Beyond Biomaterials: Engineering Bioactive Hydrogels as Immuno-Mechanobiological Niches for Osteochondral Regeneration
Abstract
1. Introduction: From Scaffold to Niche
2. The Evolving Landscape of Hydrogels for Osteochondral Regeneration
2.1. Natural Hydrogels
2.2. Synthetic Hydrogels
2.3. Hybrid and Composite Hydrogels
2.4. Functionalization for Bioactivity
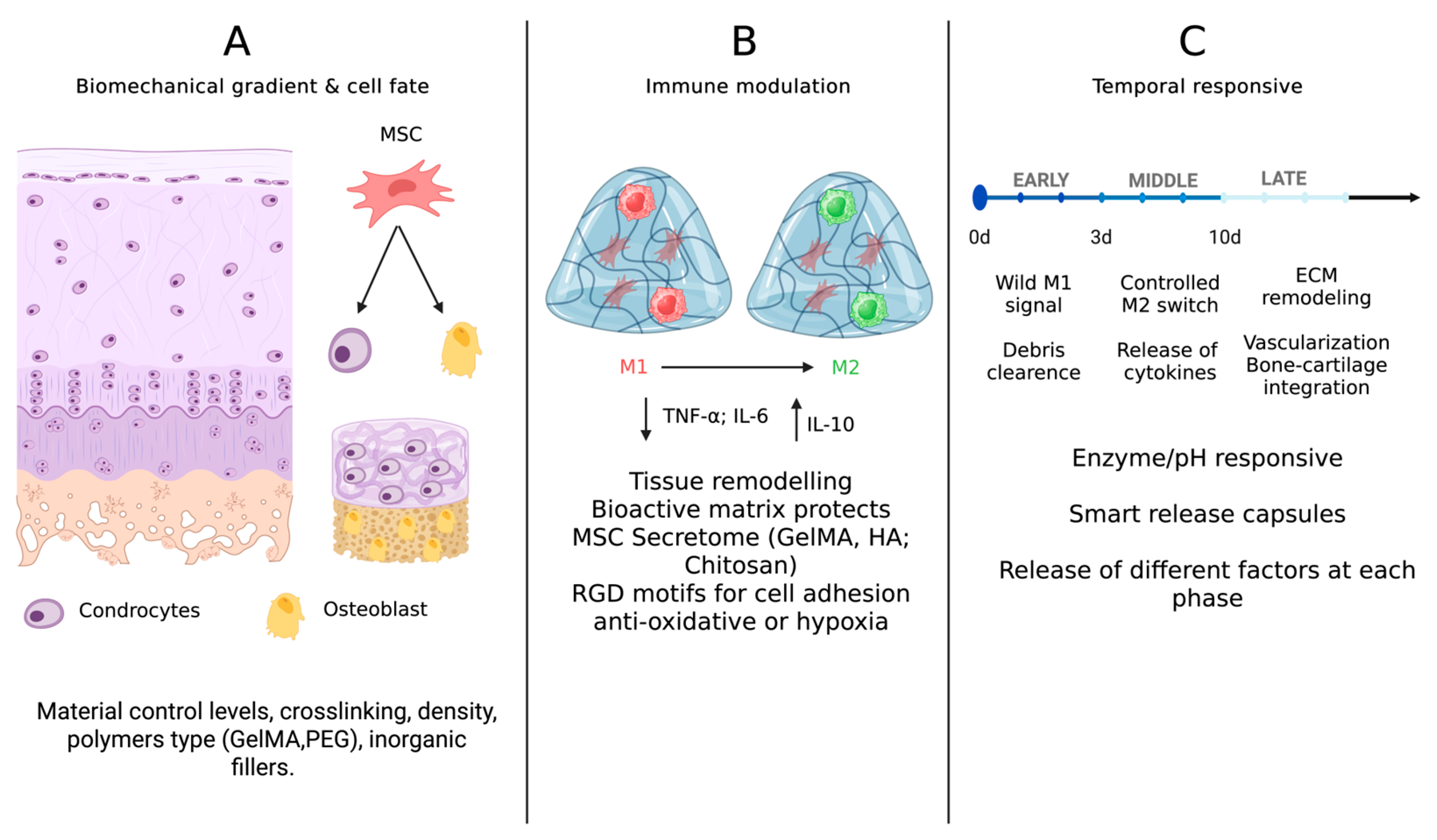
3. Immunomodulatory Hydrogels: Engineering the Healing Response
3.1. Macrophage Polarization: From Inflammation to Regeneration
3.2. MSC Secretome and Indirect Immunomodulation
3.3. Temporal Immunomodulation and “Immuno-Instructive” Materials
4. Mechanobiology in Hydrogel Design: Sculpting Cell Fate Through Force
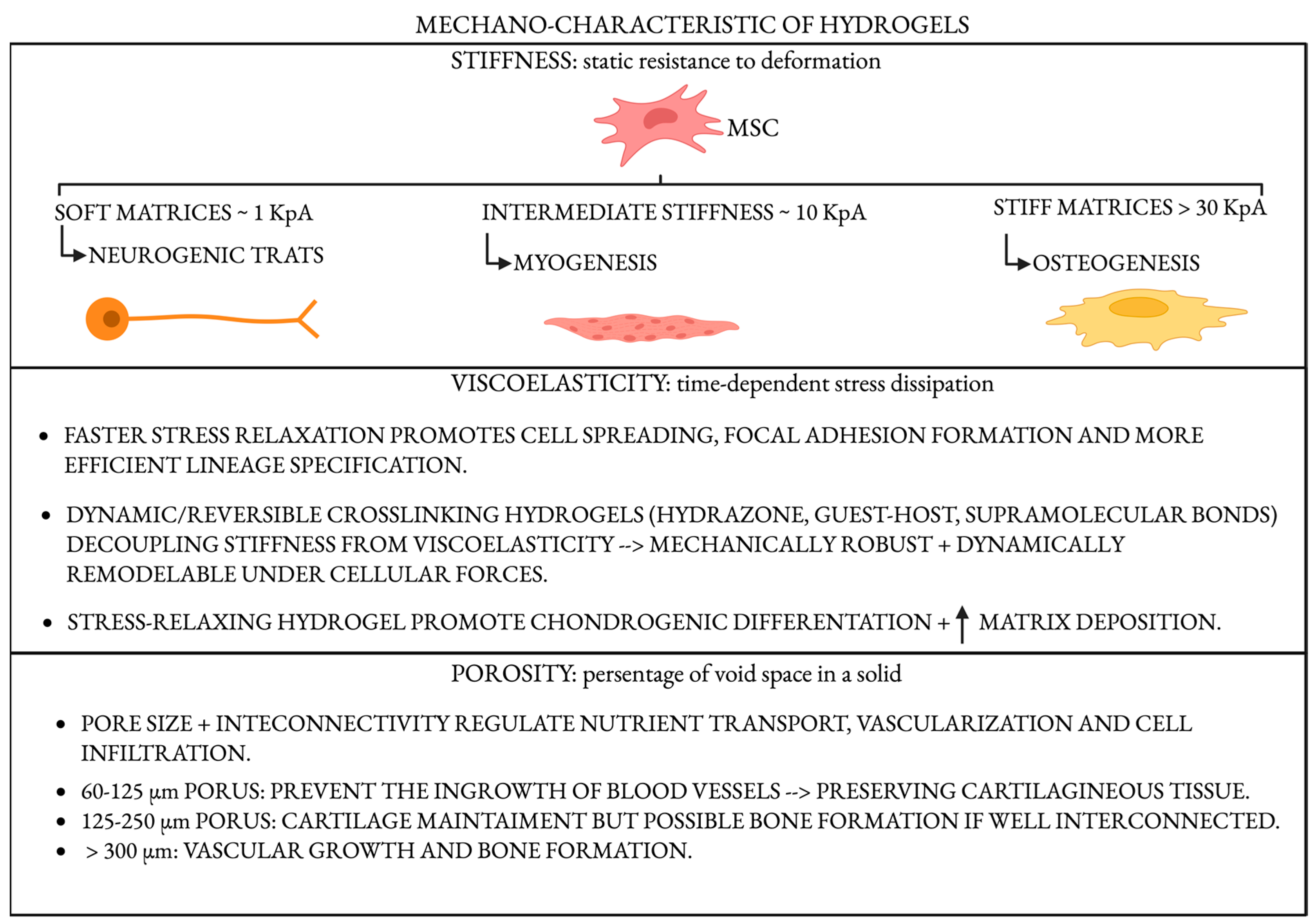
4.1. Stiffness: A Master Regulator of Lineage Commitment
4.2. Viscoelasticity and Stress Relaxation
4.3. Porosity, Architecture, and Load Transmission
5. Integrative Strategies: Toward Smart and Zonal Hydrogels
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Saris, D.B.F.; Vanlauwe, J.; Victor, J.; Haspl, M.; Bohnsack, M.; Fortems, Y.; Vandekerckhove, B.; Almqvist, K.F.; Claes, T.; Handelberg, F.; et al. Characterized Chondrocyte Implantation Results in Better Structural Repair When Treating Symptomatic Cartilage Defects of the Knee in a Randomized Controlled Trial versus Microfracture. Am. J. Sports Med. 2008, 36, 235–246. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Aslan, B.; Özkan, B.; Mutlu, H.; Üstün, E.; Budama-Kilinc, Y.; Cakir-Koc, R.; Aslan, B.; Özkan, B.; et al. Hydrogels in Regenerative Medicine. In Biomaterials in Regenerative Medicine; IntechOpen: London, UK, 2017; ISBN 978-953-51-3777-1. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-Enhanced Immunoregulation Hydrogel for Oxygenation and ROS Neutralization in Diabetic Foot Ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Li, Y.; Wang, J.; Chen, Y.; Lan, Y.; Liu, J.; Shen, J. An ATP-Activated Spatiotemporally Controlled Hydrogel Prodrug System for Treating Multidrug-Resistant Bacteria-Infected Pressure Ulcers. Bioact. Mater. 2025, 45, 301–321. [Google Scholar] [CrossRef]
- Novotná, R.; Franková, J. Materials Suitable for Osteochondral Regeneration. ACS Omega 2024, 9, 30097–30108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zha, K.; Hu, W.; Xiong, Y.; Knoedler, S.; Obed, D.; Panayi, A.C.; Lin, Z.; Cao, F.; Mi, B.; et al. Multifunctional Hydrogels: Advanced Therapeutic Tools for Osteochondral Regeneration. Biomater. Res. 2023, 27, 76. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage Polarization: An Opportunity for Improved Outcomes in Biomaterials and Regenerative Medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef]
