Tumor Organoids Grown in Mixed-Composition Hydrogels Recapitulate the Plasticity of Pancreatic Cancers
Abstract
1. Introduction
2. Results
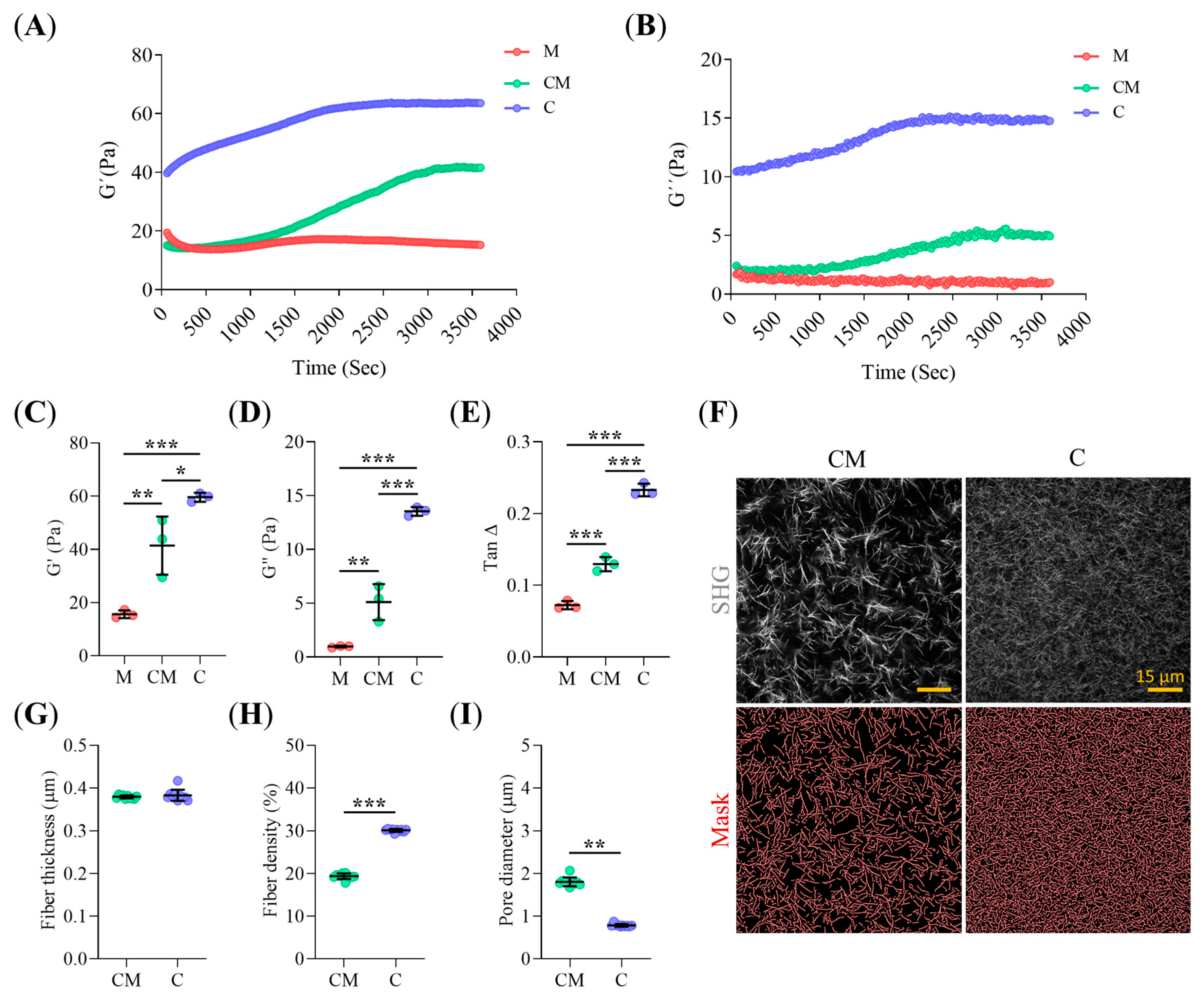
2.1. Collagen-I Content Modulates Hydrogel Micro-Architecture and Viscoelastic Behavior
2.2. Collagen-Related ECM Stiffening and TFGβ Promote Pro-Invasive Traits in Early-Stage PDAC93 Organoid Seeds
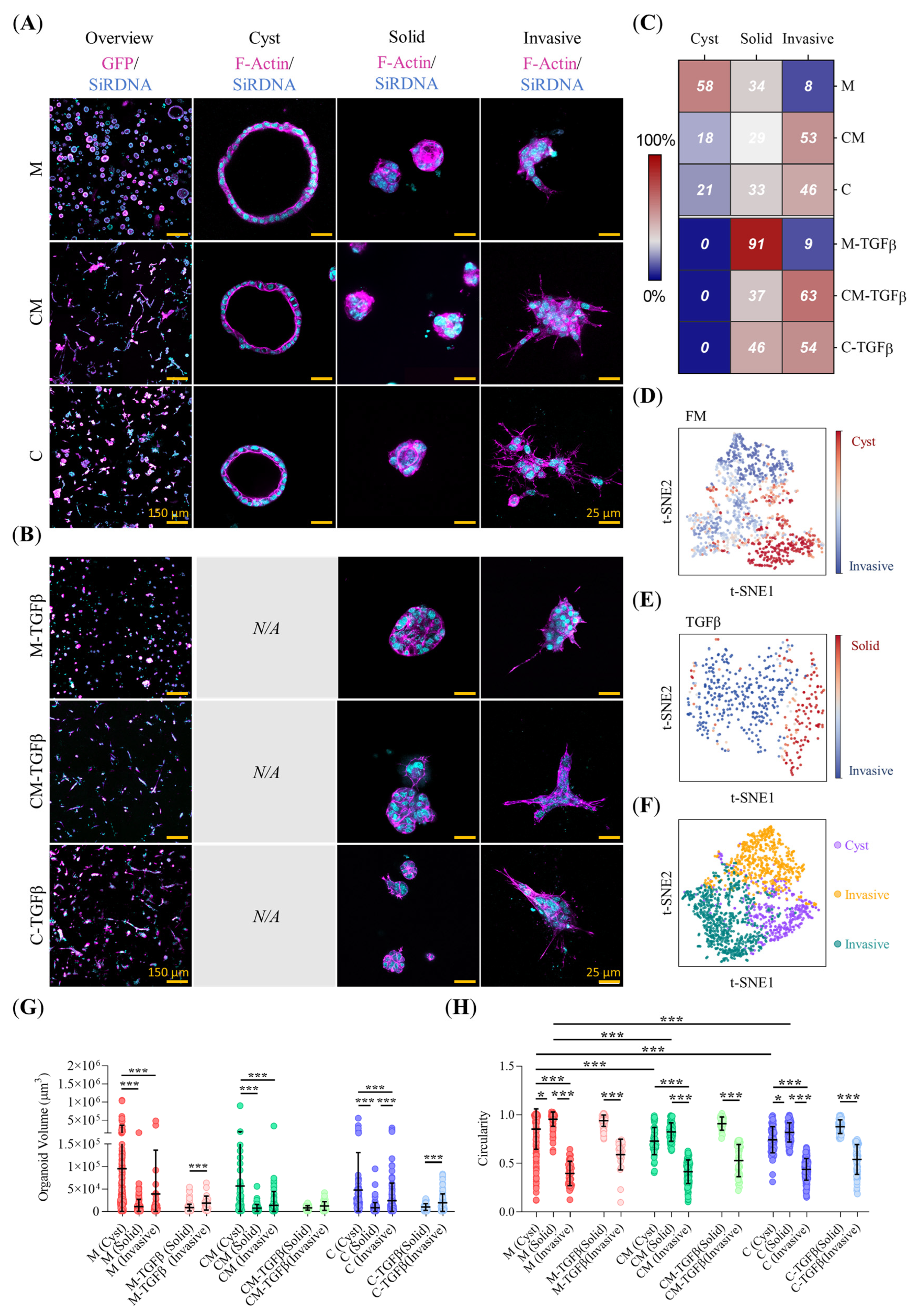
2.3. ECM Biomechanical Properties and TGFβ Modulate the Morphology of Mature PDAC93 Organoids
2.4. Clinical Subtype and EMT Status of PDAC93 Organoids Are Influenced by ECM Composition and TGFβ
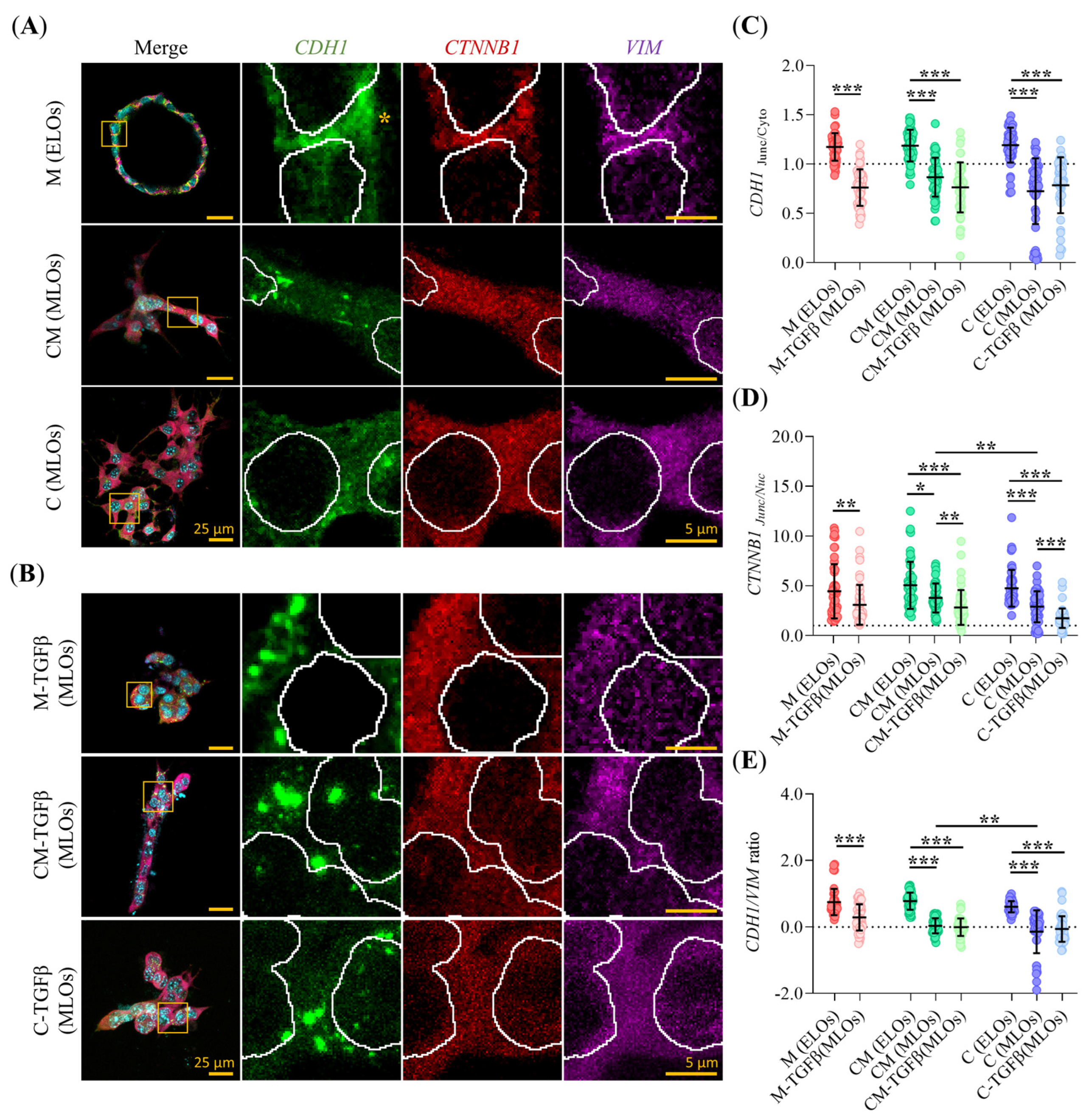
2.5. Hydrogel Composition and TGFβ Alter the Subcellular Localization of EMT Markers in PDAC93 Organoids
2.6. Invasive PDAC93 Organoid Morphologies Trigger Extensive Collagen-I Remodeling
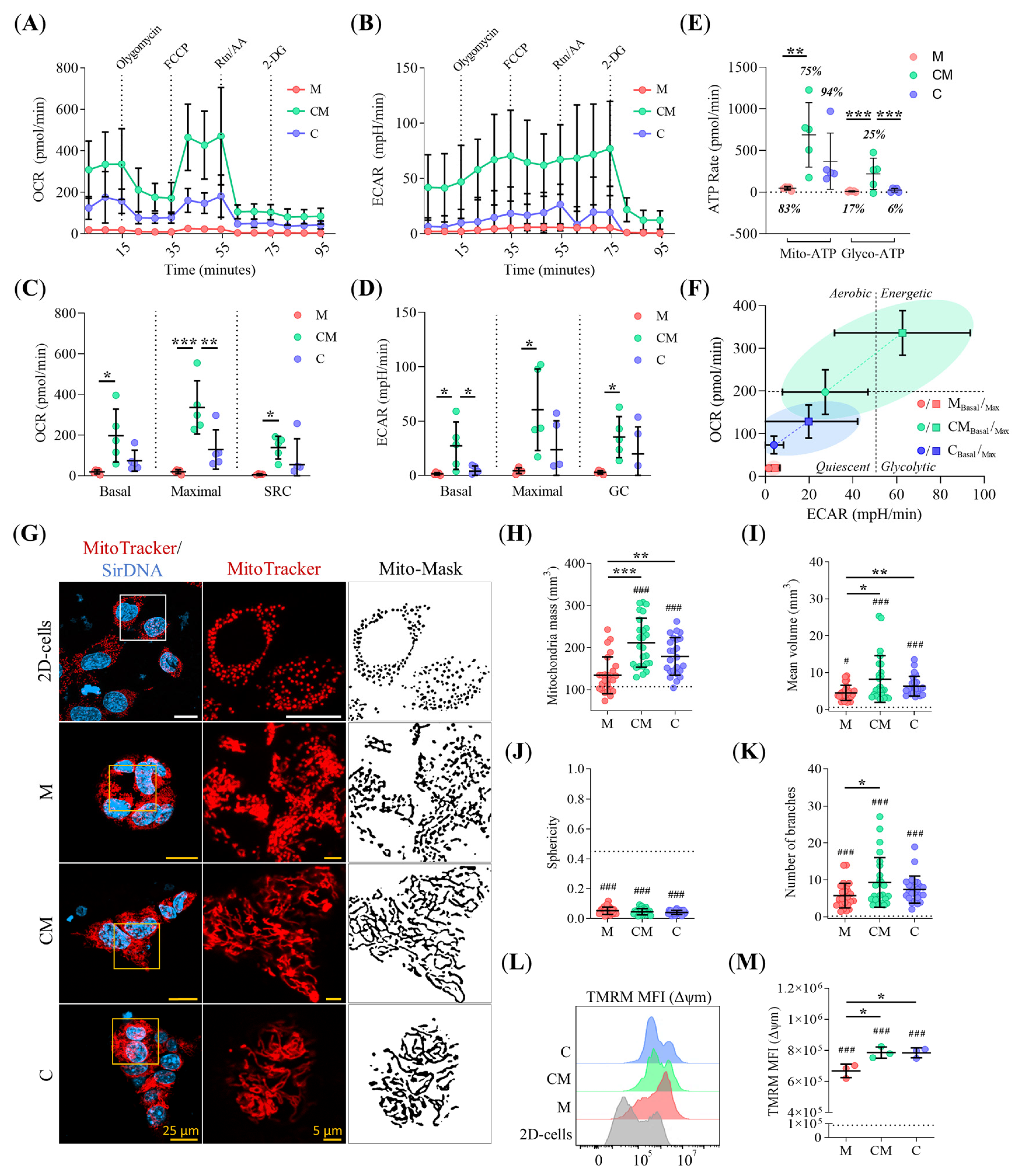
2.7. ECM Stiffening Rewires Metabolic Activity in PDAC93 Organoids
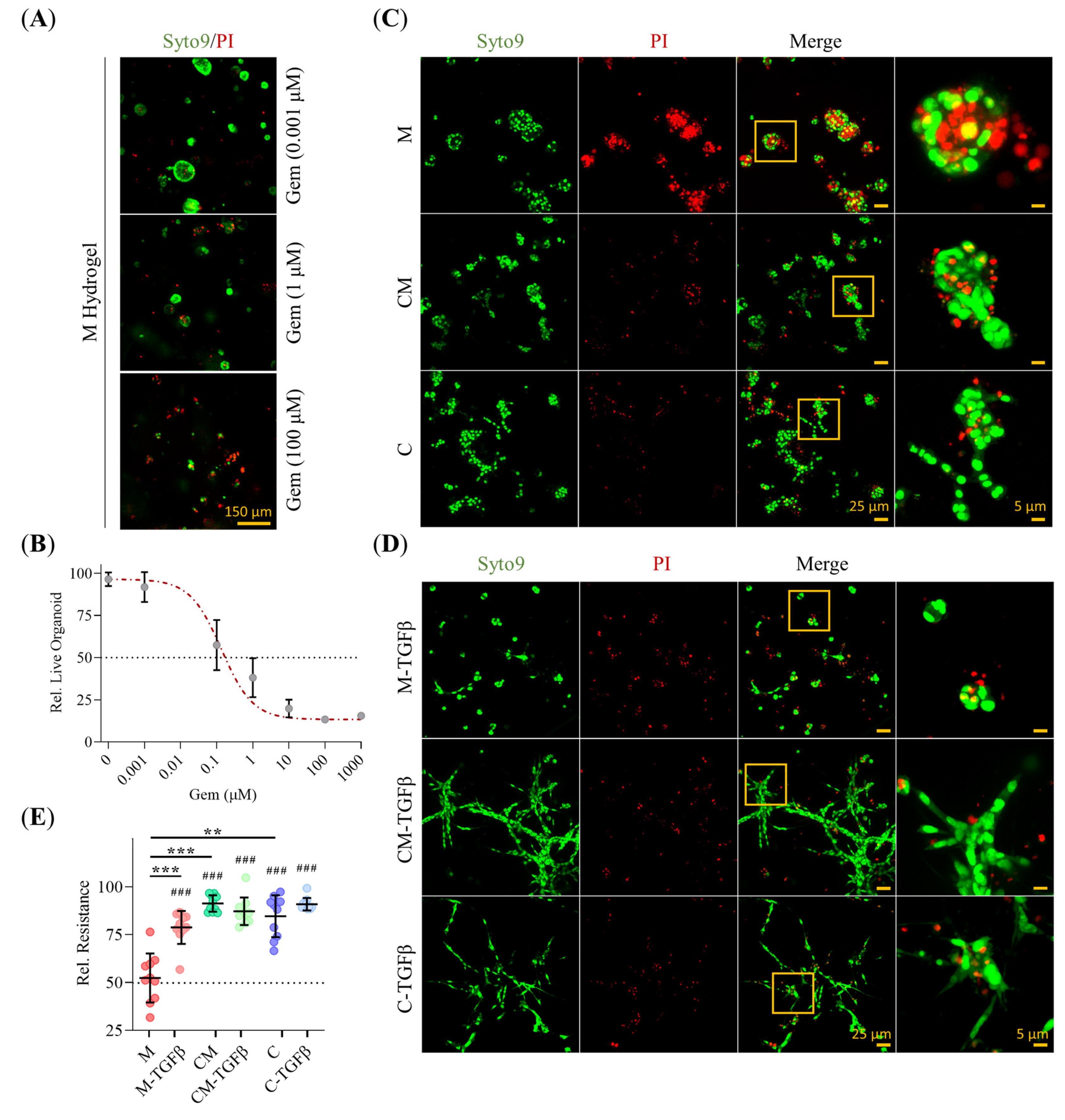
2.8. 3D-Environment Confers Adaptive GEM Resistance in PDAC93 Organoids in a Collagen-Dependent Manner
2.9. ECM Composition Drives Clinical Subtype in PDAC93 Organoid-Derived Murine Model, Enhancing Tumor Aggressiveness and GEM-Resistance
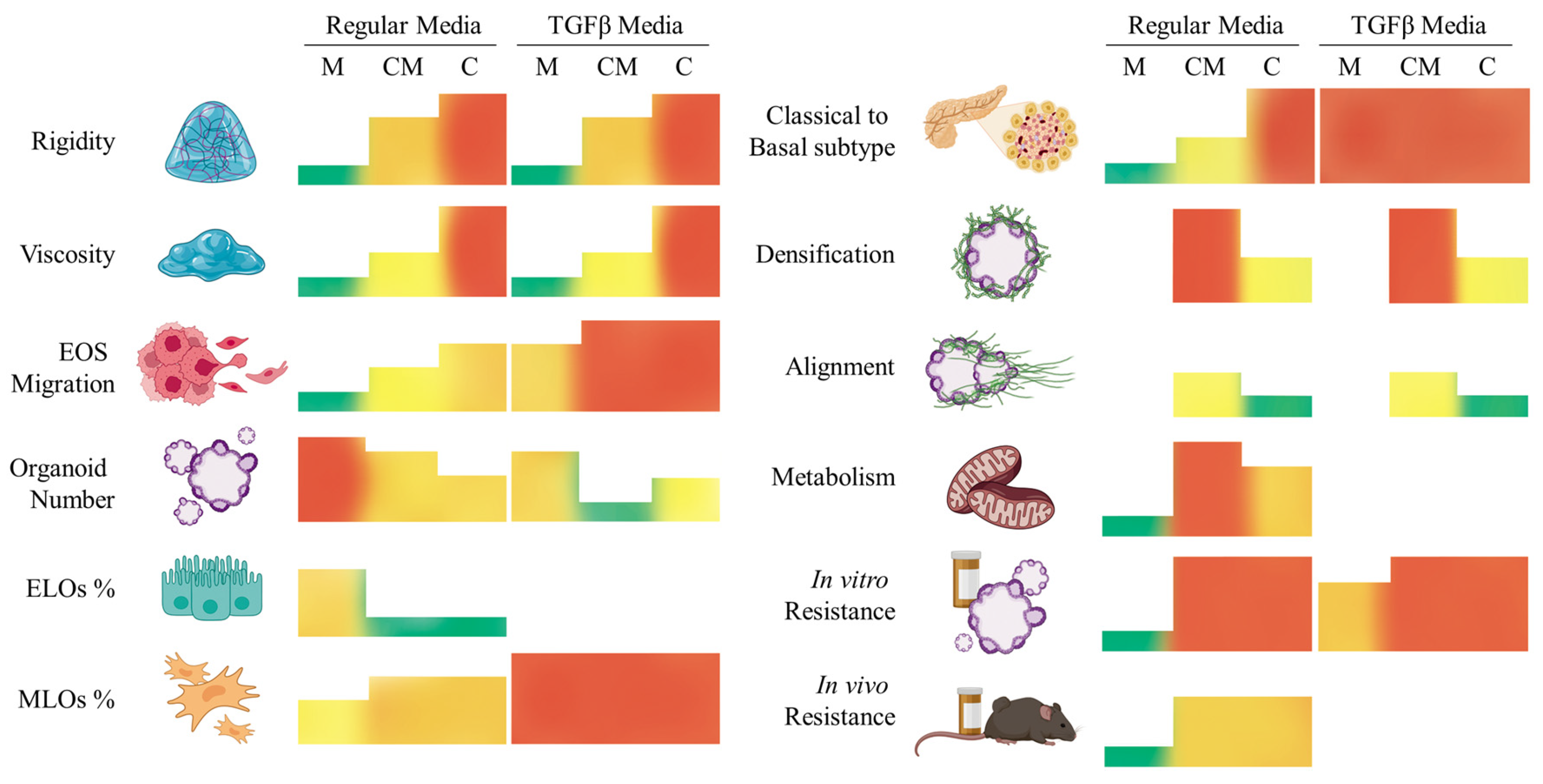
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Micro-Device Design and Fabrication
5.2. Hydrogel Fabrication
5.3. Mechanical Measurement of Stiffness
5.4. Hydrogel Morphology Measurement
5.5. Cell Line Culture and PDAC93 Organoid Generation
5.6. Generation of PDAC93-GFP Cell Lines Using Lentiviral Transduction
5.7. Time-Lapse Microscopy and Organoid Seed Tracking
5.8. Organoid Morphology Classification
5.9. Real-Time Quantitative PCR (RT-qPCR)
5.10. Immunofluorescence Staining
5.11. Collagen-I Remodeling Analysis
5.12. Mitochondrial Bioenergetics Assessment
5.13. Flow Cytometry Analysis
5.14. Cytotoxicity Assays
5.15. Mice and Subcutaneous PDAC93 Syngeneic Murine Model
5.16. Immunohistochemistry Staining
5.17. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, K.; Ohno, E.; Kawabe, N.; Kuzuya, T.; Funasaka, K.; Nakagawa, Y.; Nagasaka, M.; Ishikawa, T.; Watanabe, A.; Tochio, T.; et al. Current Status of the Diagnosis of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics 2023, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-S.; Hsu, C.-C.; Pai, V.C.; Liao, W.-Y.; Huang, S.-S.; Tan, K.-T.; Yen, C.-J.; Hsu, S.-C.; Chen, W.-Y.; Shan, Y.-S.; et al. Metronomic Chemotherapy Prevents Therapy-Induced Stromal Activation and Induction of Tumor-Initiating Cells. J. Exp. Med. 2016, 213, 2967–2988. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Dougan, S.K.; Aguirre, A.J.; Maitra, A. Translational Advances in Pancreatic Ductal Adenocarcinoma Therapy. Nat. Cancer 2022, 3, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, B.; Pignatelli, C.; Cossutta, M.; Citro, A.; Courty, J.; Piemonti, L. The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Cancers 2021, 13, 4442. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Singh, P.K. Molecular Subtypes of Pancreatic Cancer: A Proteomics Approach. Clin. Cancer Res. 2021, 27, 3272–3274. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.H.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual Microdissection Identifies Distinct Tumor- and Stroma-Specific Subtypes of Pancreatic Ductal Adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast Cancer Models: Engineering the Tumor Microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-Derived Xenograft (PDX) Models, Applications and Challenges in Cancer Research. J. Transl. Med. 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Lin, C.-C. Hydrogel Models with Stiffness Gradients for Interrogating Pancreatic Cancer Cell Fate. Bioengineering 2021, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-Generation Cancer Organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, Y. Patient-Derived Organoids in Translational Oncology and Drug Screening. Cancer Lett. 2023, 562, 216180. [Google Scholar] [CrossRef] [PubMed]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.-B.; et al. Patient-Derived Tumor Organoids: A New Avenue for Preclinical Research and Precision Medicine in Oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.J.; Mason, J.O. Mouse vs Man: Organoid Models of Brain Development & Disease. Brain Res. 2019, 1724, 146427. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Z.; Tang, Z.; Chen, Y.; Huang, M.; Liu, H.; Huang, W.; Ye, Q.; Jia, B. Research Progress, Challenges, and Breakthroughs of Organoids as Disease Models. Front. Cell Dev. Biol. 2021, 9, 740574. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Zhang, L.; Yi, C.; Guan, X.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Healthc. Mater. 2023, 12, e2202609. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The Clinical Role of the TME in Solid Cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Morales, X.; Cortés-Domínguez, I.; Ortiz-de-Solorzano, C. Modeling the Mechanobiology of Cancer Cell Migration Using 3D Biomimetic Hydrogels. Gels 2021, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Ko, E.; Kim, E.M.; Ballance, W.C.; Ito, J.D.; Chalifoux, M.; Kim, Y.J.; Bashir, R.; Kong, H. Extracellular Microenvironmental Control for Organoid Assembly. Tissue Eng. Part B Rev. 2022, 28, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Sainio, A.; Järveläinen, H. Extracellular Matrix-Cell Interactions: Focus on Therapeutic Applications. Cell Signal. 2020, 66, 109487. [Google Scholar] [CrossRef] [PubMed]
