In Situ Gel-Forming System for the Removal of Ferruginous Deposits on Nanhai I Shipwreck
Abstract
1. Introduction
2. Results and Discussion
2.1. Cleaning Solution Characterisation
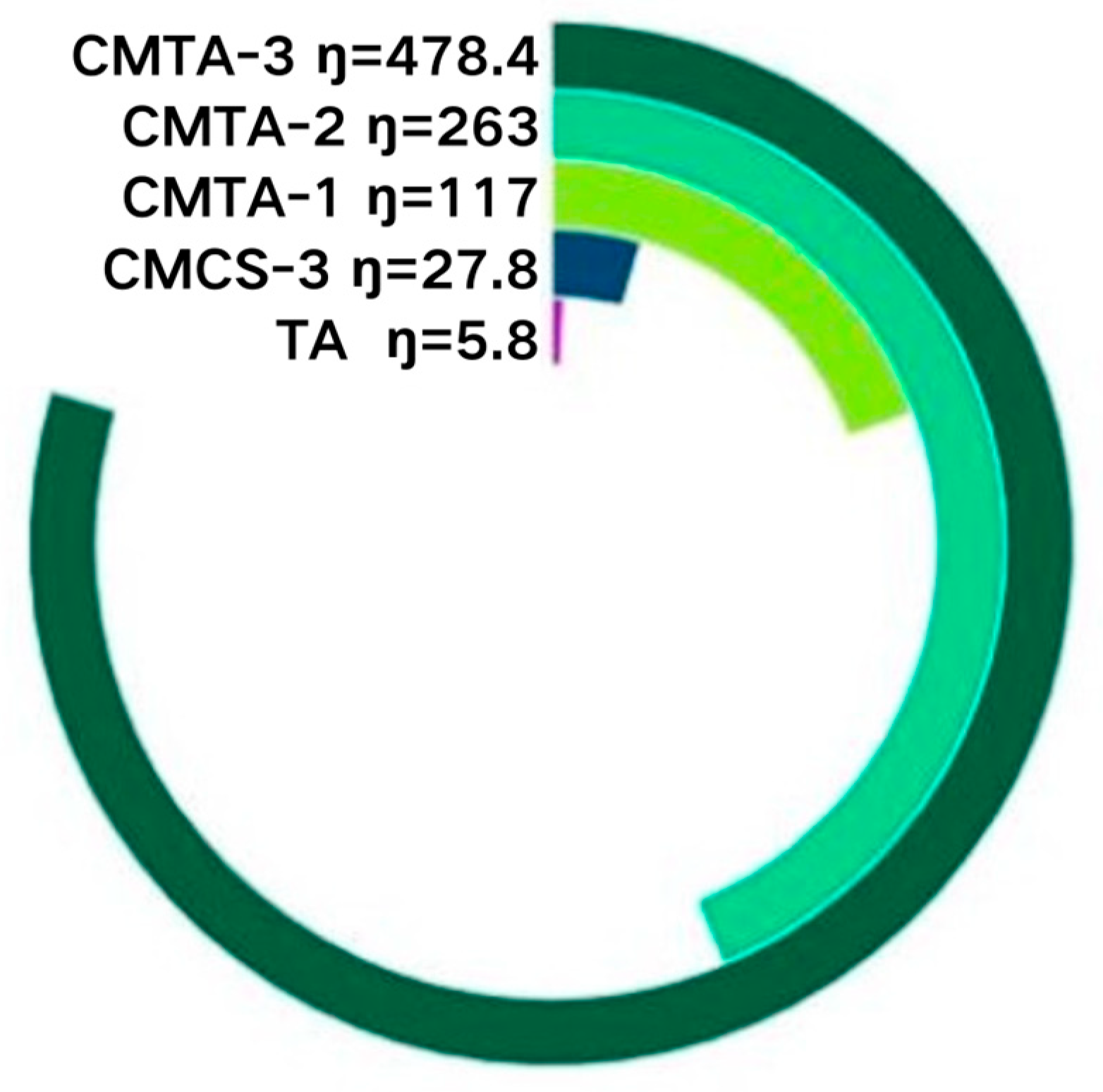
