Low-Temperature Dried Alginate/Silica Hybrid Aerogel Beads with Tunable Surface Functionalities for Removal of Lead Ions from Water
Abstract
1. Introduction
2. Results and Discussion
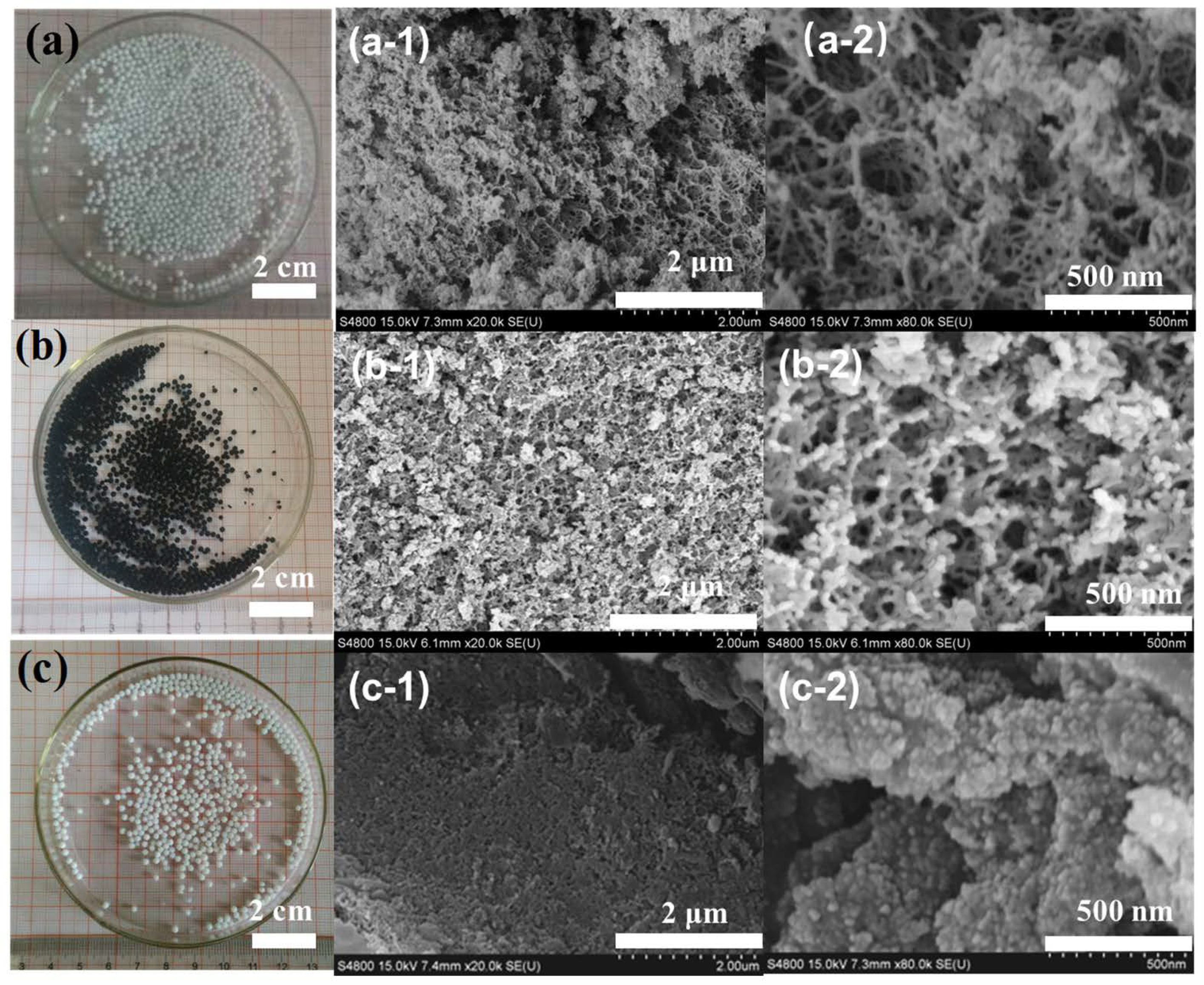
2.1. Synthesis of Hybrid Aerogel Beads
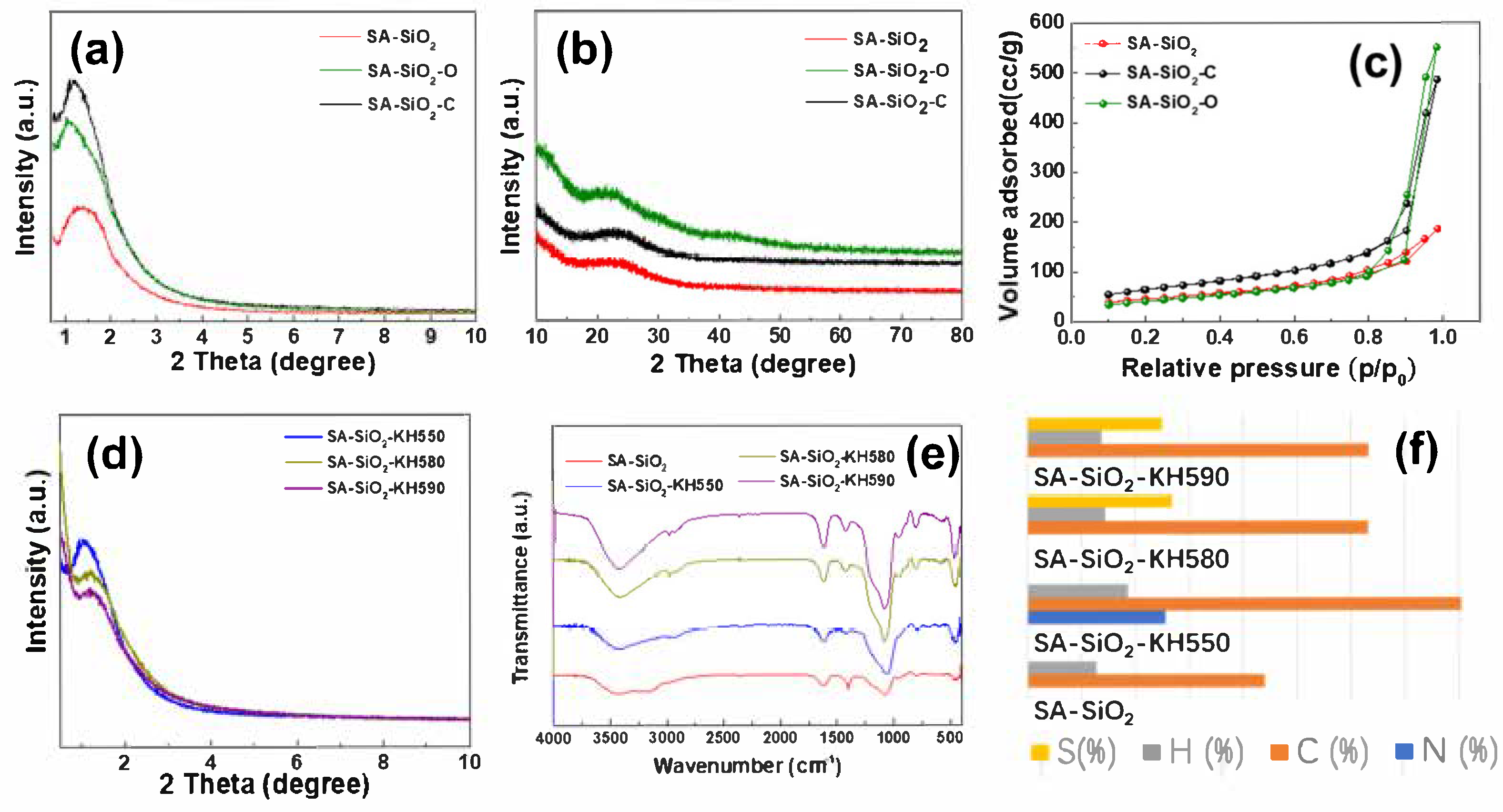
2.2. Physical and Chemical Properties of Hybrid Aerogel Beads
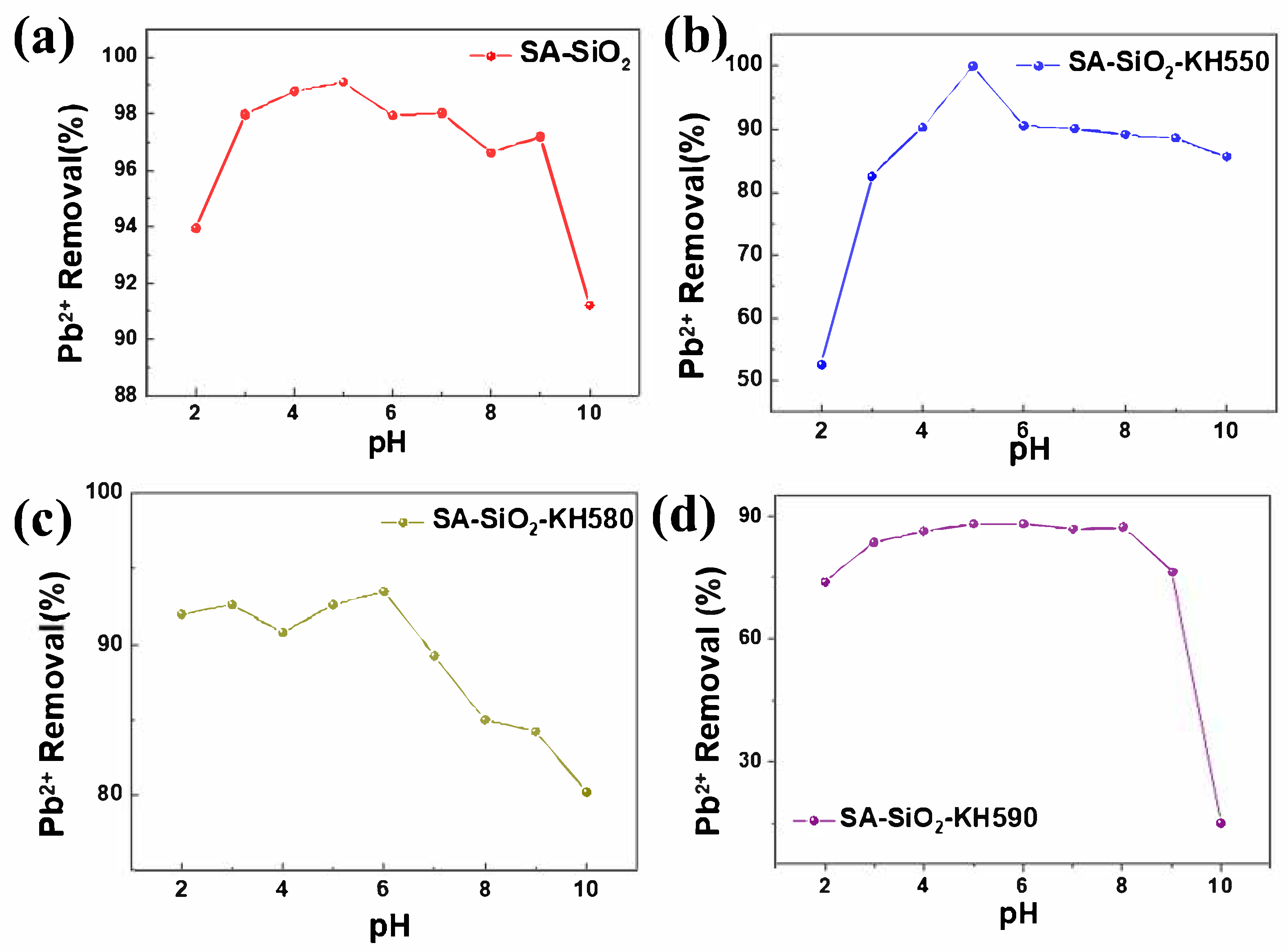
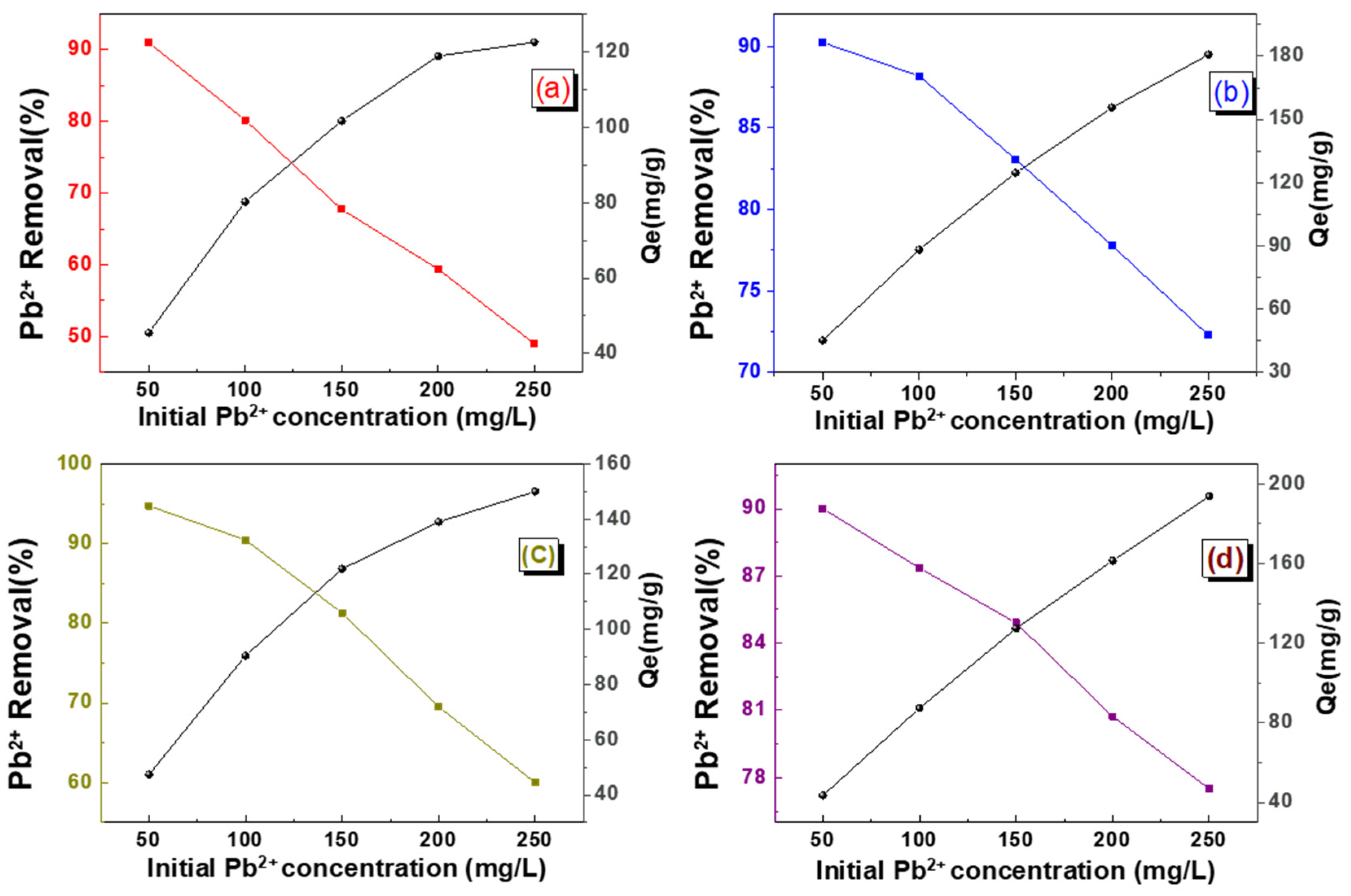
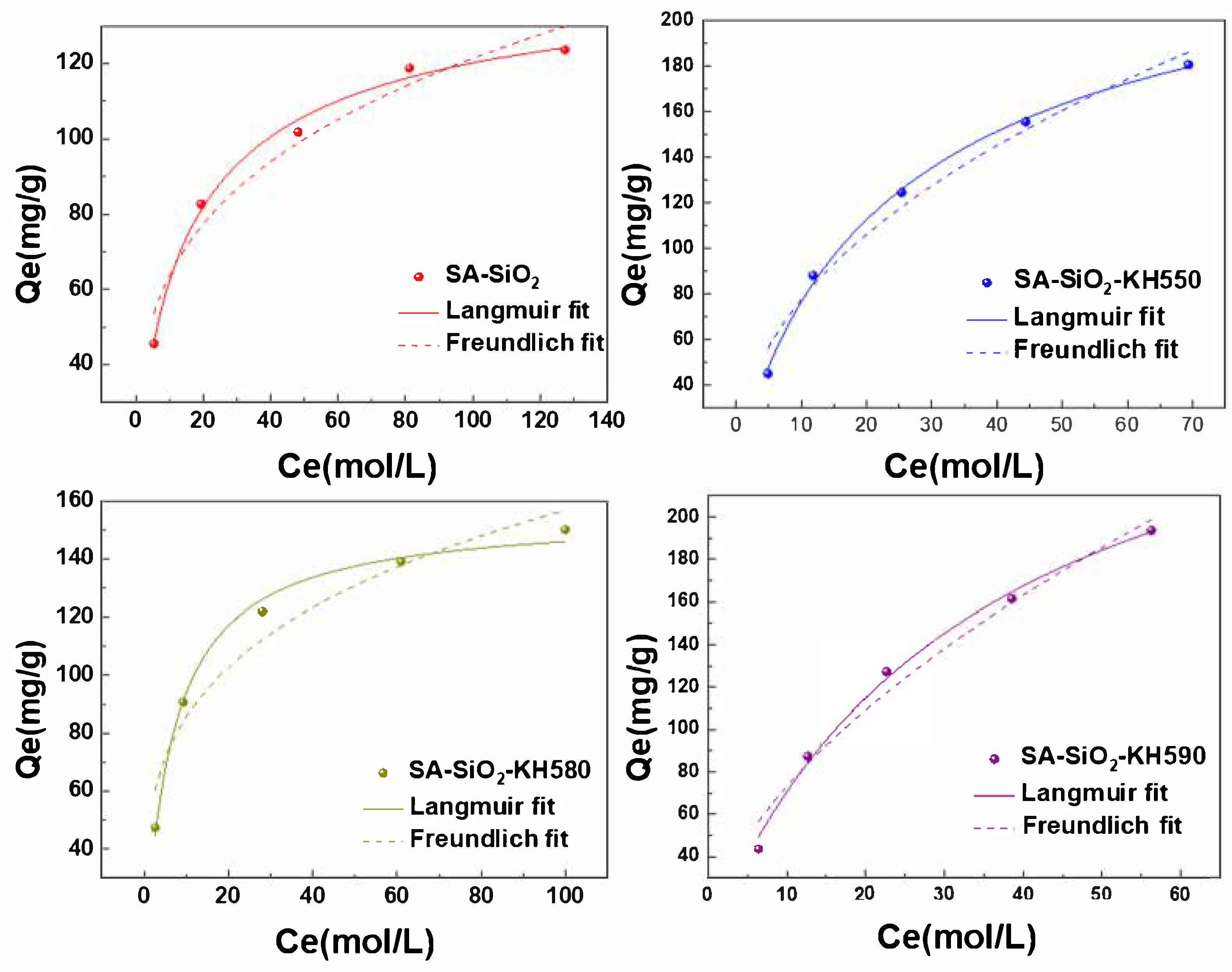
2.3. Adsorption Properties of Hybrid Aerogel Beads
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Alginate/Silica Binary Aerogel Beads (SA-SiO2)
4.3. Synthesis of Alginate/Silica Hybrid Aerogel Beads with Tunable Functional Surface
4.4. Standard Solution Curve
4.5. Material Characterizations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose-silica nanocomposite aerogels by in-situ formation of silica in cellulose gel. Angew. Chem. Int. Ed. 2012, 51, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement strategies for advanced applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate complexes and their hydrogels: A powerful toolkit for the development of next-generation sustainable functional materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shahid, M.A.; Hossain, M.T.; Sheikh, M.S.; Rahman, M.S.; Uddin, N.; Rahim, A.; Khan, R.A.; Hossain, I. Sources, extractions, and applications of alginate: A review. Discover Appl. Sci. 2024, 6, 443. [Google Scholar] [CrossRef]
