.; Vasita, R. Study of locust bean gum reinforced cyst-chitosan and oxidized dextran based semi-IPN cryogel dressing for hemostatic application. Bioact. Mater. 2018, 3, 370–384. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Yan, X.Z.; van den Beucken, J.J.; Cai, X.; Yu, N.; Jansen, J.A.; Yang, F. Periodontal tissue regeneration using enzymatically solidified chitosan hydrogels with or without cell loading. Tissue Eng. Part A 2015, 21, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.X.; Chen, X.G.; Zhao, Q.S.; Liu, C.S.; Cheng, X.J.; Wang, L.C. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J. Mater. Sci. Mater. Med. 2009, 20, 1603–1610. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Trinh, B.D.T.; Le Thi, P.; Tran, D.L.; Park, K.D.; Nguyen, D.H. Self-antibacterial chitosan/Aloe barbadensis Miller hydrogels releasing nitrite for biomedical applications. J. Ind. Eng. Chem. 2021, 103, 175–186. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Research. Part B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Işılay Özdoğan, A.; Akca, G.; Şenel, S. Development and in vitro evaluation of chitosan based system for local delivery of atorvastatin for treatment of periodontitis. Eur. J. Pharm. Sci. 2018, 124, 208–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D.; et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef]
- Ansari, S.; Diniz, I.M.; Chen, C.; Aghaloo, T.; Wu, B.M.; Shi, S.; Moshaverinia, A. Alginate/hyaluronic acid hydrogel delivery system characteristics regulate the differentiation of periodontal ligament stem cells toward chondrogenic lineage. J. Mater. Sci. Mater. Med. 2017, 28, 162. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, R.; Komasa, S.; Xue, Y.; Jin, B.; Hou, Y.; Okazaki, J.; Gao, J. Drug-Loadable Calcium Alginate Hydrogel System for Use in Oral Bone Tissue Repair. Int. J. Mol. Sci. 2017, 18, 989. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A. Gingival Mesenchymal Stem Cell (GMSC) Delivery System Based on RGD-Coupled Alginate Hydrogel with Antimicrobial Properties: A Novel Treatment Modality for Peri-Implantitis. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surfaces. B Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef]
- Tan, F.; Xu, X.; Deng, T.; Yin, M.; Zhang, X.; Wang, J. Fabrication of positively charged poly(ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold. Biomed. Mater. 2012, 7, 055009. [Google Scholar] [CrossRef]
- Xiong, X.; Xiao, W.; Zhou, S.; Cui, R.; Xu, H.H.K.; Qu, S. Enhanced proliferation and angiogenic phenotype of endothelial cells via negatively-charged alginate and chondroitin sulfate microsphere hydrogels. Biomed. Mater. 2021, 16, 025012. [Google Scholar] [CrossRef]
