Carbon Dot Integrated Cellulose-Based Green-Fluorescent Aerogel for Detection and Removal of Copper Ions in Water
Abstract
1. Introduction
2. Results and Discussions
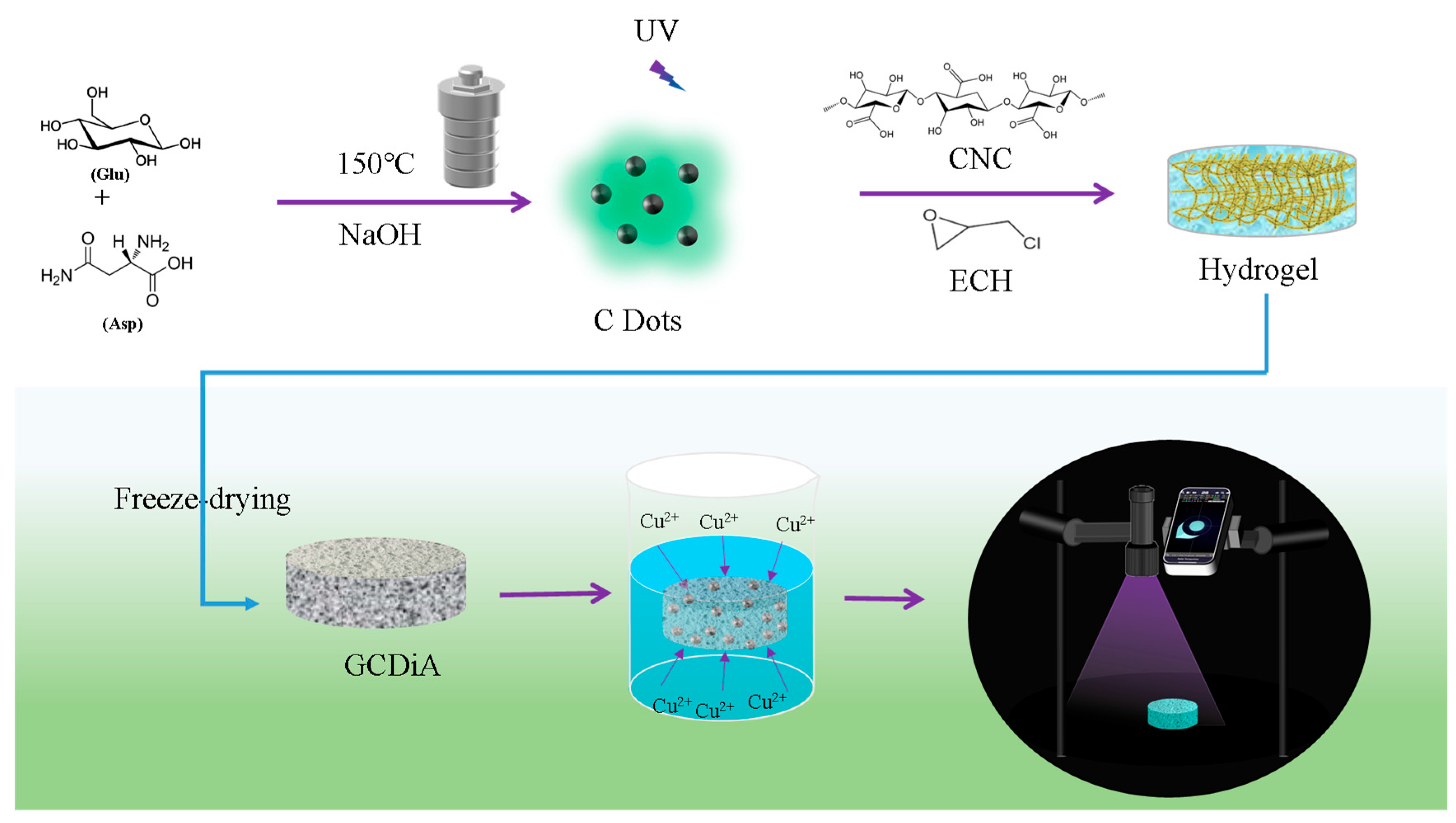
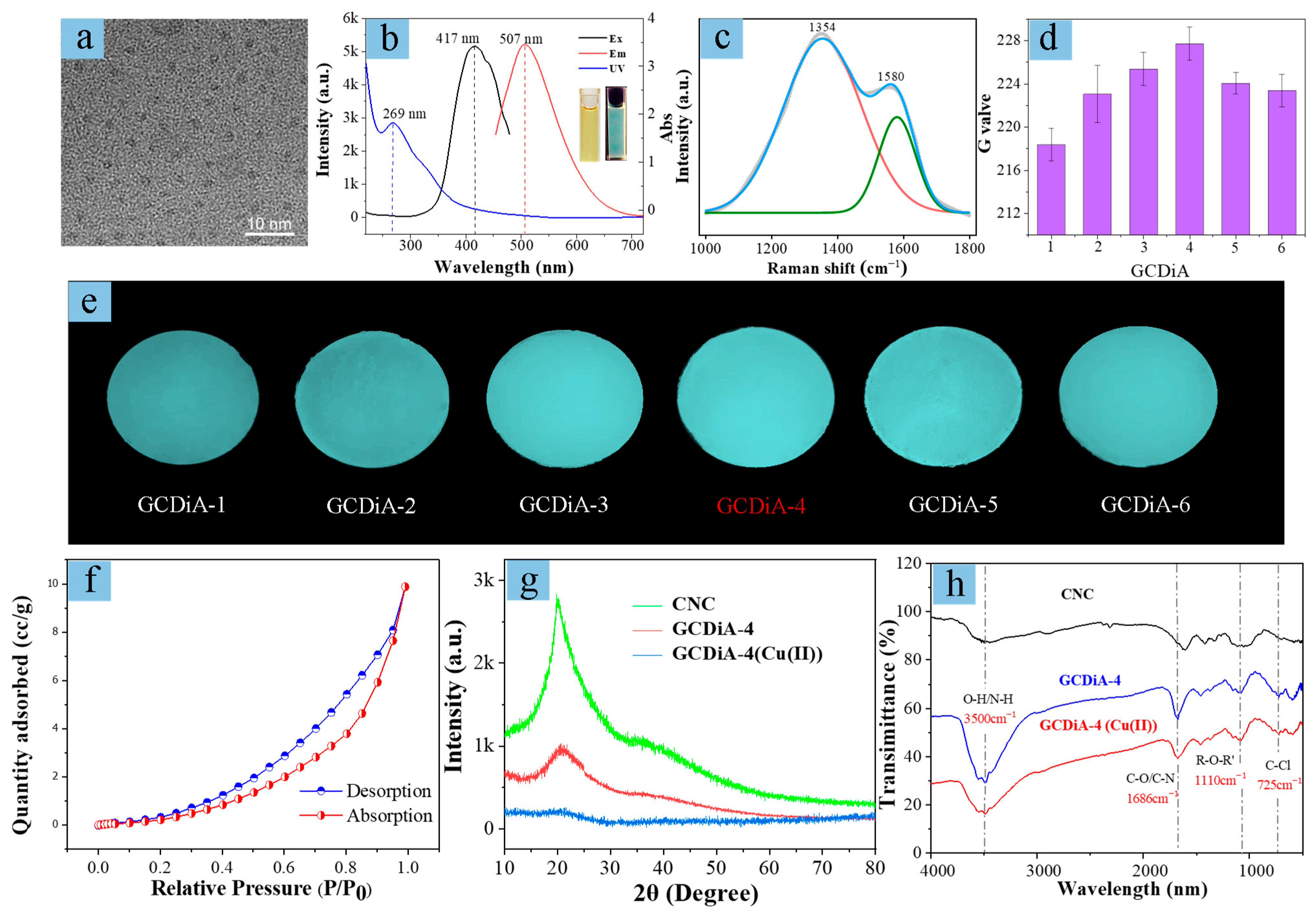
2.1. Characterization of the Novel Aerogel
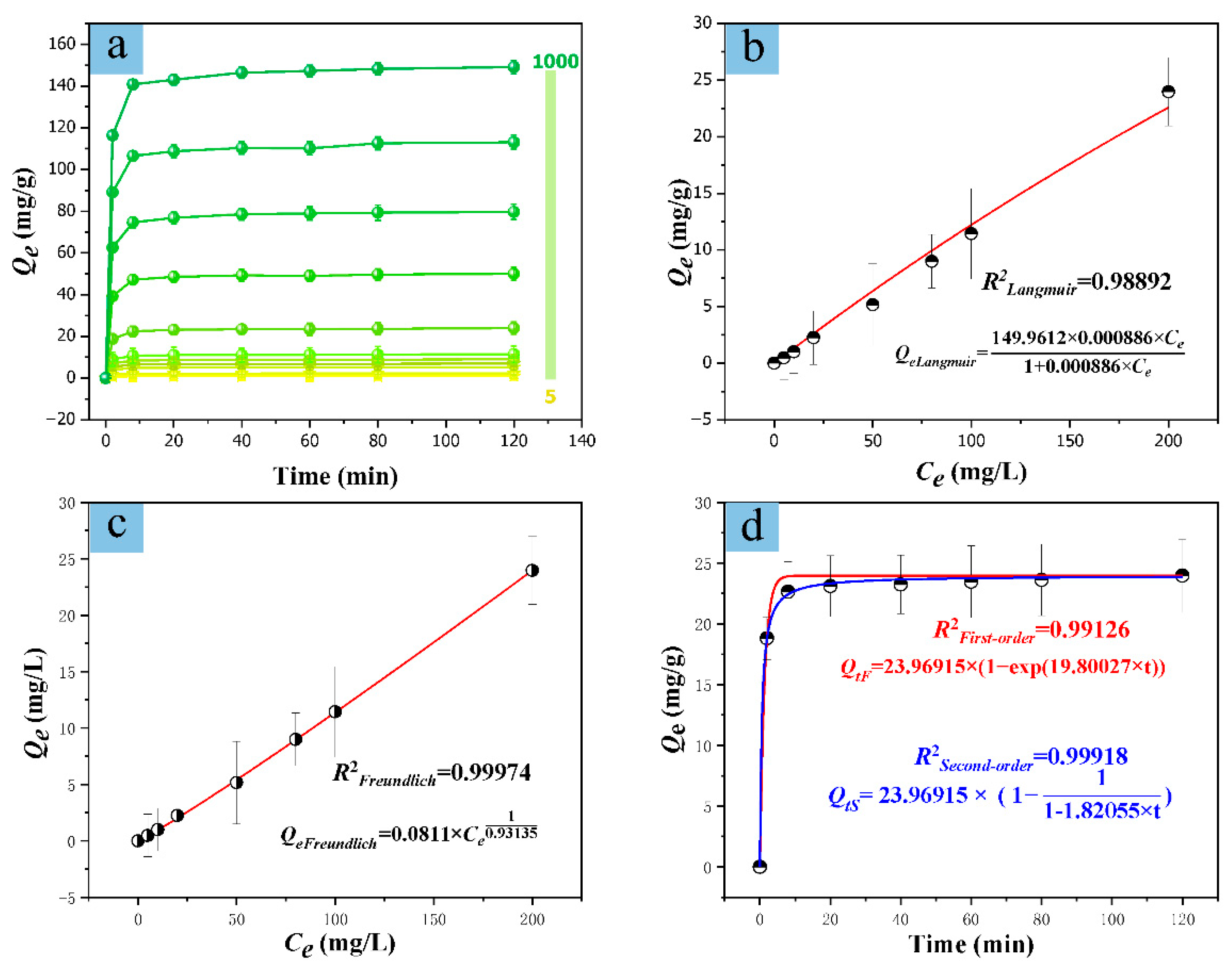
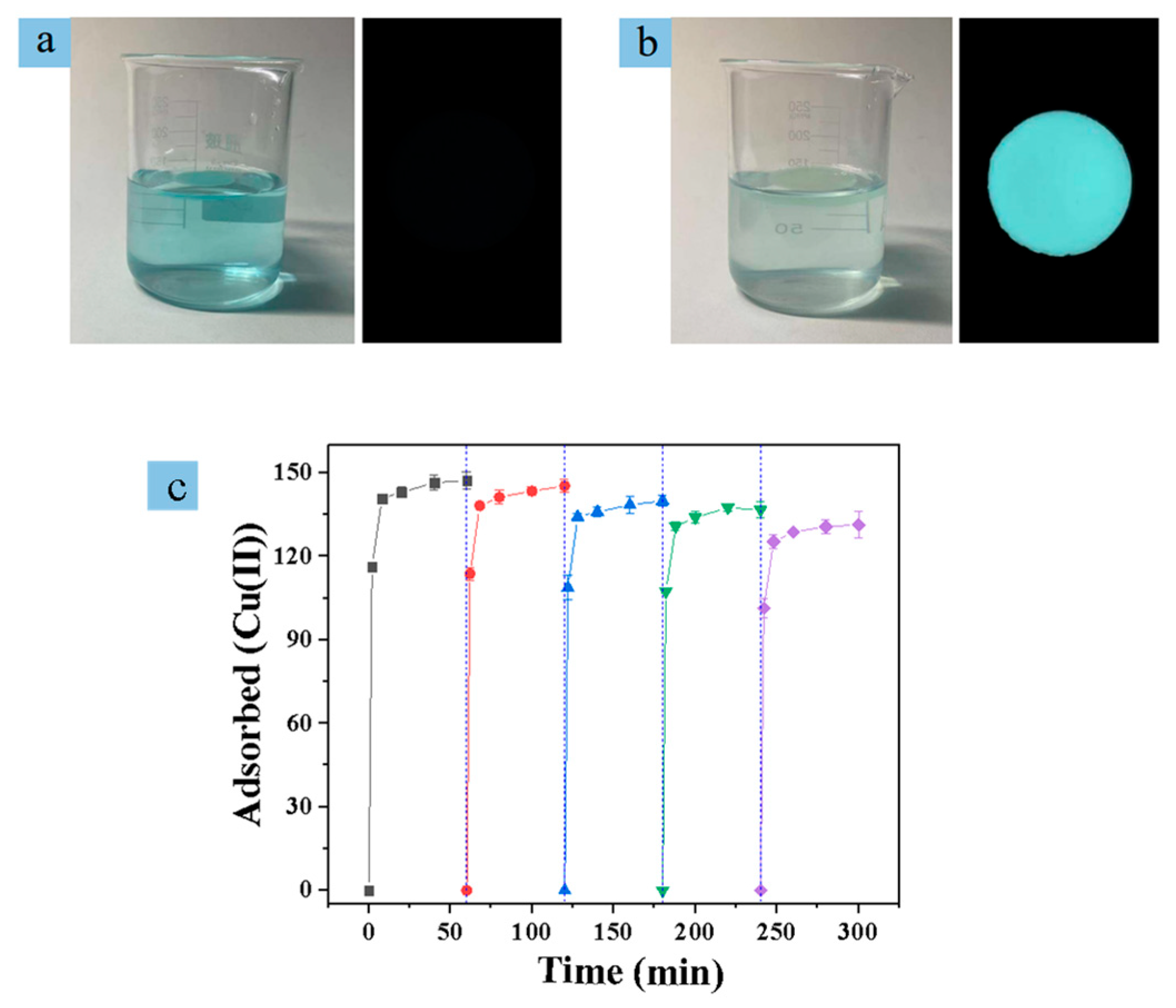
2.2. Cu(II) Adsorption and Detection
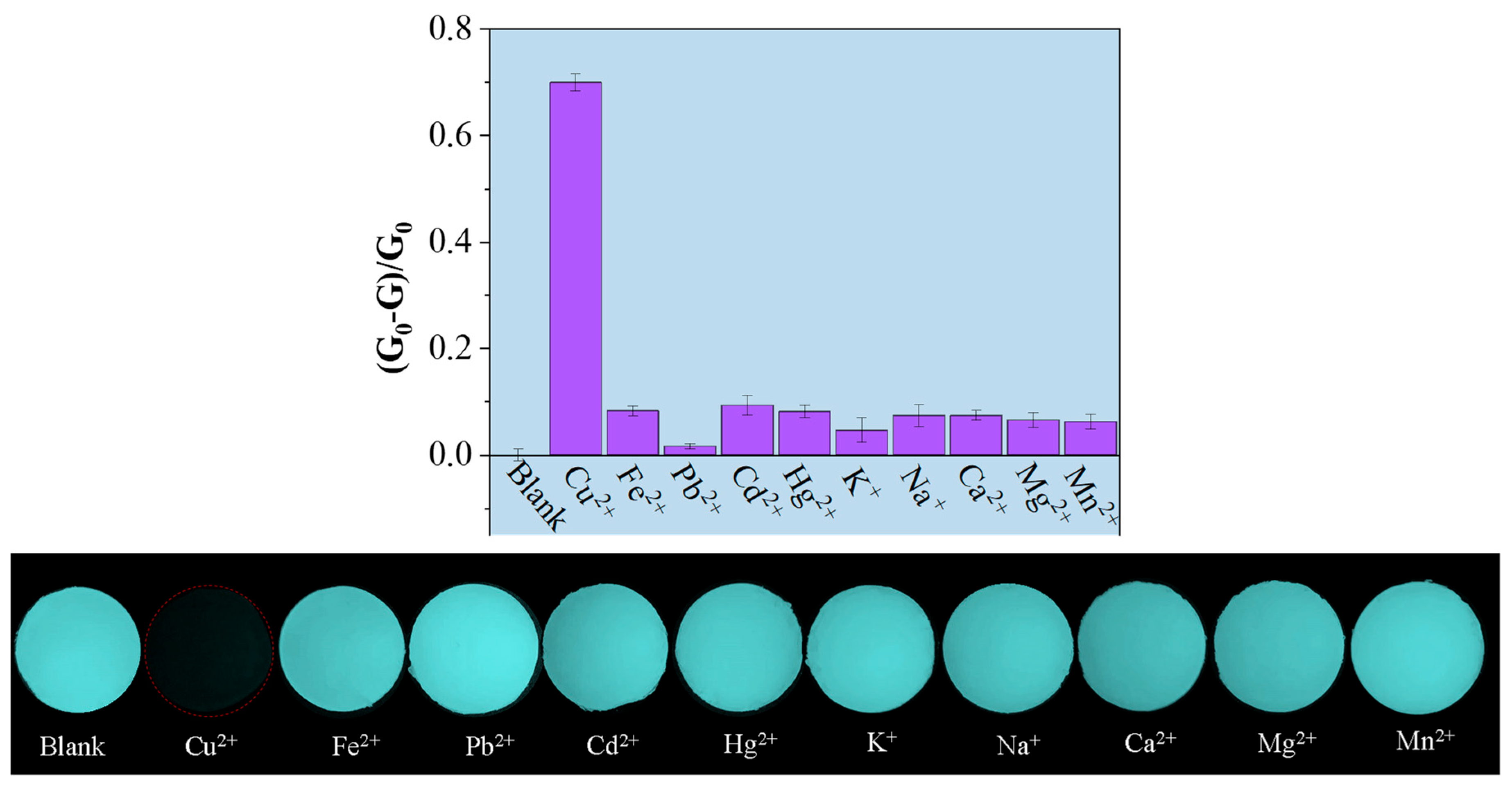
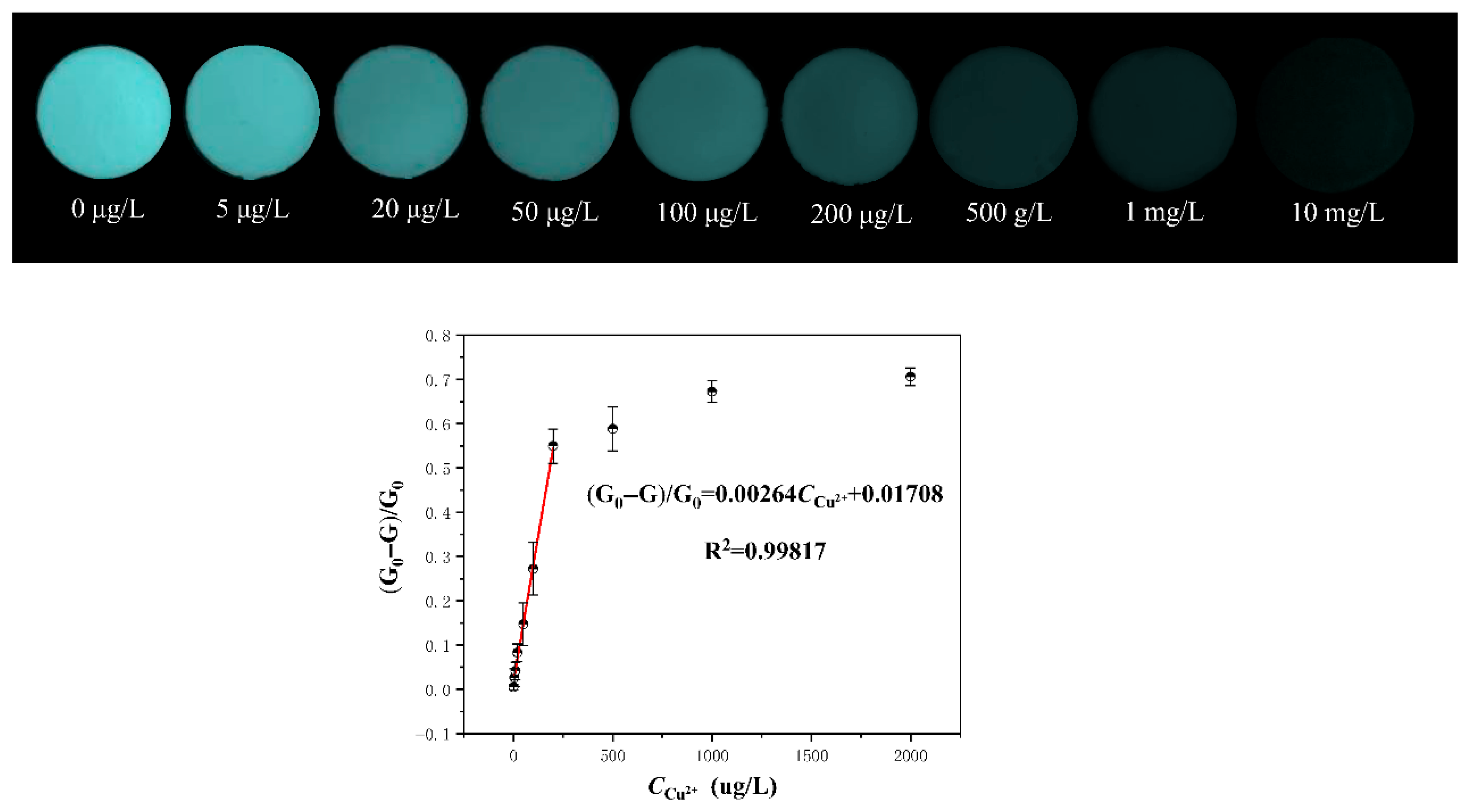
2.3. Selectivity and Sensitivity of GCDiA-4 Toward Cu(II)
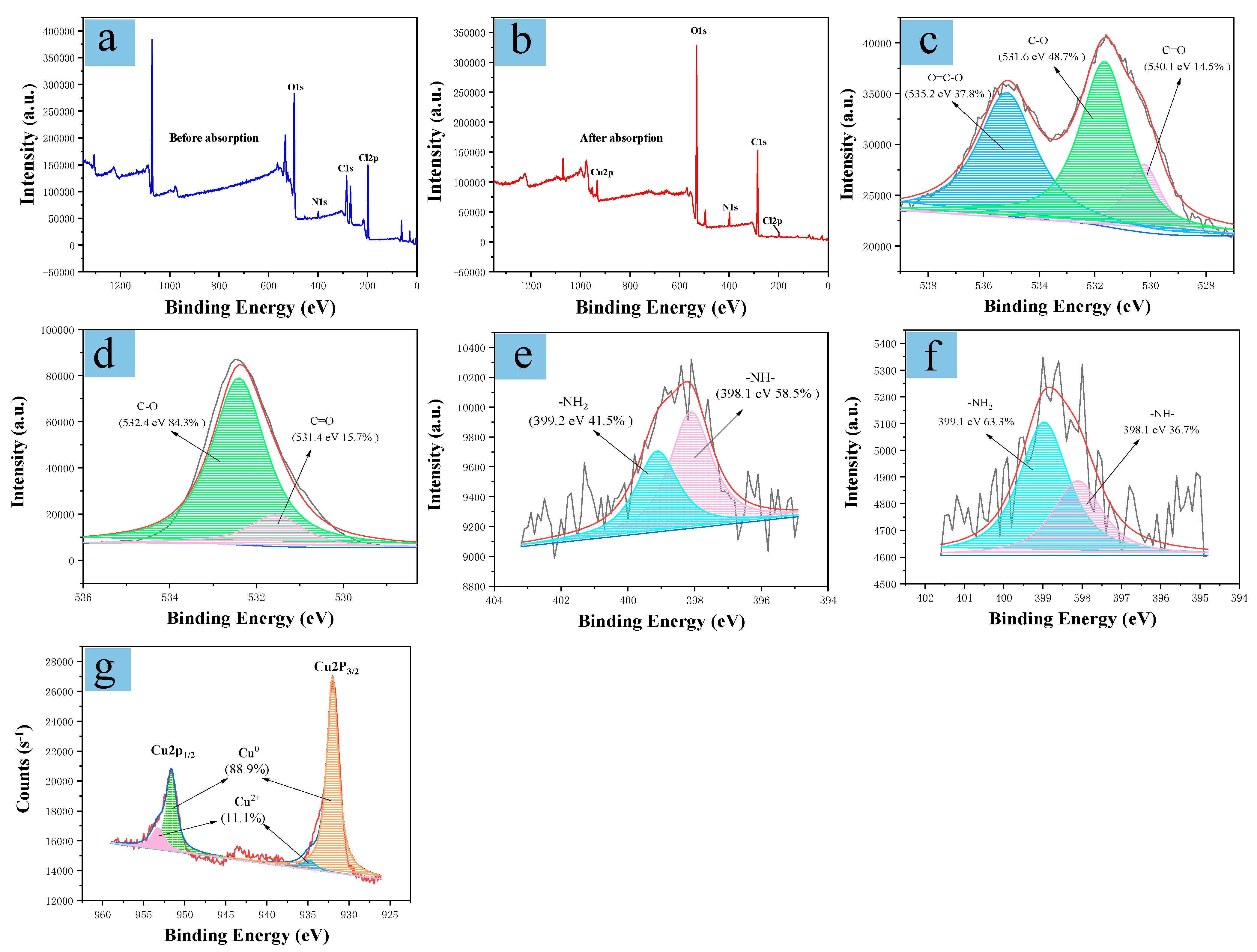
2.4. Mechanism Analysis
2.5. Electroplating Chrome Wastewater Adsorption
3. Conclusions
4. Experimental
4.1. Chemicals
4.2. Characterizations
4.3. Preparation of Green Fluorescent C Dots
4.4. Preparation of GCDiA via Cross-Linking C Dots and CNC
4.5. Batch Adsorption Experiments
4.6. Actual Wastewater Adsorption
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moutaouakkil, Y.; Mounir, R.; Ait El Cadi, M.; Lamsaouri, J.; Bousliman, Y.; Eljaoudi, R. Intoxications aux éléments traces métalliques: Nouveau concept du profil métallique. Ann. Biol. Clin. 2024, 82, 254–265. [Google Scholar]
- Więcek, S.; Paprocka, J. Disorders of Copper Metabolism in Children—A Problem too Rarely Recognized. Metabolites 2024, 14, 38. [Google Scholar] [CrossRef]
- Charkiewicz, A.E. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Curr. Issues Mol. Biol. 2024, 46, 8441–8463. [Google Scholar] [CrossRef]
- Js Cooper, G. Selective Divalent Copper Chelation for the Treatment of Diabetes Mellitus. Curr. Med. Chem. 2012, 19, 2828–2860. [Google Scholar] [CrossRef]
- Lu, J.; Gong, D.; Choong, S.Y.; Xu, H.; Chan, Y.-K.; Chen, X.; Fitzpatrick, S.; Glyn-Jones, S.; Zhang, S.; Nakamura, T.; et al. Copper(II)-selective chelation improves function and antioxidant defences in cardiovascular tissues of rats as a model of diabetes: Comparisons between triethylenetetramine and three less copper-selective transition-metal-targeted treatments. Diabetologia 2010, 53, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Pontré, B.; Pickup, S.; Choong, S.Y.; Li, M.; Xu, H.; Gamble, D.; Phillips, A.R.J.; Cowan, B.R.; Young, A.A.; et al. Treatment with a copper-selective chelator causes substantive improvement in cardiac function of diabetic rats with left-ventricular impairment. Cardiovasc. Diabetol. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.H.; Hogl, S.; Narayanan, U.; Zhang, L.; McHarg, S.; Xu, J.S.; Gong, D.M.; et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc. Diabetol. 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Peng, H.; Yu, C.; Ji, W.W.; Wang, X.; Pu, S.Y. Freezing-Induced Redistribution of Fe(II) Species within Clay Minerals for Nonlinear Variations in Hydroxyl Radical Yield and Contaminant Degradation. J. Earth Sci. 2025, 36, 1226–1235. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, J.; Meng, H.; Wang, Y.; Zhang, Y.; Li, H.; Feng, L. A paper-based microfluidic analytical device combined with home-made SPE column for the colorimetric determination of copper(II) ion. Talanta 2019, 204, 518–524. [Google Scholar] [CrossRef]
- Senpradit, Y.; Wacharasindhu, S.; Sukwattanasinitt, M. Novel highly selective quinoline-based fluorescent chemosensors for quantitative analysis of Cu(II) ion in water and food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125128. [Google Scholar] [CrossRef]
- Zha, G.; Luo, H.; Liu, Y.; Xu, B.Q.; Jiang, W.L.; Yang, B.; Liu, D.C.; Yang, H.W. An update review for refining crude selenium by sustainable vacuum distillation technology: Theory, applications, and life cycle assessment. Sep. Purif. Technol. 2025, 376, 134031. [Google Scholar] [CrossRef]
- Aldmour, S.T. Utilization of organic-rich materials for the adsorption of copper ions from aqueous environments. Results Eng. 2024, 22, 102216. [Google Scholar] [CrossRef]
- Butrin, N.; Rueangchai, N.; Noisong, P.; Sansuk, S. Synthesis of hydroxyapatite/activated carbon composite with bioactivity property and copper ion removal efficiency. Mater. Today Commun. 2024, 40, 109615. [Google Scholar] [CrossRef]
- Ozcan, D.O.; Hendekci, M.C.; Ovez, B. Enhancing the adsorption capacity of organic and inorganic pollutants onto impregnated olive stone derived activated carbon. Heliyon 2024, 10, e32792. [Google Scholar] [CrossRef]
