Enantiomeric Ratio Modulates Hierarchical Networks and Rheological Performance in Cyclohexane Bisurea Supramolecular Gels
Abstract
1. Introduction
2. Results and Discussion
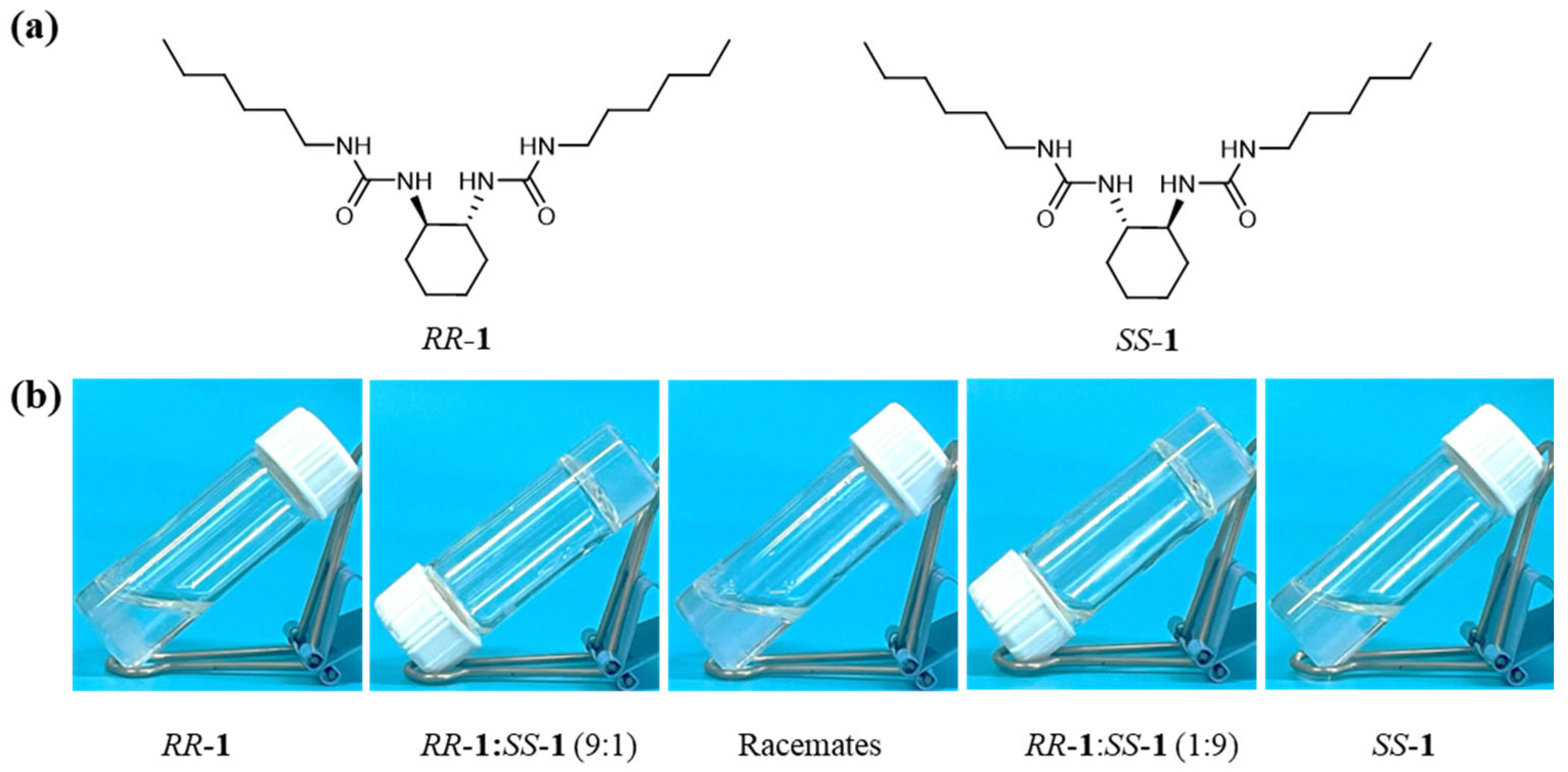
2.1. Optimal Condition for Cyclohexane Bisurea Gels
2.2. Gel Morphology Characterizations
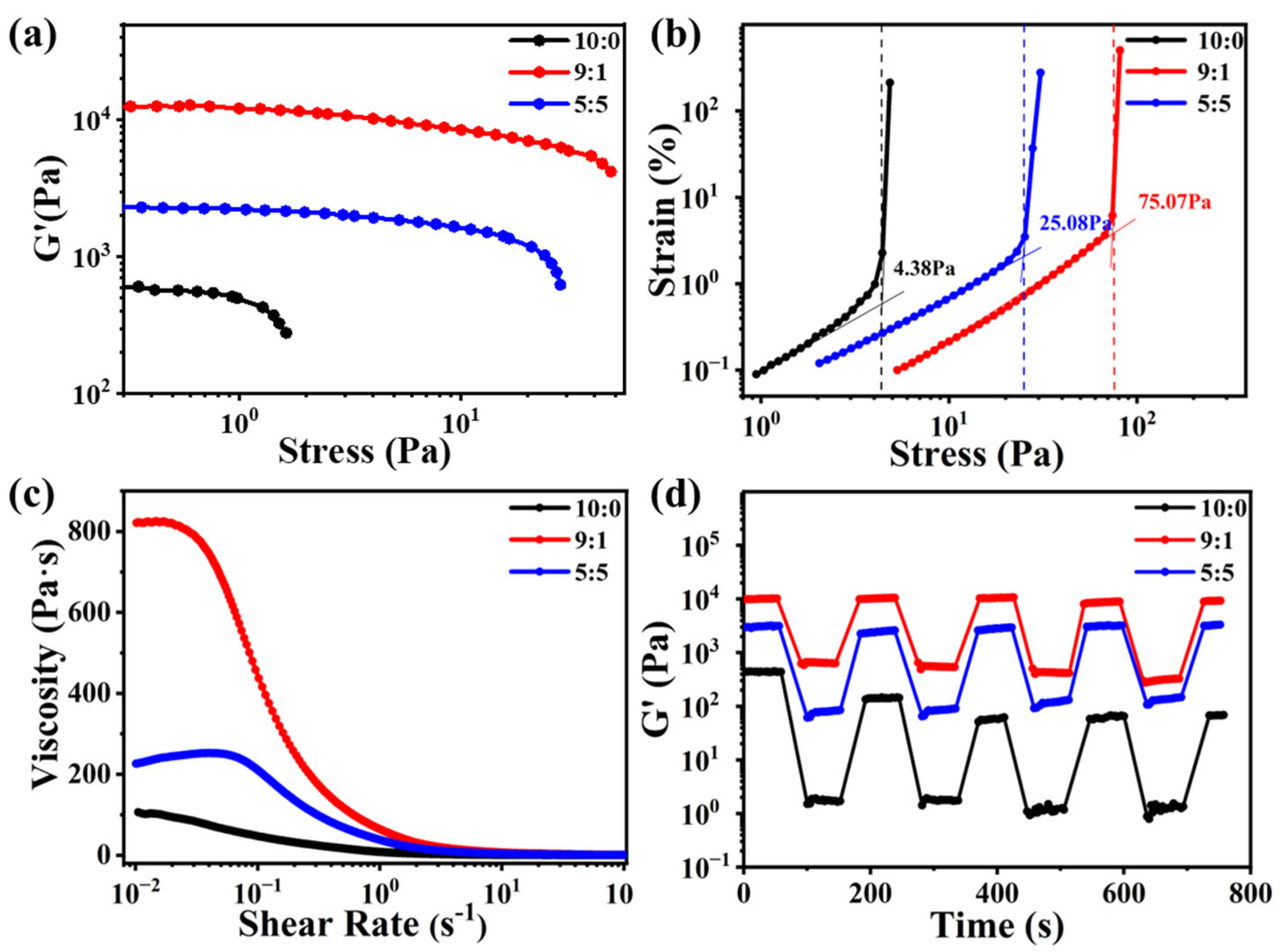
2.3. Rheological Properties Characterizations
2.4. Gelation Mechanism Characterization
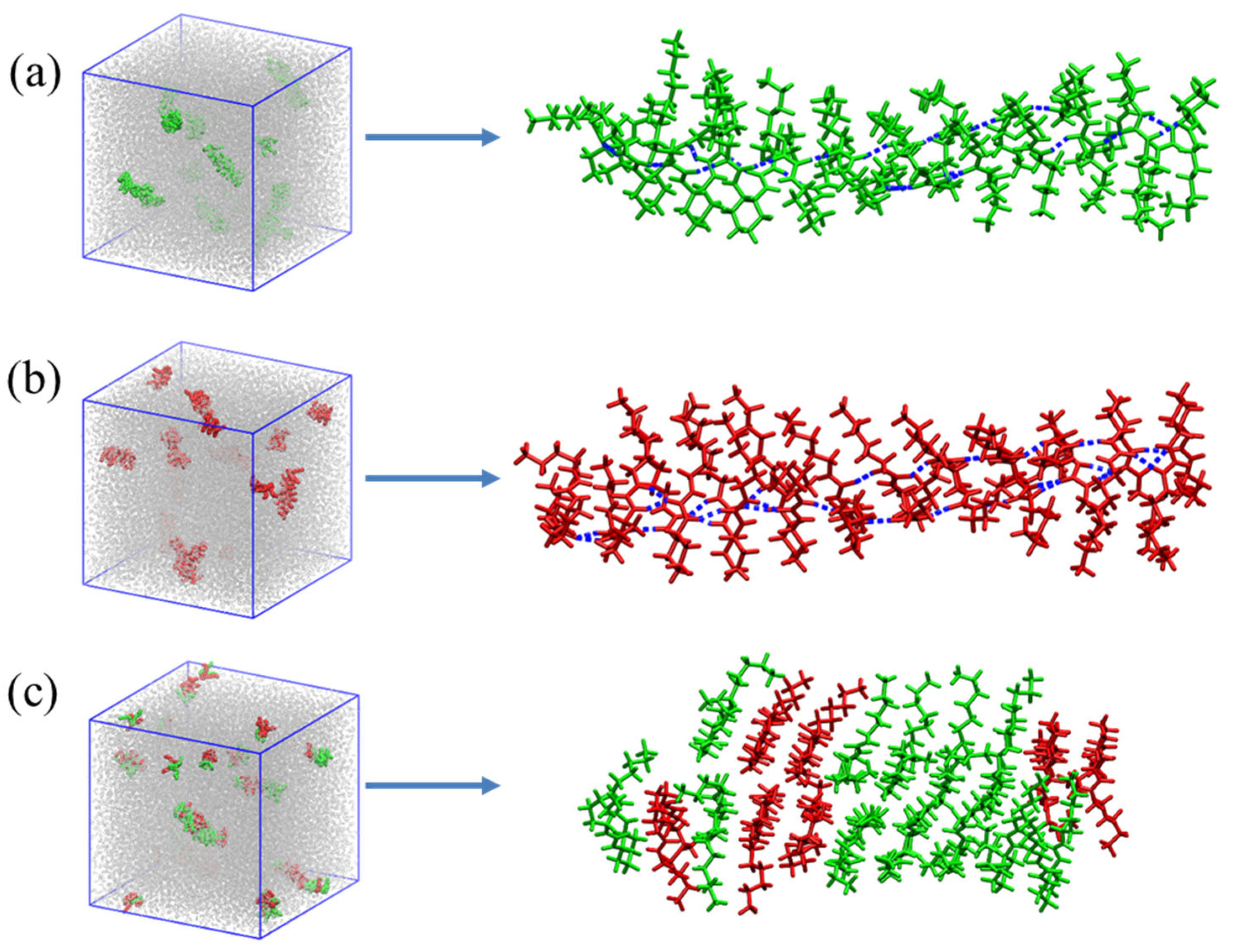
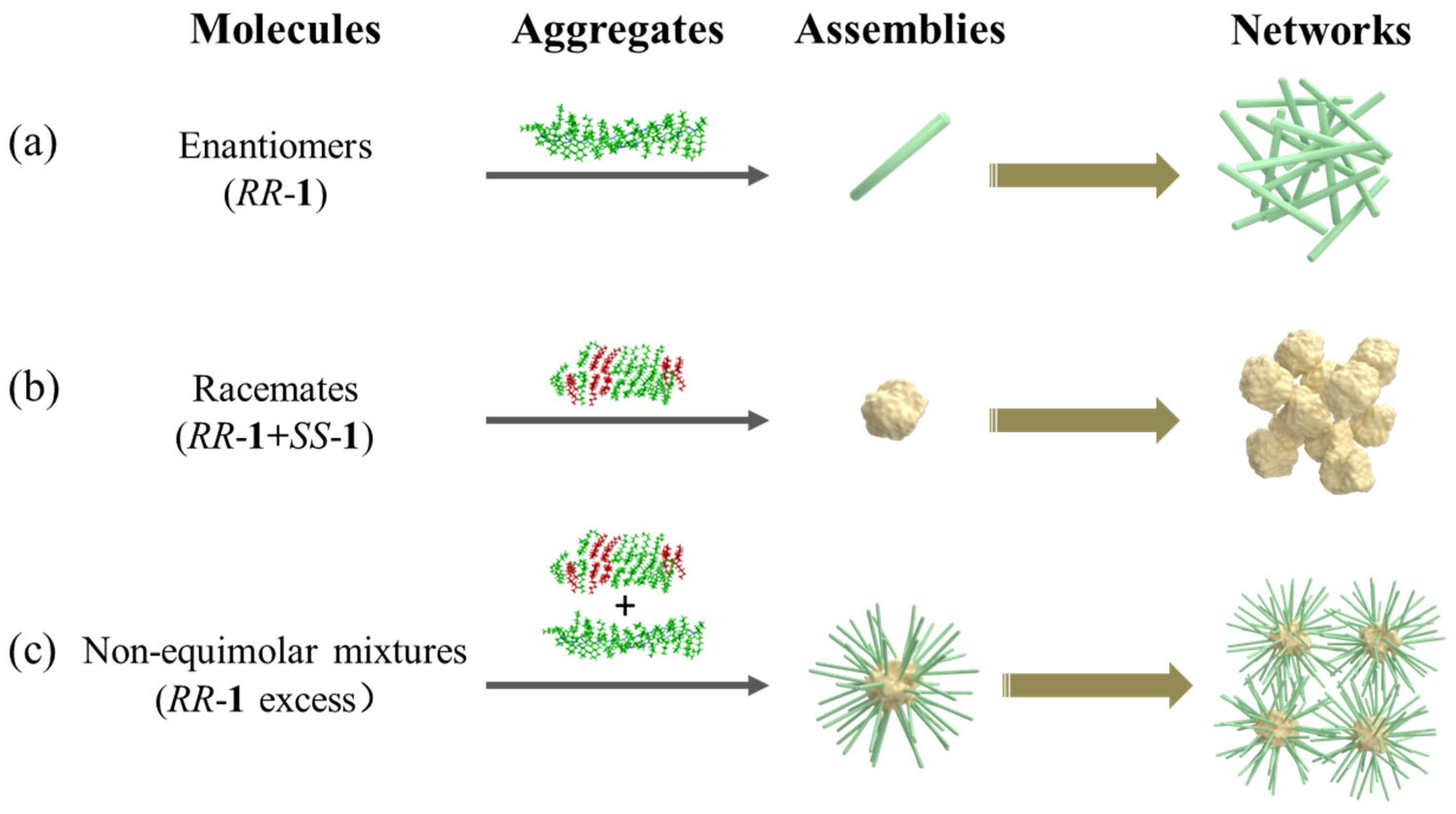
2.5. Theoretical Calculation and Mechanism
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RR-1 | 1,1’-((1R,2R)-cyclohexane-1,2-diyl)bis(3-hexylurea) |
| SS-1 | 1,1’-((1S,2S)-cyclohexane-1,2-diyl)bis(3-hexylurea) |
References
- Sun, J.; Li, Y.; Yan, F.; Liu, C.; Sang, Y.; Tian, F.; Feng, Q.; Duan, P.; Zhang, L.; Shi, X.; et al. Control over the emerging chirality in supramolecular gels and solutions by chiral microvortices in milliseconds. Nat. Commun. 2018, 9, 2599. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Shen, C.; Chen, Y.; Li, H.; Zhou, A.; Liu, K. Tuning Rheological Behaviors of Supramolecular Aqueous Gels via Charge Transfer Interactions. Langmuir 2021, 37, 14713–14723. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Zhu, X.; Fan, H.; Zhang, L.; Liu, M. Reaction induced supramolecular gelation with the evolution of circularly polarized luminescence. Mater. Chem. Front. 2022, 6, 593–599. [Google Scholar] [CrossRef]
- Du, S.; Zhu, X.; Zhang, L.; Liu, M. Switchable Circularly Polarized Luminescence in Supramolecular Gels through Photomodulated FRET. ACS Appl. Mater. Interfaces 2021, 13, 15501–15508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, W.-J.; Li, J.-L.; Wang, R.