2.1.1. Viscosity Characterisation
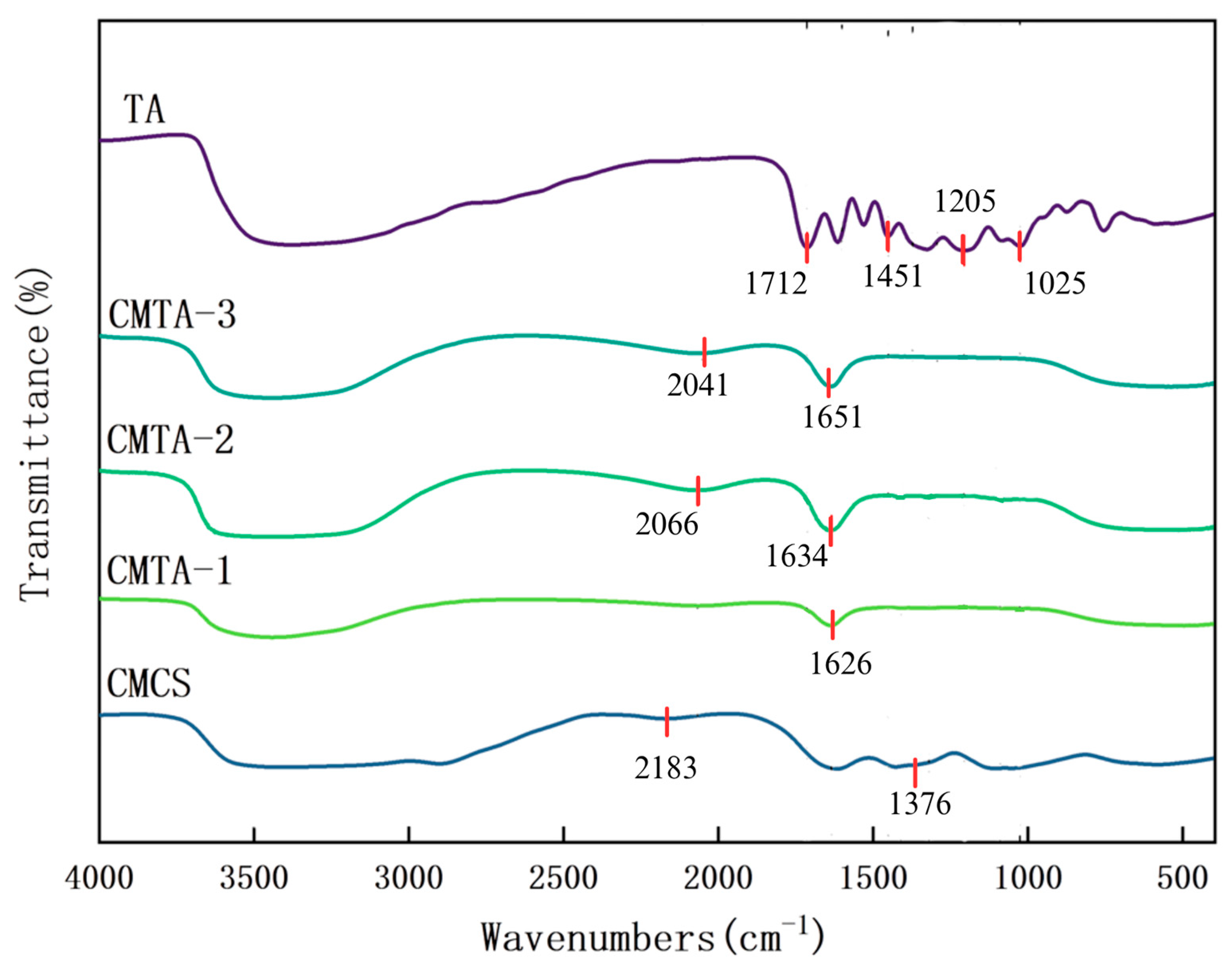
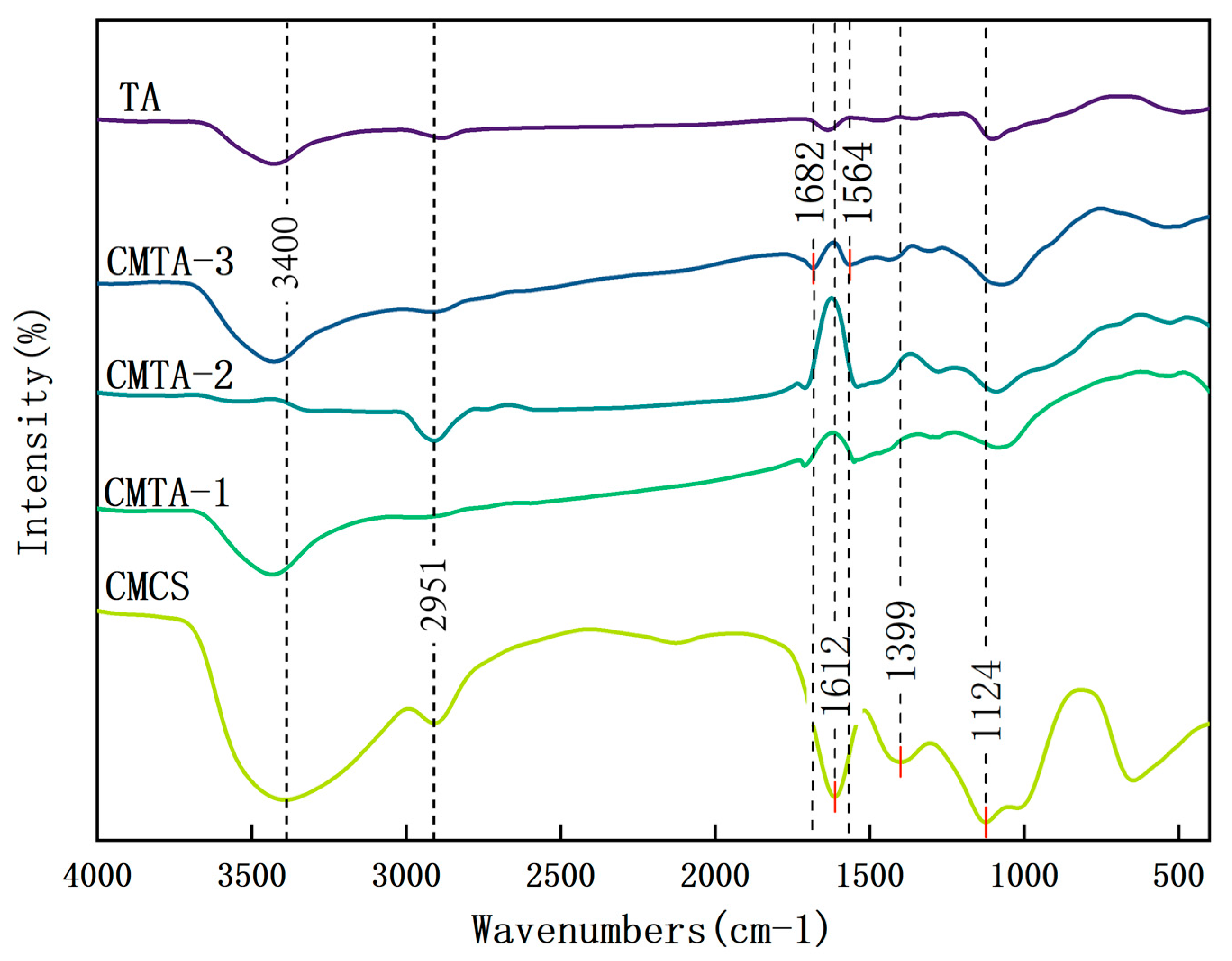