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The Use of Mixed Collagen-Matrigel Matrices of Increasing Complexity Recapitulates the Biphasic Role of Cell Adhesion in Cancer Cell Migration: ECM Sensing, Remodeling and Forces at the Leading Edge of Cancer Invasion. PLoS ONE 2020, 15, e0220019. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; Karim, S.A.; Morton, J.P.; del Río Hernández, A. Matrix Stiffness Induces Epithelial–Mesenchymal Transition and Promotes Chemoresistance in Pancreatic Cancer Cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Timbers, K.E.; Atia, L.G.; Koch, R.M.; Rana, A. TGFβ Signaling in the Pancreatic Tumor Microenvironment. Cancers 2021, 13, 5086. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of Patient-Derived Cancer Organoids for Drug-Screening Applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Qin, X.; Liu, H.; Liu, J.; Qin, J. Recent Advances in Defined Hydrogels in Organoid Research. Bioact. Mater. 2023, 28, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Anguiano, M.; Castilla, C.; Maška, M.; Ederra, C.; Peláez, R.; Morales, X.; Muñoz-Arrieta, G.; Mujika, M.; Kozubek, M.; Muñoz-Barrutia, A.; et al. Characterization of Three-Dimensional Cancer Cell Migration in Mixed Collagen-Matrigel Scaffolds Using Microfluidics and Image Analysis. PLoS ONE 2017, 12, e0171417. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Chen, Y.; Dong, S.; Zhang, C.; Liao, L.; Zhang, W. Hydrogels Mimicking the Viscoelasticity of Extracellular Matrix for Regenerative Medicine: Design, Application, and Molecular Mechanism. Chem. Eng. J. 2024, 498, 155206. [Google Scholar] [CrossRef]
- Palamaris, K.; Felekouras, E.; Sakellariou, S. Epithelial to Mesenchymal Transition: Key Regulator of Pancreatic Ductal Adenocarcinoma Progression and Chemoresistance. Cancers 2021, 13, 5532. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Corfiotti, F.; Corvaisier, M.; Vasseur, R.; Fulbert, M.; Skrzypczyk, C.; Deshorgues, A.; Gnemmi, V.; Tulasne, D.; Lahdaoui, F.; et al. Gemcitabine-induced Epithelial-mesenchymal Transition-like Changes Sustain Chemoresistance of Pancreatic Cancer Cells of Mesenchymal-like Phenotype. Mol. Carcinog. 2019, 58, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Krajina, B.A.; LeSavage, B.L.; Roth, J.G.; Zhu, A.W.; Cai, P.C.; Spakowitz, A.J.; Heilshorn, S.C. Microrheology Reveals Simultaneous Cell-Mediated Matrix Stiffening and Fluidization That Underlie Breast Cancer Invasion. Sci. Adv. 2021, 7, eabe1969. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y.; Silva, E.S.S.; Adisasmita, M.; Kim, K.S.; Jung, Y.K.; Lee, K.G.; Shin, J.H.; Choi, D. Enhancing Generation Efficiency of Liver Organoids in a Collagen Scaffold Using Human Chemically Derived Hepatic Progenitors. Ann. Hepatobiliary Pancreat. Surg. 2023, 27, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.H.; Lee, D.H.; Ko, J.; Hahn, S.; Jeong, S.Y.; Kim, H.K.; Park, E.; Choi, S.Y.; Jeong, S.; Lee, J.W.; et al. Development of Collagen-Based 3D Matrix for Gastrointestinal Tract-Derived Organoid Culture. Stem. Cells Int. 2019, 2019, 8472712. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Alam, K.; Roy, N.S.; Kaur, K.; Kaity, S.; Ravichandiran, V.; Roy, S. Exploring the Interaction between Extracellular Matrix Components in a 3D Organoid Disease Model to Replicate the Pathophysiology of Breast Cancer. J. Exp. Clin. Cancer Res. 2023, 42, 343. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jiang, Y.; Tan, H.; Liang, R. Collagen and Derivatives-Based Materials as Substrates for the Establishment of Glioblastoma Organoids. Int. J. Biol. Macromol. 2024, 254, 128018. [Google Scholar] [CrossRef] [PubMed]
- Borries, M.; Barooji, Y.F.; Yennek, S.; Grapin-Botton, A.; Berg-Sørensen, K.; Oddershede, L.B. Quantification of Visco-Elastic Properties of a Matrigel for Organoid Development as a Function of Polymer Concentration. Front. Phys. 2020, 8, 579168. [Google Scholar] [CrossRef]
- Poincloux, R.; Collin, O.; Lizárraga, F.; Romao, M.; Debray, M.; Piel, M.; Chavrier, P. Contractility of the Cell Rear Drives Invasion of Breast Tumor Cells in 3D Matrigel. Proc. Natl. Acad. Sci. USA 2011, 108, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.L.; Muñoz-Bellvís, L.; Orfao, A. Genomic Heterogeneity of Pancreatic Ductal Adenocarcinoma and Its Clinical Impact. Cancers 2021, 13, 4451. [Google Scholar] [CrossRef] [PubMed]
- Metzenmacher, M.; Zaun, G.; Trajkovic-Arsic, M.; Cheung, P.; Reissig, T.M.; Schürmann, H.; von Neuhoff, N.; O’Kane, G.; Ramotar, S.; Dodd, A.; et al. Minimally Invasive Determination of Pancreatic Ductal Adenocarcinoma (PDAC) Subtype by Means of Circulating Cell-free RNA. Mol. Oncol. 2025, 19, 357–376. [Google Scholar] [CrossRef] [PubMed]
- van Eijck, C.W.F.; Real, F.X.; Malats, N.; Vadgama, D.; van den Bosch, T.P.P.; Doukas, M.; van Eijck, C.H.J.; Mustafa, D.A.M. GATA6 Identifies an Immune-Enriched Phenotype Linked to Favorable Outcomes in Patients with Pancreatic Cancer Undergoing Upfront Surgery. Cell Rep. Med. 2024, 5, 101557. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Dardare, J.; Witz, A.; Merlin, J.