- Soudani, A.; Youcef, L.; Youcef, S.; Elbahi, S.; Toumi, K.; Saadia, G.; Sahli, A.; Soudani, N. High Performance Activated Carbon Based on Date Palm Fibers for Cu2+ Removal in Water. Chem. Afr. 2024, 7, 3903–3915. [Google Scholar] [CrossRef]
- Del Sole, R.; Fogel, A.A.; Somin, V.A.; Vasapollo, G.; Mergola, L. Evaluation of Effective Composite Biosorbents Based on Wood Sawdust and Natural Clay for Heavy Metals Removal from Water. Materials 2023, 16, 5322. [Google Scholar] [CrossRef] [PubMed]
- Vezentsev, A.I.; Gorbunova, N.M.; Sokolovskiy, P.V.; Mar’inskikh, S.G.; Chub, A.; Chau, N.H.; Greish, A.A. On the adsorption mechanism of copper ions on bentonite clay. Russ. Chem. Bull. 2022, 71, 651–655. [Google Scholar] [CrossRef]
- Abutaleb, A.; Eldoma, M.A.; Imran, M.; Taha, K.K.; Bakather, O.Y.; Zouli, N.; Hegazi, S.E.F.; Hassan, M. Active adsorption performance of planetary ball milled Saudi Arabian bentonite clay for the removal of copper ions from aqueous solution. Europhys. Lett. 2021, 135, 30005. [Google Scholar] [CrossRef]
- Vareda, J.P.; Matias, P.M.C.; Paixão, J.A.; Murtinho, D.; Valente, A.J.M.; Duraes, L. Chitosan–Silica Composite Aerogel for the Adsorption of Cupric Ions. Gels 2024, 10, 192. [Google Scholar] [CrossRef]
- Semenova, A.; Giles, L.W.; Vidallon, M.L.P.; Follink, B.; Brown, P.L.; Tabor, R.F. The structure of colloidal polyethylenimine–silica nanocomposite microparticles. Particuology 2023, 76, 86–100. [Google Scholar] [CrossRef]
- Vu, T.V.; Nguyen, M.T.; Do, T.T.; Nguyen, H.L.; Nguyen, V.A.; Nguyen, D.T. Adsorption of Copper Ions onto Poly(1,8-diaminonaphthalene)/Graphene Film for Voltammetric Determination of Pyridoxine. Electroanalysis 2022, 34, 1478–1486. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abdel-Monem, Y.K.; Hasan, A. Enhancement Adsorption of Copper and Zinc Contaminated Water Using Highly Efficient Graphene Oxide Modified with Acid-Activated Bentonite. Egypt. J. Chem. 2022, 65, 903–918. [Google Scholar] [CrossRef]
