Natural Waxes as Gelators in Edible Structured Oil Systems: A Review
Abstract
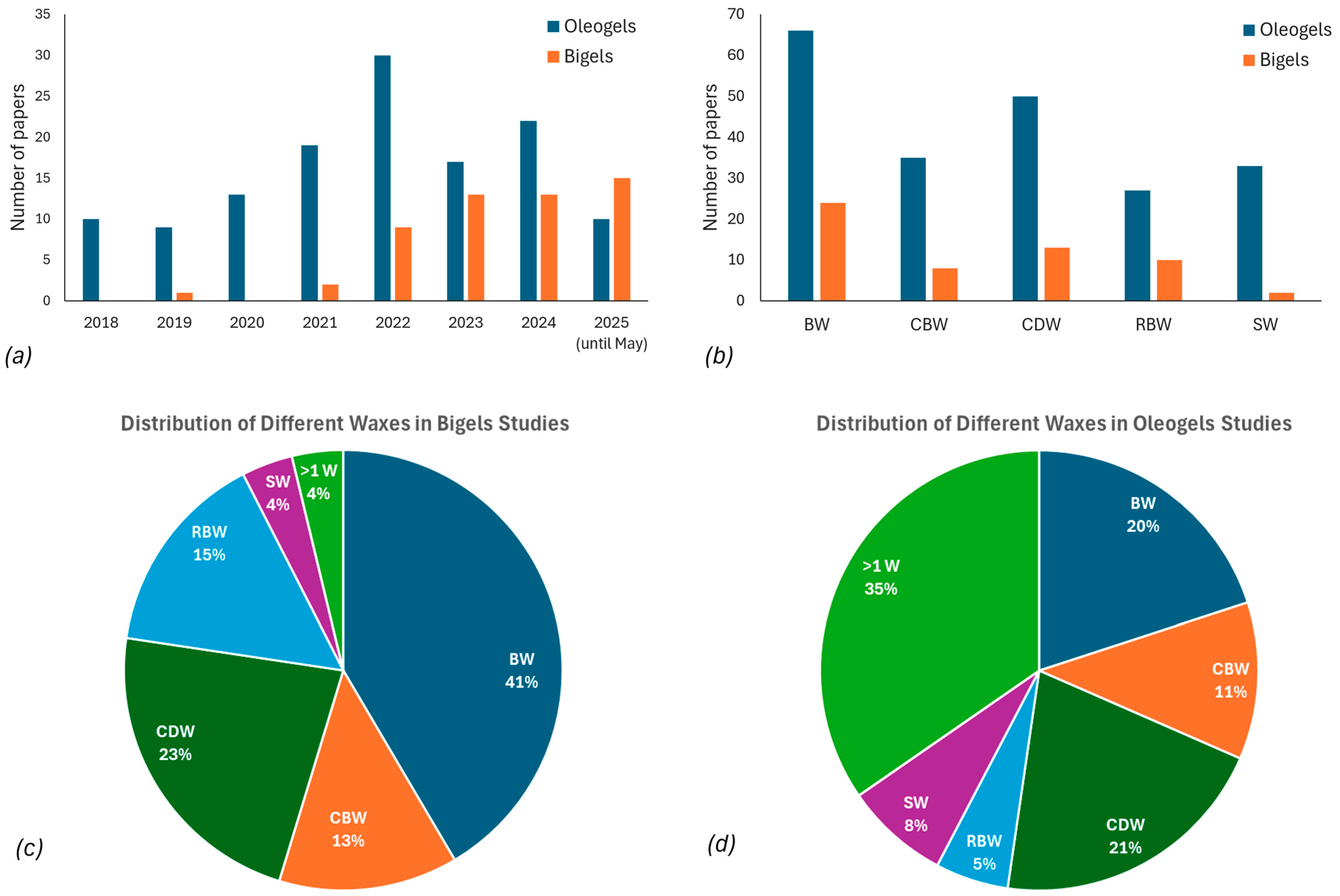
1. Introduction
2. Wax-Based Oleogels
2.1. Definition and Structure of Oleogels
2.2. Composition Design of Wax-Based Oleogels
Edible Oils
| Oleogelators | Edible Oil | Characteristics Studied and Application | Ref. |
|---|---|---|---|
| Beeswax | |||
| BW (10%), hydrocolloid blend (sodium caseinate (3.15%), guar gum (0.5%), XG (0.22%) | Sunflower oil | SFC, RM, TA, DSC, CM (Margarine formulation replacing palm oil and partially hydrogenated palm olein) | [108] |
| BW (3, 8%) | Sunflower oil | RM, DSC, OBC, CM, Potato strip analyses (Sensory, Oil Uptake) (as frying medium for potato strips) | [56] |
| BW (5%) + β-carotene (0–0.4%) | Canola oil | CM, PLM, RM, TA, DSC, FTIR | [109] |
| BW (10%) | Sunflower oil + shortening | SFC, Products properties (Gluten-free cake formulation) | [110] |
| BW (8%) | Linseed oil | Sausages properties (Pork backfat replacement in frankfurters) | [92] |
| BW (6%) | Olive, linseed, fish, and sunflower oil | PLM, TA, OBC, DSC, FTIR | [101] |
| BW (11%), EC (11%) | Blend of olive, linseed and fish oil | CM, RM, FTIR, DSC, OS (TBARS), Pate properties (Pork backfat replacement in pork liver pâtés) | [111] |
| BW (11%), EC (11%) | Blend of olive, linseed and fish oil | PLM, TA, DSC, OS (TBARS), FAC, CM, Burger properties (Pork backfat replacement in low-fat pork burgers) | [102] |
| BW (4%) | Linseed, corn, sunflower, and camellia oil | FAC, OBC, PLM, RM, DSC, FTIR, XRD | [112] |
| BW + MGs (7, 10%) (1:2) | Sunflower oil | Semi-smoked sausages properties | [113] |
| BW (4%) + β-cyclodextrins | Corn and fish oil | SEM, TGA, FTIR, Particle size, ζ-potential, SE | [114] |
| BW (5%) + WPI coating | Fish oil | RM, SEM, Encapsulation efficiency, Particle size, ζ-potential, TBARS, Micropolarity, Microviscosity (OG for stabilization and delivery of ω3 in fish oil) | [93] |
| BW (3–5%) | Sunflower oil, Medium- and long-chain triglyceride, Diacylglycerol | FAC, MGC, PLM, OBC, TA, DSC, SFC, FTIR, XRD | [115] |
| BW (10%) + Ascorbic acid or α-tocopherol (0.01–0.03%) | Canola oil | OM, TA, RM, DSC, XRD, FTIR, OBC, PV, OS (p-AV, TOTOX) | [49] |
| BW (5–10%) | Sesame oil | FAC, CM, PLM, TA, DSC, Product properties (substitute of animal fat for beef burgers) | [97] |
| BW + SHW (10%) (70:30) | Canola and linseed oil | TA, OBC, RM, DSC, CLSM, FAC, OS (PV) | [116] |
| BW, β-sito blends (10%) | Sunflower oil | PLM, CM, TA, DSC, FTIR, XRD | [117] |
| BW (3–4%) | Camellia, soybean, sunflower, and flaxseed oil | PLM, DSC, FTIR, XRD, PV (Comparison of oil types in BW-oleogel formation) | [57] |
| BW (8–9%) | Avocado, sunflower, and linseed oil (with 0.2% curcumin) | TA, OBC, RM, OS (PV, K268), Curcumin degradation kinetics, In vitro digestion, FFA/curcumin bioaccessibility | [118] |
| white BW (3–11%) | Olive, grape seed, walnut, hemp seed, and sunflower oil | SEM, OBC, PV, CM, FTIR | [88] |
| BW (6%)—combinations of hydrocarbons, monoesters, di-/triesters + FFAs + FAl (C) | Sunflower oil | PLM, TA, OBC, DSC | [119] |
| BW and BW hydrocarbons (6%) | Sunflower oil | PLM, TA, DSC (study under different cooling rates) | [58] |
| BW/StA (11.74%) (3:1) + β-sito (5%) | Sesame, rice bran oil, and blends | PLM, OBC, RM, DSC, FTIR, OS | [120] |
| BW (6%) and combinations of its fractions | Sunflower oil | OS (PV, AV, CDV, TOTOX), TA, FAC | [121] |
| BW (3%) or BW/hydrocarbon (9:1) | Sunflower oil | CM, TA, FAC, DSC, SE, Product properties (substitutes for solid fats in margarine) | [122] |
| BW (2–8%) | Peanut oil | OM, RM, TA, OS | [98] |
| Carnauba wax | |||
| CBW (6–10%) | Soybean and peanut oil | PS, PLM, OBC, DSC, SS, SE (fat replacer for ice cream) | [123] |
| CBW (4–8%) and Propolis wax (5–10%) | Safflower oil | PS, OBC, SFC, CM, FAC, PV, TPA, SE (fat substitutes in cake batters) | [80] |
| Different types of CBW (4–8%) | Soybean oil | PS, PLM, CE, TA, RM | [60] |
| CBW (5–15%) | Soybean oil | TA, OBC, SP, Product properties (as frying medium for Indian traditional snack (Mathri)) | [61] |
| CBW + Adipic acid (6%) | Soybean oil | PLM, CM, RM, FTIR, XRD, DSC, OBC, OS, Products properties (fat substitutes in cake and beef burger) | [62] |
| CBW (3–9%) | Canola oil | SFC, Product properties (fat substitutes in imitation cheese) | [70] |
| CBW + MGs (5–10%) | Canola oil | RM, DSC | [34] |
| CBW, β-sito/lecithin, EC, resveratrol (combinations) | Soybean and peanut oil | PLM, TPA, RM, DSC, FTIR, XRD, CM, OBC, SFC, in vitro, determination of bioavailability | [53] |
| CBW (6%) | Sunflower and linseed oil in various ratios | FAC, PLM, CM, OBC, DSC, FTIR, XRD, OS, Product properties (shortening substitution in cakes) | [124] |
| CBW (6%) and CBW + Adipic acid (6%) | Sunflower oil | Product properties (fat substitutes in chocolate spread) | [54] |
| CBW (8%) | Soybean oil | OBC, TPA, RM, DSC, FTIR, SP (optimize the ultrasonication conditions) | [63] |
| CBW (6%) | Acorn and soybean oil | PLM, CLSM, RM, TPA, DSC, XRD, FTIR, OBC, PS, Product properties (Chocolate spreads preparation) | [125] |
| CBW (10%) | Sunflower oil | TPA, RM, Products properties (fat replacer in pastries (bow tie cookies, cheese crackers, apple pie, cookies, jam-filled puff pastry)) | [126] |
| CBW (5–15%) | Soybean oil | OBC, TPA, RM, DSC, FTIR, SP | [64] |
| Monopalmitate + CBW (10%) | Soybean oil | OBC, SFC, DSC, NMR | [65] |
| Candelilla wax | |||
| CDW (5%) | Canola oil | Products properties (Preparation of cake (blends of canola oil oleogel/butter)) | [71] |
| CDW (1–5%) + StA (0.005–0.05%), curcumin (5%) | Groundnut oil | PLM, MGC, OBC, CM, FTIR, Raman, XRD, TA, in vitro curcumin release | [42] |
| CDW (5%) + StA (0.015%) | Groundnut oil | Product properties (different pasta samples with OG) | [127] |
| CDW (5%) + olive diacylglycerol stearin (5–35%) | Olive triacylglycerol oil | FAC, PLM, OS (PV, TBA), Product properties (Substitution of margarine, cookies) | [128] |
| CDW + GMS (10%) | Grapeseed oil | PLM, BLM, DSC, RM, TA, OBC, NMR | [29] |
| CDW + MGs + fully hydrogenated oil (5–10%) | Soybean and high-oleic sunflower oils | PLM, TA, RM, OBC, DSC, SFC (NMR) | [35] |
| CDW + Hard fats (5%) | Soybean oil | PLM, FAC, DSC, RM, OBC, SFC (NMR) | [19] |
| CDW (3–9%) | Extra-virgin linseed oil | CM, MP, TA, FTIR, Product properties (Replace fat in cookies) | [91] |
| CDW (10%), CDW + GMS (1:3), β-carotene | Sunflower oil | TA, RM, OS (PV), Product properties (applications to muffin as a shortening replacer) | [30] |
| CDW (5%) | Groundnut Oil | Product properties (substituting water with oleogel in pasta) | [96] |
| CDW + GMS (10%) | Canola oil | TA, DSC, Product properties (shortening replacer in filling creams) | [32] |
| CDW (10%) + quercetin (0.02–0.06%) | Sunflower oil | OM, FTIR, XRD, OBC, RM, CM, OS (PV), Products properties (Replace fat in meat batter and sausages) | [48] |
| CDW (3%), β-carotene | Peanut, pine nut and walnut oil | PLM, RM, TA, XRD, OBC, Product properties (β-carotene encapsulation) | [51] |
| CDW (3%) | Canola oil | Product properties (Replace solid saturated fat in sponge cake bread) | [72] |
| CDW (10–20%) + phosphorus (0–3%) | Safflower oil | RM, OBC, DSC, Evaluation of the phosphorus release | [81] |
| CDW (0.75–4%) + α-tocopherol (0.5–10%) | Canola oil | PLM, OBC, RM, TPA, DSC, NMR | [50] |
| CDW (1–8%) + MGs (0.35–0.7) or polyglycerol polyricinoleate (0.25–0.5) | High oleic safflower oil | RM, DSC, XRD, NMR | [36] |
| CDW + MGs (10%) | Walnut oil | PLM, TPA, RM, FTIR, Product properties (Replace butter in chocolate spreads) | [39] |
| CDW (0–3%), EC (0 -12%), MGs (0, 5%) | High oleic safflower oil | PLM, DSC, RM (compared with fat phase of stick, Danish, and puff pastry margarines) | [129] |
| CDW (3, 9%) | Hemp seed and olive oil | OBC, OS (PV), CM, Product properties [plant-based ice creams (oat milk, millet milk and spelt milk, sugar, oleogel and flavors)] | [88] |
| CDW (5%) + lecithin from sunflower and soya | Rice bran oil | PLM, CM, Surface Topology, FTIR, DSC, TA | [43] |
| CDW (3, 8%) and MGs (0.7%) or PGPR (0.5%) | High oleic safflower oil | PLM, SEM, TEM, XRD | [37] |
| CDW (3%) + flaxseed gum (0–0.4%) | Flaxseed oil | TA, OBC, DSC, RM, XRD | [130] |
| CDW (3–8%) | Rapeseed and linseed oil (1:1) | CM, PM, PLM, RM, PS | [131] |
| CDW or GMS (10%) | Sunflower oil | TPA, OBC, Product properties (Replace fat in Bologna Sausages) | [55] |
| CDW (3%) | Chia seed oil | OM, TA, RM, XRD | [95] |
| CDW (5%) | Canola oil | Product properties (preparation of maize tortillas) | [73] |
| Rice bran wax | |||
| RBW (2–10%) | Corn oil | PLM, TA, SFC, DSC, XRD, in vitro | [83] |
| RBW (3, 7%) | Olive, sunflower, flaxseed, soybean, and medium-chain triglyceride (MCT) oil | MGC, TA, OBC, PLM, DSC, OS | [132] |
| RBW (0.5–5%) | Sunflower oil | DSC, OBC, XRD, FTIR | [59] |
| RBW (2.5 or 10%) | Conventional and high-oleic soybean oil | Product properties (alternatives to pork fat in chicken-based bologna sausage) | [133] |
| RBW (1–11%) | Rice bran oil | TA, SFC, XRD, PLM, DSC, OS | [134] |
| RBW (0.5–25%) | Rice bran oil | WC (HPLC), DSC, PLM, RM | [18] |
| RBW (2.5 and 10%) | Soybean oil | Product properties (replace pork fat in frankfurter-type sausages) | [66] |
| Sunflower wax | |||
| SW, BEW, GMS, different ratios (6%) | Flaxseed oil | PLM, SEM, RM, DSC, OBC, OS, | [28] |
| SW (5%), Span-80 and Tween-80 (1–10 mg) | Sunflower oil | OBC, CM, PLM, DSC, FTIR, XRD, Spreadability study, curcumin release | [135] |