- Iskandar, L.; Rojo, L.; Di Silvio, L.; Deb, S. The effect of chelation of sodium alginate with osteogenic ions, calcium, zinc, and strontium. J. Biomater. Appl. 2019, 34, 573–584. [Google Scholar] [CrossRef]
- Lueckgen, A.; Garske, D.S.; Ellinghaus, A.; Desai, R.M.; Stafford, A.G.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Hydrolytically-degradable click-crosslinked alginate hydrogels. Biomaterials 2018, 181, 189–198. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992, 6, 2397–2404. [Google Scholar]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, L.; Wang, Z.; Li, P.; Feng, F.; Zheng, X. High molecular weight hyaluronic acid regulates P. gingivalis–induced inflammation and migration in human gingival fibroblasts via MAPK and NF-κB signaling pathway. Arch. Oral Biol. 2019, 98, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef]
- Babo, P.S.; Pires, R.L.; Santos, L.; Franco, A.; Rodrigues, F.; Leonor, I.; Reis, R.L.; Gomes, M.E. Platelet Lysate-Loaded Photocrosslinkable Hyaluronic Acid Hydrogels for Periodontal Endogenous Regenerative Technology. ACS Biomater. Sci. Eng. 2017, 3, 1359–1369. [Google Scholar] [CrossRef]
- Kosen, Y.; Miyaji, H.; Kato, A.; Sugaya, T.; Kawanami, M. Application of collagen hydrogel/sponge scaffold facilitates periodontal wound healing in class II furcation defects in beagle dogs. J. Periodontal Res. 2012, 47, 626–634. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168. [Google Scholar] [CrossRef]
- Janjić, K.; Agis, H.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effects of collagen membranes and bone substitute differ in periodontal ligament cell microtissues and monolayers. J. Periodonto. 2022, 93, 697–708. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In vitro evaluation of poly (vinyl alcohol)/collagen blended hydrogels for regulating human periodontal ligament fibroblasts and gingival fibroblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef]
- He, X.T.; Li, X.; Xia, Y.; Yin, Y.; Wu, R.X.; Sun, H.H.; Chen, F.M. Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats. Acta Biomater. 2019, 88, 162–180. [Google Scholar] [CrossRef]
- Pańczyszyn, E.; Jaśko, M.; Miłek, O.; Niedziela, M.; Męcik-Kronenberg, T.; Hoang-Bujnowicz, A.; Zięba, M.; Adamus, G.; Kowalczuk, M.; Osyczka, A.M.; et al. Gellan gum hydrogels cross-linked with carbodiimide stimulates vacuolation of human tooth-derived stem cells in vitro. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2021, 73, 105111. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Goncalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Anti-Inflammatory Properties of Injectable Betamethasone-Loaded Tyramine-Modified Gellan Gum/Silk Fibroin Hydrogels. Biomolecules 2020, 10, 1456. [Google Scholar] [CrossRef]
