Effects of Silica Aerogel Content on the Properties of Waterborne Organic Thermal Insulation Coatings
Abstract
1. Introduction
2. Results and Discussion
2.1. Microstructure
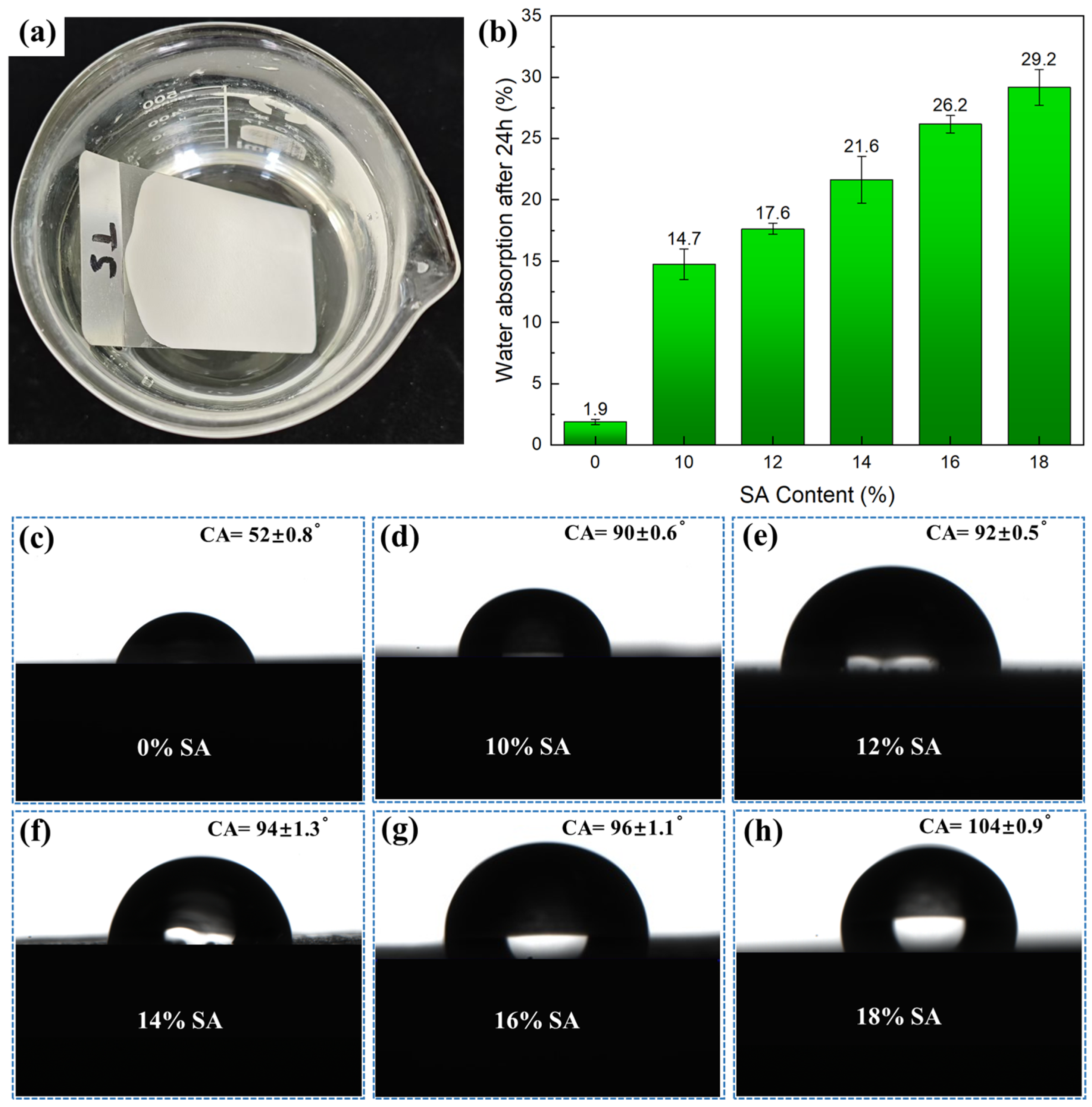
2.2. Water Resistance
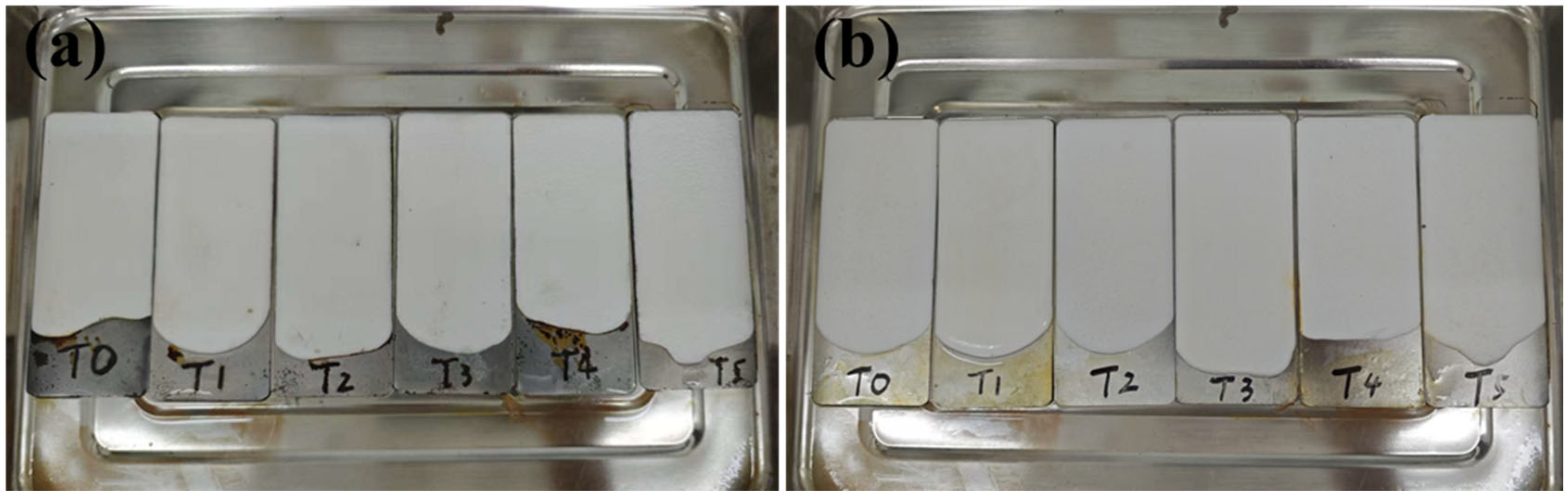
2.3. Corrosion Resistance
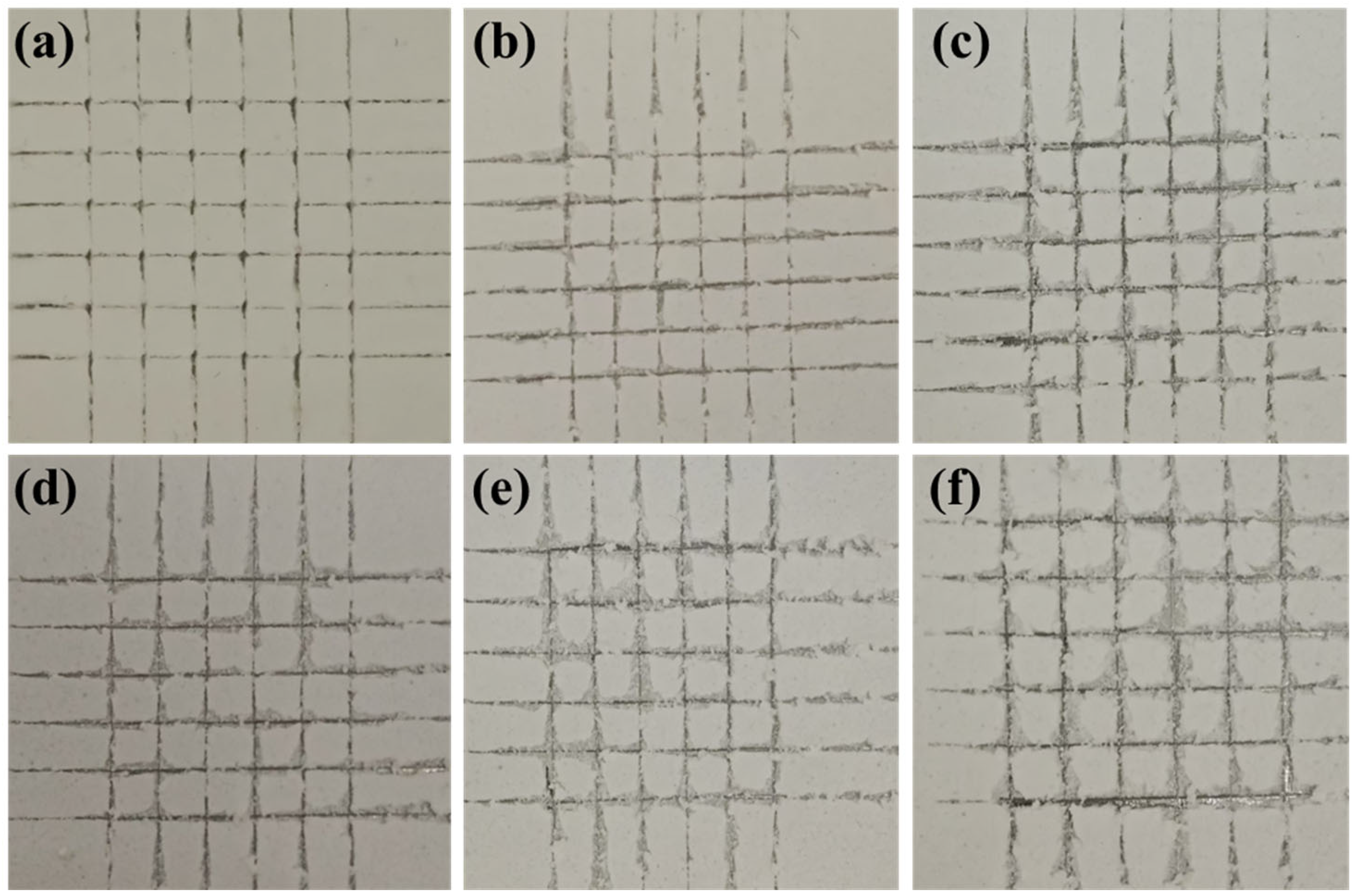
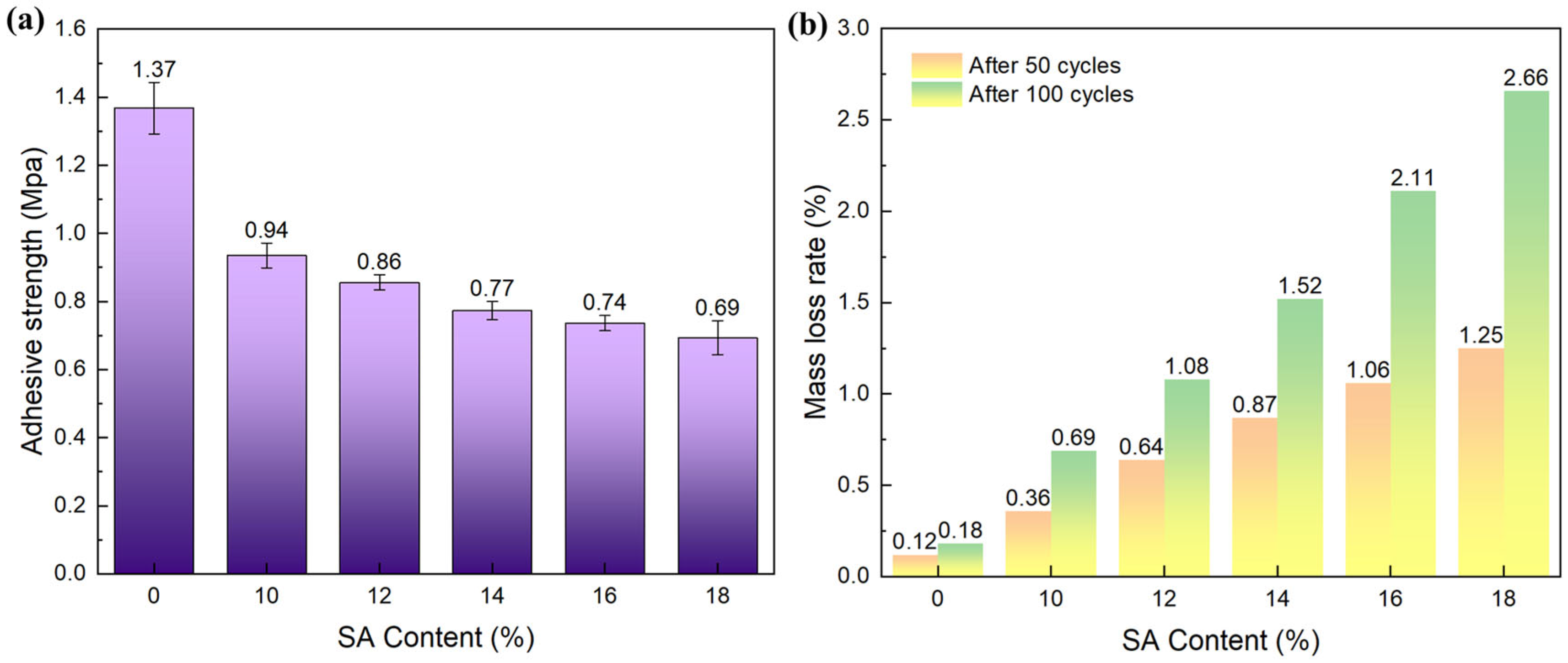
2.4. Mechanical Properties
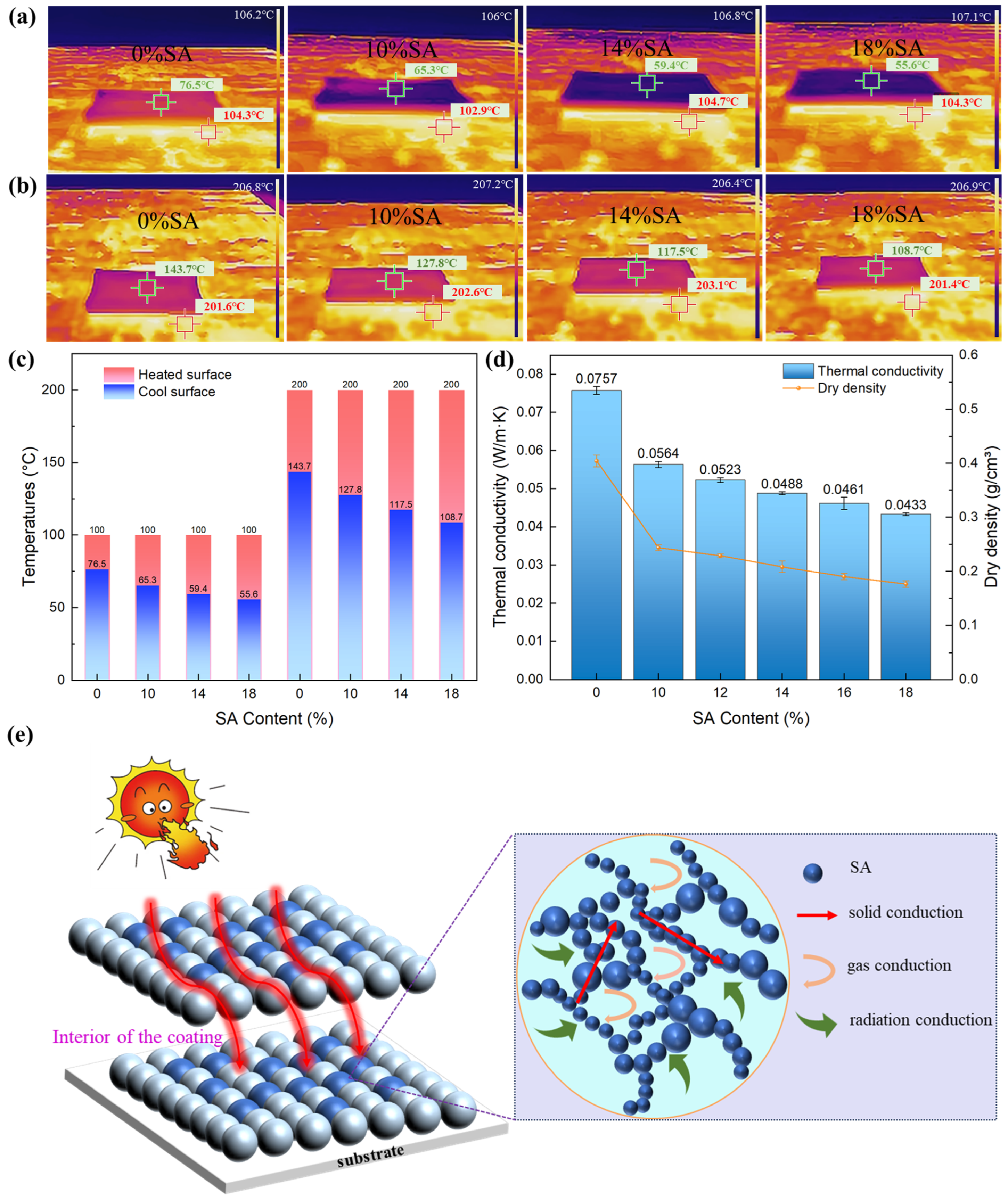
2.5. Thermal Insulation
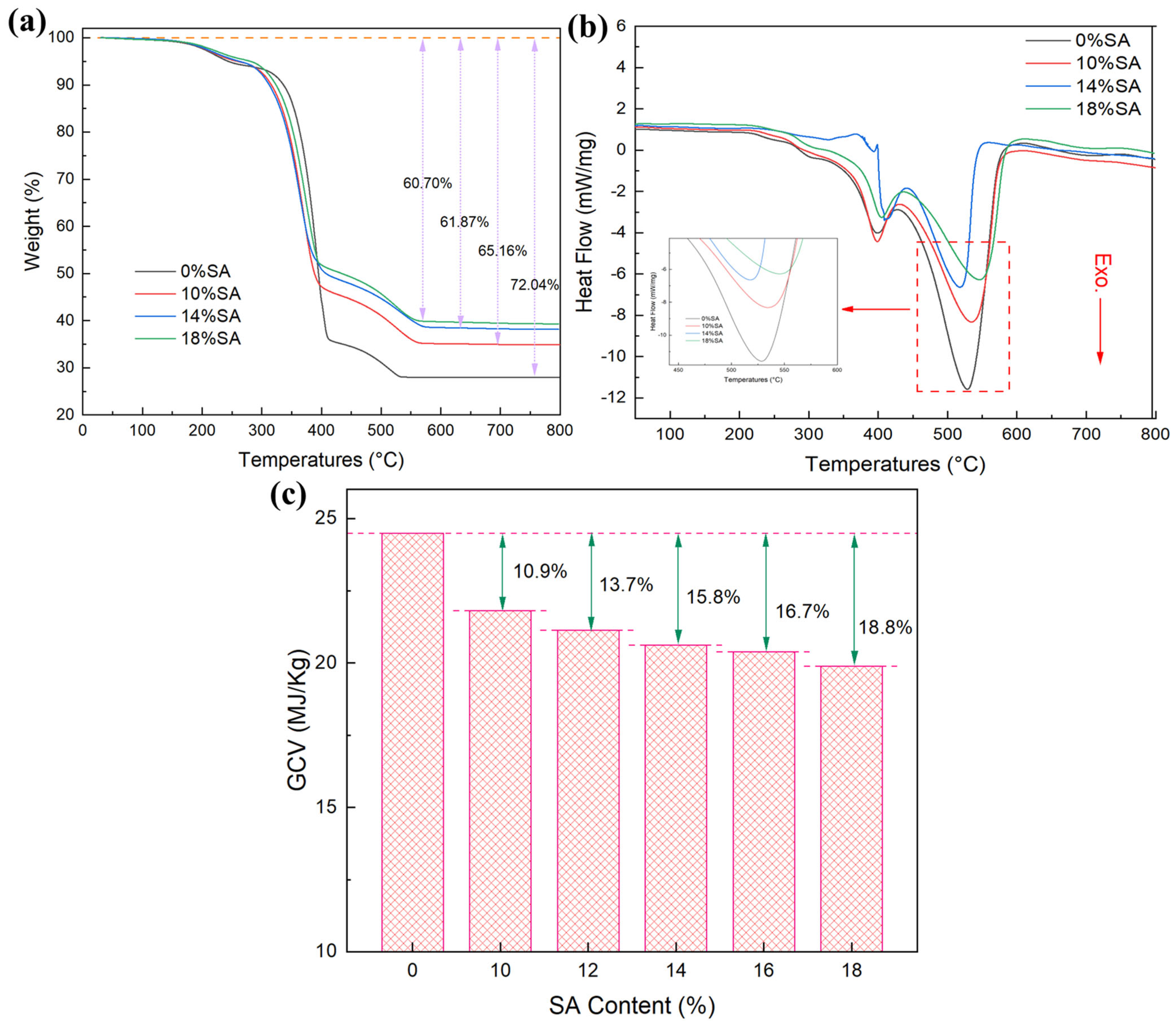
2.6. Heat Resistance
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
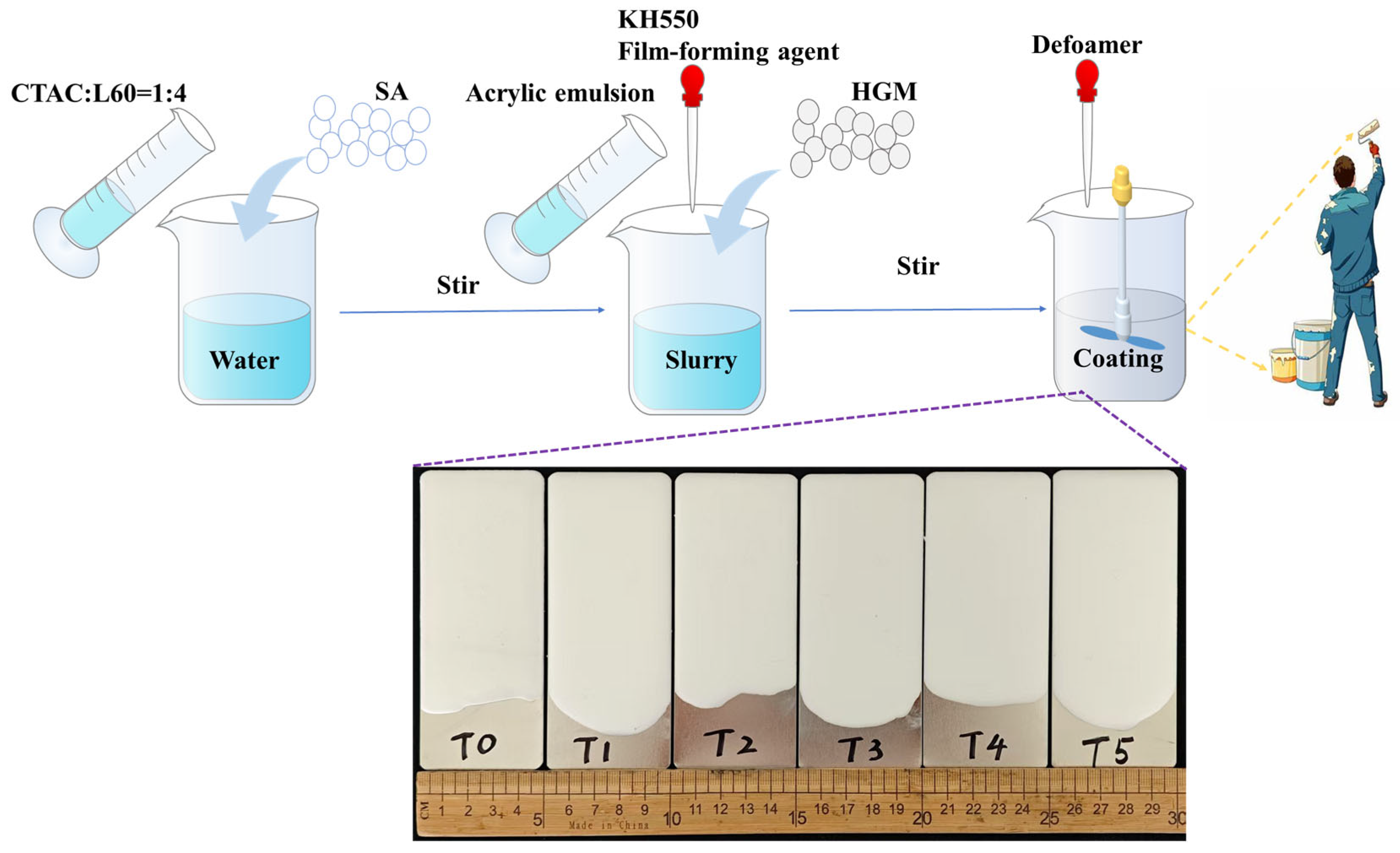
4.2. Preparation of Dispersed SA Slurry
4.3. Preparation of Thermal Insulation Coatings
4.4. Characterization Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wi, S.; Yang, S.; Yeol Yun, B.; Kim, S. Exterior Insulation Finishing System Using Cementitious Plaster/Microencapsulated Phase Change Material for Improving the Building Thermal Storage Performance. Constr. Build. Mater. 2021, 299, 123932. [Google Scholar] [CrossRef]
