Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives
Abstract
1. Introduction
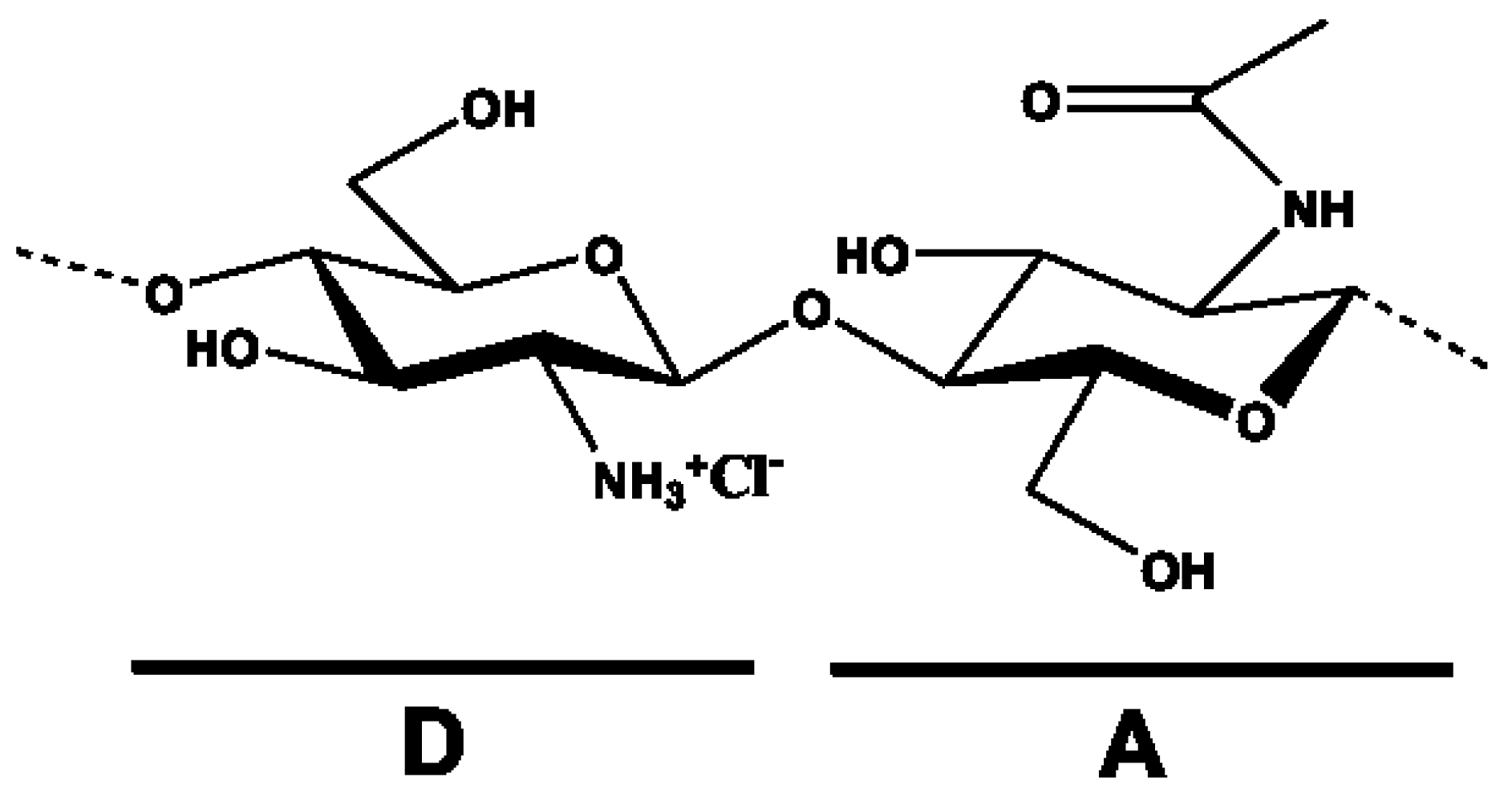
2. An Overview of Chitosan Physical/Chemical Properties and Biological Activities
3. Physical Gelation of Chitosan
4. Chitosan Gels without External Crosslinkers
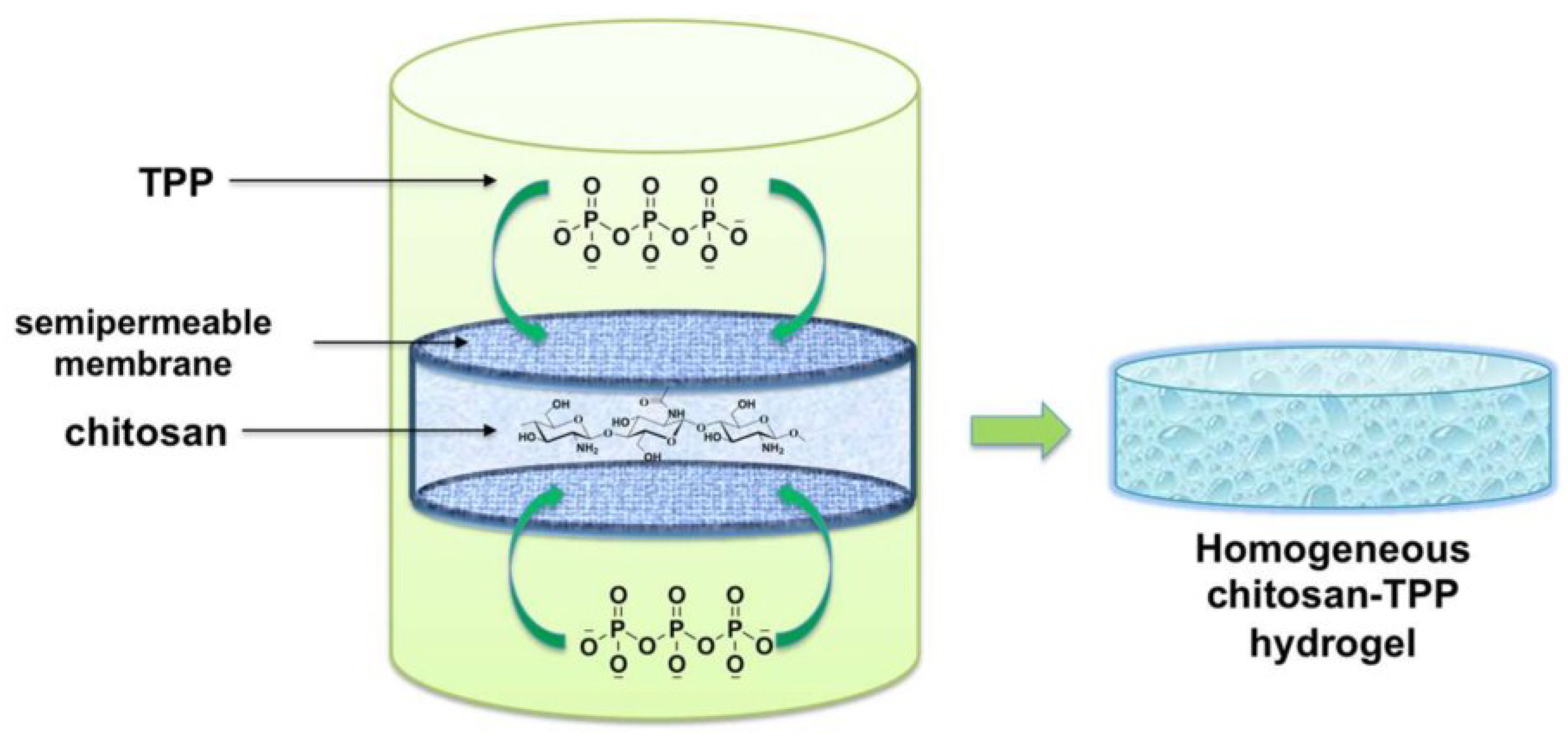
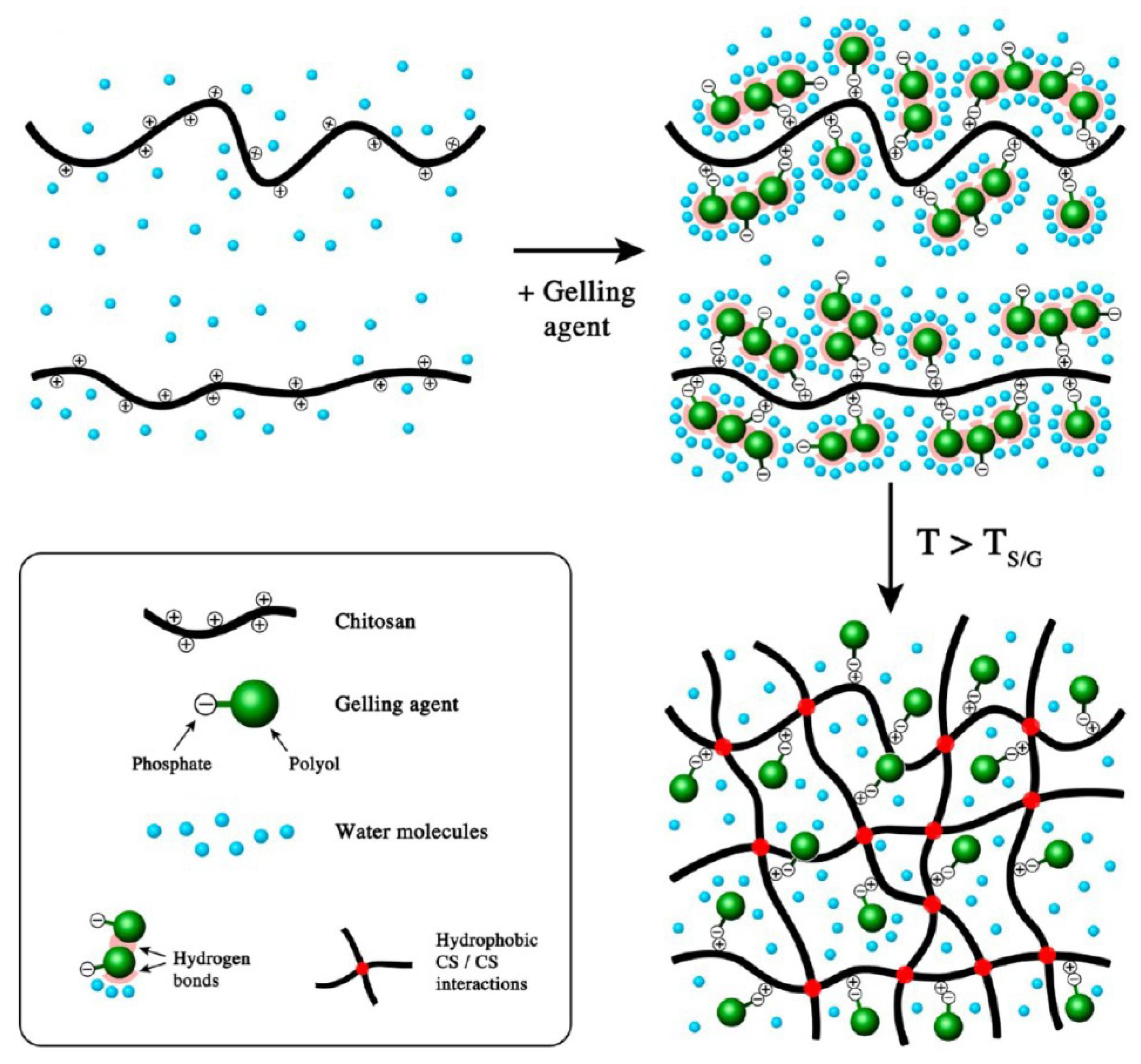
5. Ionic Chitosan Macro-Gels
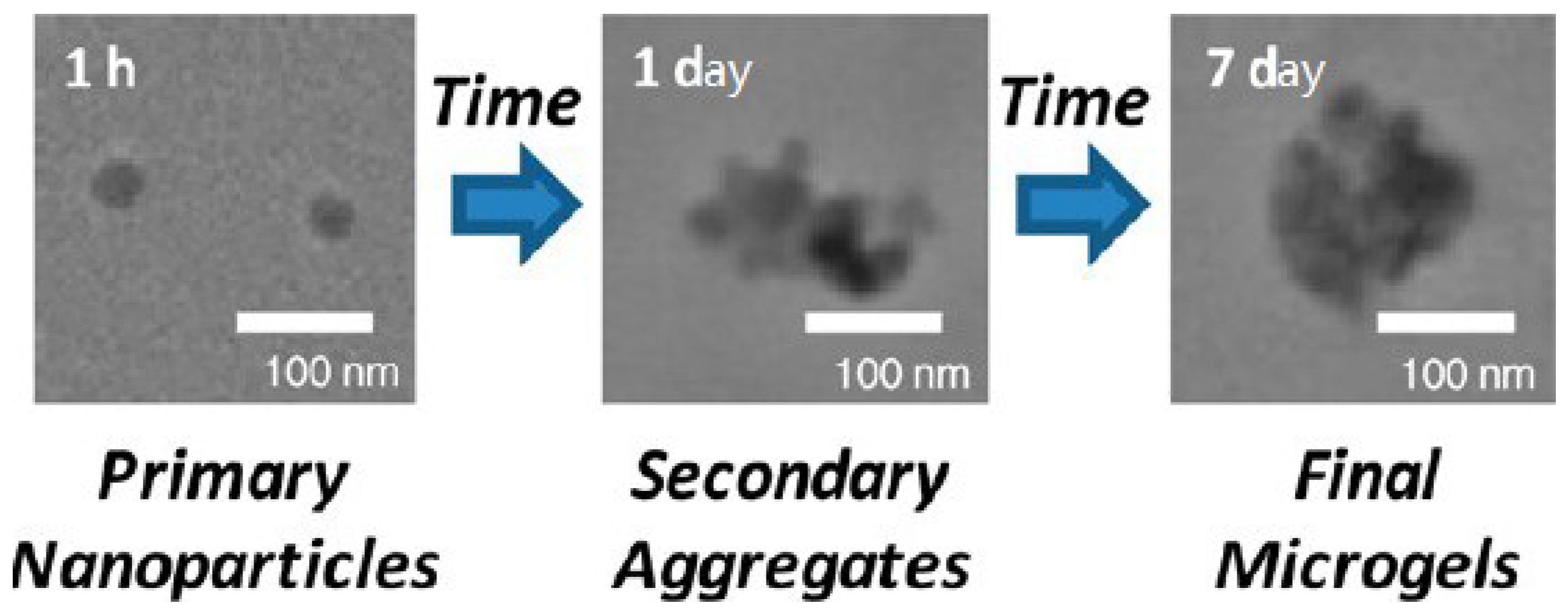
6. Ionic Chitosan Micro- and Nano-Gels
6.1. Simple Coacervation
6.2. Complex Coacervation
6.2.1. Hyaluronic Acid
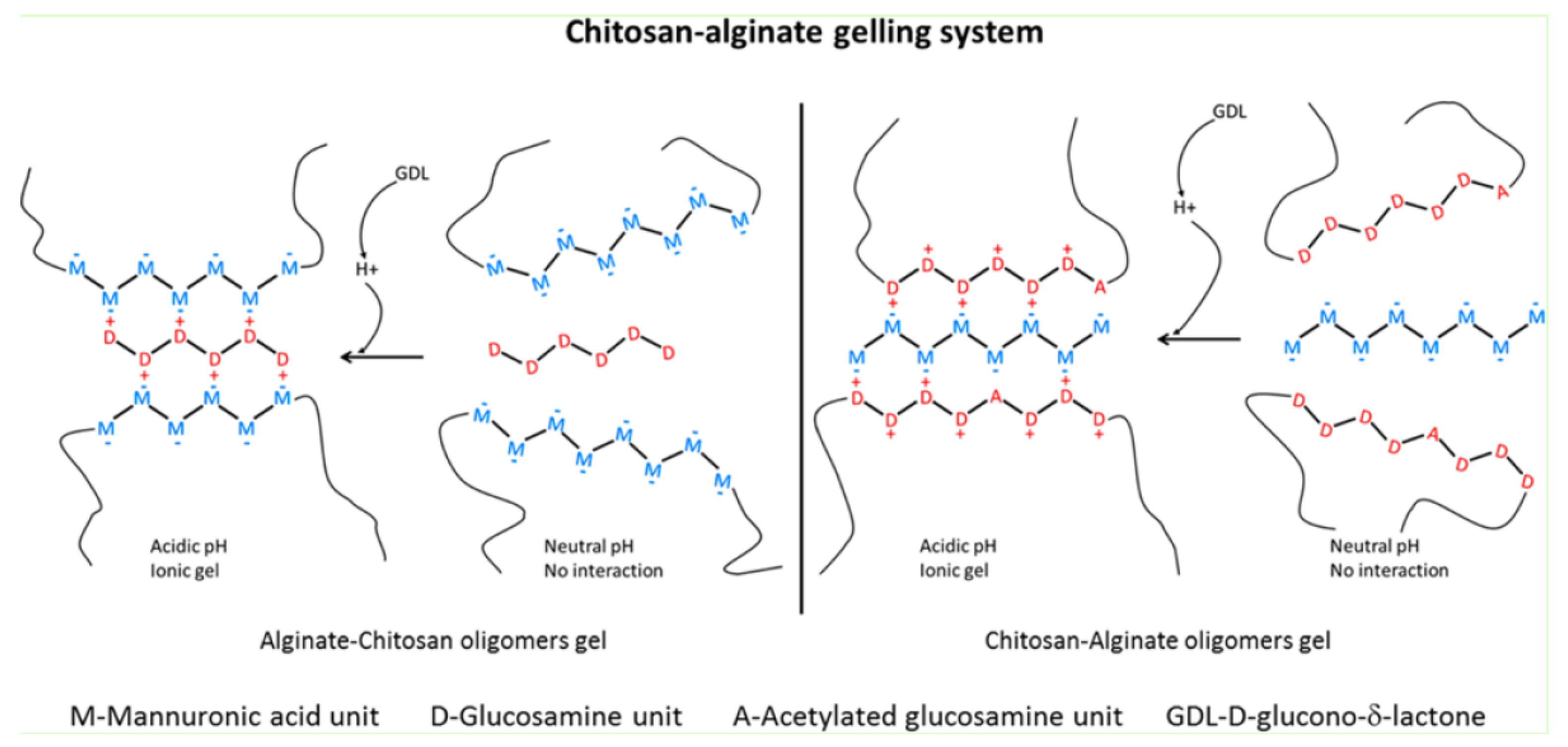
6.2.2. Alginate
6.2.3. Dextran Sulfate
6.2.4. Carrageenan
7. Thermosensitive Gels
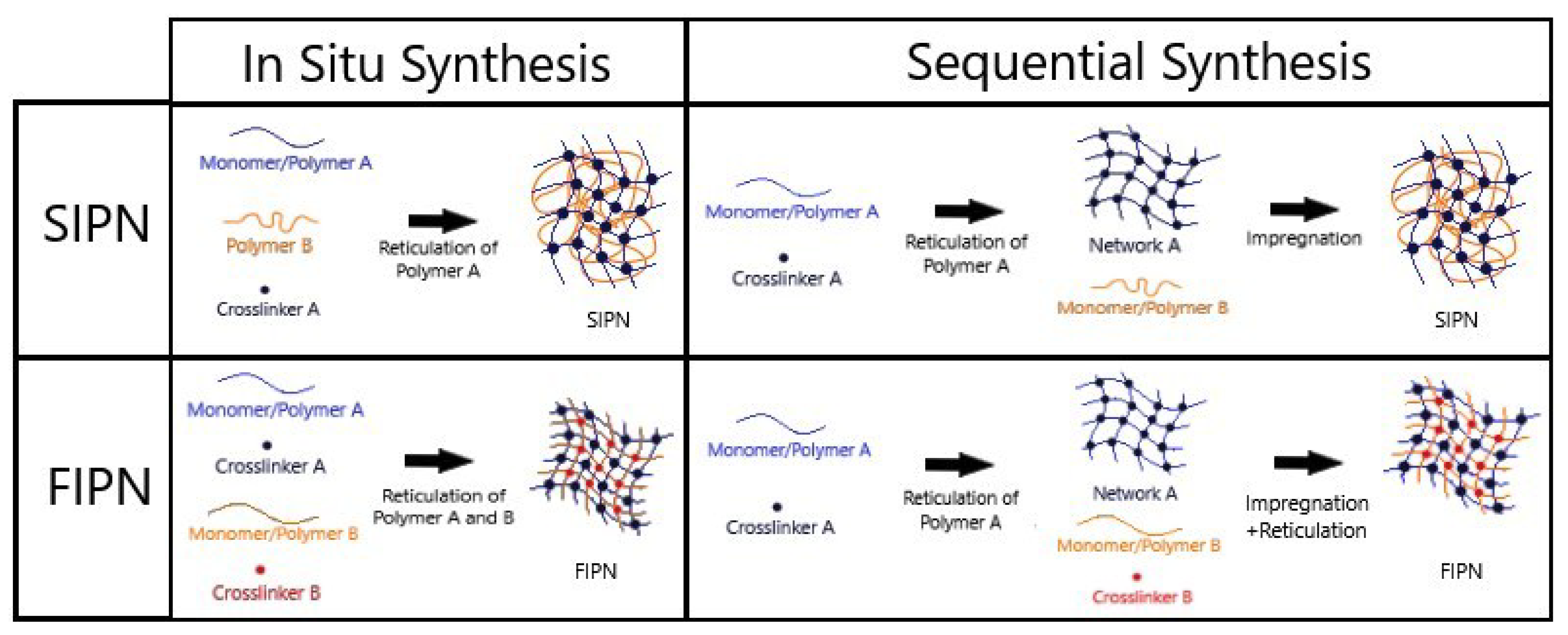
8. Interpenetrating Polymer Network (IPN)
8.1. Drug Delivery and Wound Dressing
8.2. Tridimensional Cellular Scaffolds
8.3. Bio-Electro Sensing and Soft Actuators
8.4. Tissue Engineering
9. Chitosan Derivatives
Physical Gels Based on Chitosan Derivatives
10. Lactose-Modified Chitosan (CTL)
11. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lapasin, R. Rheological characterization of hydrogels. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Pan Stanford: Singapore, 2005; pp. 83–137. [Google Scholar]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Smidsrød, O. Structure-property relationship in chitosans. In Polysaccharides: Structural Diversity and Functional Versatility; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Vårum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrød, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field n.m.r. spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, M.W.; Smidsrød, O. Hydrogen ion titration of chitosans with varying degrees of N-acetylation by monitoring induced 1H-NMR chemical shifts. Carbohydr. Polym. 1995, 26, 303–305. [Google Scholar] [CrossRef]