2.1.2. FT-IR Characterisation
2.2. Application Effects Characterisation
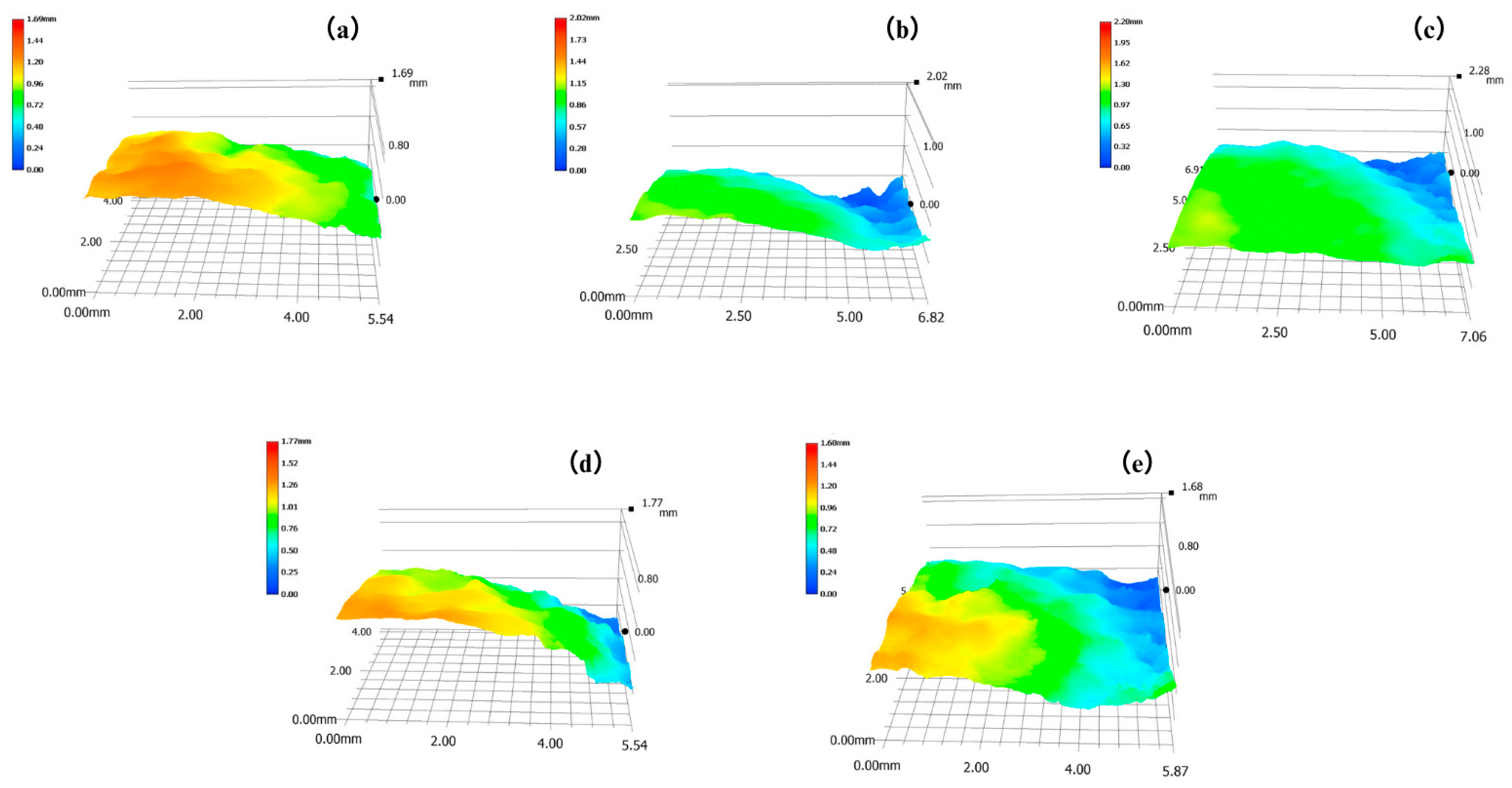