- Shen, L.; Tan, H.; Yang, Y.; He, W. Preparation and Characteristics of Paraffin/Silica Aerogel Composite Phase-Change Materials and Their Application to Cement. Constr. Build. Mater. 2023, 387, 131609. [Google Scholar] [CrossRef]
- Ibrahim, M. Limiting Windows Offset Thermal Bridge Losses Using a New Insulating Coating. Appl. Energy 2014, 123, 220–231. [Google Scholar] [CrossRef]
- Wei, L.; Shen, K.; Wei, D.; Wang, K. Simulation of Heat Transfer Performance of Silicone-Based Insulation Coatings. Int. J. Simul. Process Model. 2022, 18, 211–221. [Google Scholar] [CrossRef]
- Umate, T.B.; Sawarkar, P.D. Thermal Performance Evaluation of Aerogel-Enhanced Polyurethane Insulation Panels for Refrigerated Vehicles: A Numerical and Experimental Study. Therm. Sci. Eng. Prog. 2024, 53, 102752. [Google Scholar] [CrossRef]
- Wang, J. Silica Aerogel/Potassium Hexatitanate Whisker Composite Coated Fabric for Metallurgical Protective Clothing. Fibers Polym. 2023, 24, 243–252. [Google Scholar] [CrossRef]
- Jin, R.; Zhou, Z.; Liu, J.; Shi, B.; Zhou, N.; Wang, X.; Jia, X.; Guo, D.; Xu, B. Aerogels for Thermal Protection and Their Application in Aerospace. Gels 2023, 9, 606. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Xu, C.; Li, Z.; Yao, S.; Long, G.; Liu, Q.; Fang, C.; Wu, X. Realizing Balanced Thermal Safety and Heat Insulation in Hydrophobic Silica Aerogel Composites. Polym. Degrad. Stab. 2025, 236, 111294. [Google Scholar] [CrossRef]
- Bao, H.; Yan, C.; Wang, B.; Fang, X.; Zhao, C.Y.; Ruan, X. Double-Layer Nanoparticle-Based Coatings for Efficient Terrestrial Radiative Cooling. Sol. Energy Mater. Sol. Cells 2017, 168, 78–84. [Google Scholar] [CrossRef]
- Di, Z.; Ma, S.; Wang, H.; Guan, Z.; Lian, B.; Qiu, Y.; Jiang, Y. Modulation of Thermal Insulation and Mechanical Property of Silica Aerogel Thermal Insulation Coatings. Coatings 2022, 12, 1421. [Google Scholar] [CrossRef]
- Lee, S.W.; Lim, C.H.; Salleh, E.I.B. Reflective Thermal Insulation Systems in Building: A Review on Radiant Barrier and Reflective Insulation. Renew. Sustain. Energy Rev. 2016, 65, 643–661. [Google Scholar] [CrossRef]