- Sadtler, K.; Singh, A.; Wolf, M.T.; Wang, X.; Pardoll, D.M.; Elisseeff, J.H. Design, Clinical Translation and Immunological Response of Biomaterials in Regenerative Medicine. Nat. Rev. Mater. 2016, 1, 16040. [Google Scholar] [CrossRef]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Yi, T.; Song, S.U. Immunomodulatory Properties of Mesenchymal Stem Cells and Their Therapeutic Applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef]
- Li, Z.; Kupcsik, L.; Yao, S.-J.; Alini, M.; Stoddart, M.J. Mechanical Load Modulates Chondrogenesis of Human Mesenchymal Stem Cells through the TGF-Beta Pathway. J. Cell Mol. Med. 2010, 14, 1338–1346. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Sorboni, S.G.; Ranjbar, N.; Deyhimfar, R.; Abtahi, M.S.; Izady, M.; Kazemi, N.; Noori, A.; Pennisi, C.P. Mechanotransduction in Tissue Engineering: Insights into the Interaction of Stem Cells with Biomechanical Cues. Exp. Cell Res. 2023, 431, 113766. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Canciani, B.; Rossi, N.; Arrigoni, E.; Giorgino, R.; Sergio, M.; Aidos, L.; Di Giancamillo, M.; Herrera Millar, V.R.; Peretti, G.M.; Di Giancamillo, A.; et al. In Vitro Characterization of Human Cell Sources in Collagen Type I Gel Scaffold for Meniscus Tissue Engineering. Gels 2024, 10, 767. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-Cross-Linkable Methacrylated Gelatin and Hydroxyapatite Hybrid Hydrogel for Modularly Engineering Biomimetic Osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef]
- Zhong, Y.; Cao, X.; Huang, M.; Lei, Y.; Liu, A.-L. Biomimetic Bone Cartilage Scaffolds Based on Trilayer Methacrylated Hydroxyapatite/GelMA Composites for Full-Thickness Osteochondral Regeneration. Int. J. Biol. Macromol. 2025, 298, 139860. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Karami, P.; Laurent, A.; Philippe, V.; Applegate, L.A.; Pioletti, D.P.; Martin, R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. Int. J. Mol. Sci. 2024, 25, 9984. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical Applications and Prospects of Polylactic Acid Materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zheng, Q.; Zong, C.; Li, Q.; Li, H.; Qian, S.; Zhang, B.; Yu, L.; Pan, Z. Osteochondral Repair Using Porous Poly(Lactide-Co-glycolide)/Nano-hydroxyapatite Hybrid Scaffolds with Undifferentiated Mesenchymal Stem Cells in a Rat Model. J. Biomed. Mater. Res. 2010, 94A, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage Regeneration Using Mesenchymal Stem Cells and a PLGA–Gelatin/Chondroitin/Hyaluronate Hybrid Scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef]