-Y. Distinct kinetics of molecular gelation in a confined space and its relation to the structure and property of thin gel films. Phys. Chem. Chem. Phys. 2015, 17, 8258–8265. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Kurdtabar, M.; Mirashrafi, N.S.; Bagheri Marandi, G.; Ghobadifar, V. Synthesis and characterization of self-healable supramolecular hydrogel based on carboxymethyl cellulose for biomedical applications. Int. J. Biol. Macromol. 2024, 281, 136532. [Google Scholar] [CrossRef]
- Oliveira, C.B.P.; Carvalho, A.; Pereira, R.B.; Pereira, D.M.; Hilliou, L.; Jervis, P.J.; Martins, J.A.; Ferreira, P.M.T. New Supramolecular Hydrogels Based on Diastereomeric Dehydrotripeptide Mixtures for Potential Drug Delivery Applications. Gels 2024, 10, 629. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-Assembly of Hyperbranched Polymers and Its Biomedical Applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef]
- Roy, N.; Ghosh, S.; Dastidar, P. Supramolecular Gelators Derived from Fmoc-Amino Acid Conjugates of Mafenide for Treating Melanoma: Toward Developing Vehicle-Free Drug Delivery. ACS Appl. Bio Mater. 2025, 8, 5984–5997. [Google Scholar] [CrossRef]
- Ambekar, A.; Sahoo, J.; Singh, K. Breaking barriers in ocular drug delivery for Uveitis: Advanced drug delivery systems, challenges and future prospects. Exp. Eye Res. 2025, 257, 110456. [Google Scholar] [CrossRef]
- Pourtalebi Jahromi, L.; Rothammer, M.; Fuhrmann, G. Polysaccharide hydrogel platforms as suitable carriers of liposomes and extracellular vesicles for dermal applications. Adv. Drug Deliv. Rev. 2023, 200, 115028. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, N.; Singh, N. An Encyclopedic Compendium on Chemosensing Supramolecular Metal-Organic Gels. Chem Asian J. 2024, 19, e202400258. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Sun, B.; Yang, Q.