- Kiil, S. Quantitative Analysis of Silica Aerogel-Based Thermal Insulation Coatings. Prog. Org. Coat. 2015, 89, 26–34. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ding, Y.D.; Shu, X.; Liu, N. Preparation, Characterization and Properties of SiO2 Aerogel Composite Thermal Insulation Coating. Chem. Eng. Trans. 2016, 55, 259–264. [Google Scholar] [CrossRef]
- He, S.; Wu, X.; Zhang, X.; Sun, J.; Tian, F.; Guo, S.; Du, H.; Li, P.; Huang, Y. Preparation and Properties of Thermal Insulation Coating Based on Silica Aerogel. Energy Build. 2023, 298, 113556. [Google Scholar] [CrossRef]
- Han, D.; Wang, C.; Han, C.B.; Cui, Y.; Ren, W.R.; Zhao, W.K.; Jiang, Q.; Yan, H. Highly Optically Selective and Thermally Insulating Porous Calcium Silicate Composite SiO2 Aerogel Coating for Daytime Radiative Cooling. ACS Appl. Mater. Interfaces 2024, 16, 9303–9312. [Google Scholar] [CrossRef]
- Lei, Y.; Zheng, Q.; Zhu, H.; Dou, R.; Yuan, J.; Zhao, Y.; Peng, H.; Yu, P. A Novel Thermal Insulation and Anticorrosion Coating Based on Silica Aerogel Modification for Mild Steel: Experimental and Theoretical Studies. Mater. Today Commun. 2024, 41, 111105. [Google Scholar] [CrossRef]
- Shafi, S.; Navik, R.; Ding, X.; Zhao, Y. Improved Heat Insulation and Mechanical Properties of Silica Aerogel/Glass Fiber Composite by Impregnating Silica Gel. J. Non-Cryst. Solids 2019, 503–504, 78–83. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Yang, H.; Wang, F.; Jia, Y. Dispersion Properties of Aerogel Slurry and Application of Its Coatings in Thermal Insulation Engineering. Rev. Téc. Ing. Univ. Zulia 2016, 39, 153–161. [Google Scholar]
- Hanif, A.; Diao, S.; Lu, Z.; Fan, T.; Li, Z. Green Lightweight Cementitious Composite Incorporating Aerogels and Fly Ash Cenospheres—Mechanical and Thermal Insulating Properties. Constr. Build. Mater. 2016, 116, 422–430. [Google Scholar] [CrossRef]