- Yu, R.; Shi, Y.; Yang, D.; Liu, Y.; Qu, J.; Yu, Z.Z. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants. ACS Appl. Mater. Interfaces 2017, 9, 21809–21819. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, X. Surfactant-free synthesis of silica aerogel microspheres with hierarchically porous structure. J. Collid Interface Sci. 2018, 515, 1–9. [Google Scholar] [CrossRef]
- Zong, S.; Wei, W.; Jiang, Z.; Yan, Z.; Zhu, J.; Xie, J. Characterization and comparison of uniform hydrophilic/hydrophobic transparent silica aerogel beads: Skeleton strength and surface modification. RSC Adv. 2015, 5, 55579–55587. [Google Scholar] [CrossRef]
- Wei, W.; Hu, H.; Yin, S.; Li, Y.; Ji, X.; Xie, J. Rational fabrication of chitosan/alginate/silica ternary aerogel beads adsorbent with free separation. Micro Nano Lett. 2019, 14, 142–145. [Google Scholar] [CrossRef]
- Han, X.B.; Li, R.; Miao, P.P.; Gao, J.; Hu, G.W.; Zhao, Y.; Chen, T. Design, synthesis and adsorption evaluation of bio-based lignin/chitosan beads for congo red removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef] [PubMed]
- Boccia, A.C.; Neagu, M.; Pulvirenti, A. Bio-based aerogels for the removal of heavy metal ions and Oils from Water: Novel solutions for environmental remediation. Gels 2024, 10, 32. [Google Scholar] [CrossRef]