- Valderrama, P.; Jung, R.E.; Thoma, D.S.; Jones, A.A.; Cochran, D.L. Evaluation of parathyroid hormone bound to a synthetic matrix for guided bone regeneration around dental implants: A histomorphometric study in dogs. J. Periodontol. 2010, 81, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Chin, S.Y.; Poh, Y.C.; Kohler, A.C.; Sia, S.K. An Additive Manufacturing Technique for the Facile and Rapid Fabrication of Hydrogel-based Micromachines with Magnetically Responsive Components. J. Vis. Exp. JoVE 2018, 137, e56727. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.K.; Jung, U.W.; Thoma, D.S.; Hämmerle, C.H.F.; Jung, R.E. Osteogenic efficacy of BMP-2 mixed with hydrogel and bone substitute in peri-implant dehiscence defects in dogs: 16 weeks of healing. Clin. Oral Implant. Res. 2018, 29, 300–308. [Google Scholar] [CrossRef]
- Isaac, A.; Jivan, F.; Xin, S.; Hardin, J.; Luan, X.; Pandya, M.; Diekwisch, T.G.H.; Alge, D.L. Microporous Bio-orthogonally Annealed Particle Hydrogels for Tissue Engineering and Regenerative Medicine. ACS Biomater. Sci. Eng. 2019, 5, 6395–6404. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Nguyen, T.; Benoit, D.S.W. Matrix Control of Periodontal Ligament Cell Activity Via Synthetic Hydrogel Scaffolds. Tissue Eng. Part A 2021, 27, 733–747. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, N.; Zhang, T.; Sun, Q.; Han, B.; Yu, T. A Tetra-PEG Hydrogel Based Aspirin Sustained Release System Exerts Beneficial Effects on Periodontal Ligament Stem Cells Mediated Bone Regeneration. Front. Chem. 2019, 7, 682. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Chen, X.; Bai, S.; Li, B.; Liu, H.; Wu, G.; Liu, S.; Zhao, Y. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016, 11, 4707–4718. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Mansur, H.S.; Costa, E.D.S.; Mansur, A.A.P.; Barbosa-Stancioli, E.F. Cytocompatibility evaluation in cell-culture systems of chemically crosslinked chitosan/PVA hydrogels. Mater. Sci. Eng. C 2009, 29, 1574–1583. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Zhang, S.; Wang, B.; Shang, L.; Shao, J.; Lin, M.; Cui, Y.; Sun, S.; Ge, S. 6-Bromoindirubin-3′-oxime Promotes Osteogenic Differentiation of Periodontal Ligament Stem Cells and Facilitates Bone Regeneration in a Mouse Periodontitis Model. ACS Biomater. Sci. Eng. 2021, 7, 232–241. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Gruber, R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 52–69. [Google Scholar] [CrossRef]