| SW (5%), Span-60 and stearyl alcohol (1–10 mg) | Sunflower oil | OBC, CM, PLM, TA, DSC, FTIR, XRD, curcumin release | [46] |
| SW (5%), Span-80, Span-60, Tween-80, and stearyl alcohol (0.05–0.015%) | Sunflower oil | FAC, CLSM, DSC, Raman, Properties of probiotic (as growth modulator of probiotics) | [45] |
| SW, SW + MGs (6–12%) | Olive, sunflower, sesame, and soybean oil | CM, PLM, TA, DSC, FTIR, OS | [136] |
| SW (3–7%) | Soybean oil from 3 types of seeds | PLM, RM, TA, DSC, SFC | [137] |
| SW (3, 7%) | Olive, canola, corn, soybean, grapeseed, sacha inchi, chia seed, and flaxseed oil | FAC, TocA, TPCA, FFA, TA, DSC, WC | [100] |
| SW (5%), MGs (5%) | Rapeseed oil | TA, Product properties (as frying medium for French fries) | [85] |
| SW, EC, and MGs individual or in mixtures (5–10%) | Rapeseed oil | OBC, TA, SEM, RA, Product properties (Cookie Preparation) | [33] |
| SW (10%) + thyme and cumin (1%) | Virgin olive oil | OBC, SFC, CM, FFA, XRD, DSC, RM, Volatile Compound Analysis, SE, Consumer Tests | [47] |
| Different/combined waxes | |||
| BW, CBW, SW (6–10%) | Moringa, tiger nut and garden cress oil | OBC, Total phenolic content, FAC, OS, TPA, CM, DSC | [104] |
| BW, CBW, SHW, SW, MGs (7–14%) | Laurel oil | TA, PLM, XRD, TGA, NIR | [38] |
| BW, RBW (3–10%) | Safflower oil | OBC, SFC, CTD, CM, FAC, OS (PV, FFA, K232, K270), Product properties (shortening replacers in cakes) | [82] |
| BW (4–8%), CBW (4–8%) | Pumpkin seed oil, sunflower oil (for comparison) | DSC, RM, TA, SFC, OBC | [138] |
| BW, CBW (6%) | Pumpkin, hemp, almond, rice, sesame, and grapeseed oil | FAC, DSC, RM, SFC, OBC, CM | [105] |
| SW, BW (1–15%) | Eucalyptus, lavender, lemon peel and tea tree essential oils | OBC, OS, CM, TA, XRD, DSC, TGA | [139] |
| SW, BW (5–15%) | Black cumin seed, St. John’s Wort, and grape seed oils | OBC, CM, TA, XRD, DSC, Volatile composition | [107] |
| RBW, CDW, SW, and BW together with MGs (tot. conc. 15%) | Olive, sunflower, sesame, and soybean oil | CM, PLM, TA, MP, FTIR | [26] |
| BW, RBW, SW, StA, Octadecanol, γ-β, and EC (10%) | Sunflower oil | PS, CTD, DSC, PLM, OBC, RM | [41] |
| RAW, RBW, SW, BW, MGs, γ-β (5–15%) | Medium-chain triacylglycerides oil | PS, PLM, OBC, RM | [40] |
| SW, RBW, CDW, and BW (2–4%) various binary wax blends (1:1, 1:3, and 3:1 w/w) | Olive oil | DSC, TA, PLM, OBC, FAC | [78] |
| CDW (3, 7%) or RBW (5, 7%) | Hemp oil | SFC, FFA, FAC, OS (PV, CDV, TBARS), Product properties (Replace animal fat in meat patties) | [89] |
| CDW + BW (3–7%) | Soybean oil | TA, DSC, SFC, Product properties (Margarine formulation) | [67] |
| SW (1–1.5%), RBW (8–10%) | Sunflower, mustard, soybean, sesame, groundnut, rice bran, palm, and coconut oil | DSC, RM, SEM, XRD, CTD, OBC, SFC | [106] |
| RBW, SW (0.5–1%) | Soybean oil | DSC, Product properties (Replace solid milk fat in Swiss cheese) | [140] |
| BW (3%), RBW (1–9%), SW (1–9%), CW (3%) | Fish oil | OS (PV, CDV, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels), DSC, NMR, TA, CM | [137] |
| RBW, SW, CDW, BW (3–7%) | Cold-pressed hempseed oil | FAC, TA, SFC, OM, DSC, Product properties (fat replacement in margarines and spreads) | [141] |
| CDW, CBW, BW (10%) | Insect oil (from Tenebrio molitor larvae) | DSC, RM, TA, OBC, PV, OS, Product properties (shortening replacement in cookies) | [142] |
| SW, CDW, BW (white and yellow), MGs (5%) | Rapeseed oil | TA, OBC, CM, OS, OM, PLM | [86] |
| BW, CBW, CDW, RBW, sitosterol, pea protein, and XG, (9%) | Hemp seed oil | Dough and cookies properties (Replacing margarine in cookies) | [90] |
| CBW, BW, CDW, RBW (5%), MGs (7, 12%), and mixtures wax (1–5%) with MGs (7%) | Sunflower oil | MGC, PLM, TA, OBC, DSC, RM, FTIR, in vitro | [25] |
| SW, CBW, CDW, BW, BEW, FW + Soybean lecithin (LEC), various ratios FW:LEC (0–100%) (1.75–7%) | Sunflower oil | DSC, RM, PLM, SEM, Raman | [44] |
| White/Yellow BW (5%), CDW (2–3%), RBW (2%), EC (8%), MG (5%) | High-oleic rapeseed oil | TA, OBC, DSC (Short-dough biscuits reformulated to replace palm oil with OG) | [87] |
| BW (10%), SW (7%), CBW (7%) | black cumin oil | DSC, PLM, OBC, RM, TA, OS | [99] |
| CDW, BW, RBW, CBW (1–10%) | Rice bran oil | MGC, PLM, TA, XRD, Product properties (replace fat in cookies | [76] |
| SBW, BW, CBW, binary mixtures (0–9%) | Sunflower oil | TA, CM, SP, FAC, PLM, FTIR, XRD | [143] |
| CDW, BW, CBW, EC with diff. mass ratios (10%) | Corn oil | PS, TPA, OBC, CM, FTIR, DSC, RM, PLM | [84] |
| BW, RBW (5 and 9%) | Grape seed, hemp seed, olive, sunflower, and walnut oil | RM, DSC, TGA, TPA, Dough properties (Dough preparation using oleogels) | [144] |
| CDW, BW (6–12%) | Virgin coconut and mustard oils | OBC, CM, CLSM, RM, TPA, FTIR, SP | [145] |
| BW, CDW, SW, and RBW (4, 10%) | Canola and sunflower oil | Oil purification and increment of polar oil components, RM, TA, DSC, BLM | [146] |
| BW, CDW, CBW (0.5–5%) | Camellia oil and medium chain triglycerides | MGC, OBC, TA, DSC, XRD, PLM, FTIR | [147] |
| BW, CDW, CBW (1–6%) | Extra virgin olive oil | PCM, OS (PV), TA, DSC, RM | [79] |
| BW, CBW, CDW and RBW (5%) | Soybean oil | OBC, CTD, PLM, RM, Characteristics of chips (Deep-frying potato chips) | [68] |
| CBW, GMS, β-sito/BW (10%), and β-sito: lecithin (16%) | Sunflower oil | TA, OBC, RM, Product properties (tender dough products using oleogels) | [31] |
| BW (3%), CBW (6%), EC (4, 8%) and mixtures | Rice bran oil | PS, PLM, CLSM, RM, TA, OBC, DSC, XRD, FTIR | [77] |
| CBW + BW different ratios (4%) | Rice bran oil | PS, PLM, TA, RM, OBC, DSC, XRD, OS | [148] |
| CDW or CDW + BW (1:1) (3%) | Canola oil | RM, XRD, OBC (potential to mimic commercial margarine) | [74] |
| SW, BW, hydrolyzes SW and BW, combinations (ratios 0–100) (8, 12%) | Canola oil | Composition of waxes, BFM, SEM, DSC, RM, TA | [75] |
| SW, RBW, BW, CDW, SCW, and CBW (10%) | Canola oil and medium-chain triglycerides oil | DSC, BFM, PLM, TA, RM | [149] |
| BW, CDW, SW (5%) and binary mixtures | Soybean oil | DSC, phase contrast light microscopy, SFC, TA | [150] |
| BW, SW, CDW, RBW (0.5–2%) | Fully hydrogenated cottonseed oil | OBC, FAC, SEM, Properties of peanut butter (waxes as stabilizers in peanut butter) | [21] |
| BW + CDW + SW, different ratios (5%) | Soybean oil | WA (HPLC), MGC, XRD, DSC, TA, PLM, SFC, NMR, RM | [69] |
| Tea wax, RSW, orange peel wax, rose wax, and BEW, compared to SW (1–25%) | Sunflower oil | CTD, OBC, SFC, CM, PV, DSC, PLM, XRD, RM, SE | [151] |
| SW, BW (10%) | Flaxseed oil, Tallow fat (melted, filtered, and stored) | CM, FFA, DSC, Product properties (Production of the sucuk samples) | [152] |
| RBW, CW, BW, CBW (6%) | Rice bran oil | Rice cooking properties | [153] |
2.3. Physicochemical and Structural Characterization of Oleogels
2.3.1. Microstructural Analysis
2.3.2. Rheological and Structural Properties
2.3.3. Thermal Properties
2.3.4. FTIR Analysis and X-Ray Diffraction
2.3.5. Oil Binding Capacity
2.3.6. Oxidative Stability
3. Wax-Based Bigels
3.1. Definition and Structure of Bigels
| Oleogel | Hydrogel | OG:HG Ratio | Characteristics Studied and Application | Ref. | |
|---|---|---|---|---|---|
| Oleogelator | Edible Oil | Hydrogelator | |||
| Beeswax | |||||
| BW (2%) | Algae oil | Gellan gum (2%) | 20:80, 40:60, 50:50, 60:40, 80:20 | PLM, CLSM, RM, TPA, FTIR, XRD, NMR, 3D-PA 3D printing | [159] |
| BW (10%) | Soybean oil | Polyglycerol polyricinoleate (3%) | 20:80, 40:60, 50:50, 60:40, 80:20 | PS, OM, PLM, CLSM, RM, TPA, FTIR, 3D-PA, 3D printing | [160] |
| BW (5%) | Canola oil | Sweet potato starch (10%) or Chayote tuber starch (10%) | 30:70, 40:60, 50:50 | Microscopy, RM, TPA, LBC (oil), XRD, FTIR | [161] |
| BW (5%) | Sunflower oil | Gel (10%), XG (1%), Agar (15%) | 5:95, 10:90, 20:80 | PS, BLM, SEM, RM, TPA, FTIR, 3D-PA 3D printing | [162] |
| BW (20%) | Grape seed oil | SA (2%) | 99:1, 95:5, 90:10 | PLM, TPA, RM, DSC, XRD, SFC, OS (PV), SE Compound chocolate | [163] |
| BW (10%), GMS (2%) | Soybean oil | Gellan gum (3%) | 30:70, 60:40, 65:35, 70:30, 80:20 | PLM, CLSM, RM, FTIR, SS, 3D printing-Prepared foams | [164] |
| BW (10%) | Sesame oil | SA (3%), Whey protein (25%) | Not reported | FAC, PLM, FTIR, OS (PV, RA, DPPH), Microbiological characteristics, Chemical analysis, SE Cinnamon oil and probiotic strains, -Butter spread | [165] |
| BW (6%) | Sunflower oil | Tapioca starch (5–10%) | 25:75, 40:60, 50:50, 60:40, 75:25 | PLM, RM, TPA, DSC, FTIR, SB, in vitro digestion, particle size | [166] |
| BW (0–3%) + SL (0–1%) | Soybean oil | Flaxseed gum (1%) | 90:10 to 30:70 | RM, DSC, FTIR, CLSM, probiotic viability, in vitro digestion, FFA Encapsulated probiotics | [167] |
| BW (0–12%) + DGs (5%) | Soybean DAG oil | Hydroxypropyl methyl cellulose (2%) | 50:50 | OM, CLSM, DSD, TPA, RM, DSC, FTIR, Product properties-Bread | [168] |
| BW (8–12%) | Soybean oil | Cellulose nanofibres (2%) | 30:70, 40:60, 50:50, 60:40, 70:30 | PS, CLSM, LBC (oil), RM | [169] |
| BW (3%, 6%) | Μedium chain triglycerides oil | SA (2%) | 50:50, 80:20, 90:10, 95:5, 99:1 | BFM, TPA, RM, XRD | [170] |
| BW (5%) | Sunflower oil | Agar (15%) or Gel (10%), + XA (2%) | 20:80, 30:70, 40:60 | RM, TPA, 3D-printing | [171] |
| BW (12%) + Plant Sterol Esters (8%) | Diacylglycerol corn oil | Gel (5%) + Whey protein isolate (5%) | 20:80 to 80:20 | PS, CM, OM, PLM, CLSM, TPA, DSC, FTIR, XRD, SE, LBC (oil and water), OS (PV, TBA) | [172] |