- Yağci, Ö.; Eker Gümüş, B.; Taşdemir, M. Thermal, Structural and Dynamical Mechanical Properties of Hollow Glass Sphere-Reinforced Polypropylene Composites. Polym. Bull. 2021, 78, 3089–3101. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Wen, S.; Wang, C.; Zhao, Z.; Li, W. Zinc Phosphate Coated Modified Hollow Glass Beads and Their Thermal Insulation and Anticorrosion Performance in Coatings. Ceram. Int. 2021, 47, 23507–23517. [Google Scholar] [CrossRef]
- Xing, Z.; Ke, H.; Wang, X.; Zheng, T.; Qiao, Y.; Chen, K.; Zhang, X.; Zhang, L.; Bai, C.; Li, Z. Investigation of the Thermal Conductivity of Resin-Based Lightweight Composites Filled with Hollow Glass Microspheres. Polymers 2020, 12, 518. [Google Scholar] [CrossRef]
- Niazi, P.; Karevan, M.; Javanbakht, M. Mechanical and Thermal Insulation Performance of Hollow Glass Microsphere (HGMS)/Fumed Silica/Polyester Microcomposite Coating. Prog. Org. Coat. 2023, 176, 107387. [Google Scholar] [CrossRef]
- Zeng, Q.; Mao, T.; Li, H.; Peng, Y. Thermally Insulating Lightweight Cement-Based Composites Incorporating Glass Beads and Nano-Silica Aerogels for Sustainably Energy-Saving Buildings. Energy Build. 2018, 174, 97–110. [Google Scholar] [CrossRef]
- Ji, S.; Gui, H.; Guan, G.; Zhou, M.; Guo, Q.; Tan, M.Y.J. Molecular Design and Copolymerization to Enhance the Anti-Corrosion Performance of Waterborne Acrylic Coatings. Prog. Org. Coat. 2021, 153, 106140. [Google Scholar] [CrossRef]
- Yang, J.; Huang, D.; Feng, Z.; He, H.; Jin, M. Effect of Aerogel Incorporation on Water Content Distribution and Capillary Water Absorption in Pore Size Intervals of Cementitious Composites. J. Mater. Sci. 2024, 59, 13756–13771. [Google Scholar] [CrossRef]
- Karim, A.N.; Johansson, P.; Sasic Kalagasidis, A. Increasing Water Absorptivity of an Aerogel-Based Coating Mortar in Subsequent Wetting and Drying. Gels 2022, 8, 764. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Vashchuk, A.; Klapiszewski, Ł. Research Development in Silica Aerogel Incorporated Cementitious Composites—A Review. Polymers 2022, 14, 1456. [Google Scholar] [CrossRef]
- HG/T 3344-2012; Determination of Water Absorption of Paint Film. Chemical Industry Press: Beijing, China, 2012.