- Flores-Gómez, J.; Romero-Arellano, V.H.; Vazquez-Lepe, M.; Martínez-Gómez, Á.d.J.; Morales-Rivera, J. Modeling and optimization of the adsorption of Cr (VI) in a chitosan-resole aerogel using response surface methodology. Gels 2023, 9, 197. [Google Scholar] [CrossRef]
- Xia, S.; Cui, C.; Li, D.; Wang, L.J. Preparation of sodium alginate/quaternary ammonium-functionalized chitosan adsorbents: For the removal of high-molecular-weight invert sugar alkaline degradation products. Int. J Biol. Macromol. 2025, 303, 140550. [Google Scholar] [CrossRef]
- Elsayed, I.; Schueneman, G.T.; El-Giar, E.M.; Hassan, E.B. Amino-functionalized cellulose nanofiber/lignosulfonate new aerogel adsorbent for the removal of dyes and heavy Metals from Wastewater. Gels 2023, 9, 154. [Google Scholar] [CrossRef]
- Iskandar, M.A.; Yahya, E.B.; Abdul Khalil, H.P.S.; Rahman, A.A.; Ismail, M.A. Recent Progress in modification strategies of nanocellulose-based aerogels for oil absorption application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef]
- Sadat Fazel, S.; Jonoobi, M.; Pourtahmasi, K.; Sepahvand, S.; Ashori, A. Enhancing the oil adsorption properties of cellulose nanofiber aerogels through chemical modification. J. Polym. Environ. 2024, 32, 1304–1313. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, T.; Xiao, S.; He, H.; Yang, J.; Qin, Z. Preparation and adsorption performance of functionalization cellulose-based composite aerogel. Int. J Biol. Macromol. 2022, 211, 1–14. [Google Scholar] [CrossRef]
- Hou, Y.; Zhong, X.; Ding, Y.; Zhang, S.; Shi, F.; Hu, J. Alginate-based aerogels with double catalytic activity sites and high mechanical strength. Carbohydr. Polym. 2020, 245, 116490. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M.; Yin, H.; Liu, W. Separation and purification of polyprenols from Ginkgo biloba leaves by silver ion anchored on imidazole-based ionic liquid functionalized mesoporous MCM-41 sorbent. Food Chem. 2024, 450, 139284. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Basu, H.; Bassan, M.K.T.; Singhal, R.K. Thiol functionalised silica microsphere loaded polymeric hydrogel: Development of a novel hybrid sorbent for removal of lead and cadmium. Chemosphere 2022, 286, 131659. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Tang, M.; Ma, H.; Gui, Y.; Tian, X.; Quan, F.; Song, X.; Xia, Y. Alginate-based hierarchical porous carbon aerogel for high-performance supercapacitors. J. Alloys Compd. 2018, 749, 517–522. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel based on an alginate–Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Tanimu, A.; Ganiyu, S.A.; Kozhevnikov, I.; Alhooshani, K. In-situ activation of NiMo catalyst based on support surface-bound thiols: A green approach to catalyst sulfidation and improved activity. Arab. J. Chem. 2021, 14, 103030. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, T.; Ni, X.; Xia, J.; Suo, H.; Yan, L.; Zou, B. Immobilized lipase based on SBA-15 adsorption and gel embedding for catalytic synthesis of isoamyl acetate. Food Biosci. 2024, 60, 104427. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Bao, Q.; Zheng, J.; Deng, D.; Duan, Y.; Shen, L. The physicochemical characterization, equilibrium, and kinetics of heavy metal ions adsorption from aqueous solution by arrowhead plant (Sagittaria trifolia L.) stalk. J. Food Biochem. 2018, 42, e12448. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Hou, B.; Hao, C.; Li, X.; Wu, J. Construction of a lignosulfonate–lysine hydrogel for the adsorption of heavy metal ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef]
- Fan, X.; Peng, L.; Wang, X.; Han, S.; Yang, L.; Wang, H.; Hao, C. Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Ind. Crop. Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Han, S.; Xie, H.; Zhang, L.; Wang, X.; Zhong, Y.; Shen, Y.; Wang, H.; Hao, C. High-performance polyethylenimine-functionalized lignin/silica porous composite microsphere for the removal of hexavalent chromium, phosphate and Congo red from aqueous solutions. Ind. Crop. Prod. 2023, 194, 116289. [Google Scholar] [CrossRef]
- Shao, Z.; Xing, C.; Xue, M.; Fang, Y.; Li, P. Selective removal of Pb (II) from yellow rice wine using magnetic carbon-based adsorbent. J. Sci. Food Agric. 2023, 103, 6929–6939. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhai, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. A simple and sensitive electrochemical sensing based on amine-functionalized metal–organic framework and polypyrrole composite for detection of lead ions in meat samples. J. Food Meas. Charact. 2024, 18, 5813–5825. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.; Zhang, T.; Chen, Q.; Qiu, F.; Peng, Y.; Tang, S. Fabrication of functional biomass carbon aerogels derived from sisal fibers for application in selenium extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W. Sponge effect of aerated concrete on phosphorus adsorption-desorption from agricultural drainage water in rainfall. Soil Water Res. 2020, 15, 220–227. [Google Scholar] [CrossRef]
- Buema, G.; Segneanu, A.-E.; Herea, D.-D.; Grozescu, I. Gels for water remediation: Current research and perspectives. Gels 2024, 10, 585. [Google Scholar] [CrossRef]
- Tordi, P.; Gelli, R.; Tamantini, S.; Bonini, M. Alginate crosslinking beyond calcium: Unlocking the potential of a range of divalent cations for fiber formation. Int. J. Biol. Macromol. 2025, 306, 141196. [Google Scholar] [CrossRef]