-L.; Bochnakian, A.; Toussaint, P.; Gilson, P.; Harlé, A. Epithelial to Mesenchymal Transition in Patients with Pancreatic Ductal Adenocarcinoma: State-of-the-Art and Therapeutic Opportunities. Pharmaceuticals 2021, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Gagliano, N. E-Cadherin in Pancreatic Ductal Adenocarcinoma: A Multifaceted Actor during EMT. Cells 2020, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Bajor, D.L.; Norgard, R.J.; Sahmoud, A.; Bhagwat, N.; Pham, M.N.; Cornish, T.C.; Iacobuzio-Donahue, C.A.; Vonderheide, R.H.; Stanger, B.Z. Metastatic Progression Is Associated with Dynamic Changes in the Local Microenvironment. Nat. Commun. 2016, 7, 12819. [Google Scholar] [CrossRef] [PubMed]
- Arensman, M.D.; Kovochich, A.N.; Kulikauskas, R.M.; Lay, A.R.; Yang, P.-T.; Li, X.; Donahue, T.; Major, M.B.; Moon, R.T.; Chien, A.J.; et al. WNT7B Mediates Autocrine Wnt/β-Catenin Signaling and Anchorage-Independent Growth in Pancreatic Adenocarcinoma. Oncogene 2014, 33, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kwon, Y.; Jang, M.; Park, M.; Kim, J.; Cho, S.; Jang, D.G.; Lee, W.-B.; Jung, S.H.; Choi, H.J.; et al. β-Catenin Activation down-Regulates Cell-Cell Junction-Related Genes and Induces Epithelial-to-Mesenchymal Transition in Colorectal Cancers. Sci. Rep. 2019, 9, 18440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morris, J.P.; Yan, W.; Schofield, H.K.; Gurney, A.; Simeone, D.M.; Millar, S.E.; Hoey, T.; Hebrok, M.; Pasca di Magliano, M. Canonical Wnt Signaling Is Required for Pancreatic Carcinogenesis. Cancer Res. 2013, 73, 4909–4922. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.U.; Bansal, S.; Kaur, P.; Jain, S.K.; Altadil, T.; Hinzman, C.P.; Li, Y.; Moulton, J.; Singh, B.; Bansal, S.; et al. TGFβ Drives Metabolic Perturbations during Epithelial Mesenchymal Transition in Pancreatic Cancer: TGFβ Induced EMT in PDAC. Cancers 2021, 13, 6204. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.H.; Ungewiss, C.; Tong, P.; Byers, L.A.; Wang, J.; Canales, J.R.; Villalobos, P.A.; Uraoka, N.; Mino, B.; Behrens, C.; et al. ZEB1 Induces LOXL2-Mediated Collagen Stabilization and Deposition in the Extracellular Matrix to Drive Lung Cancer Invasion and Metastasis. Oncogene 2017, 36, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.E.; Weinberg, S.H.; Lemmon, C.A. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2019, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, H.; Webster, M.R.; Behera, R.; Jimenez Valencia, A.M.; Wirtz, D.; Weeraratna, A.T.; Shenoy, V.B. Modeling the Two-Way Feedback between Contractility and Matrix Realignment Reveals a Nonlinear Mode of Cancer Cell Invasion. Proc. Natl. Acad. Sci. USA 2017, 114, E1617–E1626. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Increased Stiffness of the Tumor Microenvironment in Colon Cancer Stimulates Cancer Associated Fibroblast-Mediated Prometastatic Activin A Signaling. Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.E.; St Johnston, D. Apical–Basal Polarity and the Control of Epithelial Form and Function. Nat. Rev. Mol. Cell Biol. 2022, 23, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, T.R.; Røsland, G.V.; Tronstad, K.J.; Søreide, K.; Hagland, H.R. Metabolic Flux Analysis of 3D Spheroids Reveals Significant Differences in Glucose Metabolism from Matched 2D Cultures of Colorectal Cancer and Pancreatic Ductal Adenocarcinoma Cell Lines. Cancer Metab. 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Karasinska, J.M.; Topham, J.T.; Johal, D.; Kalloger, S.; Metcalfe, A.; Warren, C.S.; Miyagi, A.; Tao, L.V.; Kevorkova, M.; et al. Pancreatic Cancer Tumor Organoids Exhibit Subtype-Specific Differences in Metabolic Profiles. Cancer Metab. 2024, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Fuentes-Retamal, S.; Palominos, C.; Rodríguez-Lucart, Y.A.; López-Torres, C.; Araya-Maturana, R. Extracellular Matrix Signals as Drivers of Mitochondrial Bioenergetics and Metabolic Plasticity of Cancer Cells During Metastasis. Front. Cell Dev. Biol. 2021, 9, 751301. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Carmona, C.A.; Dalla Pozza, E.; Ambrosini, G.; Errico, A.; Dando, I. Divergent Roles of Mitochondria Dynamics in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-H.; Zhu, Y.-H.; Yang, J.; Ji, P.-X.; Zhao, R.-K.; Duan, Z.-H.; Yao, H.-F.; Jia, Q.-Y.; Yin, Y.-F.; Hu, L.-P.; et al. A CLIC1 Network Coordinates Matrix Stiffness and the Warburg Effect to Promote Tumor Growth in Pancreatic Cancer. Cell Rep. 2024, 43, 114633. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.-K.; Weaver, V.M. Fibrosis and Cancer: A Strained Relationship. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends. Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Loessner, D.; Avery, V.M. Addressing the Tumour Microenvironment in Early Drug Discovery: A Strategy to Overcome Drug Resistance and Identify Novel Targets for Cancer Therapy. Drug Discov. Today 2021, 26, 663–676. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer Drug Resistance Induced by EMT: Novel Therapeutic Strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Du, Y.; Zhao, X.; Wang, C. Tumor Microenvironment and Metabolic Remodeling in Gemcitabine-based Chemoresistance of Pancreatic Cancer. Cancer Lett. 2021, 521, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Okesola, B.O.; Osuna de la Peña, D.; Li, W.; Lin, M.; Trabulo, S.; Tatari, M.; Lawlor, R.T.; Scarpa, A.; Wang, W.; et al. A Self-Assembled 3D Model Demonstrates How Stiffness Educates Tumor Cell Phenotypes and Therapy Resistance in Pancreatic Cancer. Adv. Healthc. Mater. 2024, 13, e2301941. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhu, S.; Gong, T.; Wu, D.; Zhao, Y.; Yan, J.; Dai, C.; Huang, Y.; Yang, Y.; Guo, Y. Matrix Stiffness Triggers Chemoresistance through Elevated Autophagy in Pancreatic Ductal Adenocarcinoma. Biomater. Sci. 2023, 11, 7358–7372. [Google Scholar] [CrossRef] [PubMed]