- Hasnidawani, J.N.; Hassan, N.A.; Norita, H.; Samat, N.; Bonnia, N.N.; Surip, S.N. ZnO Nanoparticles for Anti-Corrosion Nanocoating of Carbon Steel. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2017; Volume 894, pp. 76–80. [Google Scholar] [CrossRef]
- Shen, X.; Mao, T.; Li, C.; Mao, F.; Xue, Z.; Xu, G.; Amirfazli, A. Durable Superhydrophobic Coatings Based on CNTs-SiO2gel Hybrids for Anti-Corrosion and Thermal Insulation. Prog. Org. Coat. 2023, 181, 107602. [Google Scholar] [CrossRef]
- Bakhtiarvand, M.; Ghaffari, M.; Bahlakeh, G. The Efficient Acrylic-Based Coating for Corrosion Protection of Aluminum Surface Utilizing Surface-Modified ZnO Nanoparticles: Combined Electrochemical Studies and DFT Computation. J. Appl. Electrochem. 2025, 55, 1309–1328. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Duan, Y.; Chen, J.; Yao, S.; Peng, L.; Chen, W.; Menshutina, N.; Liu, M. A Review of Silica Aerogel Based Thermal Insulation Coatings: Preparation, Properties and Applications. Prog. Org. Coat. 2025, 208, 109449. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.; Li, X.; Jiang Yin, X.; Djati Utomo, H. Development of Water-Based Thermal Insulation Paints Using Silica Aerogel Made from Incineration Bottom Ash. Energy Build. 2022, 259, 111866. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Solorzano, E.; De Saja, J.A.; Dumon, M.; Rodríguez-Pérez, M.A. Experimental Validation of the Knudsen Effect in Nanocellular Polymeric Foams. Polymer 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Yang, H.; Ye, F. Microtexture, Microstructure Evolution, and Thermal Insulation Properties of Si3N4/Silica Aerogel Composites at High Temperatures. RSC Adv. 2022, 12, 12226–12234. [Google Scholar] [CrossRef]
- He, F.; Qi, Z.; Zhen, W.; Wu, J.; Huang, Y.; Xiong, X.; Zhang, R. Thermal Conductivity of Silica Aerogel Thermal Insulation Coatings. Int. J. Thermophys. 2019, 40, 92. [Google Scholar] [CrossRef]
- Li, F.-F. Comprehensive Review of Recent Research Advances on Flame-Retardant Coatings for Building Materials: Chemical Ingredients, Micromorphology, and Processing Techniques. Molecules 2023, 28, 1842. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Nong, Z.-S.; Man, T.-N.; Lu, S.-W.; Dong, L.-H. SiO2 Aerogel Coating with Organic Montmorillonite Compounded with Flame Retardants: A Strategy for Design of a Multifunctional Thermal Barrier Coating. J. Mater. Res. Technol. 2024, 30, 318–331. [Google Scholar] [CrossRef]
- Li, Z.; Yao, S.; Zhou, F.; Hu, M.; Shen, K.; Liu, M.; Fan, C.; Wu, X. Preparing Hydrophobic Silica Aerogels with Superior Thermal Insulation and Fire Safety by Introducing Sepiolite. Constr. Build. Mater. 2025, 461, 139905. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Z.; Li, Z.; Duan, Y.; Yang, Y.; Zhou, F.; Chen, J.; Liu, Q.; Wu, X. Synthesis of High-Performance Hydrophobic Silica Aerogel Composites with Excellent Flame Retardant and Thermal Insulating Properties. J. Alloys Compd. 2025, 1027, 180529. [Google Scholar] [CrossRef]
- Li, Z. Binary Surfactant-Optimized Silica Aerogel Slurries for Building Materials: Effects of Formulation and Content. Constr. Build. Mater. 2024, 438, 137161. [Google Scholar] [CrossRef]
- GB/T 9286–2021; Paints and Varnishes—Cross-Cut Test. Standardization Administration of China (SAC): Beijing, China, 2021.
- GB/T 5210-2006; Paints and Varnishes—Pull-Off Test for Adhesion. Standardization Administration of China (SAC): Beijing, China, 2006.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, D.; Yao, S.; Duan, Y.; Chen, J.; Liu, M.; Liu, T.; Li, Z. Effects of Silica Aerogel Content on the Properties of Waterborne Organic Thermal Insulation Coatings. Gels 2025, 11, 547. https://doi.org/10.3390/gels11070547
Chen Z, Li D, Yao S, Duan Y, Chen J, Liu M, Liu T, Li Z. Effects of Silica Aerogel Content on the Properties of Waterborne Organic Thermal Insulation Coatings. Gels. 2025; 11(7):547. https://doi.org/10.3390/gels11070547
Chicago/Turabian StyleChen, Zikang, Dingwei Li, Shengjie Yao, Yumin Duan, Jiahui Chen, Miao Liu, Taoying Liu, and Zhi Li. 2025. "Effects of Silica Aerogel Content on the Properties of Waterborne Organic Thermal Insulation Coatings" Gels 11, no. 7: 547. https://doi.org/10.3390/gels11070547
APA StyleChen, Z., Li, D., Yao, S., Duan, Y., Chen, J., Liu, M., Liu, T., & Li, Z. (2025). Effects of Silica Aerogel Content on the Properties of Waterborne Organic Thermal Insulation Coatings. Gels, 11(7), 547. https://doi.org/10.3390/gels11070547