-P.; Wei, T.-B.; Zhang, Y.-M. A novel supramolecular metallogel-based high-resolution anion sensor array. Chem. Commun. 2015, 51, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Wei, T.-B.; Li, H.; Zhang, Y.-M. Rationally introduce multi-competitive binding interactions in supramolecular gels: A simple and efficient approach to develop multi-analyte sensor array. Chem. Sci. 2016, 7, 5341–5346. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Fang, H.; Qu, W.-J.; Yang, H.-H.; He, J.-X.; Yao, H.; Lin, Q.; Wei, T.-B.; Zhang, Y.-M. A self-assembled supramolecular gel constructed by phenazine derivative and its application in ultrasensitive detection of cyanide. Dye. Pigment. 2020, 174, 108066. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Zhang, T.; Zhou, L.-P.; Cai, L.-X.; Wu, L.-X.; Sun, Q.-F.; Cheng, X.-Y. Self-Healable Poly(vinyl alcohol)—Metal–Organic Hybrid Gel Electrolyte for Flexible Capacitor Sensors. ACS Appl. Polym. Mater. 2022, 4, 6487–6494. [Google Scholar] [CrossRef]
- Cicek, M.O.; Durukan, M.B.; Yıldız, B.; Keskin, D.; Doganay, D.; Çınar Aygün, S.; Cakir, M.P.; Unalan, H.E. Ultra-Sensitive Bio-Polymer Iontronic Sensors for Object Recognition from Tactile Feedback. Adv. Mater. Technol. 2023, 8, 2300322. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Jia, L.; Zhang, Q.; Xu, X. Stretchable and Self-Healing Integrated All-Gel-State Supercapacitors Enabled by a Notch-Insensitive Supramolecular Hydrogel Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 36028–36036. [Google Scholar] [CrossRef]