- Strand, S.P.; Tømmeraas, K.; Vårum, K.M.; Østgaard, K. Electrophoretic light scattering studies of chitosans with different degrees of N-acetylation. Biomacromolecules 2001, 2, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling concept combining chitosan and alginate-proof of principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Salt-assisted mechanistic analysis of chitosan/tripolyphosphate micro- and nanogel formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Kondo, S.; Ohe, Y. Chitosan gel: A novel polysaccharide gel. Polymer 1975, 16, 2716. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Chitosan gels: 1. Study of reaction variables. Int. J. Biol. Macromol. 1980, 2, 73–77. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Chitosan gels: 2. Mechanism of gelation. Int. J. Biol. Macromol. 1980, 2, 78–80. [Google Scholar] [CrossRef]
- Vachoud, L.; Zydowicz, N.; Domard, A. Formation and characterisation of a physical chitin gel. Carbohydr. Res. 1997, 302, 169–177. [Google Scholar] [CrossRef]
- Hirano, S.; Ohe, Y.; Ono, H. Selective N-acylation of chitosan. Carbohydr. Res. 1976, 47, 315–320. [Google Scholar] [CrossRef]
- Vachoud, L.; Zydowicz, N.; Domard, A. Physicochemical behaviour of chitin gels. Carbohydr. Res. 2000, 326, 295–304. [Google Scholar] [CrossRef]
- Gérentes, P.; Vachoud, L.; Doury, J.; Domard, A. Study of a chitin-based gel as injectable material in periodontal surgery. Biomaterials 2002, 23, 1295–1302. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in aqueous solution without cross-linking agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan hydrogels for the regeneration of infarcted myocardium: Preparation, physicochemical characterization, and biological evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Viton, C.; Domard, A. Physico-chemical studies of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Boucard, N.; Viton, C.; Domard, A. New aspects of the formation of physical hydrogels of chitosan in a hydroalcoholic medium. Biomacromolecules 2005, 6, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.G.; Tahiri, K.; Montembault, A.S.; Domard, A.J.; Corvol, M.-T.M. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials 2011, 32, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Malaise, S.; Delmond, S.; Fricain, J.-C.; Siadous, R.; Schlaubitz, S.; Laurichesse, E.; Amédée, J.; Montembault, A.; David, L.; et al. Physicochemical modulation of chitosan-based hydrogels induces different biological responses: Interest for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Terbojevich, M.; Carraro, C.; Cosani, A.; Marsano, E. Solution studies of the chitin-lithium chloride-N,N-di-methylacetamide system. Carbohydr. Res. 1988, 180, 73–86. [Google Scholar] [CrossRef]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-based networks from homogeneous chitosan-tripolyphosphate hydrogels: Synthesis and characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the correlation between the microscopic structure and properties of phosphate-cross-linked chitosan gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic hydrogel based on chitosan cross-linked with 6-phosphogluconic trisodium salt as a drug delivery system. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Vårum, K.M.; Moen, E.; Gynnild, H.; Smidsrød, O. Chitosan cross-linked with Mo(VI) polyoxyanions: A new gelling system. Biomaterials 1992, 13, 635–638. [Google Scholar] [CrossRef]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 2009, 10, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Vårum, K.M.; Marsich, E. Butyrate-loaded chitosan/hyaluronan nanoparticles: A suitable tool for sustained inhibition of ROS release by activated neutrophils. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. Determining the colloidal behavior of ionically cross-linked polyelectrolytes with isothermal titration calorimetry. J. Phys. Chem. B 2013, 117, 9548–9557. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. On the kinetics of chitosan/tripolyphosphate micro- and nanogel aggregation and their effects on particle polydispersity. J. Colloid Interface Sci. 2017, 486, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors affecting the stability of chitosan/tripolyphosphate micro- and nanogels: Resolving the opposing findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef]
- Lee, D.; Powers, K.; Baney, R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr. Polym. 2004, 58, 371–377. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Oh, M.; Allen, C.; Kumacheva, E. Monodisperse chitosan nanoparticles for mucosal drug delivery. Biomacromolecules 2004, 5, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.; Abdul Majeed, S.; Taju, G.; Nambi, K.S.N.; Sundar Raj, N.; Madan, N.; Farook, M.A.; Rajkumar, T.; Gopinath, D.; Sahul Hameed, A.S. Chitosan tripolyphosphate (CS/TPP) nanoparticles: Preparation, characterization and application for gene delivery in shrimp. Acta Trop. 2013, 128, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; RemunanLopez, C.; VilaJato, J.L.; Alonso, M.J.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan ethylene oxide propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Sanchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Janes, K.A.; Alonso, M.J. Depolymerized chitosan nanoparticles for protein delivery: Preparation and characterization. J. Appl. Polym. Sci. 2003, 88, 2769–2776. [Google Scholar] [CrossRef]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 2005, 295, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Abul Kalam, M.; Khan, A.A.; Khan, S.; Almalik, A.; Alshamsan, A. Optimizing indomethacin-loaded chitosan nanoparticle size, encapsulation, and release using Box-Behnken experimental design. Int. J. Biol. Macromol. 2016, 87, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lapitsky, Y. Analysis of chitosan/tripolyphosphate micro- and nanogel yields is key to understanding their protein uptake performance. J. Colloid Interface Sci. 2017, 494, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V. V Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Manzoor, S.; Rehman, A.; Rahman, A.; Nasir, H.; Imran, M. Antihypertensive nano-ceuticales based on chitosan biopolymer: Physico-chemical evaluation and release kinetics. Carbohydr. Polym. 2016, 142, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Umerska, A.; Paluch, K.J.; Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Exploring the assembly process and properties of novel crosslinker-free hyaluronate-based polyelectrolyte complex nanocarriers. Int. J. Pharm. 2012, 436, 75–87. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Delair, T. Stabilization of chitosan/hyaluronan colloidal polyelectrolyte complexes in physiological conditions. Carbohydr. Polym. 2015, 119, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Donati, I.; Paoletti, S.; Marsich, E. Chitosan acetylation degree influences the physical properties of polysaccharide nanoparticles: implication for the innate immune cells response. submitted.