- Gregori, A.; Bergonzini, C.; Capula, M.; de Mercado, R.R.; Danen, E.H.J.; Giovannetti, E.; Schmidt, T. Altered Mechanobiology of PDAC Cells with Acquired Chemoresistance to Gemcitabine and Paclitaxel. Cancers 2024, 16, 3863. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Jadhao, M.; Liao, W.-T.; Chang, W.-T.; Hung, C.-T.; Chiu, C.-C. Culprits of PDAC Resistance to Gemcitabine and Immune Checkpoint Inhibitor: Tumour Microenvironment Components. Front. Mol. Biosci. 2022, 9, 1020888. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Estrella, J.S.; Katz, M.H.; Kim, M.; Rashid, A.; Lee, J.E.; Maitra, A.; Wistuba, I.I.; Wolff, R.A.; Varadhachary, G.R.; et al. Expression of Epithelial-Mesenchymal Transition Markers in Treated Pancreatic Ductal Adenocarcinoma. Pancreas 2019, 48, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Masuo, H.; Kubota, K.; Shimizu, A.; Notake, T.; Miyazaki, S.; Yoshizawa, T.; Sakai, H.; Hayashi, H.; Soejima, Y. Increased Mitochondria Are Responsible for the Acquisition of Gemcitabine Resistance in Pancreatic Cancer Cell Lines. Cancer Sci. 2023, 114, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating Extracellular Matrix Stiffness: A Strategic Approach to Boost Cancer Immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Yapp, D.T.; Wong, M.Q.; Kyle, A.H.; Valdez, S.M.; Tso, J.; Yung, A.; Kozlowski, P.; Owen, D.A.; Buczkowski, A.K.; Chung, S.W.; et al. The Differential Effects of Metronomic Gemcitabine and Antiangiogenic Treatment in Patient-Derived Xenografts of Pancreatic Cancer: Treatment Effects on Metabolism, Vascular Function, Cell Proliferation, and Tumor Growth. Angiogenesis 2016, 19, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Amaveda, H.; Mora, M.; García-Aznar, J.M. Combined Experimental and Computational Characterization of Crosslinked Collagen-Based Hydrogels. PLoS ONE 2018, 13, e0195820. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An Open and Extensible Platform for Single-Particle Tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Royer, L.A.; Steinbach, P.; Schmidt, D.; Dibrov, A.; Schmidt, U.; Weigert, M.; Maghelli, N.; Tomancak, P.; Jug, F.; et al. CLIJ: GPU-Accelerated Image Processing for Everyone. Nat. Methods 2020, 17, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated Library and Plugins for Mathematical Morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–273. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2012, 12, 2825–2830. [Google Scholar]