- Gu, C.; Peng, Y.; Li, J.; Wang, H.; Xie, X.Q.; Cao, X.; Liu, C.S. Supramolecular G4 Eutectogels of Guanosine with Solvent-Induced Chiral Inversion and Excellent Electrochromic Activity. Angew. Chem. Int. Ed. 2020, 59, 18768–18773. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ginnaram, S.; Wu, C.-H.; Lu, H.-W.; Pu, Y.-F.; Wu, J.I.; Gupta, D.; Lai, Y.-C.; Lin, H.-C. Fully self-healable, highly stretchable, and anti-freezing supramolecular gels for energy-harvesting triboelectric nanogenerator and self-powered wearable electronics. Nano Energy 2021, 90, 106525. [Google Scholar] [CrossRef]
- Li, P.; Liao, M.; Cui, S.; Li, J.; Ye, L.; Yang, Y.; Wang, C.; Wang, B.; Peng, H. Dynamically Resettable Electrode-Electrolyte Interface through Supramolecular Sol-Gel Transition Electrolyte for Flexible Zinc Batteries. Angew. Chem. Int. Ed. 2023, 62, e202300705. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Chen, H.; Chen, L.; Liu, S.; Zhang, X. Supramolecular hydrogels mediated by cucurbit[6]uril-modified Fe3O4 with self-healing, photothermal responsiveness and stretchability for flexible electronics. Colloids Surf. Physicochem. Eng. Aspects 2024, 694, 134042. [Google Scholar] [CrossRef]
- Gupta, B.; Kalyan Samanta, S.; Singh, R. Recent advances in conducting gels for flexible and stretchable smart electronic devices: A comprehensive review. Mater. Today 2024, 80, 681–709. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Liu, M. Gelation and self-assembly of glutamate bolaamphiphiles with hybrid linkers: Effect of the aromatic ring and alkyl spacers. Soft Matter 2009, 5, 1066–1073. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Liu, X.Y. Gelation with Small Molecules: From Formation Mechanism to NanostructureArchitecture. Top. Curr. Chem. 2005, 256, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, X.Y. Architecture of Supramolecular Soft Functional Materials: From Understanding to Micro-/Nanoscale Engineering. Adv. Funct. Mater. 2010, 20, 3196–3216. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Qin, M.; Cao, Y.; Wang, W. An enzyme-assisted nanoparticle crosslinking approach to enhance the mechanical strength of peptide-based supramolecular hydrogels. Chem. Commun. 2013, 49, 8653–8655. [Google Scholar] [CrossRef] [PubMed]
- Noteborn, W.E.M.; Zwagerman, D.N.H.; Talens, V.S.; Maity, C.; van der Mee, L.; Poolman, J.M.; Mytnyk, S.; van Esch, J.H.; Kros, A.; Eelkema, R.; et al. Crosslinker-Induced Effects on the Gelation Pathway of a Low Molecular Weight Hydrogel. Adv. Mater. 2017, 29, 1603769. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Lovrak, M.; le Sage, V.A.A.; Zhang, K.; Guo, X.; Eelkema, R.; Mendes, E.; van Esch, J.H. Biomimetic Strain-Stiffening Self-Assembled Hydrogels. Angew. Chem. Int. Ed. 2020, 59, 4830–4834. [Google Scholar] [CrossRef]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, X.; Wang, L.; Wang, H.; Liang, G.; Zhang, J.; Long, J.; Yang, Z. Rational Design of a Tetrameric Protein to Enhance Interactions between Self-Assembled Fibers Gives Molecular Hydrogels. Angew. Chem. Int. Ed. 2012, 51, 4388–4392. [Google Scholar] [CrossRef]
- Raeburn, J.; Zamith Cardoso, A.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5169. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Rosa, M.; Diaferia, C.; Fernandes, C. A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels. Gels 2024, 10, 507. [Google Scholar] [CrossRef]
- Sathaye, S.; Mbi, A.; Sonmez, C.; Chen, Y.; Blair, D.L.; Schneider, J.P.; Pochan, D.J. Rheology of peptide- and protein-based physical hydrogels: Are everyday measurements just scratching the surface? WIREs Nanomed. Nanobiotechnol. 