- Tong, L.; Shi, G.; Liu, Q.; Qian, Z.; Li, J.; Zhang, K.; Zhu, Y.; Fang, Y.; Sha, L.; Bai, L.; et al. Fabrication and Evaluation of 3D Printed PLGA/nHA/GO Scaffold for Bone Tissue Engineering. Sci. Rep. 2025, 15, 12446. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiaowen, Y.; Yang, Y.; Liu, L.; Sun, Y.; Liu, Y.; Yin, L.; Chen, Z. Osteogenic and Anti-Inflammatory Effect of the Multifunctional Bionic Hydrogel Scaffold Loaded with Aspirin and Nano-Hydroxyapatite. Front. Bioeng. Biotechnol. 2023, 11, 1105248. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhou, F.; Yang, X.; Zhao, J.; Zhao, Y.; Yuan, X. A Pilot Study of Conically Graded Chitosan–Gelatin Hydrogel/PLGA Scaffold with Dual-delivery of TGF-β1 and BMP-2 for Regeneration of Cartilage–Bone Interface. J. Biomed. Mater. Res. 2015, 103, 1344–1353. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Y.; Zhu, T.; Gao, S.; Ye, K.; Zhou, F.; Qiu, D.; Wang, X.; Tian, Y.; Qu, X. Biphasic Double-Network Hydrogel With Compartmentalized Loading of Bioactive Glass for Osteochondral Defect Repair. Front. Bioeng. Biotechnol. 2020, 8, 752. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic Differentiation of Mesenchymal Stem Cells from Bone Marrow: Differentiation-Dependent Gene Expression of Matrix Components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Mohan, N.; Dormer, N.H.; Caldwell, K.L.; Key, V.H.; Berkland, C.J.; Detamore, M.S. Continuous Gradients of Material Composition and Growth Factors for Effective Regeneration of the Osteochondral Interface. Tissue Eng. Part A 2011, 17, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Behre, A.; Fainor, M.; Seims, K.B.; Chow, L.W. Spatial Organization of Biochemical Cues in 3D-Printed Scaffolds to Guide Osteochondral Tissue Engineering. Biomater. Sci. 2021, 9, 6813–6829. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Bai, H.; Li, R.; Shang, J.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Wang, J.; et al. Sustained Release of VEGF to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front. Bioeng. Biotechnol. 2021, 9, 757767. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Deponti, D.; Modina, S.; Tessaro, I.; Domeneghini, C.; Peretti, G.M. Age-Related Modulation of Angiogenesis-Regulating Factors in the Swine Meniscus. J. Cell. Mol. Med. 2017, 21, 3066–3075. [Google Scholar] [CrossRef]