| BW (20%) | Corn oil | Soy protein isolate (20%) | 5:95, 10:90, 15:85, 20:80, 25:75, 30:70 | CLSM, SEM, RM, NMR, XRD, 3D printing | [173] |
| BW (15%) | Corn oil | κC + XG (1:1) (1.5%) | 20:80, 30:70, 50:50, 70:30, 80:20 | CLSM, PLM, RM, XRD, 3D printing | [174] |
| BW (10%) | Canola oil | SA or carboxymethylcellulose (3%) | 50:50 | FAC, OS (PV, AV), CLSM, TPA, RM, XRD, LBC, Product properties-Cookies | [175] |
| BW (4–8%), GMS (4–8%) | Corn oil | κC (0.75%) and Tween 20 (0.5%) | 50:50 | CLSM, DSD, RM, TPA, DSC, XRD, FTS | [176] |
| BW (6%) + glyceride monooleate (2%) | Sunflower oil | Agar (0.5–2%) | 90:10, 80:20, 70:30, 60:40 | RM, CLSM, UV/Vis, stability of bigel films BG films for fresh meat | [177] |
| BW (10%) | Corn germ oil | Myofibrillar protein | 10:90, 30:70, 50:50, 70:30, 90:10 | CLSM, TPA, RM, DSC, FTIR, XRD | [178] |
| BW (20%) | Soybean oil | κC (2%) + starch (10%) | 75:25, 50:50, 25:75 | OM, RM, TPA, DSC, FTIR, SB, FTS | [179] |
| BW (1%) + GMS (1%), + lycopene (0.1%) | Soybean oil | Gellan gum (0.3%) | 10:90 to 60:40 (w/w) | CLSM, TPA, RM, DSC, FTIR, SB, in vitro lycopene release profile Designed for lycopene encapsulation and controlled release | [180] |
| Carnauba wax | |||||
| CBW (10%) + SL (0.5–1.5%) | Sunflower and olive pomace oil | Gel (5%), Col (15.6%), Agar (2.5%), and combinations | 60:40 | CM, TA, RM, LBC (water and oil), OS (PV), AC Lingonberry pomace, Edible spreads | [181] |
| CBW (9.3%) + SL (0.6%) | Sunflower and olive pomace oil | Col (40, 60%) | 40:60, 50:50, 60:40 | CM, TPA, RM Dysphagia product | [182] |
| CBW (15%) + SL (2%), + chlorophyll extract (2 types) | Sunflower oil | Agar (5%) + XG (1%) | 80:20, 60:40, 40:60, 20:80 | PS, CM, CLSM, RM, FTIR, LBC, DA, 3D-printing | [183] |
| CBW (7%) | Canola oil, Thyme essential oil (0.5–2%) | CPI (15%), microbial transglutaminase | 50:50, 40:60, 30:70, 20:80, 10:90 | CM, PLM, RM, FTIR, DSC, LBC (water and oil), AC | [184] |
| CBW (8%) | Canola oil | AG (4%) | 90:10 | OM, CLSM, TA, RM, DSC, FTIR, XRD, LBC, OS | [185] |
| CBW (10%) | Rice bran oil | ιC (3%) | 40:60, 50:50, 60:40 | PS, CM, TPA, RM, DSC, FTIR, XRD 3D-printing | [186] |
| CBW (9%) | Sunflower oil and olive pomace oil | Col (60%) + SDF (from cranberry and sea buckthorn berry pomace) (1.34%) | 25:75 | PS, RM, Viability of probiotic cells, Product properties Encapsulation probiotics, Butter spread | [187] |
| Candelilla wax | |||||
| CDW (5%) | Canola oil | GCS (5%) | 20:80, 40:60, 60:40 | OM, RM, FTIR | [109] |
| CDW (5%) | Canola oil | GCS (5%) | 50:50 | Dough and cookies properties Shortening substitute for cookies | [52] |
| CDW (7.5%) | Soybean oil | Egg whites (5–10%) | 80:20, 60:40, 40:60, 20:80 | CLSM, PLM, RM, TPA, DSC, Emulsions Stability | [188] |
| CDW (15%), MGs (15%), CDW + MGs (7.5 + 7.5%) | Olive, sunflower, sesame, and soybean oil | Agar (1–4%), κC (0.5–2%), and combinations of them | 80:20, 60:40, 40:60, 20:80 | CM, OM, PLM, DSD, TPA, DSC, FTIR | [189] |
| CDW (5%) | Corn oil | Potato protein isolate (15–25%) | 30:70, 10:90 | CLSM, RM, TPA, DSC, in vitro digestion Encapsulated curcumin | [190] |
| CDW (4%) | Walnut oil | Potato starch (3.3%) | 2:1, 1:1, 1:2, 1:3, 1:4 | SFC, FTIR, XRD, NMR Margarine | [191] |
| GMS (10%) + CDW (2–8%), Paprika oleoresin (0.3%) | Canola oil | Guar gum (0.5%) | 2:8 | Encapsulation efficiency, CLSM, PO released, CM, RM Phenoxyethanol (0.55%) or caprylyl glycol (0.45%) addition | [192] |
| γ-β (8%, 3/2), MGs (8%), or CDW (8%) + span 65 (0.7%) | Walnut oil | Chitosan, Sodium tripolyphosphate | 40:60, 50:50, 60:40, 70:30, 80:20 | SEM, CLSM, RM, TPA, DSC, FTIR, XRD, LBC, SFC, SE Spread replacement | [193] |
| CDW (6%) | Canola oil | Puratein C (15%) | 30:70, 40:60, 50:50, 60:40 | Protein characterization, CHNS elemental analysis, LSM, TPA, RM, FTIR, DSC, TGA Transglutaminase (0–35%) | [194] |
| CDW (8%) + Sucrose ester (0.66%) | Canola oil | XG (0.5%) | 55:45, 65:35, 75:25, 85:15 | CLSM, RM, TPA, DSC, LBC | [195] |
| CDW (3%) + MGs or SL (1%) | High oleic acid sunflower seed oil | Fish Gel (5%) | 30:70, 50:50, 70:30 | PLM, RM, TPA, 3D-PA 3D-printing | [196] |
| CDW (3%) + MGs or SL (1%) | High oleic acid sunflower seed oil | Fish Gel (5%) | 30:70, 50:50, 70:30 | CSLM, XRD, FTIR, NMR, 3D-PA, in vitro digestion, HPLC Encapsulated with catechin (0.1% in HG) and quercetin (0.1% in OG) | [197] |
| Rice bran wax | |||||
| RBW (7.5%) | Soybean oil | Gel (10%) | 40:60, 50:50, 60:40, 70:30 | PS, CLSM, RM, DSC, LBC (oil and water), OS (PV), FTS | [198] |
| RBW (2%) + GMS (1%) | Corn oil | SA (2%) | 60:40, 50:50, 40:60, 30:70, 20:80, 10:90 | BFM, RM, TPA, Product properties-Dough and baked bread | [199] |
| RBW (7.5%) | Soybean oil | Gel (7–8%) | 70:30, 60:40 | Sausage properties Fat replacement in sausage | [200] |
| RBW (9%) + MGs (0–2%) | Soybean oil | SA (1%) + κC (0.5%) | 70:30, 80:20 | CLSM, RM, DSC, LBC, NMR | [201] |
| RBW (10%) | Soybean oil | Gel (5–10%) | 50:50, 40:60, 30:70, 20:80 | CSLM, RM, DSC, FTIR | [202] |
| RBW (10%) + DGs (0–3%) | Soybean oil | Gel (7%) | 60:40, 70:30, 80:20 | CSLM, TPA, FTIR, NMR, LBC | [203] |
| RBW (8%, 9%) | Walnut oil | Guar gum (1.8%) | 30:70, 50:50, 70:30 | OM, RM, TPA, FTIR, OBC, Propyl paraben (0.02% w/w) (antimicrobial) | [204] |
| RBW (1–7%) | Sunflower oil | Pea protein (4%) and carboxymethyl cellulose (0.3%) | 75:25 | CLSM, RM, TPA, FTS Transglutaminase (0.1–0.4%) | [205] |
| Sunflower wax | |||||
| SW (6–12%), SW + MGs (6–12%) | Olive oil | Agar (2%) + κC (1%) | 80:20, 60:40, 40:60, 20:80 | CM, OM, PLM, DSD, DSC, TPA, FTIR, SB, LBC (water and oil), OS (PV) | [206] |
| SW (5%) | Soybean oil | Spirulina platensis protein (1%) + XG (1%) | 20:80, 40:60, 50:50, 54:46, 56:44, 58:42, 60:40, 80:20 | OM, PLM, CLSM, RM, TPA, FTIR, 3D-PA, 3D-printing | [207] |
| Different/combined waxes | |||||
| CDW, CBW, RBW, BW (12–20%) + MGs (1%) | Canola oil | XG (1%) | 80:20 | PLM, TPA, SFC, Product properties-Croissant preparation | [208] |
| BW (6%) + RBW (4%) | Soybean oil | Gel (10%) | 80:20, 60:40, 50:50, 40:60, 20:80 | OM, RM, TPA, DSC, FTIR, FTS | [209] |
3.2. Composition Design of Wax-Based Bigels
3.2.1. Edible Oils
3.2.2. Hydrogelators
3.2.3. Oleogel-to-Hydrogel Ratio
3.3. Physicochemical and Structural Characterization of Bigels
3.3.1. Physical and Sensory Properties
3.3.2. Microstructure Analysis
3.3.3. Rheological and Textural Properties
3.3.4. Thermal Properties
3.3.5. Fourier Transform Infrared Spectroscopy (FTIR) and X-Ray Diffraction (XRD)
3.3.6. Swelling Behavior
3.3.7. Liquid Binding Capacity
3.3.8. Storage Stability
Oxidative Stability
Freeze–Thaw Stability
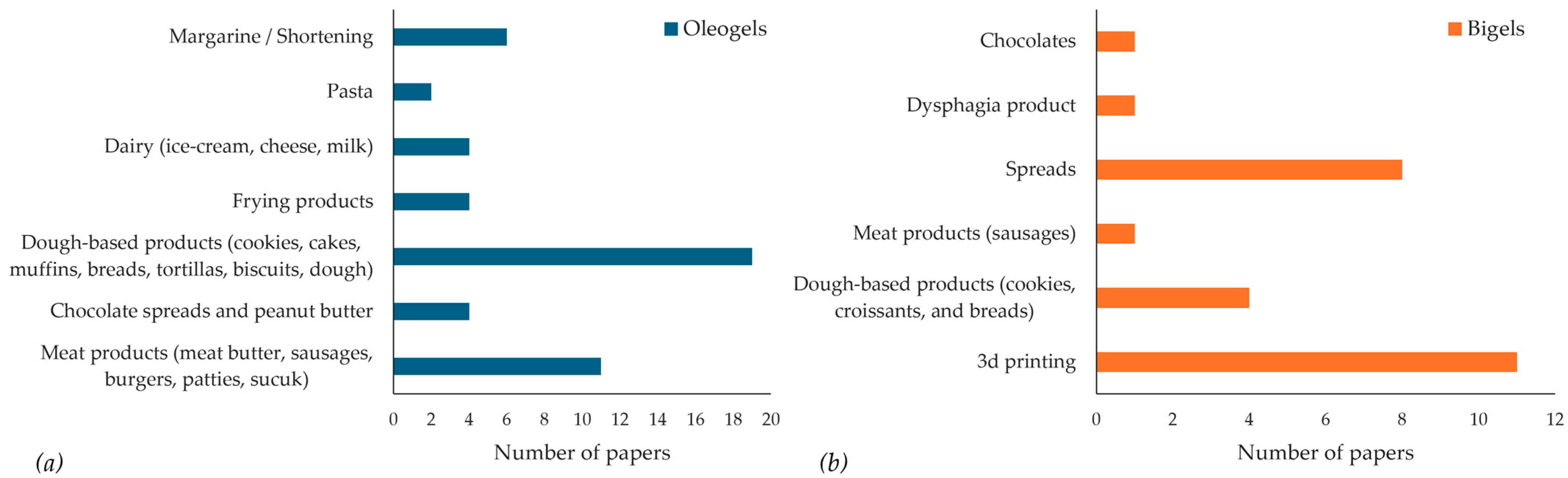
4. Applications of Natural Wax-Based Gelators in Food Systems
4.1. Applications of Natural Wax-Based Bigels
4.2. Applications of Natural Wax-Based Oleogels
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a Promising Structured Oil for Decreasing Saturated Fatty Acid Concentrations: Production and Food-Based Applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation. In FAO Food and Nutrition Paper; FAO: Rome, Italy, 2010; pp. 1–166. [Google Scholar]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health Effects of Trans-Fatty Acids: Experimental and Observational Evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef] [PubMed]
- WHO. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 29 April 2020).
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and External Factors Affecting the Crystallization, Gelation and Applicability of Wax-Based Oleogels in Food Industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible Oil Structuring: An Overview and Recent Updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Perța-Crișan, S.; Ursachi, C.-Ș.; Chereji, B.-D.; Tolan, I.; Munteanu, F.-D. Food-Grade Oleogels: Trends in Analysis, Characterization, and Applicability. Gels 2023, 9, 386. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. Edible Oleogels: Structure and Health Implications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 0-12-814271-5. [Google Scholar]
- Zampouni, K.; Dimakopoulou-Papazoglou, D.; Katsanidis, E. Food-Grade Bigel Systems: Formulation, Characterization, and Applications for Novel Food Product Development. Gels 2024, 10, 712. [Google Scholar] [CrossRef]
- Thakur, D.; Singh, A.; Suhag, R.; Dhiman, A.; Chauhan, D.S. Oleogelation Based on Plant Waxes: Characterization and Food Applications. J. Food Sci. Technol. 2023, 60, 2927–2944. [Google Scholar] [CrossRef]
- Mandu, C.C.; Barrera-Arellano, D.; Santana, M.H.A.; Fernandes, G.D. Waxes Used as Structuring Agents for Food Organogels: A Review. Grasas Aceites 2020, 71, e344. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Shirvani, A.; Elmizadeh, A.; Marangoni, A.G. Wax-Based Delivery Systems: Preparation, Characterization, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2994–3030. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and Physical Properties of Plant Wax Crystal Networks and Their Relationship to Oil Binding Capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Sintang, M.D.B.; Danthine, S.; Van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: A Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2017, 12, 97–108. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids: Chemistry, Nutrition, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1-315-15185-5. [Google Scholar]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba Wax Uses in Food—A Review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; del Pilar Medina-Herrera, N.; Castro-López, C.; Martínez-Ávila, G.C.G. Candelilla Wax: Prospective Suitable Applications within the Food Field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and Rheology of Oleogels Made from Rice Bran Wax and Rice Bran Oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Interactions between Candelilla Wax and Saturated Triacylglycerols in Oleogels. Food Res. Int. 2019, 121, 900–909. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of Oleogels Based on Edible Lipid Materials. Curr. Opin. Colloid Interface 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Felker, F.C.; Hwang, H.-S. Physical Properties of Beeswax, Sunflower Wax, and Candelilla Wax Mixtures and Oleogels. J. Am. Oil Chem. Soc. 2019, 96, 1125–1142. [Google Scholar] [CrossRef]