- Poličar, P.G.; Stražar, M.; Zupan, B. OpenTSNE: A Modular Python Library for t-SNE Dimensionality Reduction and Embedding. J. Stat. Softw. 2024, 109, 1–30. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Desousa, B.R.; Kim, K.K.; Jones, A.E.; Ball, A.B.; Hsieh, W.Y.; Swain, P.; Morrow, D.H.; Brownstein, A.J.; Ferrick, D.A.; Shirihai, O.S.; et al. Calculation of ATP Production Rates Using the Seahorse XF Analyzer. EMBO Rep. 2023, 24, e56380. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uygun, B.E.; Geerts, S.; Ozer, S.; Scalf, M.; Gilpin, S.E.; Ott, H.C.; Yarmush, M.L.; Smith, L.M.; Welham, N.V.; et al. Proteomic Analysis of Naturally-Sourced Biological Scaffolds. Biomaterials 2016, 75, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nyga, A.; Muñoz, J.J.; Dercksen, S.; Fornabaio, G.; Uroz, M.; Trepat, X.; Baum, B.; Matthews, H.K.; Conte, V. Oncogenic RAS Instructs Morphological Transformation of Human Epithelia via Differential Tissue Mechanics. Sci. Adv. 2021, 7, eabg6467. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Chaudhry, A.; Shi, R.; Luciani, D.S. A Pipeline for Multidimensional Confocal Analysis of Mitochondrial Morphology, Function, and Dynamics in Pancreatic β-Cells. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E87–E101. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorzabal-Bellido, I.; Morales, X.; Cortés-Domínguez, I.; Esparza, M.; Grande, L.; Castillo, P.; Larumbe, S.; Monteserín, M.; Narayanan, S.; Ponz-Sarvise, M.; et al. Tumor Organoids Grown in Mixed-Composition Hydrogels Recapitulate the Plasticity of Pancreatic Cancers. Gels 2025, 11, 562. https://doi.org/10.3390/gels11070562
Sorzabal-Bellido I, Morales X, Cortés-Domínguez I, Esparza M, Grande L, Castillo P, Larumbe S, Monteserín M, Narayanan S, Ponz-Sarvise M, et al. Tumor Organoids Grown in Mixed-Composition Hydrogels Recapitulate the Plasticity of Pancreatic Cancers. Gels. 2025; 11(7):562. https://doi.org/10.3390/gels11070562
Chicago/Turabian StyleSorzabal-Bellido, Ioritz, Xabier Morales, Iván Cortés-Domínguez, Maider Esparza, Lucía Grande, Pedro Castillo, Silvia Larumbe, María Monteserín, Shruthi Narayanan, Mariano Ponz-Sarvise, and et al. 2025. "Tumor Organoids Grown in Mixed-Composition Hydrogels Recapitulate the Plasticity of Pancreatic Cancers" Gels 11, no. 7: 562. https://doi.org/10.3390/gels11070562
APA StyleSorzabal-Bellido, I., Morales, X., Cortés-Domínguez, I., Esparza, M., Grande, L., Castillo, P., Larumbe, S., Monteserín, M., Narayanan, S., Ponz-Sarvise, M., Vicent, S., & Ortiz-de-Solórzano, C. (2025). Tumor Organoids Grown in Mixed-Composition Hydrogels Recapitulate the Plasticity of Pancreatic Cancers. Gels, 11(7), 562. https://doi.org/10.3390/gels11070562