- Herrera Millar, V.R.; Canciani, B.; Mangiavini, L.; Filipe, J.F.S.; Aidos, L.; Pallaoro, M.; Peretti, G.M.; Pocar, P.; Modina, S.C.; Di Giancamillo, A. Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells. Biomedicines 2022, 10, 2415. [Google Scholar] [CrossRef]
- Krishna, D.V.; Srinivas, V.L.S.V.; Chandan, P.B.; Sankar, M.R.; Reddy, T.N. Smart Hydrogels for Tissue Engineering Applications. In Metal Additive Manufacturing; Rajasekar, R., Mostafaei, A., Mogana Priya, C., Sathish Kumar, P., Eds.; Wiley: Hoboken, NJ, USA, 2025; pp. 497–526. ISBN 978-1-394-28762-8. [Google Scholar]
- Song, Z.; Cheng, Y.; Chen, M.; Xie, X. Macrophage Polarization in Bone Implant Repair: A Review. Tissue Cell 2023, 82, 102112. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The Role of Macrophage Phenotype in Vascularization of Tissue Engineering Scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, R.; Bai, L.; Cai, Y.; Lei, M.; Bao, C.; Lin, S.; Ji, S.; Liu, C.; Qu, X. Extracellular Matrix-Mimetic Immunomodulatory Hydrogel for Accelerating Wound Healing. Adv. Healthc. Mater. 2023, 12, 2301264. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tian, W.; Wang, S.; Liu, X.; Wang, Z.; Hou, L.; Ge, J.; Zhang, X.; He, Z.; Wang, X. TSG-6 Secreted by Bone Marrow Mesenchymal Stem Cells Attenuates Intervertebral Disc Degeneration by Inhibiting the TLR2/NF-κB Signaling Pathway. Lab. Investig. 2018, 98, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.Y.; Martin, K.E.; García, J.R.; Johnson, C.T.; Theriault, H.S.; Han, W.M.; Zhou, D.W.; Botchwey, E.A.; García, A.J. Integrin-Specific Hydrogels Modulate Transplanted Human Bone Marrow-Derived Mesenchymal Stem Cell Survival, Engraftment, and Reparative Activities. Nat. Commun. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Cai, Y.; Shi, X.; Chen, J.; Li, D.; Zhang, P.; Liu, J. Macrophage-Mediated Immunomodulation in Biomaterial-Assisted Bone Repair: Molecular Insights and Therapeutic Prospects. Chem. Eng. J. 2024, 488, 150631. [Google Scholar] [CrossRef]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a Pro-Regenerative Biomaterial Scaffold Microenvironment Requires T Helper 2 Cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved Mesenchymal Stromal Cells Display Impaired Immunosuppressive Properties as a Result of Heat-Shock Response and Impaired Interferon-γ Licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Lei, L.; Wen, Z.; Cao, M.; Zhang, H.; Ling, S.K.