- da Silva Carneiro, J.S.; da Costa Leite, D.A.; de Castro, G.; Franca, J.R.; Botelho, L.; Soares, J.R.; de Oliveira, J.E.; Melo, L.C.A. Biochar-graphene oxide composite is efficient to adsorb and deliver copper and zinc in tropical soil. J. Clean. Prod. 2022, 360, 132170. [Google Scholar] [CrossRef]
- Sariboga, R.; Sarioglu, O.F. Applications of Cellulose-Based Nanomaterials for Sustainability and Therapeutics: A Review. Chem. Bio. Eng. Rev. 2024, 11, e202300069. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Saliu, O.D.; Ramontja, J.; Adeniyi, A.G. Advances in the extraction, classification, modification, emerging and advanced applications of crystalline cellulose: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100337. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Marimuthu, T.; Chee, C.Y.; Sulaiman, N.M.N. A review on the use of cellulose nanomaterials for wastewater remediation of heavy metal ions. Int. J. Environ. Sci. Technol. 2023, 20, 3421–3436. [Google Scholar] [CrossRef]
- Li, F.; Xie, Z.; Wen, J.; Tang, T.; Jiang, L.; Hu, G.H.; Li, M. Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions. Int. J. Mol. Sci. 2023, 24, 8922. [Google Scholar] [CrossRef]
- Xiaorui, K.; Cong, Z.; Pin, X.; Du, Z.W.; Cai, Z.J. Copper ion-imprinted bacterial cellulose for selectively removing heavy metal ions from aqueous solution. Cellulose 2022, 29, 4001–4019. [Google Scholar] [CrossRef]
- Sarkhel, R.; Ganguly, P.; Das, P.; Bhowal, A.; Sengupta, S. Synthesis of biodegradable PVA/cellulose polymer composites and their application in dye removal. Environ. Qual. Manag. 2023, 32, 313–323. [Google Scholar]
- Jung, S.; Kim, J.; Bang, J.; Jung, M.; Park, S.; Yun, H.; Kwak, H.W. pH-sensitive cellulose/chitin nanofibrillar hydrogel for dye pollutant removal. Carbohydr. Polym. 2023, 317, 121090. [Google Scholar] [CrossRef]
- Ashori, A.; Chiani, E.; Shokrollahzadeh, S.; Madadi, M.; Sun, F.B.; Zhang, X.M. Cellulose-Based Aerogels for Sustainable Dye Removal: Advances and Prospects. J. Polym. Environ. 2024, 32, 6149–6181. [Google Scholar] [CrossRef]
- Pant, A.; Jain, R.; Ahammad, S.Z.; Ali, S.W. Removal of antibiotic resistance genes from wastewater using diethylaminoethyl cellulose as a promising adsorbent. J. Water Process Eng. 2023, 55, 104109. [Google Scholar] [CrossRef]