- Choi, I.; Lee, Y.; Lyu, J.S.; Lee, J.S.; Han, J. Characterization of ionically crosslinked alginate films: Effect of different anion-based metal cations on the improvement of water-resistant properties. Food Hydrocoll. 2022, 131, 107785. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]


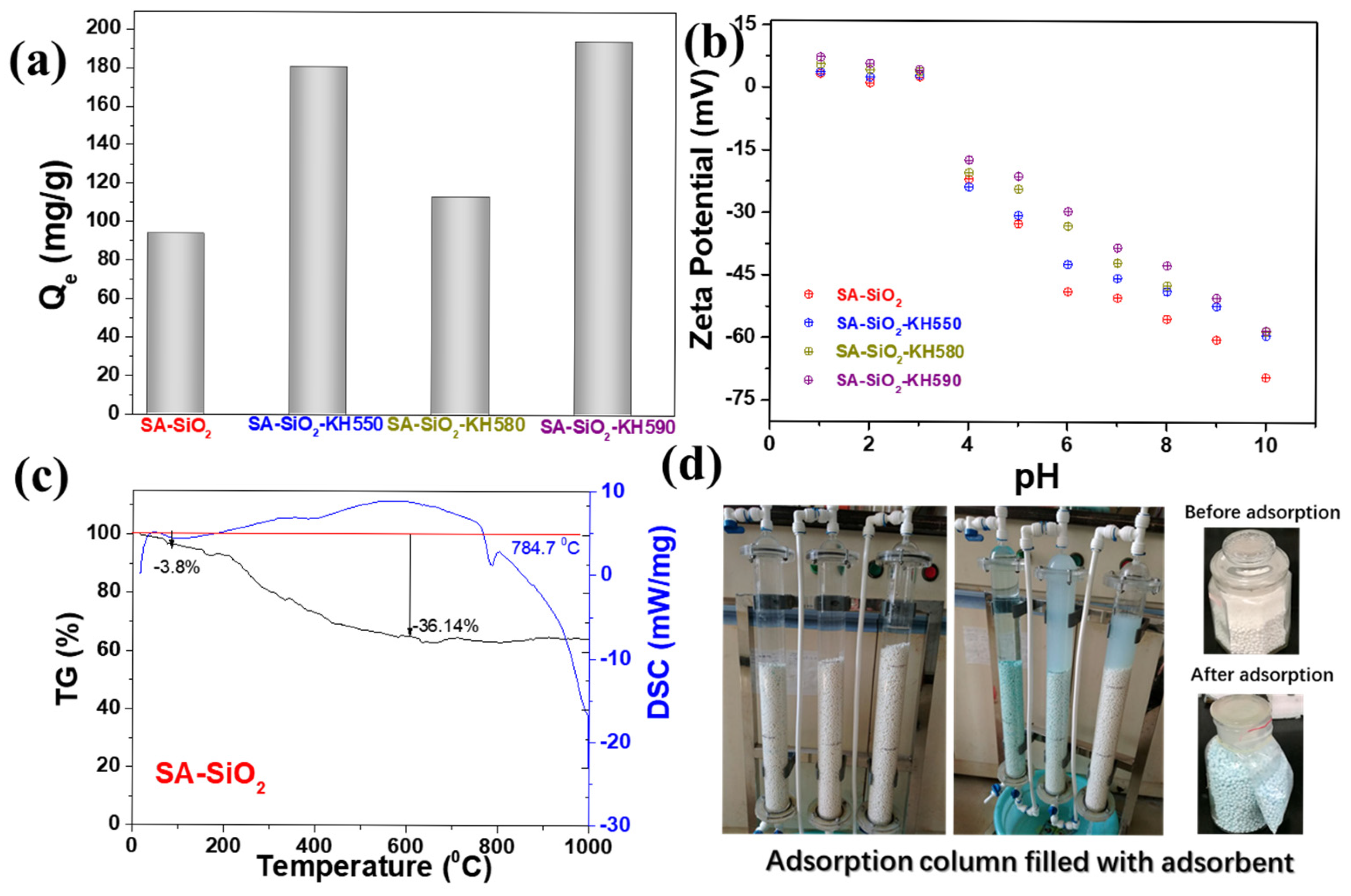
| Physical Property | SA-SiO2 | SA-SiO2-C | SA-SiO2-O | SA-SiO2-KH550 | SA-SiO2-KH580 | SA-SiO2-KH590 |
|---|---|---|---|---|---|---|
| Packing density (g/cm3) | 0.160 | 0.155 | 0.148 | 0.177 | 0.171 | 0.174 |
| Porosity (%) | 91.67 | 92.67 | 92.44 | 90.67 | 90.64 | 89.94 |
| Oil absorption (mL/g) | 3.45 | 2.95 | 3.05 | 2.88 | 2.81 | 2.87 |
| Water absorption (mL/g) | 2.88 | 2.80 | 2.78 | 2.63 | 2.59 | 2.79 |
| Shape and appearance | Spheroid Yellow | Spheroid Black | Spheroid Milky white | Spheroid Milky white | Spheroid Milky white | Spheroid Milky white |
| BET surface area (m2/g) | 160.8 | 210.8 | 154.4 | 125.2 | 157.1 | 157.2 |
| Pore volume (cm3/g) | 0.54 | 0.75 | 0.95 | 0.36 | 0.51 | 0.64 |
| Average pore diameter (nm) | 6.6 | 4.3 | 3.4 | 3.1 | 3.4 | 3.4 |
| Adsorption Isotherm Model | Constant | SA-SiO2 | SA-SiO2-KH550 | SA-SiO2-KH580 | SA-SiO2-KH590 |
|---|---|---|---|---|---|
| Langmuir equation | R | 0.992 | 0.996 | 0.991 | 0.994 |