-K.; Fu, B.S.-C.; Qin, L.; Xu, J.; Yung, P.S.-H. The Emerging Role of Piezo1 in the Musculoskeletal System and Disease. Theranostics 2024, 14, 3963–3983. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.-H. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS ONE 2015, 10, e0135519. [Google Scholar] [CrossRef] [PubMed]
- Knapik, D.M.; Perera, P.; Nam, J.; Blazek, A.D.; Rath, B.; Leblebicioglu, B.; Das, H.; Wu, L.C.; Hewett, T.E.; Agarwal, S.K.; et al. Mechanosignaling in Bone Health, Trauma and Inflammation. Antioxid. Redox Signal. 2014, 20, 970–985. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N.; et al. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Mauck, R.L.; Burdick, J.A. Local Nascent Protein Deposition and Remodelling Guide Mesenchymal Stromal Cell Mechanosensing and Fate in Three-Dimensional Hydrogels. Nat. Mater. 2019, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore Size Directs Bone Marrow Stromal Cell Fate and Tissue Regeneration in Nanofibrous Macroporous Scaffolds by Mediating Vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-Temperature Photothermal and Oxidative Bacteria Killing and Acceleration of Wound Healing by Polydopamine-Assisted Au-Hydroxyapatite Nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, 1906423. [Google Scholar] [CrossRef]
- Ng, K.W.; Ateshian, G.A.; Hung, C.T. Zonal Chondrocytes Seeded in a Layered Agarose Hydrogel Create Engineered Cartilage with Depth-Dependent Cellular and Mechanical Inhomogeneity. Tissue Eng. Part A 2009, 15, 2315–2324. [Google Scholar] [CrossRef]
- Scalzone, A.; Cerqueni, G.; Wang, X.-N.; Ferreira-Duarte, A.; Dalgarno, K.; Mattioli-Belmonte, M.; Gentile, P. An In Vitro Engineered Osteochondral Model as Tool to Study Osteoarthritis Environment. Adv. Healthc. Mater. 2023, 12, e2202030. [Google Scholar] [CrossRef]
- Morouço, P.; Fernandes, C.; Lattanzi, W. Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. J. Funct. Biomater. 2021, 12, 17. [Google Scholar] [CrossRef]
- Nedrelow, D.S.; Townsend, J.M.; Detamore, M.S. Osteochondral Regeneration With Anatomical Scaffold 3D -Printing—Design Considerations for Interface Integration. J. Biomed. Mater. Res. 2025, 113, e37804. [Google Scholar] [CrossRef] [PubMed]