2014, 7, 34–68. [Google Scholar] [CrossRef]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure–Function Correlation. Chem. Eur. J. 2017, 24, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Qi, Z.; Cao, Y.; Li, R.; Wu, J.; Tang, R.; Gao, Y.; Du, R.; Liu, M. Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications. Pharmaceutics 2025, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Ruochong, Z.; Dongmei, W.; Xinshao, C.; Minghuan, W.; Xiaodong, H.; Qi, D.; Xuefeng, X.; Meirong, C.; Litian, H. Recent development in friction of supramolecular gel lubricant: From mechanisms to applications. Nanotechnology 2025, 36, 182003. [Google Scholar] [CrossRef]
- Panja, S.; Fuentes-Caparrós, A.M.; Cross, E.R.; Cavalcanti, L.; Adams, D.J. Annealing Supramolecular Gels by a Reaction Relay. Chem. Mater. 2020, 32, 5264–5271. [Google Scholar] [CrossRef]
- Ben Messaoud, G.; Le Griel, P.; Hermida-Merino, D.; Roelants, S.L.K.W.; Soetaert, W.; Stevens, C.V.; Baccile, N. pH-Controlled Self-Assembled Fibrillar Network Hydrogels: Evidence of Kinetic Control of the Mechanical Properties. Chem. Mater. 2019, 31, 4817–4830. [Google Scholar] [CrossRef]
- Wang, R.Y.; Liu, X.Y.; Xiong, J.Y.; Li, J.L. Real-time observation of fiber network formation in molecular organogel: Supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 2006, 110, 7275–7280. [Google Scholar] [CrossRef]
- Colquhoun, C.; Draper, E.R.; Schweins, R.; Marcello, M.; Vadukul, D.; Serpell, L.C.; Adams, D.J. Controlling the network type in self-assembled dipeptide hydrogels. Soft Matter 2017, 13, 1914–1919. [Google Scholar] [CrossRef]
- Laishram, R.; Sarkar, S.; Seth, I.; Khatun, N.; Aswal, V.K.; Maitra, U.; George, S.J. Secondary Nucleation-Triggered Physical Cross-Links and Tunable Stiffness in Seeded Supramolecular Hydrogels. J. Am. Chem. Soc. 2022, 144, 11306–11315. [Google Scholar] [CrossRef]