- Li, N.; Tao, K.; Xia, W.; Yu, C.W.; Yang, H. A novel cellulose/lignin/montmorillonite ternary hybrid aerogel for efficiently adsorptive removal of antibiotics from water. Chem. Eng. J. 2023, 466, 143265. [Google Scholar] [CrossRef]
- Fu, T.; Wu, S.; Zhao, M.; Zheng, X.Y.; Wang, Z.Q.; Jin, Z.M.; Fan, C.Z. Preparation and application of cattail residue-based magnetic cellulose composites for tetracycline antibiotics adsorption. Process Saf. Environ. Prot. 2024, 189, 598–611. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, Y.; Gao, R.; Chen, Y.X.; Xiong, K.X.; Ye, J.; Zheng, Q.; Fang, Z.X.; Pang, G.S.; Lee, H.J. Functional Aerogels Composed of Regenerated Cellulose and Tungsten Oxide for UV Detection and Seawater Desalination. Gels 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, G.-A.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Radulescu, M.; Grumezescu, A.M.; Nicolae, C.L. Advancements in Aerogel Technology for Antimicrobial Therapy: A Review. Nanomaterials 2024, 14, 1110. [Google Scholar] [CrossRef]
- Teow, Y.H.; Kam, L.M.; Mohammad, A.W. Synthesis of cellulose hydrogel for copper (II) ions adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, S.D.; Zhang, Y.J.; Li, J.; Wu, A.G. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Xiao, K.; Tian, T.; Pan, M.P. Carbon aerogel monoliths from polymers: A review. J. Clean. Prod. 2024, 437, 140736. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Sun, M.; Duan, H.J.; Zhang, H.J.; Li, S.P. Preparation of Lignocellulosic Aerogel and Its Flame Retardant Modification. Prog. Chem. 2024, 36, 586–600. [Google Scholar]
- Rai, N.; Chauhan, I. A review on polysaccharide based aerogel synthesis and their applications. Polym. Plast. Technol. Mater. 2024, 63, 2122–2140. [Google Scholar] [CrossRef]
- Jeong, Y.; Patel, R.; Patel, M. Biopolymer-Based Biomimetic Aerogel for Biomedical Applications. Biomimetics 2024, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Ju, X.; Ma, H.; Meng, S.Q.; Liu, Y.X.; Wang, J.W.; Wang, D.C.; Yang, Z.Y. Aerogel-based carbon capture materials: Research progress and application prospects. Sep. Purif. Technol. 2025, 354, 128794. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, H.; Jiao, S.; Guo, Z.N.; Zhu, D.; Zhao, X.F. A Turn-on Biosensor-Based Aptamer-Mediated Carbon Quantum Dots Nanoaggregate for Acetamiprid Detection in Complex Samples. Food Anal. Methods 2019, 12, 668–676. [Google Scholar] [CrossRef]