- Mou, J.; Liu, Z.; Liu, J.; Lu, J.; Zhu, W.; Pei, D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: Preparation, characterization and evaluation. Drug Deliv. 2019, 26, 179–187. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, Y.; Chen, Y.; Liu, Y.; Tang, C.; Qu, X. Injectable Adhesive Hydrogel through a Microcapsule Cross-Link for Periodontitis Treatment. ACS Appl. Bio Mater. 2019, 2, 5985–5994. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Berglundh, T.; Al-Nawas, B.; Araujo, M.; Bartold, P.M.; Bouchard, P.; Chapple, I.; Gruber, R.; Lundberg, P.; Sculean, A.; et al. Biological factors involved in alveolar bone regeneration: Consensus report of Working Group 1 of the 15(th) European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 6–11. [Google Scholar] [CrossRef] [PubMed]
- Divband, B.; Aghazadeh, M.; Al-Qaim, Z.H.; Samiei, M.; Hussein, F.H.; Shaabani, A.; Shahi, S.; Sedghi, R. Bioactive chitosan biguanidine-based injectable hydrogels as a novel BMP-2 and VEGF carrier for osteogenesis of dental pulp stem cells. Carbohydr. Polym. 2021, 273, 118589. [Google Scholar] [CrossRef]
- Fujihara, C.; Kanai, Y.; Masumoto, R.; Kitagaki, J.; Matsumoto, M.; Yamada, S.; Kajikawa, T.; Murakami, S. Fibroblast growth factor-2 inhibits CD40-mediated periodontal inflammation. J. Cell. Physiol. 2019, 234, 7149–7160. [Google Scholar] [CrossRef] [PubMed]
- He, X.T.; Wu, R.X.; Xu, X.Y.; Wang, J.; Yin, Y.; Chen, F.M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018, 71, 132–147. [Google Scholar] [CrossRef]
- Hegedűs, O.; Juriga, D.; Sipos, E.; Voniatis, C.; Juhász, Á.; Idrissi, A.; Zrínyi, M.; Varga, G.; Jedlovszky-Hajdú, A.; Nagy, K.S. Free thiol groups on poly(aspartamide) based hydrogels facilitate tooth-derived progenitor cell proliferation and differentiation. PLoS ONE 2019, 14, e0226363. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, Y.; Qiu, J.; Zhang, Z.; Jin, Z.; Cao, M.; Jiao, Y.; Yang, H. Local SDF-1α application enhances the therapeutic efficacy of BMSCs transplantation in osteoporotic bone healing. Heliyon 2020, 6, e04347. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, M.; Hai, Z.; Wu, C.; Lin, J.; Kuang, W.; Tang, H.; Huang, Y.; Chen, X.; Liang, G. Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS Nano 2019, 13, 5616–5622. [Google Scholar] [CrossRef]