- Silva, R.C.; Ferdaus, M.J.; Foguel, A.; da Silva, T.L. Oleogels as a Fat Substitute in Food: A Current Review. Gels 2023, 9, 180. [Google Scholar] [CrossRef]
- Cui, X.; Saleh, A.S.M.; Yang, S.; Wang, N.; Wang, P.; Zhu, M.; Xiao, Z. Oleogels as Animal Fat and Shortening Replacers: Research Advances and Application Challenges. Food Rev. Int. 2023, 39, 5233–5254. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Gultekin Subasi, B.; Saricaoglu, B.; Karabulut, G.; Capanoglu, E. Exploring the Frontier of Bioactive Oleogels in Recent Research. Trends Food Sci. Technol. 2024, 151, 104613. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Wang, M.; Bi, Y.; Zhang, H.; Xu, X. Development and Characterization of Compound Oleogels Based on Monoglycerides and Edible Waxes. ACS Food Sci. Technol. 2022, 2, 302–314. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Giannakaki, F.; Katsanidis, E. Structural and Physical Characteristics of Mixed-Component Oleogels: Natural Wax and Monoglyceride Interactions in Different Edible Oils. Gels 2023, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chem. Soc. 2012, 89, 639–647. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-O.; Hwang, H.-S.; Jeong, S.; Kim, S.; Lee, S. The Thermal, Rheological, and Structural Characterization of Grapeseed Oil Oleogels Structured with Binary Blends of Oleogelator. J. Food Sci. 2020, 85, 3432–3441. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of Antioxidant-Fortified Oleogel and Its Application as a Solid Fat Replacer to Muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef]
- Tanislav, A.E.; Pușcaș, A.; Păucean, A.; Mureșan, A.E.; Semeniuc, C.A.; Mureșan, V.; Mudura, E. Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products. Gels 2022, 8, 317. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, H.-S.; Jeong, S.; Lee, S. Utilization of Oleogels with Binary Oleogelator Blends for Filling Creams Low in Saturated Fat. LWT 2022, 155, 112972. [Google Scholar] [CrossRef]
- Schubert, M.; Erlenbusch, N.; Wittland, S.; Nikolay, S.; Hetzer, B.; Matthäus, B. Rapeseed Oil Based Oleogels for the Improvement of the Fatty Acid Profile Using Cookies as an Example. Eur. J. Lipid Sci. Technol. 2022, 124, 2200033. [Google Scholar] [CrossRef]
- Pakseresht, S.; Tehrani, M.M.; Farhoosh, R.; Koocheki, A. Rheological and Thermal Properties of Reinforced Monoglyceride-Carnauba Wax Oleogels. J. Sci. Food Agric. 2023, 103, 4184–4194. [Google Scholar] [CrossRef]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Physical Properties of Candelilla Wax, Monoacylglycerols, and Fully Hydrogenated Oil Oleogels. J. Am. Oil Chem. Soc. 2018, 95, 797–811. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.D.; Sánchez-Becerril, M.; Marangoni, A.G.; Toro-Vazquez, J.F.; Ornelas-Paz, J.J.; Ibarra-Junquera, V. Structuration, Elastic Properties Scaling, and Mechanical Reversibility of Candelilla Wax Oleogels with and without Emulsifiers. Food Res. Int. 2019, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Becerril, M.; Marangoni, A.G.; Perea-Flores, M.J.; Cayetano-Castro, N.; Martínez-Gutiérrez, H.; Andraca-Adame, J.A.; Pérez-Martínez, J.D. Characterization of the Micro and Nanostructure of the Candelilla Wax Organogels Crystal Networks. Food Struct. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Ayvaz, H.; Albayrak, E.; Öğütcü, M. Rapid Identification of Organogelator Types in Oleogel Using a Near-Infrared Spectroscopy-Based SIMCA Model. Int. J. Food Sci. Technol. 2025, 60, vvae007. [Google Scholar] [CrossRef]
- Pușcaș, A.; Tanislav, A.E.; Mureșan, A.E.; Fărcaș, A.C.; Mureșan, V. Walnut Oil Oleogels as Milk Fat Replacing System for Commercially Available Chocolate Butter. Gels 2022, 8, 613. [Google Scholar] [CrossRef]
- Fayaz, G.; Polenghi, O.; Giardina, A.; Cerne, V.; Calligaris, S. Structural and Rheological Properties of Medium-Chain Triacylglyceride Oleogels. Int. J. Food Sci. Technol. 2021, 56, 1040–1047. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Bharti, D.; Dhal, S.; Sahu, D.; Behera, H.; Sahoo, M.; Kim, D.; Jarzębski, M.; Anis, A.; Mohanty, B.; et al. Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels. ChemEngineering 2023, 7, 96. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Okuro, P.K.; Tavernier, I.; Bin Sintang, M.D.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic Interactions between Lecithin and Fruit Wax in Oleogel Formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef]
- Bharti, D.; Kulanthaivel, S.; Mishra, P.; Jain, N.; Pal, K.; Banerjee, I. Emulsifier-Modified Sunflower Oil-Sunflower Wax Oleogel as Growth Modulator of Probiotics. BBA Adv. 2025, 7, 100147. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Demirci, Ş. Preparation and Evaluation of Virgin Olive Oil Oleogels Including Thyme and Cumin Spices with Sunflower Wax. Gels 2021, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Han, J.H.; Keum, D.H.; Kothuri, V.; Shin, D.-M.; Han, S.G. Quercetin-Loaded Candelilla Wax/Sunflower Oil Oleogels: Structural, Sensory, and Storage Properties, and Application as Fat Replacer in Emulsion-Type Sausage. Food Chem. 2025, 479, 143847. [Google Scholar] [CrossRef] [PubMed]
- Millao, S.; Quilaqueo, M.; Contardo, I.; Rubilar, M. Enhancing the Oxidative Stability of Beeswax–Canola Oleogels: Effects of Ascorbic Acid and Alpha-Tocopherol on Their Physical and Chemical Properties. Gels 2025, 11, 43. [Google Scholar] [CrossRef]
- Peixoto, V.O.D.S.; Brito, G.B.; Chrisman, E.C.A.N.; do Amaral Vendramini, A.L.; Ract, J.N.R.; Rodrigues, J.G.P.; Dias, M.L.; Magalhães, A.; do Rosário, D.K.A.; Conte-Junior, C.A.; et al. Tailoring Candelilla Wax-Based Oleogels Loaded with α-Tocopherol to Mimic Rheological Properties of Solid Food Fats and to Increase Nutritional Value of Food. J. Am. Oil Chem. Soc. 2025, 102, 279–293. [Google Scholar] [CrossRef]
- Li, L.; Taha, A.; Geng, M.; Zhang, Z.; Su, H.; Xu, X.; Pan, S.; Hu, H. Ultrasound-Assisted Gelation of β-Carotene Enriched Oleogels Based on Candelilla Wax-Nut Oils: Physical Properties and in-Vitro Digestion Analysis. Ultrason. Sonochem. 2021, 79, 105762. [Google Scholar] [CrossRef]
- Barragán-Martínez, L.P.; Román-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Impact of Fat Replacement by a Hybrid Gel (Canola Oil/Candelilla Wax Oleogel and Gelatinized Corn Starch Hydrogel) on Dough Viscoelasticity, Color, Texture, Structure, and Starch Digestibility of Sugar-Snap Cookies. Int. J. Gastron. Food Sci. 2022, 29, 100563. [Google Scholar] [CrossRef]
- Qiu, H.; Qu, K.; Zhang, H.; Eun, J.-B. Characterization and Comparison of Physical Properties and in Vitro Simulated Digestion of Multi-Component Oleogels with Different Molecular Weights Prepared by the Direct Method. Food Hydrocoll. 2023, 142, 108850. [Google Scholar] [CrossRef]
- Roufegarinejad, L.; Habibzadeh Khiabani, A.; Konar, N.; Toofighi, S.; Rasouli Pirouzian, H. Carnauba Wax and Adipic Acid Oleogels as an Innovative Strategy for Cocoa Butter Alternatives in Chocolate Spreads. J. Food Sci. Technol. 2024, 61, 331–339. [Google Scholar] [CrossRef]
- Tanislav, A.E.; Cornea, A.A.; Radu, E.D.; Țibulcă, D.; Mureșan, V.; Mudura, E. Candelilla Wax and Glycerol Monostearate-Based Oleogels as Animal Fat Substitutes in Bologna Sausages. Gels 2024, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Aydeniz Guneser, B.; Yılmaz, E.; Uslu, E.K. Sunflower Oil–Beeswax Oleogels Are Promising Frying Medium for Potato Strips. Eur. J. Lipid Sci. Technol. 2021, 123, 2100063. [Google Scholar] [CrossRef]
- Pang, M.; Shi, Z.; Lei, Z.; Ge, Y.; Jiang, S.; Cao, L. Structure and Thermal Properties of Beeswax-Based Oleogels with Different Types of Vegetable Oil. Grasas Aceites 2020, 71, e380. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Vorobiova, I.; Kochetkova, A. A Study of the Quantitative Relationship between Yield Strength and Crystal Size Distribution of Beeswax Oleogels. Gels 2022, 8, 39. [Google Scholar] [CrossRef]
- Modupalli, N.; Thangaraju, S.; Naik, G.M.; Rawson, A.; Natarajan, V. Assessment of Physicochemical, Functional, Thermal, and Phytochemical Characteristics of Refined Rice Bran Wax. Food Chem. 2022, 396, 133737. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Serna-Saldívar, S.O.; Pérez-Carrillo, E.; Jordânia Silva, T.; Barrera-Arellano, D.; Buitimea-Cantúa, N.E. Effect of Quality of Carnauba Wax (Copernica cerífera) on Microstructure, Textural, and Rheological Properties of Soybean Oil-Based Organogels. LWT 2021, 136, 110267. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Khare, A.; Lal, A.B.; Bebartta, R.P. Utilising Oleogel as a Frying Medium for Deep Fried Indian Traditional Product (Mathri) to Reduce Oil Uptake. J. Indian Chem. Soc. 2022, 99, 100378. [Google Scholar] [CrossRef]