- Wang, D.; Lin, B.; Cao, Y.; Guo, M.L.; Yu, Y. A Highly Selective and Sensitive Fluorescence Detection Method of Glyphosate Based on an Immune Reaction Strategy of Carbon Dot Labeled Antibody and Antigen Magnetic Beads. J. Agric. Food Chem. 2016, 64, 6042–6050. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Farshbaf, M.; Soodabeh, D.; Fariborz, R.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.Y.; He, P.; Ding, G.Q.; Xie, X.M.; Kang, Z.H.; Jiang, M.H. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Li, F.; Yang, D.; Xu, H. Non-Metal-Heteroatom-Doped Carbon Dots: Synthesis and Properties. Chem. A Eur. J. 2019, 25, 1165–1176. [Google Scholar] [CrossRef]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Su, L.; Xing, Z.; Wang, D.; Xu, G.H.; Ren, S.X.; Fang, G.Z. Mechanical Properties Research and Structural Characterization of Alkali Lignin/Poly (vinyl alcohol) Reaction Films. Bio. Res. 2013, 8, 3532–3543. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S.G. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Yang, H.-R.; Li, S.-S.; Yang, C.; An, Q.D.; Zhai, S.R.; Xiao, Z.Y. Bi-layered hollow amphoteric composites: Rational construction and ultra-efficient sorption performance for anionic Cr(VI) and cationic Cu(II) ions. J. Colloid Interface Sci. 2022, 607, 556–567. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Xu, Y.; Yang, L.X.; Wang, M.H.; Xia, Y.Y.; Wang, Y.; Tang, Y.; Qiao, C.; Lin, Y. Fabrication of fluorescence sensor based on semi-covalent dummy molecularly imprinted silica on silane-modified carbon quantum dot for highly selective and sensitive detection of bisphenol A in spirits. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134292. [Google Scholar] [CrossRef]