- Barkow, P.; Waletzko-Hellwig, J.; Abroug, N.; Polley, C.; Schöbel, L.; Boccaccini, A.; Bader, R.; Seitz, H. Development and Characterization of a Gradient Scaffold for Osteochondral Defects. Trans. Addit. Manuf. Meets Med. 2025, 7, 2112. [Google Scholar] [CrossRef]
- Brown, W.E.; Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Engineering Large, Anatomically Shaped Osteochondral Constructs with Robust Interfacial Shear Properties. npj Regen. Med. 2021, 6, 42. [Google Scholar] [CrossRef]
- Fu, J.-N.; Wang, X.; Yang, M.; Chen, Y.-R.; Zhang, J.-Y.; Deng, R.-H.; Zhang, Z.-N.; Yu, J.-K.; Yuan, F.-Z. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front. Bioeng. Biotechnol. 2022, 9, 812383. [Google Scholar] [CrossRef]
- Fang, L.; Lin, X.; Xu, R.; Liu, L.; Zhang, Y.; Tian, F.; Li, J.J.; Xue, J. Advances in the Development of Gradient Scaffolds Made of Nano-Micromaterials for Musculoskeletal Tissue Regeneration. Nano-Micro Lett. 2025, 17, 75. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
| Category | Advantages | Disadvantages |
|---|---|---|
| Natural Hydrogels |
|
|
| Synthetic Hydrogels |
|
|
| Hybrid and Composite Hydrogels |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeraro, F.; Herrera Millar, V.R.; Aidos, L.; Sergio, M.; Impieri, L.; Peretti, G.M.; Mangiavini, L.; Di Giancamillo, A.; Rossi, N. Beyond Biomaterials: Engineering Bioactive Hydrogels as Immuno-Mechanobiological Niches for Osteochondral Regeneration. Gels 2025, 11, 658. https://doi.org/10.3390/gels11080658
Semeraro F, Herrera Millar VR, Aidos L, Sergio M, Impieri L, Peretti GM, Mangiavini L, Di Giancamillo A, Rossi N. Beyond Biomaterials: Engineering Bioactive Hydrogels as Immuno-Mechanobiological Niches for Osteochondral Regeneration. Gels. 2025; 11(8):658. https://doi.org/10.3390/gels11080658
Chicago/Turabian StyleSemeraro, Francesca, Valentina Rafaela Herrera Millar, Lucia Aidos, Mirko Sergio, Lorenzo Impieri, Giuseppe Michele Peretti, Laura Mangiavini, Alessia Di Giancamillo, and Nicolò Rossi. 2025. "Beyond Biomaterials: Engineering Bioactive Hydrogels as Immuno-Mechanobiological Niches for Osteochondral Regeneration" Gels 11, no. 8: 658. https://doi.org/10.3390/gels11080658
APA StyleSemeraro, F., Herrera Millar, V. R., Aidos, L., Sergio, M., Impieri, L., Peretti, G. M., Mangiavini, L., Di Giancamillo, A., & Rossi, N. (2025). Beyond Biomaterials: Engineering Bioactive Hydrogels as Immuno-Mechanobiological Niches for Osteochondral Regeneration. Gels, 11(8), 658. https://doi.org/10.3390/gels11080658