- Aliasl khiabani, A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and Characterization of Carnauba Wax/Adipic Acid Oleogel: A New Reinforced Oleogel for Application in Cake and Beef Burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef]
- Sejwar, H.; Singh, A.; Kumar, N.; Srivastava, S.; Upadhyay, A.; Dar, A.H. Effect of Ultrasonication on the Properties of Carnauba Wax-Based Soybean Oleogel. Appl. Acoust. 2024, 216, 109729. [Google Scholar] [CrossRef]
- Thakur, D.; Singh, A.; Prabhakar, P.K.; Meghwal, M.; Upadhyay, A. Optimization and Characterization of Soybean Oil-Carnauba Wax Oleogel. LWT 2022, 157, 113108. [Google Scholar] [CrossRef]
- Yang, S.; Yang, G.; Chen, X.; Chen, J.; Liu, W. Interaction of Monopalmitate and Carnauba Wax on the Properties and Crystallization Behavior of Soybean Oleogel. Grain Oil Sci. Technol. 2020, 3, 49–56. [Google Scholar] [CrossRef]
- Wolfer, T.L.; Acevedo, N.C.; Prusa, K.J.; Sebranek, J.G.; Tarté, R. Replacement of Pork Fat in Frankfurter-Type Sausages by Soybean Oil Oleogels Structured with Rice Bran Wax. Meat Sci. 2018, 145, 352–362. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Winkler-Moser, J.K. Properties of Margarines Prepared from Soybean Oil Oleogels with Mixtures of Candelilla Wax and Beeswax. J. Food Sci. 2020, 85, 3293–3302. [Google Scholar] [CrossRef]
- Tan, T.-H.; Tong, S.-C.; Ooi, E.Z.H.; Quek, W.P.; Chan, E.-S.; Manja, M.; Wang, Y.; Tang, T.-K.; Lee, Y.-Y. Comparing Frying Performance of Oleogels Prepared from Various Wax Types under Repeated Frying Cycles. J. Sci. Food Agric. 2025, 105, 5458–5469. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Hwang, H.-S.; Felker, F.C.; Byars, J.A.; Peterson, S.C. Increasing the Firmness of Wax-Based Oleogels Using Ternary Mixtures of Sunflower Wax with Beeswax: Candelilla Wax Combinations. J. Am. Oil Chem. Soc. 2023, 100, 387–402. [Google Scholar] [CrossRef]
- Moon, K.; Choi, K.-O.; Jeong, S.; Kim, Y.-W.; Lee, S. Solid Fat Replacement with Canola Oil-Carnauba Wax Oleogels for Dairy-Free Imitation Cheese Low in Saturated Fat. Foods 2021, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ramirez, J.; Vernon-Carter, E.J.; Carrera-Tarela, Y.; Garcia, A.; Roldan-Cruz, C. Effects of Candelilla Wax/Canola Oil Oleogel on the Rheology, Texture, Thermal Properties and in Vitro Starch Digestibility of Wheat Sponge Cake Bread. LWT 2020, 130, 109701. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Trujillo-Ramírez, D.; Bustos-Vázquez, G.; Cervantes-Arista, C. The Use of Candelilla Wax/Canola Oil Oleogel in the Formulation of Sponge Cake Bread Improves Morphostructural and Sensory Properties. Discov. Food 2024, 4, 160. [Google Scholar] [CrossRef]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Meraz, M.; Bello-Perez, L.A.; Garcia-Diaz, S. Canola Oil/Candelilla Wax Oleogel Improves Texture, Retards Staling and Reduces in Vitro Starch Digestibility of Maize Tortillas. J. Sci. Food Agric. 2020, 100, 1238–1245. [Google Scholar] [CrossRef]
- Werner-Cárcamo, E.R.; Soleimaniam, Y.; Macias-Rodriguez, B.A.; Rubilar, M.; Marangoni, A.G. Mechanical Properties of Wax-Oleogels: Assessing Their Potential to Mimic Commercial Margarine Functionality under Small and Large Deformations. Food Res. Int. 2024, 189, 114579. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Hetzer, B.; Flöter, E. Characterization of Oleogels Based on Waxes and Their Hydrolyzates. Eur. J. Lipid Sci. Technol. 2021, 123, 2000345. [Google Scholar] [CrossRef]
- Pang, M.; Kang, S.; Liu, L.; Ma, T.; Zheng, Z.; Cao, L. Physicochemical Properties and Cookie-Making Performance as Fat Replacer of Wax-Based Rice Bran Oil Oleogels. Gels 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Multicomponent Oleogels Prepared with High- and Low-Molecular-Weight Oleogelators: Ethylcellulose and Waxes. Foods 2023, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Dobson, S.; Marangoni, A.G. Hardness, Plasticity, and Oil Binding Capacity of Binary Mixtures of Natural Waxes in Olive Oil. Curr. Res. Food Sci. 2022, 5, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Martins, A.J.; Fasolin, L.H.; Vicente, A.A. Modulation and Characterization of Wax-Based Olive Oil Organogels in View of Their Application in the Food Industry. Gels 2021, 7, 12. [Google Scholar] [CrossRef]
- Baştürk, A.; Badem, Ş.; Ceylan, M.M. Propolis and Carnauba Wax-Based Safflower Oil Oleogels as Fat Substitutes in Cakes: Production, Oxidative Stability, and Characterization. Eur. J. Lipid Sci. Technol. 2023, 125, 2200213. [Google Scholar] [CrossRef]
- Olmos-Oropeza, G.; Aguilar-Zárate, M.; Saavedra-Leos, M.Z.; Martínez-Juárez, L.G.; Toro-Vazquez, J.F.; Sánchez-Macías, A.; López-Martínez, L.A. Development of Candelilla Wax Oleogels as a Medium of Controlled Release of Phosphorus in an In Vitro Model. Appl. Sci. 2021, 11, 3815. [Google Scholar] [CrossRef]
- Badem, Ş.; Baştürk, A. Oxidative Stability and Characterization of Oleogels Obtained from Safflower Oil-Based Beeswax and Rice Bran Wax and Their Effect on the Quality of Cake Samples. J. Am. Oil Chem. Soc. 2023, 100, 635–649. [Google Scholar] [CrossRef]
- Dent, T.; Hallinan, R.; Chitchumroonchokchai, C.; Maleky, F. Rice Bran Wax Structured Oleogels and in Vitro Bioaccessibility of Curcumin. J. Am. Oil Chem. Soc. 2022, 99, 299–311. [Google Scholar] [CrossRef]
- Qu, K.; Ma, J.; Zhang, H.; Li, X. Characterization of Construction and Physical Properties of Composite Oleogel Based on Single Low Molecular Weight Wax and Polymer Ethyl Cellulose. LWT 2024, 192, 115722. [Google Scholar] [CrossRef]
- Nikolay, S.; Schubert, M.; Erlenbusch, N.; Weber, L.; Smit, I.; Matthäus, B. Rapeseed Oil-Based Oleogels with Sunflower Wax and Monoacylglycerols as Alternatives to Conventional Frying Fats and Oils for Deep-Fat Frying of French Fries. Eur. J. Lipid Sci. Technol. 2025, 127, e202400005. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed Oil in New Application: Assessment of Structure of Oleogels Based on Their Physicochemical Properties and Microscopic Observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of High-Oleic Rapeseed Oil Oleogels on the Quality of Short-Dough Biscuits and Fat Migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef]
- Ropciuc, S.; Ghinea, C.; Leahu, A.; Prisacaru, A.E.; Oroian, M.A.; Apostol, L.C.; Dranca, F. Development and Characterization of New Plant-Based Ice Cream Assortments Using Oleogels as Fat Source. Gels 2024, 10, 397. [Google Scholar] [CrossRef]
- Hamidioglu, I.; Alenčikienė, G.; Dzedulionytė, M.; Zabulionė, A.; Bali, A.; Šalaševičienė, A. Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties. Foods 2022, 11, 4030. [Google Scholar] [CrossRef] [PubMed]
- Leahu, A.; Ghinea, C.; Ropciuc, S.; Damian, C. Textural, Color, and Sensory Analysis of Cookies Prepared with Hemp Oil-Based Oleogels. Gels 2025, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Flores-García, C.L.; Medina-Herrera, N.; Rodríguez-Romero, B.A.; Martínez-Ávila, G.C.; Rojas, R.; Meza-Carranco, Z. Impact of Fat Replacement by Using Organic-Candelilla-Wax-Based Oleogels on the Physicochemical and Sensorial Properties of a Model Cookie. Gels 2023, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards Replacing Pork Backfat with a Linseed Oleogel in Frankfurter Sausages and Its Evaluation on Physicochemical, Nutritional, and Sensory Characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef]
- Lee, M.C.; Tan, C.; Abbaspourrad, A. Combination of Internal Structuring and External Coating in an Oleogel-Based Delivery System for Fish Oil Stabilization. Food Chem. 2019, 277, 213–221. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Fhaner, M.; Winkler-Moser, J.K.; Liu, S.X. Oxidation of Fish Oil Oleogels Formed by Natural Waxes in Comparison with Bulk Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700378. [Google Scholar] [CrossRef]
- Trujillo-Ramírez, D.; Reyes, I.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Chia Seed Oil-Candelilla Wax Oleogels Structural Features and Viscoelasticity Are Enhanced by Annealing. LWT 2022, 153, 112433. [Google Scholar] [CrossRef]
- Kabi, S.R.; Sahu, D.; Jarzebski, M.; Anis, A.; Kim, D.; Nayak, A.K.; Pal, K. Impact of Groundnut Oil/Candelilla Wax Oleogel Replacement on Physicochemical Properties of Whole Wheat Pasta. Starch-Stärke 2025, 77, 2400002. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of Sesame Oil Oleogels Based on Beeswax and Application as Partial Substitutes of Animal Fat in Beef Burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Sowiński, M.; Szymańska, I.; Żbikowska, K.; Marciniak-Łukasiak, K.; Werpachowski, W. Analysis of Stability, Rheological and Structural Properties of Oleogels Obtained from Peanut Oil Structured with Yellow Beeswax. Gels 2022, 8, 448. [Google Scholar] [CrossRef]
- Orhan, N.O.; Eroglu, Z. Structural Characterization and Oxidative Stability of Black Cumin Oil Oleogels Prepared with Natural Waxes. J. Food Process. Preserv. 2022, 46, e17211. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Kim, S.; Winkler-Moser, J.K. Unsaturation and Polar Compounds of Vegetable Oils Affect the Properties of Sunflower Wax-Oleogels. Eur. J. Lipid Sci. Technol. 2024, 126, 2300205. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The Influence of Edible Oils’ Composition on the Properties of Beeswax-Based Oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. Assessment of a Healthy Oil Combination Structured in Ethyl Cellulose and Beeswax Oleogels as Animal Fat Replacers in Low-Fat, PUFA-Enriched Pork Burgers. Food Bioprocess Technol. 2019, 12, 1068–1081. [Google Scholar] [CrossRef]