- Geng, B.; Wang, H.; Wu, S.; Ru, J.; Tong, C.C.; Chen, Y.F.; Liu, H.Z.; Wu, S.C.; Liu, X.Y. Surface-Tailored Nanocellulose Aerogels with Thiol-Functional Moieties for Highly Efficient and Selective Removal of Hg(II) Ions from Water. ACS Sustain. Chem. Eng. 2017, 5, 11715–11726. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, G.; Luo, Q.; Xiao, T.; Zuo, Y.F.; Guo, X.; Xu, D.; Wu, Y.Q. A 3D net-like structured fluorescent aerogel based on carboxy-methylated cellulose nanofibrils and carbon dots as a highly effective adsorbent and sensitive optical sensor of Cr(vi). Environ. Sci. Nano 2020, 7, 773–781. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Azari, S.; Losic, D. Graphene-Diatom Silica Aerogels for Efficient Removal of Mercury Ions from Water. ACS Appl. Mater. Interfaces 2015, 7, 11815–11823. [Google Scholar] [CrossRef]
- Gil, A.; Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S. Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: Effects of aging time, Fe loading quantity and co-existing ions. Environ. Sci. Pollut. Res. 2018, 25, 2814–2829. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Shainy, F. Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. J. Colloid Interface Sci. 2015, 456, 22–31. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.-L.; Sun, Y. Manipulating the morphology of nanoscale zero-valent iron on pumice for removal of heavy metals from wastewater. Chem. Eng. J. 2015, 263, 55–61. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Seif, A.; Ahmadi, T.S.; Zargar, B.; Rashidi, F.; Rouzbahani, G.M. Thermodynamic and kinetic studies for the adsorption of Hg(II) by nano-TiO2 from aqueous solution. Adv. Powder Technol. 2012, 23, 148–156. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Pudza, M.Y.; Zentou, H. Efficient removal of Cu(ii) from aqueous systems using enhanced quantum yield nitrogen-doped carbon nanodots. RSC Adv. 2020, 10, 14979–14990. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Mei, Y.; Wu, Y.; Lin, H.J.; Li, Y.L. Effective Detection of Cu(II) Ions Based on Carbon Dots@Exfoliated Layered Double Hydroxides Composites Fluorescence Probe. J. Fluoresc. 2025, 35, 1441–1456. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, B. One-Pot Synthesis of Nitrogen-Doped Graphene Quantum Dots and Their Applications in Bioimaging and Detecting Copper Ions in Living Cells. ACS Omega 2023, 8, 27333–27343. [Google Scholar] [CrossRef]