- van Esch, J.H.; Schoonbeek, F.; de Loos, M.; Kooijman, H.; Spek, A.L.; Kellogg, R.M.; Feringa, B.L. Cyclic Bis-Urea Compounds as Gelators for Organic Solvents. Chem. Eur. J. 1999, 5, 937–950. [Google Scholar] [CrossRef]
- Hanabusa, K.; Yamada, M.; Kimura, M.; Shirai, H. Prominent Gelation and Chiral Aggregation of Alkylamides Derived from trans-1,2-Diaminocyclohexane. Angew. Chem. Int. Ed. 1996, 35, 1949–1951. [Google Scholar] [CrossRef]
- Brinksma, J.; Feringa, B.L.; Kellogg, R.M.; Vreeker, R.; van Esch, J. Rheology and Thermotropic Properties of Bis-Urea-Based Organogels in Various Primary Alcohols. Langmuir 2000, 16, 9249–9255. [Google Scholar] [CrossRef]
- de Loos, M.; van Esch, J.; Kellogg, R.M.; Feringa, B.L. Chiral Recognition in Bis-Urea-Based Aggregates and Organogels through Cooperative Interactions. Angew. Chem. Int. Ed. 2001, 40, 613–616. [Google Scholar] [CrossRef]
- Zweep, N.; Hopkinson, A.; Meetsma, A.; Browne, W.R.; Feringa, B.L.; van Esch, J.H. Balancing Hydrogen Bonding and van der Waals Interactions in Cyclohexane-Based Bisamide and Bisurea Organogelators. Langmuir 2009, 25, 8802–8809. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, T.; Liu, M. Tuning the Gelation Ability of Racemic Mixture by Melamine: Enhanced Mechanical Rigidity and Tunable Nanoscale Chirality. Langmuir 2014, 30, 10772–10778. [Google Scholar] [CrossRef]
- Čaplar, V.; Frkanec, L.; Vujičić, N.Š.; Žinić, M. Positionally Isomeric Organic Gelators: Structure–Gelation Study, Racemic versus Enantiomeric Gelators, and Solvation Effects. Chem. Eur. J. 2010, 16, 3066–3082. [Google Scholar] [CrossRef]
- McAulay, K.; Dietrich, B.; Su, H.; Scott, M.T.; Rogers, S.; Al-Hilaly, Y.K.; Cui, H.; Serpell, L.C.; Seddon, A.M.; Draper, E.R.; et al. Using chirality to influence supramolecular gelation. Chem. Sci. 2019, 10, 7801–7806. [Google Scholar] [CrossRef]