- Ropciuc, S.; Dranca, F.; Oroian, M.A.; Leahu, A.; Codină, G.G.; Prisacaru, A.E. Structuring of Cold Pressed Oils: Evaluation of the Physicochemical Characteristics and Microstructure of White Beeswax Oleogels. Gels 2023, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.A.; Kamel, R.M.; Salama, M.A.; Abdin, M.; Salama, Y.A.; Elsayed, M. Oleogel-Based Fat Structuring: Functional, Oxidative, and Thermal Stability of Moringa Seed, Tiger Nut, and Garden Cress Oils with Various Waxes. Int. J. Food Sci. Technol. 2025, 60, vvaf080. [Google Scholar] [CrossRef]
- Borriello, A.; Antonella Miele, N.; Masi, P.; Aiello, A.; Cavella, S. Effect of Fatty Acid Composition of Vegetable Oils on Crystallization and Gelation Kinetics of Oleogels Based on Natural Wax. Food Chem. 2022, 375, 131805. [Google Scholar] [CrossRef]
- Holey, S.A.; Sekhar, K.P.C.; Mishra, S.S.; Kanjilal, S.; Nayak, R.R. Effect of Oil Unsaturation and Wax Composition on Stability, Properties and Food Applicability of Oleogels. J. Am. Oil Chem. Soc. 2021, 98, 1189–1203. [Google Scholar] [CrossRef]
- Çokay, H.; Uzkuç, N.M.Ç.; Yüceer, Y.K.; Öğütcü, M. Comparative Analysis of Essential Oil Oleogels Containing Beeswax and Sunflower Wax with Petrolatum Gels. Eur. J. Lipid Sci. Technol. 2024, 126, 2300055. [Google Scholar] [CrossRef]
- Abdolmaleki, K.; Alizadeh, L.; Nayebzadeh, K.; Baranowska, H.M.; Kowalczewski, P.Ł.; Mousavi Khaneghah, A. Potential Application of Hydrocolloid-Based Oleogel and Beeswax Oleogel as Partial Substitutes of Solid Fat in Margarine. Appl. Sci. 2022, 12, 12136. [Google Scholar] [CrossRef]
- Barragán-Martínez, L.P.; Molina-Rodríguez, A.; Román-Guerrero, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Effect of Starch Gelatinization on the Morphology, Viscoelasticity, and Water Structure of Candelilla Wax–Canola Oil–Starch Hybrid Gels. J. Food Process. Preserv. 2022, 46, e16520. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B. Utilization of Beeswax Oleogel-Shortening Mixtures in Gluten-Free Bakery Products. J. Am. Oil Chem. Soc. 2019, 96, 545–554. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of Ethyl Cellulose and Beeswax Oleogels and Their Suitability as Fat Replacers in Healthier Lipid Pâtés Development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Liu, Y.; Xu, Y.; Tan, C.-P. Crystal Network Structure and Stability of Beeswax-Based Oleogels with Different Polyunsaturated Fatty Acid Oils. Food Chem. 2022, 381, 131745. [Google Scholar] [CrossRef]
- Igenbayev, A.; Ospankulova, G.; Amirkhanov, S.; Aldiyeva, A.; Temirova, I.; Amirkhanov, K. Substitution of Pork Fat with Beeswax-Structured Oleogels in Semi-Smoked Sausages. Appl. Sci. 2023, 13, 5312. [Google Scholar] [CrossRef]
- Lee, M.C.; Jiang, X.; Brenna, J.T.; Abbaspourrad, A. Oleogel-Structured Composite for the Stabilization of Ω3 Fatty Acids in Fish Oil. Food Funct. 2018, 9, 5598–5606. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Jiang, X.; Yang, G.; Zhou, S. Effect of Different Molecular Compositions of Oils on Beeswax-Based Oleogels. Eur. J. Lipid Sci. Technol. 2025, 127, e70015. [Google Scholar] [CrossRef]
- Morales, E.; Iturra, N.; Contardo, I.; Quilaqueo, M.; Franco, D.; Rubilar, M. Fat Replacers Based on Oleogelation of Beeswax/Shellac Wax and Healthy Vegetable Oils. LWT 2023, 185, 115144. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Cao, L.; Shi, Z.; Lei, Z.; Jiang, S. Structure and Thermal Properties of β-Sitosterol-Beeswax-Sunflower Oleogels. Int. J. Food Sci. Technol. 2020, 55, 1900–1908. [Google Scholar] [CrossRef]
- Ramírez-Carrasco, P.; Alemán, A.; González, E.; Gómez-Guillén, M.C.; Robert, P.; Giménez, B. Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels. Foods 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Sarkisyan, V.; Frolova, Y.; Sobolev, R.; Kochetkova, A. On the Role of Beeswax Components in the Regulation of Sunflower Oil Oleogel Properties. Food Biophys. 2023, 18, 262–272. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Fawzia, S.; Mundree, S.; Madhujith, T.; Karim, A. Investigation of the Influence of Minor Components and Fatty Acid Profile of Oil on Properties of Beeswax and Stearic Acid-Based Oleogels. Food Res. Int. 2024, 184, 114213. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Makarenko, M.; Kochetkova, A. Effect of Beeswax and Combinations of Its Fractions on the Oxidative Stability of Oleogels. Food Biosci. 2022, 48, 101744. [Google Scholar] [CrossRef]
- Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Kochetkova, A. Waxy Oleogels for Partial Substitution of Solid Fat in Margarines. Gels 2023, 9, 683. [Google Scholar] [CrossRef]
- Airoldi, R.; da Silva, T.L.T.; Ract, J.N.R.; Foguel, A.; Colleran, H.L.; Ibrahim, S.A.; da Silva, R.C. Potential Use of Carnauba Wax Oleogel to Replace Saturated Fat in Ice Cream. J. Am. Oil Chem. Soc. 2022, 99, 1085–1099. [Google Scholar] [CrossRef]
- Roufegarinejad, L.; Dehghani, S.; Bakhshi, S.; Toker, O.S.; Pirouzian, H.R.; Khiabani, A.H. Oleogelation of Sunflower-Linseed Oils with Carnauba Wax as an Innovative Strategy for Shortening Substitution in Cakes. Food Chem. 2024, 437, 137745. [Google Scholar] [CrossRef]
- Shahamati, M.; Ahmadi, P.; Tabibiazar, M.; Fazelioskouei, T.; Azadmard-Damirchi, S.; Zargaraan, A. Characterization of Acorn Oil and Its Application on Carnauba Wax-Based Oleogel and Chocolate Spread. Int. J. Biol. Macromol. 2024, 260, 129571. [Google Scholar] [CrossRef]
- Tanislav, A.E.; Șandru, B.; Man, S.M.; Pușcaș, A.; Mureșan, A.E.; Păucean, A.; Mureșan, V.; Mudura, E. Investigating the Complete Replacement of Conventional Fat with Oleogel on the Structural Behavior of Five Different Pastry Products. Eur. Food Res. Technol. 2024, 250, 1933–1947. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Dhal, S.; Sahu, D.; Jarzębski, M.; Anis, A.; Kim, D.; Pal, K. Study of Microstructure, Texture, and Cooking Qualities of Reformulated Whole Wheat Flour Pasta by Substituting Water with Stearic Acid–Candelilla Wax–Groundnut Oil Oleogel. ChemEngineering 2024, 8, 51. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W. The Lipid-Amylose Complexes Enhance Resistant Starch Content in Candelilla Wax-Based Oleogels Cookies. Int. J. Biol. Macromol. 2024, 278, 134804. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.K.; Pérez-Martínez, J.D.; Gallegos-Infante, J.A.; Toro-Vazquez, J.F.; Ornelas-Paz, J.J. Rheological Properties of Ethyl Cellulose-Monoglyceride-Candelilla Wax Oleogel Vis-a-Vis Edible Shortenings. Carbohydr. Polym. 2021, 252, 117171. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, Y.; Zhu, J.; Gao, Y.; Li, Q.; Du, S.; Yu, X. Effect of Flaxseed Gum on the Brittleness of Oleogels Based on Candelilla Wax. RSC Adv. 2022, 12, 30734–30741. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, I.; Żbikowska, A.; Onacik-Gür, S. Candelilla Wax-Based Oleogels versus Palm Oil: Evaluation of Physical Properties of Innovative and Conventional Lipids Using Optical Techniques. J. Sci. Food Agric. 2022, 102, 2309–2320. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Kim, S.; Winkler-Moser, J.K.; Liu, S.X. Effect of Vegetable Oil Unsaturation and Minor Polar Components on the Physical Properties of Rice Bran Wax Oleogels. J. Food Sci. 2025, 90, e17613. [Google Scholar] [CrossRef]
- Tarté, R.; Paulus, J.S.; Acevedo, N.C.; Prusa, K.J.; Lee, S.-L. High-Oleic and Conventional Soybean Oil Oleogels Structured with Rice Bran Wax as Alternatives to Pork Fat in Mechanically Separated Chicken-Based Bologna Sausage. LWT 2020, 131, 109659. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Zhou, Q.; Jiang, L.; Wang, L.; Dai, Y.; Yu, D.; Elfalleh, W. Crude Wax Extracted from Rice Bran Oil Improves Oleogel Properties and Oxidative Stability. Eur. J. Lipid Sci. Technol. 2021, 123, 2000091. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of Biodegradable Hydrophilic and Hydrophobic Emulsifiers on the Oleogels Containing Sunflower Wax and Sunflower Oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Zampouni, K.; Prodromidis, P.; Moschakis, T.; Katsanidis, E. Microstructure, Physical Properties, and Oxidative Stability of Olive Oil Oleogels Composed of Sunflower Wax and Monoglycerides. Gels 2024, 10, 195. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Gillman, J.D.; Winkler-Moser, J.K.; Kim, S.; Singh, M.; Byars, J.A.; Evangelista, R.L. Properties of Oleogels Formed with High-Stearic Soybean Oils and Sunflower Wax. J. Am. Oil Chem. Soc. 2018, 95, 557–569. [Google Scholar] [CrossRef]
- Borriello, A.; Masi, P.; Cavella, S. Novel Pumpkin Seed Oil-Based Oleogels: Development and Physical Characterization. LWT 2021, 152, 112165. [Google Scholar] [CrossRef]
- Çokay, H.; Öğütcü, M. Determining the Structure and Stability of Essential Oil-Sunflower Wax and Beeswax Oleogels. J. Am. Oil Chem. Soc. 2023, 100, 993–1002. [Google Scholar] [CrossRef]