| Qmax (mg·g−1) | 132.7 | 229.6 | 155.5 | 208.3 | |
| KL(L·mg−1) | 0.087 | 0.049 | 0.152 | 0.029 | |
| Freundlich equation | R | 0.945 | 0.974 | 0.932 | 0.971 |
| KF (mg·g−1) | 33.38 | 27.45 | 46.71 | 19.32 | |
| 1/n | 0.281 | 0.452 | 0.263 | 0.578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Yang, S.; Zhu, Z.; Lu, J.; Zhang, B.; Zhang, M.; Wei, W. Low-Temperature Dried Alginate/Silica Hybrid Aerogel Beads with Tunable Surface Functionalities for Removal of Lead Ions from Water. Gels 2025, 11, 397. https://doi.org/10.3390/gels11060397
Wei J, Yang S, Zhu Z, Lu J, Zhang B, Zhang M, Wei W. Low-Temperature Dried Alginate/Silica Hybrid Aerogel Beads with Tunable Surface Functionalities for Removal of Lead Ions from Water. Gels. 2025; 11(6):397. https://doi.org/10.3390/gels11060397
Chicago/Turabian StyleWei, Jiuqi, Shilong Yang, Zhicheng Zhu, Jialu Lu, Bencong Zhang, Mingmei Zhang, and Wei Wei. 2025. "Low-Temperature Dried Alginate/Silica Hybrid Aerogel Beads with Tunable Surface Functionalities for Removal of Lead Ions from Water" Gels 11, no. 6: 397. https://doi.org/10.3390/gels11060397
APA StyleWei, J., Yang, S., Zhu, Z., Lu, J., Zhang, B., Zhang, M., & Wei, W. (2025). Low-Temperature Dried Alginate/Silica Hybrid Aerogel Beads with Tunable Surface Functionalities for Removal of Lead Ions from Water. Gels, 11(6), 397. https://doi.org/10.3390/gels11060397