- Patterson, A.K.; El-Qarra, L.H.; Smith, D.K. Chirality-directed hydrogel assembly and interactions with enantiomers of an active pharmaceutical ingredient. Chem. Commun. 2022, 58, 3941–3944. [Google Scholar] [CrossRef]
- Ghosh, D.; Farahani, A.D.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Unraveling the Self-Assembly Modes in Multicomponent Supramolecular Gels Using Single-Crystal X-ray Diffraction. Chem. Mater. 2020, 32, 3517–3527. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. J. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A Well-Behaved Electrostatic Potential based Method Using Charge Restraints for Deriving Atomic Charges: The RESP Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an Axternal Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, S.; Jiang, Y.; Song, A.; Jiang, J. Enantiomeric Ratio Modulates Hierarchical Networks and Rheological Performance in Cyclohexane Bisurea Supramolecular Gels. Gels 2025, 11, 821. https://doi.org/10.3390/gels11100821
Hua S, Jiang Y, Song A, Jiang J. Enantiomeric Ratio Modulates Hierarchical Networks and Rheological Performance in Cyclohexane Bisurea Supramolecular Gels. Gels. 2025; 11(10):821. https://doi.org/10.3390/gels11100821
Chicago/Turabian StyleHua, Shaoshuai, Yuqian Jiang, Andong Song, and Jian Jiang. 2025. "Enantiomeric Ratio Modulates Hierarchical Networks and Rheological Performance in Cyclohexane Bisurea Supramolecular Gels" Gels 11, no. 10: 821. https://doi.org/10.3390/gels11100821
APA StyleHua, S., Jiang, Y., Song, A., & Jiang, J. (2025). Enantiomeric Ratio Modulates Hierarchical Networks and Rheological Performance in Cyclohexane Bisurea Supramolecular Gels. Gels, 11(10), 821. https://doi.org/10.3390/gels11100821