- Huang, H.; Hallinan, R.; Maleky, F. Comparison of Different Oleogels in Processed Cheese Products Formulation. Int. J. Food Sci. Technol. 2018, 53, 2525–2534. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Kim, S.; Winkler-Moser, J.K.; Lee, S.; Liu, S.X. Feasibility of Hemp Seed Oil Oleogels Structured with Natural Wax as Solid Fat Replacement in Margarine. J. Am. Oil Chem. Soc. 2022, 99, 1055–1070. [Google Scholar] [CrossRef]
- Kim, D.; Oh, I. The Characteristic of Insect Oil for a Potential Component of Oleogel and Its Application as a Solid Fat Replacer in Cookies. Gels 2022, 8, 355. [Google Scholar] [CrossRef]
- Pino, N.A.; Marchetti, L.; Lorenzo, G. Impact of Binary Mixtures of Natural Waxes in Mechanical Properties and Microstructure of Oleogels. J. Sci. Food Agric. 2024, 104, 6157–6165. [Google Scholar] [CrossRef]
- Ropciuc, S.; Dranca, F.; Oroian, M.A.; Leahu, A.; Prisacaru, A.E.; Spinei, M.; Codină, G.G. Characterization of Beeswax and Rice Bran Wax Oleogels Based on Different Types of Vegetable Oils and Their Impact on Wheat Flour Dough Technological Behavior during Bun Making. Gels 2024, 10, 194. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, V.; Sharma, R.; Singh, B. Virgin Coconut and Mustard Oleogels as Affected by Beeswax and Candelilla Wax: Functional, Textural, Rheological and Morphological Characteristics. Int. J. Food Sci. Technol. 2023, 58, 3293–3302. [Google Scholar] [CrossRef]
- Scharfe, M.; Niksch, J.; Flöter, E. Influence of Minor Oil Components on Sunflower, Rice Bran, Candelilla, and Beeswax Oleogels. Eur. J. Lipid Sci. Technol. 2022, 124, 2100068. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, L.; Kang, S.; Jiang, S.; Pang, M. Influence of Wax Type on Characteristics of Oleogels from Camellia Oil and Medium Chain Triglycerides. Int. J. Food Sci. Technol. 2022, 57, 2003–2014. [Google Scholar] [CrossRef]
- Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Binary Wax Oleogels: Improving Physical Properties and Oxidation Stability through Substitution of Carnauba Wax with Beeswax. J. Food Sci. 2024, 89, 4372–4388. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Brykczynski, H.; Flöter, E. Wax-Based Oleogels—Properties in Medium Chain Triglycerides and Canola Oil. Eur. J. Lipid Sci. Technol. 2022, 124, 2100114. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Byars, J.A.; Singh, M.; Hwang, H.-S. Evaluation of Beeswax, Candelilla Wax, Rice Bran Wax, and Sunflower Wax as Alternative Stabilizers for Peanut Butter. J. Am. Oil Chem. Soc. 2019, 96, 1235–1248. [Google Scholar] [CrossRef]
- Yilmaz, E.; Keskin Uslu, E.; Öz, C. Oleogels of Some Plant Waxes: Characterization and Comparison with Sunflower Wax Oleogel. J. Am. Oil Chem. Soc. 2021, 98, 643–655. [Google Scholar] [CrossRef]
- Yılmaz, E.; Toksöz, B. Flaxseed Oil-Wax Oleogels Replacement for Tallowfat in Sucuk Samples Provided Higher Concentrations of Polyunsaturated Fatty Acids and Aromatic Volatiles. Meat Sci. 2022, 192, 108875. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, L.; Wang, Y.; Kang, S.; Cao, L.; Pang, M. Oleogel for Improving the Texture and Flavor in Rice Cooking: Preparation from Natural Wax and Rice Bran Oil and Characterization. J. Cereal Sci. 2024, 118, 103955. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A Unique Class of Materials for Drug Delivery Applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Farzana, W.; Mahesh, S.; Sharma, S.; Syed, I.; Abdi, G.; Upadhyay, R. A Comprehensive Review on Bigels as a Potential Replacement to Solid Fat in Food Applications. J. Food Qual. 2025, 2025, 2483241. [Google Scholar] [CrossRef]
- Francavilla, A.; Corradini, M.G.; Joye, I.J. Bigels as Delivery Systems: Potential Uses and Applicability in Food. Gels 2023, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Greco, V.; Baldino, N.; de Cindio, B.; Fischer, P.; Gabriele, D. The Effects of Intermolecular Interactions on the Physical Properties of Organogels in Edible Oils. J. Colloid Interface Sci. 2016, 483, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.; Li, J.; Duan, Z.; Fan, L. Bigels as Emerging Biphasic Systems: Properties, Applications, and Prospects in the Food Industry. Food Hydrocoll. 2024, 154, 110089. [Google Scholar] [CrossRef]
- Chao, E.; Yan, X.; Fan, L. Fabrication of Edible Inks for 3D Printing as a Dysphagia Food: An Emerging Application of Bigels. Food Hydrocoll. 2024, 157, 110463. [Google Scholar] [CrossRef]
- Chen, Z.; Bian, F.; Cao, X.; Shi, Z.; Meng, Z. Novel Bigels Constructed from Oleogels and Hydrogels with Contrary Thermal Characteristics: Phase Inversion and 3D Printing Applications. Food Hydrocoll. 2023, 134, 108063. [Google Scholar] [CrossRef]
- De Los Santos-Trinidad, J.; Pérez-Alonso, C.; Cruz-Sosa, F.; Román-Guerrero, A. Effect of the Use of Sweet Potato or Chayote Tuber Starch Hydrogels on the Physical Properties of Oleogel-in-Hydrogel (Bigels) Systems. Int. J. Food Sci. Technol. 2023, 58, 6871–6880. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Neves, B.V.; Mazzo, T.M.; Longo, E.; Jacob-Lopez, E.; Zepka, L.Q.; de Rosso, V.V. Bigels as Potential Inks for Extrusion-Based 3d Food Printing: Effect of Oleogel Fraction on Physical Characterization and Printability. Food Hydrocoll. 2023, 144, 108986. [Google Scholar] [CrossRef]
- Ghorghi, Z.B.; Yeganehzad, S.; Hesarinejad, M.A.; Faezian, A.; Kutsenkova, V.; Gao, Z.; Nishinari, K.; Nepovinnykh, N. Fabrication of Novel Hybrid Gel Based on Beeswax Oleogel: Application in the Compound Chocolate Formulation. Food Hydrocoll. 2023, 140, 108599. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Song, X.; Meng, Z. Stabilization Models of Foams Prepared from Whippable Bigels: Crystal Absorption and Droplet Stability. Food Hydrocoll. 2024, 147, 109383. [Google Scholar] [CrossRef]
- Hashemi, B.; Varidi, M.; Jafari, S.M. Fabrication and Characterization of Novel Whey Protein-Based Bigels as Structured Materials with High-Mechanical Properties. Food Hydrocoll. 2023, 145, 109082. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Xiao, Y.; Guo, R.; Liu, X.; Zhang, H.; Bi, Y.; Xu, X. Fabrication and Characterization of Novel Food-Grade Bigels Based on Interfacial and Bulk Stabilization. Foods 2023, 12, 2546. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, F.; Yuan, S.; Dai, W.; Yin, L.; Dai, Q.; Li, Z.; Liu, H.; Guo, Q.; Zhu, Q. Development of Novel Lactobacillus Plantarum-Encapsulated Bigel Based on Soy Lecithin-Beeswax Oleogel and Flaxseed Gum Hydrogel for Enhanced Survival during Storage and Gastrointestinal Digestion. Food Hydrocoll. 2025, 163, 111052. [Google Scholar] [CrossRef]
- Lin, X.; Liu, F.; Ma, Z.; Li, X.; Li, Y. Investigating the Impact of Beeswax Addition and Diacylglycerol Profiles on Bigel Properties and Application in Bread: Insights on Intermolecular Interaction Mechanisms. Food Hydrocoll. 2025, 160, 110838. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; Lu, S.; Xu, B.; Bai, C.; Ma, T.; Song, Y. Cellulose Nanofiber/Beeswax Based Bigels: Study of Phase Inversion and Regulatory Properties. Food Hydrocoll. 2025, 168, 111494. [Google Scholar] [CrossRef]
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid Gels: Influence of Oleogel/Hydrogel Ratio on Rheological and Textural Properties. Food Res. Int. 2019, 116, 1298–1305. [Google Scholar] [CrossRef]
- Neves, B.V.; Fernandes, A.S.; Bonsanto, F.P.; Capriles, V.D.; Braga, A.R.C.; de Rosso, V.V. Exploring Different Food-Grade Bigel Systems for Delivering Bioactive Carotenoids: Part 2—Potential for Extrusion-Based 3D Printing. Food Hydrocoll. 2025, 167, 111428. [Google Scholar] [CrossRef]
- Pang, M.; Xu, L.; Ge, Y.; Cheng, J.; Zhang, Z.; Cao, L. Fabrication of Beeswax/Plant Sterol Ester-Gelatin/Whey Protein Isolate Bigels with Dual Gelation Effects as Substitutes for Traditional Solid Fats. Food Hydrocoll. 2024, 157, 110458. [Google Scholar] [CrossRef]
- Qiu, R.; Qiu, G.; Zhao, P.; Awais, M.; Fan, B.; Huang, Y.; Tong, L.; Wang, L.; Liu, L.; Wang, F. Regulation of Rheological Properties of Soy Protein Isolate-Beeswax Based Bigel Inks for High-Precision 3D Printing. Food Hydrocoll. 2024, 153, 110052. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, K.; Tian, H.; Liu, X.; Liu, G.; Hu, Z.; Zhao, L. Analysis on the Printability and Rheological Characteristics of Bigel Inks: Potential in 3D Food Printing. Food Hydrocoll. 2022, 129, 107675. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, H.; Mo, Y.; Gao, Y.; Mao, L. Structural Characterization of Hydrogel-Oleogel Biphasic Systems as Affected by Oleogelators. Food Res. Int. 2022, 158, 111536. [Google Scholar] [CrossRef]
- Zhai, X.; Sun, Y.; Cen, S.; Wang, X.; Zhang, J.; Yang, Z.; Li, Y.; Wang, X.; Zhou, C.; Arslan, M.; et al. Anthocyanins-Encapsulated 3D-Printable Bigels: A Colorimetric and Leaching-Resistant Volatile Amines Sensor for Intelligent Food Packaging. Food Hydrocoll. 2022, 133, 107989. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, Z.; Zhang, L.; Zong, W.; Fan, W. Bigels Based on Myofibrillar Protein Hydrogel and Beeswax Oleogel: Micromorphology, Mechanical Property, and Rheological Properties. Int. J. Biol. Macromol. 2025, 309, 142962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, B.; Wu, A.; Hu, Z.; Liu, J.; Wang, Y.; Liu, H. Preparation of a Bigel System Based on K-Carrageenan Hydrogel and Beeswax Oleogel and the Effect of Starch on the Bigel Properties. LWT 2024, 205, 116516. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and Characterization of Novel Bigels Based on Monoglyceride-Beeswax Oleogel and High Acyl Gellan Gum Hydrogel for Lycopene Delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef] [PubMed]