- Qu, L.; Dubey, N.; Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Xu, J.; Castilho, R.M.; Bottino, M.C. Metformin-loaded nanospheres-laden photocrosslinkable gelatin hydrogel for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2021, 116, 104293. [Google Scholar] [CrossRef]
- Shi, W.; Guo, S.; Liu, L.; Liu, Q.; Huo, F.; Ding, Y.; Tian, W. Small Extracellular Vesicles from Lipopolysaccharide-Preconditioned Dental Follicle Cells Promote Periodontal Regeneration in an Inflammatory Microenvironment. ACS Biomater. Sci. Eng. 2020, 6, 5797–5810. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021, 126, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Khandan, A. 14—Safety, regulatory issues, long-term biotoxicity, and the processing environment. In Nanobiomaterials Science, Development and Evaluation; Razavi, M., Thakor, A., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 261–279. [Google Scholar]
- Cui, P.; Pan, P.; Qin, L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact. Mater. 2023, 19, 487–498. [Google Scholar] [CrossRef]
- Farazin, A.; Aghadavoudi, F.; Motififard, M.; Saber-Samandari, S.; Khandan, A. Nanostructure, Molecular Dynamics Simulation and Mechanical Performance of PCL Membranes Reinforced with Antibacterial Nanoparticles. J. Appl. Comput. Mech. 2021, 7, 1907–1915. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl. Mater. Interfaces 2020, 12, 54497–54506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef] [PubMed]
- Mustfa, S.A.; Maurizi, E.; McGrath, J.; Chiappini, C. Nanomedicine Approaches to Negotiate Local Biobarriers for Topical Drug Delivery. Adv. Ther. 2020, 4, 2000160. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Sun, M.; Cheng, Z.; Jia, W.; Jiao, K.; Wang, S.; Jiang, K.; Yang, Y.; Dai, Z.; et al. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 2022, 146, 37–48. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.; Wang, Y.; Wu, J. Polydopamine/puerarin nanoparticle-incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Ou, Q.; Zhang, S.; Fu, C.; Yu, L.; Xin, P.; Gu, Z.; Cao, Z.; Wu, J.; Wang, Y. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 2021, 19, 237. [Google Scholar] [CrossRef]
- Yin, L.H.; Cheng, W.X.; Qin, Z.S.; Sun, K.M.; Zhong, M.; Wang, J.K.; Gao, W.Y.; Yu, Z.H. Effects of ginsenoside Rg-1 on the proliferation and osteogenic differentiation of human periodontal ligament stem cells. Chin. J. Integr. Med. 2015, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, S.; Yang, X.; Wu, J.; Kirk, T.B.; Xu, J.; Xu, A.; Xue, W. Injectable and Self-Healing Hydrogels with Double-Dynamic Bond Tunable Mechanical, Gel-Sol Transition and Drug Delivery Properties for Promoting Periodontium Regeneration in Periodontitis. ACS Appl. Mater. Interfaces 2021, 13, 61638–61652. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Ashrafihelan, J.; Salehi, R.; Aghazadeh, Z.; Rezabakhsh, A.; Hassanzadeh, A.; Firouzamandi, M.; Heidarzadeh, M.; Rahbarghazi, R.; Aghazadeh, M.; et al. In vivo evaluation of biocompatibility and immune modulation potential of poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone)-gelatin hydrogels enriched with nano-hydroxyapatite in the model of mouse. J. Biomater. Appl. 2021, 35, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.J.; Min, K.S. Effects of proanthocyanidin, a crosslinking agent, on physical and biological properties of collagen hydrogel scaffold. Restor. Dent. Endod. 2016, 41, 296–303. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef]
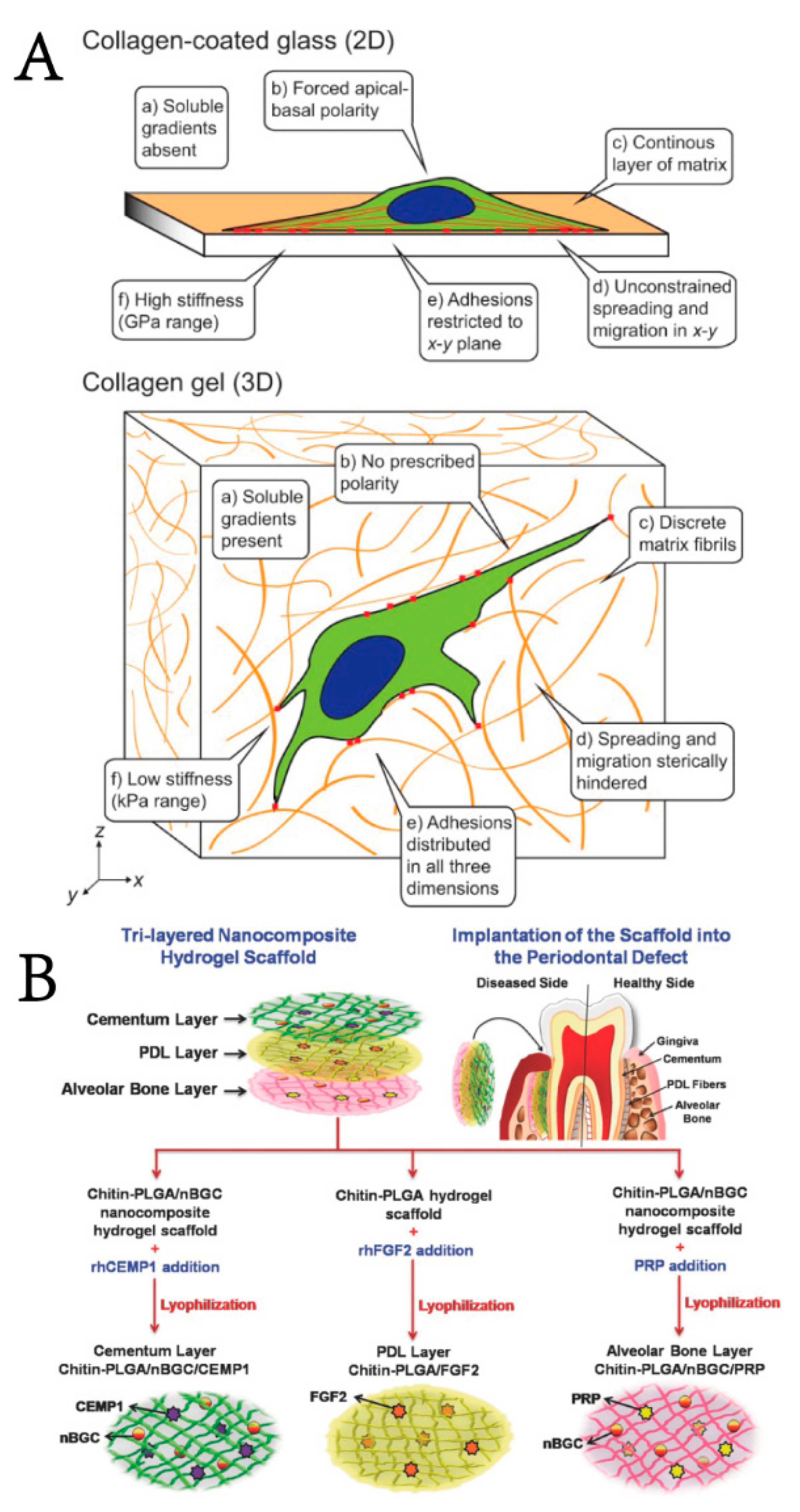
- Madl, C.M.; Heilshorn, S.C. Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annu. Rev. Biomed. Eng. 2018, 20, 21–47. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Dangaria, S.J.; Ito, Y.; Walker, C.; Druzinsky, R.; Luan, X.; Diekwisch, T.G. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differ. Res. Biol. Divers. 2009, 78, 79–90. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng. Part A 2014, 20, 611–621. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Mekhemar, M.K.; Beck-Broichsitter, B.E.; Bähr, T.; Hegab, M.; Receveur, J.; Heneweer, C.; Becker, S.T.; Wiltfang, J.; Dörfer, C.E. Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J. Clin. Periodontol. 2015, 42, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Zahid, S.; Ikram, F.; Maqbool, M.; Chaudhry, A.A.; Rahim, M.I.; Schmidt, F.; Goerke, O.; Khan, A.S.; Rehman, I.U. Tri-layered functionally graded membrane for potential application in periodontal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109812. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Petit, C.; Batool, F.; Stutz, C.; Anton, N.; Klymchenko, A.; Vandamme, T.; Benkirane-Jessel, N.; Huck, O. Development of a thermosensitive statin loaded chitosan-based hydrogel promoting bone healing. Int. J. Pharm. 2020, 586, 119534. [Google Scholar] [CrossRef]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Li, D.D.; Pan, J.F.; Ji, Q.X.; Yu, X.B.; Liu, L.S.; Li, H.; Jiao, X.J.; Wang, L. Characterization and cytocompatibility of thermosensitive hydrogel embedded with chitosan nanoparticles for delivery of bone morphogenetic protein-2 plasmid DNA. J. Mater. Sci. Mater. Med. 2016, 27, 134. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
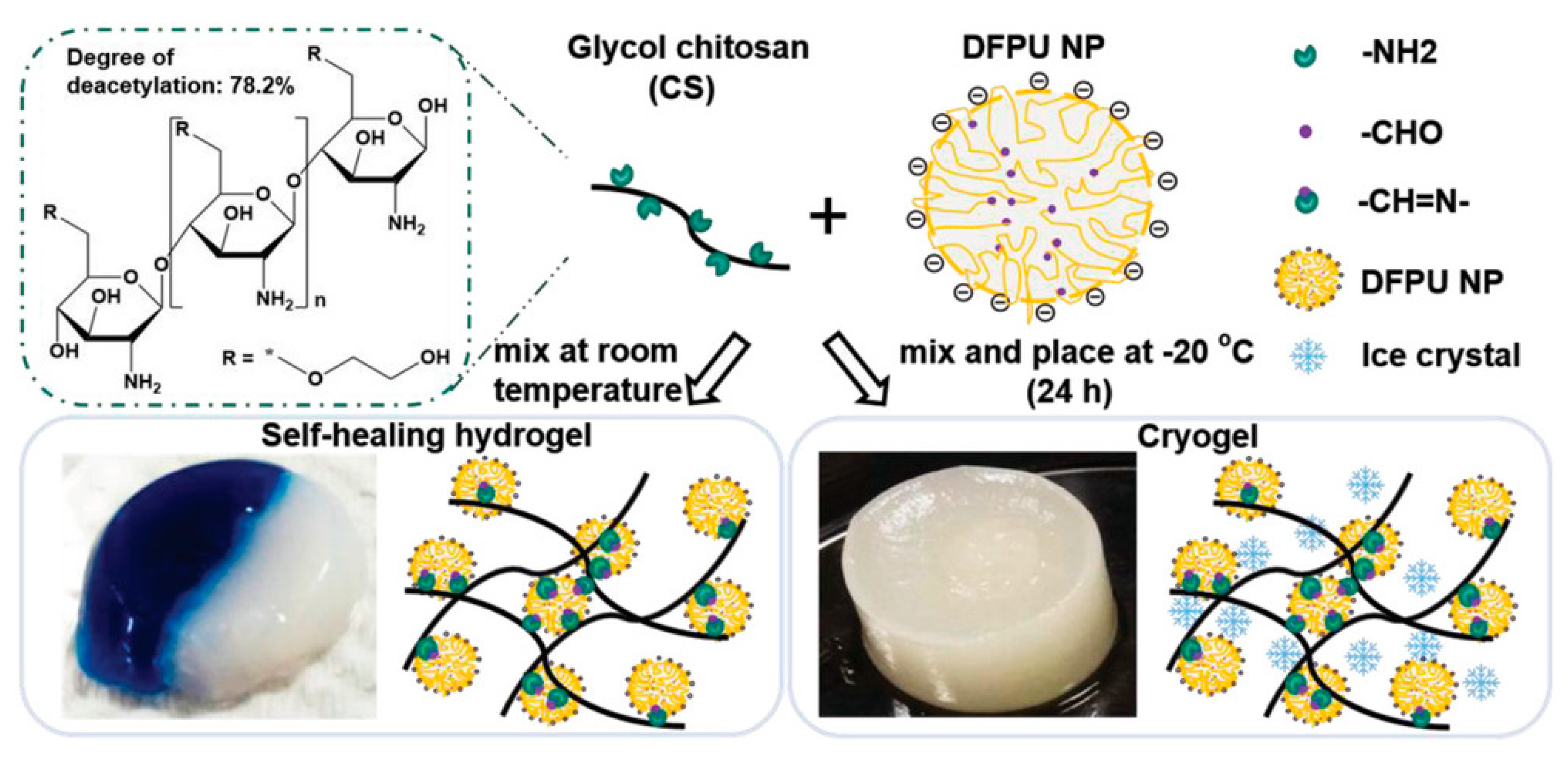