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Parajó, Y.; D’Angelo, I.; Welle, A.; Garcia-Fuentes, M.; Alonso, M.J. Hyaluronic acid/Chitosan nanoparticles as delivery vehicles for VEGF and PDGF-BB. Drug Deliv. 2010, 17, 596–604. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Design of novel polysaccharidic nanostructures for gene delivery. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic acid (HA) presentation as a tool to modulate and control the receptor-mediated uptake of HA-coated nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.X.; Tang, Z.Y. The prognostic molecular markers in hepatocellular carcinoma. World J. Gastroenterol. 2002, 8, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Rios de la Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/hyaluronic acid nanoparticles: Rational design revisited for RNA delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Alradwan, I.; Majrashi, M.A.; Alsaffar, B.A.; Algarni, A.T.; Alsuabeyl, M.S.; Alrabiah, H.; Tirelli, N.; Alhasan, A.H. Cellular responses of hyaluronic acid coated-chitosan nanoparticles. Toxicol. Res. 2018. [Google Scholar] [CrossRef]
- Nasti, A.; Zaki, N.M.; De Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Day, P.J.; Tirelli, N. HA-coated chitosan nanoparticles for CD44-mediated nucleic acid delivery. Macromol. Biosci. 2013, 13, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Bianchini, C.; Forte, M.M.C.; Kist, T.B.L. Influence of the composition and preparation method on the morphology and swelling behavior of alginate-chitosan hydrogels. Carbohydr. Polym. 2008, 74, 283–289. [Google Scholar] [CrossRef]
- Costalat, M.; David, L.; Delair, T. Reversible controlled assembly of chitosan and dextran sulfate: A new method for nanoparticle elaboration. Carbohydr. Polym. 2014, 102, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Costalat, M.; Alcouffe, P.; David, L.; Delair, T. Macro-hydrogels versus nanoparticles by the controlled assembly of polysaccharides. Carbohydr. Polym. 2015, 134, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Drogoz, A.; David, L.; Domard, A.; Charles, M.H.; Verrier, B.; Delair, T. Polysaccharide-based vaccine delivery systems: Macromolecular assembly, interactions with antigen presenting cells, and in vivo immunomonitoring. J. Biomed. Mater. Res. Part A 2010, 93, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Da Costa, A.M.R.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remuñán-López, C.; Grenha, A. Hybrid nanosystems based on natural polymers as protein carriers for respiratory delivery: Stability and toxicological evaluation. Carbohydr. Polym. 2015, 123, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Boisclair, J.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Chitosan/glucose 1-phosphate as new stable in situ forming depot system for controlled drug delivery. Eur. J. Pharm. Biopharm. 2014, 88, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological study of chitosan/polyol-phosphate systems: Influence of the polyol part on the thermo-induced gelation mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Filion, D.; Buschmann, M.D. Heat-induced transfer of protons from chitosan to glycerol phosphate produces chitosan precipitation and gelation. Biomacromolecules 2008, 9, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.T.; Han, H.; Larson, I.; Dass, C.R.; Dunstan, D.E. Chitosan-dibasic orthophosphate hydrogel: A potential drug delivery system. Int. J. Pharm. 2009, 371, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Gurny, R.; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Chang, H.W.; Yu, H.C.; Lin, Y.S.; Tsai, Y.D. Effect of chitosan characteristics and solution conditions on gelation temperatures of chitosan/2-glycerophosphate/nanosilver hydrogels. Carbohydr. Polym. 2011, 84, 1337–1343. [Google Scholar] [CrossRef]
- Ganji, F.; Abdekhodaie, M.J.; Ramazani, A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J. Sol-Gel Sci. Technol. 2007, 42, 47–53. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Bastrop, M.; Hvilsom, A.; Contri, R.V.; Mäder, K. Characterization of thermosensitive chitosan-based hydrogels by rheology and electron paramagnetic resonance spectroscopy. Eur. J. Pharm. Biopharm. 2008, 68, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, G.; Leroux, J.-C.; Damas, J.; Adam, A. Biocompatibility of thermosensitive chitosan-based hydrogels: An in vivo experimental approach to injectable biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Sun, J.; Légaré, A.; McKee, M.D.; Buschmann, M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartil. 2005, 13, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Dai, L.; Man, Z.; Yuan, L.; Chen, H.; Zhou, C.; et al. A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials 2014, 35, 9608–9619. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Hu, X.; Dai, L.; Zhu, J.; Man, Z.; Chen, H.; Zhou, C.; Ao, Y. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Dessì, M.; Borzacchiello, A.; Mohamed, T.H.A.; Abdel-Fattah, W.I.; Ambrosio, L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Man, Z.; Dai, L.; Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Zhang, J.; Fu, X.; et al. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Starnes, T.; Ko, J.W.K.; Laurencin, C.T. Development of injectable thermogelling chitosan-inorganic phosphate solutions for biomedical applications. Biomacromolecules 2007, 8, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, X.; Wang, X.; Shi, S.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Gel-sol-gel thermo-gelation behavior study of chitosan-inorganic phosphate solutions. Eur. J. Pharm. Biopharm. 2010, 75, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, G.; Gu, S.; Hu, Z.; Yao, P. Novel interpenetrating polymer network sulfonated poly (phthalazinone ether sulfone ketone)/polyacrylic acid proton exchange membranes for fuel cell. J. Memb. Sci. 2007, 295, 80–87. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Naeem, S.; Ramesh, S.; Ramesh, K. pH responsive N-succinyl chitosan/Poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Tinoco, D.; Ortega, A.; Burillo, G.; Nucleares, I.D.C.; Nacional, U.; De México, A.; Universitaria, C. Different hydrogel architectures synthesized by gamma radiation based on chitosan and N,N-dimethylacrylamide. MRS Commun. 2018. [Google Scholar] [CrossRef]
- Borgogna, M.; Marsich, E.; Donati, I.; Paoletti, S.; Travan, A. Hydrogels. In Polysaccharide Hydrogels Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Pan Stanford: Singapore, 2016. [Google Scholar]
- Wang, Y.; Zhang, X.; Qiu, D.; Li, Y.; Yao, L.; Duan, J. Ultrasonic assisted microwave synthesis of poly (Chitosan-co-gelatin)/polyvinyl pyrrolidone IPN hydrogel. Ultrason. Sonochem. 2018, 40, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.M. Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Treenate, P.; Monvisade, P.; Yamaguchi, M. Development of hydroxyethylacryl chitosan/alginate hydrogel films for biomedical application. J. Polym. Res. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Hernández, P.; Lucero-Acuña, A.; Gutiérrez-Valenzuela, C.A.; Moreno, R.; Esquivel, R. Systematic evaluation of pH and thermoresponsive poly(n-isopropylacrylamide-chitosan-fluorescein) microgel. E-Polymers 2017, 17, 399–408. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, S.R.; Kim, N.G.; Kim, S.I. Swelling behavior of semi-interpenetrating polymer network hydrogels based on chitosan and poly(acryl amide). J. Macromol. Sci. Part A 2005, 42, 1073–1083. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Gao, X.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Semi-interpenetrating polymer network hydrogels based on water-soluble N-carboxylethyl chitosan and photopolymerized poly (2-hydroxyethyl methacrylate). Carbohydr. Polym. 2009, 75, 293–298. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Gao, C. Chemically cross-linked chitosan hydrogel loaded with gelatin for chondrocyte encapsulation. Biotechnol. J. 2011, 6, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.J.; Kim, I.Y.; Shin, M.S.; Kim, S.I. Electric stimuli responses to poly(vinyl alcohol)/chitosan interpenetrating polymer network hydrogel in NaCl solutions. J. Appl. Polym. Sci. 2002, 86, 2285–2289. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.I.L.; Shin, S.R.; Kim, S.I. Electrical Behavior of Chitosan and poly(hydroxyethyl/rmethacrylate) hydrogel in the contact system. J. Appl. Polym. Sci. 2004, 92, 915–919. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, W.; Li, X.; Zeng, J.; Wu, L. Direct electrochemistry and electrocatalysis of hemoglobin entrapped in semi-interpenetrating polymer network hydrogel based on polyacrylamide and chitosan. Bioelectrochemistry 2007, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Duan, H.; Zhu, L.; Li, G.; Ban, Q.; Lucia, L.A. A semi-interpenetrating network polyampholyte hydrogel simultaneously demonstrating remarkable toughness and antibacterial properties. New J. Chem. 2016, 40, 10520–10525. [Google Scholar] [CrossRef]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, K.; Wang, Y.P.; Zhang, S.T. Preparation and PH responsive behavior of poly(vinyl alcohol)-chitosan- poly(acrylic acid) full-IPN hydrogels. J. Bioact. Compat. Polym. 2007, 22, 207–218. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rabea, E.I.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Diolosà, M.; Donati, I.; Turco, G.; Cadenaro, M.; Di Lenarda, R.; Breschi, L.; Paoletti, S. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules 2014, 15, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Nah, J.W.; Cho, M.-H.; Park, T.G.; Cho, C.S. Receptor-mediated gene delivery into antigen presenting cells using mannosylated chitosan/DNA nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.; Xiong, W.; Wang, H.; Sun, Y.; Liu, H. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, L.; Yu, Z.; Feng, J.; Yu, Q. Role of mannose receptor in oligochitosan-mediated stimulation of macrophage function. Int. Immunopharmacol. 2005, 5, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Xie, S.; Cao, J.; Ge, H.; Xu, M.; Zhang, L.; Zhou, J. Quaternized chitosan/poly(acrylic acid) polyelectrolyte complex hydrogels with tough, self-recovery, and tunable mechanical properties. Macromolecules 2016, 49, 1049–1059. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Recillas-Mota, M.; Fernández-Quiroz, D. Chitosan-based thermosensitive materials. In Biological Activities and Application of Marine Polysaccharides; InTechOpenLimited: London, UK, 2017. [Google Scholar]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Hu, J.W. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Cheng, T.H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, J.; Fan, H.; Zhang, X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr. Polym. 2012, 88, 46–53. [Google Scholar] [CrossRef]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Bharani, S.; Patil, S. Layer-by-layer self-assembly of modified hyaluronic acid/chitosan based on hydrogen bonding. Biomacromolecules 2009, 10, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, S.; Donati, I.; Marsich, E. Polymer Mixtures of Anionic and Cationic Polysaccharides and Use Thereof. U.S. Patent US8951991B2, WO/2007/135116A1, EP2021408B1, 21 May 2007. [Google Scholar]
- Donati, I.; Borgogna, M.; Turello, E.; Cesàro, A.; Paoletti, S. Tuning supramolecular structuring at the nanoscale level: Nonstoichiometric soluble complexes in dilute mixed solutions of alginate and lactose-modified chitosan (chitlac). Biomacromolecules 2007, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Haug, I.J.; Scarpa, T.; Borgogna, M.; Draget, K.I.; Skjåk-Braek, G.; Paoletti, S. Synergistic effects in semidilute mixed solutions of alginate and lactose-modified chitosan (chitlac). Biomacromolecules 2007, 8, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Feresini, M.; Travan, A.; Marsich, E.; Lapasin, R.; Paoletti, S. Polysaccharide-based polyanion–polycation–polyanion ternary systems. a preliminary analysis of interpolyelectrolyte interactions in dilute solutions. Biomacromolecules 2011, 12, 4044–4056. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Travan, A.; Feresini, M.; Lapasin, R.; Paoletti, S.; Donati, I. Polysaccharide-based polyanion-polycation-polyanion ternary systems in the concentrated regime and hydrogel form. Macromol. Chem. Phys. 2013, 214, 1309–1320. [Google Scholar] [CrossRef]
- D’Amelio, N.; Esteban, C.; Coslovi, A.; Feruglio, L.; Uggeri, F.; Villegas, M.; Benegas, J.; Paoletti, S.; Donati, I. Insight into the molecular properties of Chitlac, a chitosan derivative for tissue engineering. J. Phys. Chem. B 2013, 117, 13578–13587. [Google Scholar] [CrossRef] [PubMed]
- Esteban, C.; Donati, I.; Pantano, S.; Villegas, M.; Benegas, J.; Paoletti, S. Dissecting the conformational determinants of chitosan and chitlac oligomers. Biopolymers 2018, 109. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Stredanska, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Marcon, P.; Marsich, E.; Vetere, A.; Mozetic, P.; Campa, C.; Donati, I.; Vittur, F.; Gamini, A.; Paoletti, S. The role of Galectin-1 in the interaction between chondrocytes and a lactose-modified chitosan. Biomaterials 2005, 26, 4975–4984. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Borgogna, M.; Donati, I.; Mozetic, P.; Strand, B.L.; Salvador, S.G.; Vittur, F.; Paoletti, S. Alginate/lactose-modified chitosan hydrogels: A bioactive biomaterial for chondrocyte encapsulation. J. Biomed. Mater. Res. A 2008, 84, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Foulc, M.-P.; Moritz, N.; Aro, H.T.; Paoletti, S. Polysaccharide-coated thermosets for orthopedic applications: From material characterization to in vivo tests. Biomacromolecules 2012, 13, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gao, X.; Sun, J.; Xiao, C.; Hu, X. Double stimulus-induced stem cell aggregation during differentiation on a biopolymer hydrogel substrate. Chem. Commun. 2013, 49. [Google Scholar] [CrossRef] [PubMed]
- Medelin, M.; Porrelli, D.; Aurand, E.R.; Scaini, D.; Travan, A.; Borgogna, M.A.; Cok, M.; Donati, I.; Marsich, E.; Scopa, C.; et al. Exploiting natural polysaccharides to enhance in vitro bio-constructs of primary neurons and progenitor cells. Acta Biomater. 2018, 73, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Furlani, F.; Cok, M.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Boric acid induced transient cross-links in lactose-modified chitosan (chitlac). Biomacromolecules 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Cok, M.; Sacco, P.; Porrelli, D.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Mimicking mechanical response of natural tissues. Strain hardening induced by transient reticulation in lactose-modified chitosan (chitlac). Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex coacervates between a lactose-modified chitosan and hyaluronic acid as radical-scavenging drug carriers. 2018; submitted. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. https://doi.org/10.3390/gels4030067
Sacco P, Furlani F, De Marzo G, Marsich E, Paoletti S, Donati I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels. 2018; 4(3):67. https://doi.org/10.3390/gels4030067
Chicago/Turabian StyleSacco, Pasquale, Franco Furlani, Gaia De Marzo, Eleonora Marsich, Sergio Paoletti, and Ivan Donati. 2018. "Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives" Gels 4, no. 3: 67. https://doi.org/10.3390/gels4030067
APA StyleSacco, P., Furlani, F., De Marzo, G., Marsich, E., Paoletti, S., & Donati, I. (2018). Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels, 4(3), 67. https://doi.org/10.3390/gels4030067