- Baltuonytė, G.; Eisinaitė, V.; Kazernavičiūtė, R.; Vinauskienė, R.; Jasutienė, I.; Leskauskaitė, D. Novel Formulation of Bigel-Based Vegetable Oil Spreads Enriched with Lingonberry Pomace. Foods 2022, 11, 2213. [Google Scholar] [CrossRef]
- Eisinaitė, V.; Jasutienė, I.; Vinauskienė, R.; Leskauskaitė, D. Development of Bigel Based Dysphagia-Oriented Products, Structured with Collagen and Carnauba Wax: Characterisation and Rheological Behaviour. Int. J. Food Sci. Technol. 2023, 58, 145–153. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Jacob-Lopez, E.; Zepka, L.Q.; Roca, M.; de Rosso, V.V. Bioactive Compound-Loaded Food-Grade Bigels: (I) Characterization and Study of Colorimetry and 3d-Printing Capability. Food Hydrocoll. 2025, 168, 111486. [Google Scholar] [CrossRef]
- Oyom, W.; Strange, J.; Nowlin, K.; Tukur, P.; Ferdaus, M.J.; Faraji, H.; Tahergorabi, R. Development and Characterization of Bigel Systems as Carriers for Thyme Essential Oil Utilizing Hydrogel from Chicken Processing By-Products for Food Applications. Int. J. Biol. Macromol. 2025, 292, 139222. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Morales, E.; Rubilar, M.; Contardo, I. Evaluation of Mixing Temperature in the Preparation of Plant-Based Bigels. Gels 2024, 10, 725. [Google Scholar] [CrossRef]
- Swathika, B.S.; Santhoshkumar, P.; Moses, J.A.; Sinija, V.R. Novel Three-Dimensional-Printed ι-Carrageenan–Carnauba Wax Bigel Formulation for Codelivery Applications. ACS Food Sci. Technol. 2025, 5, 1183–1192. [Google Scholar] [CrossRef]
- Tamašauskaitė, L.; Minelgaitė, V.; Šipailienė, A.; Vinauskienė, R.; Eisinaitė, V.; Leskauskaitė, D. Bigel Matrix Loaded with Probiotic Bacteria and Prebiotic Dietary Fibers from Berry Pomace Suitable for the Development of Probiotic Butter Spread Product. Gels 2024, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-García, I.; Salgado-Cruz, M.D.; García-Hernández, A.B.; Gutiérrez-López, G.F.; Hernández-Sánchez, H.; Camacho-Díaz, B.H.; Alamilla-Beltrán, L. Egg White-Based Gels with Candelilla Wax: A Study of Rheological, Mechanical, Calorimetric and Microstructural Properties. Gels 2024, 10, 733. [Google Scholar] [CrossRef]
- Giannakaki, F.; Dimakopoulou-Papazoglou, D.; Zampouni, K.; Moschakis, T.; Katsanidis, E. Design and Characterization of Bigels Composed of Agar, κ-Carrageenan, Candelilla Wax, and Monoglycerides. Int. J. Biol. Macromol. 2025, 308, 142422. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Zhou, H.; Vicente, A.A.; Pinheiro, A.C.; McClements, D.J. Plant-Based Bigels for Delivery of Bioactive Compounds: Influence of Hydrogel: Oleogel Ratio and Protein Concentration on Their Physicochemical Properties. Food Hydrocoll. 2024, 150, 109721. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Y.; Lu, Q.; Jiang, Y.; Jin, H.; Li, Q.; Yu, X. Development and Evaluation of a Novel Margarine Using Starch Hydrogel Combined Edible Wax Oleogel Bigels. J. Food Eng. 2025, 388, 112360. [Google Scholar] [CrossRef]
- Lee, G.R.; Baek, Y.; Jeong, E.; Lee, H.G. Development and Characterization of a Novel Bigel System Based on Candelilla Wax Oleogel and Guar Gum Hydrogel for Heat-Triggered Release Properties. Food Hydrocoll. 2024, 152, 109892. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Zhang, Y.; Shen, Y.; Deng, X.; Wang, F. Novel Bigels Based on Walnut Oil Oleogel and Chitosan Hydrogel: Preparation, Characterization, and Application as Food Spread. Int. J. Biol. Macromol. 2024, 260, 129530. [Google Scholar] [CrossRef]
- Moguiliansky, S.; Friedman, N.; Davidovich-Pinhas, M. The Effect of Transglutaminase on the Structure and Texture of Plant-Protein Based Bigel. Food Hydrocoll. 2025, 162, 110981. [Google Scholar] [CrossRef]
- Vershkov, B.; Davidovich-Pinhas, M. The Effect of Preparation Temperature and Composition on Bigel Performance as Fat Replacers. Food Funct. 2023, 14, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Hu, H.; Huang, Q.; Lu, X. Development and Characterization of Food-Grade Bigel System for 3D Printing Applications: Role of Oleogel/Hydrogel Ratios and Emulsifiers. Food Hydrocoll. 2023, 139, 108565. [Google Scholar] [CrossRef]
- Xie, D.; Hu, H.; Huang, Q.; Lu, X. Influence of Oleogel/Hydrogel Ratios and Emulsifiers on Structural and Digestion Properties of Food-Grade 3D Printed Bigels as Carriers for Quercetin and Catechin. Food Hydrocoll. 2023, 144, 108948. [Google Scholar] [CrossRef]
- Cho, K.; Tarté, R.; Acevedo, N.C. Development and Characterization of the Freeze-Thaw and Oxidative Stability of Edible Rice Bran Wax-Gelatin Biphasic Gels. LWT 2023, 174, 114330. [Google Scholar] [CrossRef]
- Han, L.; Chen, F.; Qiu, Y.; Gao, J.; Zhu, Q.; Wu, T.; Wang, P.; Zhang, M. Development and Characterization of Hydrogel-in-Oleogel (Bigel) Systems and Their Application as a Butter Replacer for Bread Making. J. Sci. Food Agric. 2024, 104, 1920–1927. [Google Scholar] [CrossRef]
- Kibler, N.D.; Acevedo, N.C.; Cho, K.; Zuber-McQuillen, E.A.; Carvajal, Y.B.; Tarté, R. Novel Biphasic Gels Can Mimic and Replace Animal Fat in Fully-Cooked Coarse-Ground Sausage. Meat Sci. 2022, 194, 108984. [Google Scholar] [CrossRef]
- Nutter, J.; Shi, X.; Lamsal, B.; Acevedo, N.C. Designing and Characterizing Multicomponent, Plant-Based Bigels of Rice Bran Wax, Gums, and Monoglycerides. Food Hydrocoll. 2023, 138, 108425. [Google Scholar] [CrossRef]
- Saffold, A.C.; Acevedo, N.C. Development of Novel Rice Bran Wax/Gelatin-Based Biphasic Edible Gels and Characterization of Their Microstructural, Thermal, and Mechanical Properties. Food Bioprocess Technol. 2021, 14, 2219–2230. [Google Scholar] [CrossRef]
- Saffold, A.C.; Acevedo, N.C. The Effect of Mono-Diglycerides on the Mechanical Properties, Microstructure, and Physical Stability of an Edible Rice Bran Wax–Gelatin Biphasic Gel System. J. Am. Oil Chem. Soc. 2022, 99, 1033–1043. [Google Scholar] [CrossRef]
- Shakouri, S.; Tehrani, M.M.; Koocheki, A.; Farhoosh, R.; Abdolshahi, A. The Development and Characterization of Edible Bigel a Hydrogel/Oleogel Structure Based on Guar Gum, Walnut Oil and Rice Bran Wax for Using as Fat Replacer. J. Polym. Environ. 2025, 33, 2058–2071. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Zhang, Q.; Wang, Y.; Jiao, A.; Jin, Z. Development and Characterisation of a Novel Bigel Based on Pea Protein Hydrogel and Rice Bran Wax Oleogel: Enhancement of Rheological Properties and Freeze-Thaw Stability. Int. J. Biol. Macromol. 2024, 282, 136606. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulou-Papazoglou, D.; Zampouni, K.; Moschakis, T.; Katsanidis, E. Novel Plant-Based Bigels Formulated with Sunflower Wax, Monoglycerides, Agar and κ-Carrageenan. Food Hydrocoll. 2025, 166, 111341. [Google Scholar] [CrossRef]
- Guo, J.; Gu, X.; Du, L.; Meng, Z. Spirulina Platensis Protein Nanoparticle-Based Bigels: Dual Stabilization, Phase Inversion, and 3D Printing. Food Hydrocoll. 2023, 135, 108160. [Google Scholar] [CrossRef]
- Steinkellner, C.; Ohlmeyer, M.; Franke, K. Effect of Margarine Replacement by a Low-Saturated Fat Bigel on Puff Pastry Quality. Food Bioproc. Technol. 2025, 18, 3981–3992. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Wu, A.; Li, J.; Tang, J.; Wang, Y.; Hu, Z. Gelatin-Wax-Based Bigel System: Innovative Research on a Low-Calorie Fat Substitute. LWT 2025, 224, 117836. [Google Scholar] [CrossRef]
- Hashim, A.F.; El-Sayed, S.M.; El-Sayed, H.S. Bigel Formulations Based on Sesame Oleogel with Probiotics Alginate Hydrogel: A Novel Structure for Nutritious Spreadable Butter. Int. J. Biol. Macromol. 2023, 242, 124782. [Google Scholar] [CrossRef]
| Wax | Composition | Melting Point (°C) | Source | Ref. |
|---|---|---|---|---|
| Beeswax (BW) | Wax esters: 60–80%, Hydrocarbons: 10–25%, Free fatty acid: 10–15%, Free fatty alcohol: 0–5% | 61–65 | Animal (secretion by honeybees) | [14,15] |
| Carnauba wax (CBW) | Wax esters: 50–70%, Hydrocarbons: 1.5–3%, Free fatty acid: 3–6%, Free fatty alcohol: 15–30%, Resins/others: 6.5–10% | 80–85 | Plant (leaves of Copernicia prunifera) | [13,16] |
| Candelilla wax (CDW) | Wax esters: 20–30%, Hydrocarbons: 60–65%, Free fatty acid: 7–10%, Free fatty alcohol: 10–15% | 68–73 | Plant (stems/leaves of Euphorbia antisyphilitica) | [13,14,17] |
| Rice bran wax (RBW) | Wax esters: 90–97%, Free fatty acid: 3–6%, Resins/others: 3–8% | 78–82 | Plant (by-product of rice bran oil refining) | [13,14,18] |
| Sunflower wax (SW) | Wax esters: 96–97%, Free fatty acid: 0–1%, Resins/others: 0–3% | 75–80 | Plant (sunflower seed oil processing) | [13,14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimakopoulou-Papazoglou, D.; Zampouni, K.; Katsanidis, E. Natural Waxes as Gelators in Edible Structured Oil Systems: A Review. Gels 2025, 11, 656. https://doi.org/10.3390/gels11080656
Dimakopoulou-Papazoglou D, Zampouni K, Katsanidis E. Natural Waxes as Gelators in Edible Structured Oil Systems: A Review. Gels. 2025; 11(8):656. https://doi.org/10.3390/gels11080656
Chicago/Turabian StyleDimakopoulou-Papazoglou, Dafni, Konstantina Zampouni, and Eugenios Katsanidis. 2025. "Natural Waxes as Gelators in Edible Structured Oil Systems: A Review" Gels 11, no. 8: 656. https://doi.org/10.3390/gels11080656
APA StyleDimakopoulou-Papazoglou, D., Zampouni, K., & Katsanidis, E. (2025). Natural Waxes as Gelators in Edible Structured Oil Systems: A Review. Gels, 11(8), 656. https://doi.org/10.3390/gels11080656