- Lin, T.W.; Hsu, S.H. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Adv. Sci. 2020, 7, 1901388. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Rezaeifard, S.; Naeimi, M.; Ghasemi, S.; Mohammadi-Samani, S.; Welland, M.E.; Tayebi, L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam). Int. J. Nanomed. 2019, 14, 6901–6915. [Google Scholar] [CrossRef]
- Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl-Riboflavin (GelMA-RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef]
- Leung, B.; Dharmaratne, P.; Yan, W.; Chan, B.C.L.; Lau, C.B.S.; Fung, K.P.; Ip, M.; Leung, S.S.Y. Development of thermosensitive hydrogel containing methylene blue for topical antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 203, 111776. [Google Scholar] [CrossRef]
- Kariu, T.; Nakao, R.; Ikeda, T.; Nakashima, K.; Potempa, J.; Imamura, T. Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. J. Periodontal Res. 2017, 52, 89–96. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrínyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Z.; Chen, D.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Poly(aspartic acid) based self-healing hydrogels with antibacterial and light-emitting properties for wound repair. Colloids Surf. B Biointerfaces 2021, 200, 111568. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, X. Host-Guest Chemistry in Supramolecular Theranostics. Theranostics 2019, 9, 3041–3074. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, M.; Huang, X.; Zhang, X.; Lu, C.; Song, J.; Yang, H. A bioinspired mineral-organic composite hydrogel as a self-healable and mechanically robust bone graft for promoting bone regeneration. Chem. Eng. J. 2021, 413, 127512. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Lv, J.; Yang, Y.; Cheng, G.; Guo, S.; Liu, C.; Ding, Y. Advances of Hydrogel Therapy in Periodontal Regeneration—A Materials Perspective Review. Gels 2022, 8, 624. https://doi.org/10.3390/gels8100624
Li M, Lv J, Yang Y, Cheng G, Guo S, Liu C, Ding Y. Advances of Hydrogel Therapy in Periodontal Regeneration—A Materials Perspective Review. Gels. 2022; 8(10):624. https://doi.org/10.3390/gels8100624
Chicago/Turabian StyleLi, Maoxue, Jiaxi Lv, Yi Yang, Guoping Cheng, Shujuan Guo, Chengcheng Liu, and Yi Ding. 2022. "Advances of Hydrogel Therapy in Periodontal Regeneration—A Materials Perspective Review" Gels 8, no. 10: 624. https://doi.org/10.3390/gels8100624
APA StyleLi, M., Lv, J., Yang, Y., Cheng, G., Guo, S., Liu, C., & Ding, Y. (2022). Advances of Hydrogel Therapy in Periodontal Regeneration—A Materials Perspective Review. Gels, 8(10), 624. https://doi.org/10.3390/gels8100624