- Li, A. Preparation of green fluorescent carbon dots and their application indetection of Cu2+. Chin. J. Anal. Lab. 2018, 37, 1057–1061. [Google Scholar]
| Material | Ions | Maximum Adsorption (mg/g) | Ref. |
|---|---|---|---|
| Alkaline-modified biomass pectin | Cu(II) | 105.00 | [59] |
| Sodium alginate-polyamine adsorbent | Cu(II) | 91.00 | [60] |
| Polyacrylamide/chitosan adsorbent | Cu(II) | 32.46 | [61] |
| SDS-coated magnetic chitosan | Cu(II) | 50.74 | [62] |
| Itaconic acid-grafted magnetite nanocellulose composites | Cu(II) | 32.00 | [63] |
| Ionic liquid-modified microcrystalline cellulose Ion-imprinted magnetic chitosan beads | Cu(II) Cu(II) | 138.30 78.10 | [64] [65] |
| Nitrogen-doped carbon dots (N-CDs) from carboxymethyl cellulose | Cu(II) | 185.00 | [66] |
| Carbon dots@exfoliated layered double hydroxides (CDs@LDH) | Cu(II) | ~150.00 | [67] |
| Nitrogen-doped graphene quantum dots (N-GQDs) | Cu(II) | ~120.00 | [68] |
| This work | Cu(II) | 149.62 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, G.; Peng, C.; Yu, J.; Cao, J.; Peng, S.; Zhao, T.; Xu, D. Carbon Dot Integrated Cellulose-Based Green-Fluorescent Aerogel for Detection and Removal of Copper Ions in Water. Gels 2025, 11, 655. https://doi.org/10.3390/gels11080655
Fu G, Peng C, Yu J, Cao J, Peng S, Zhao T, Xu D. Carbon Dot Integrated Cellulose-Based Green-Fluorescent Aerogel for Detection and Removal of Copper Ions in Water. Gels. 2025; 11(8):655. https://doi.org/10.3390/gels11080655
Chicago/Turabian StyleFu, Guanyan, Chenzhan Peng, Jiangrong Yu, Jiafeng Cao, Shilin Peng, Tian Zhao, and Dong Xu. 2025. "Carbon Dot Integrated Cellulose-Based Green-Fluorescent Aerogel for Detection and Removal of Copper Ions in Water" Gels 11, no. 8: 655. https://doi.org/10.3390/gels11080655
APA StyleFu, G., Peng, C., Yu, J., Cao, J., Peng, S., Zhao, T., & Xu, D. (2025). Carbon Dot Integrated Cellulose-Based Green-Fluorescent Aerogel for Detection and Removal of Copper Ions in Water. Gels, 11(8), 655. https://doi.org/10.3390/gels11080655