2.2.1. Three-Dimensional Super Depth-of-Field Microscope (3D-SDF)
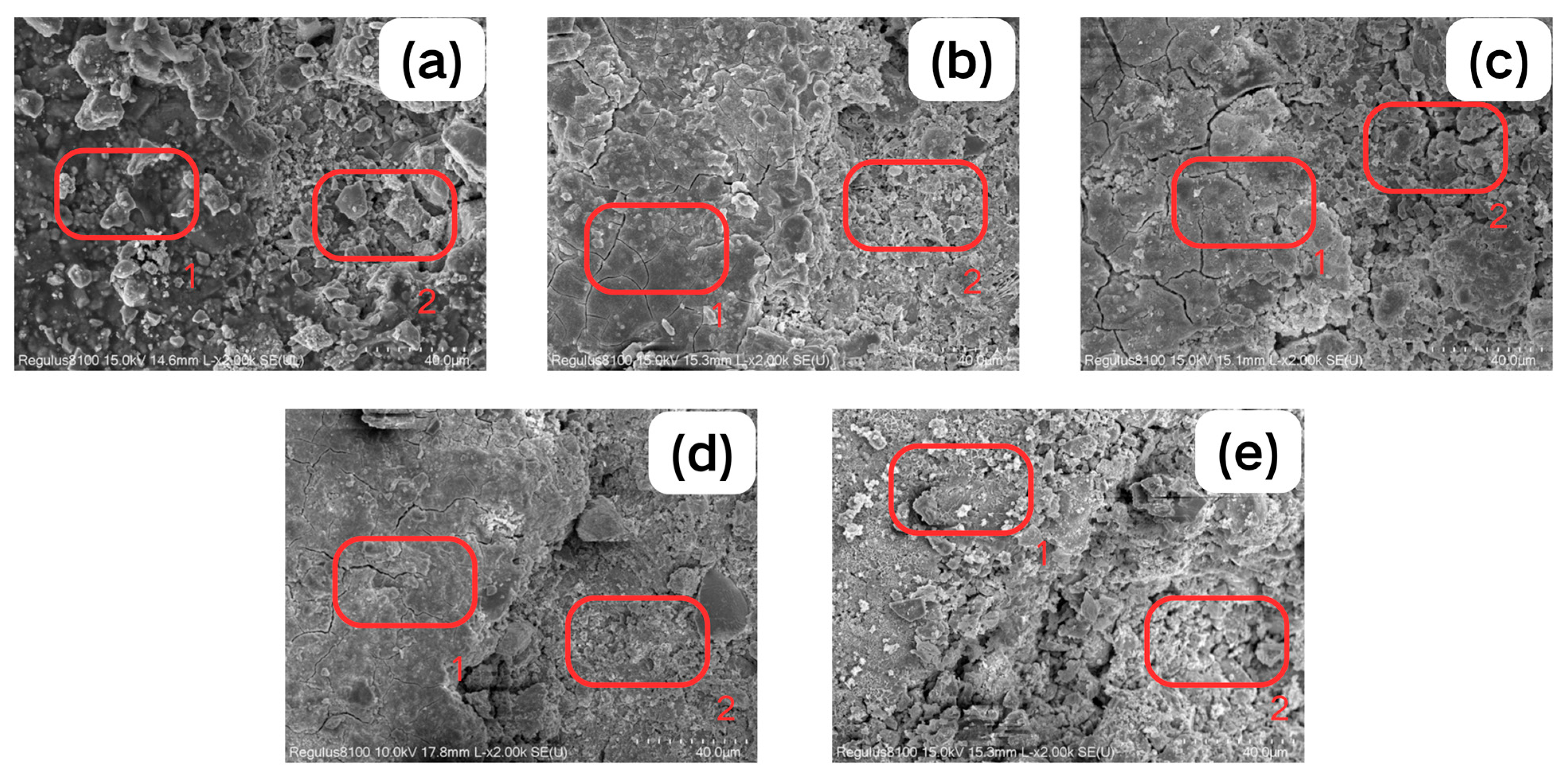
2.2.2. SEM-EDS
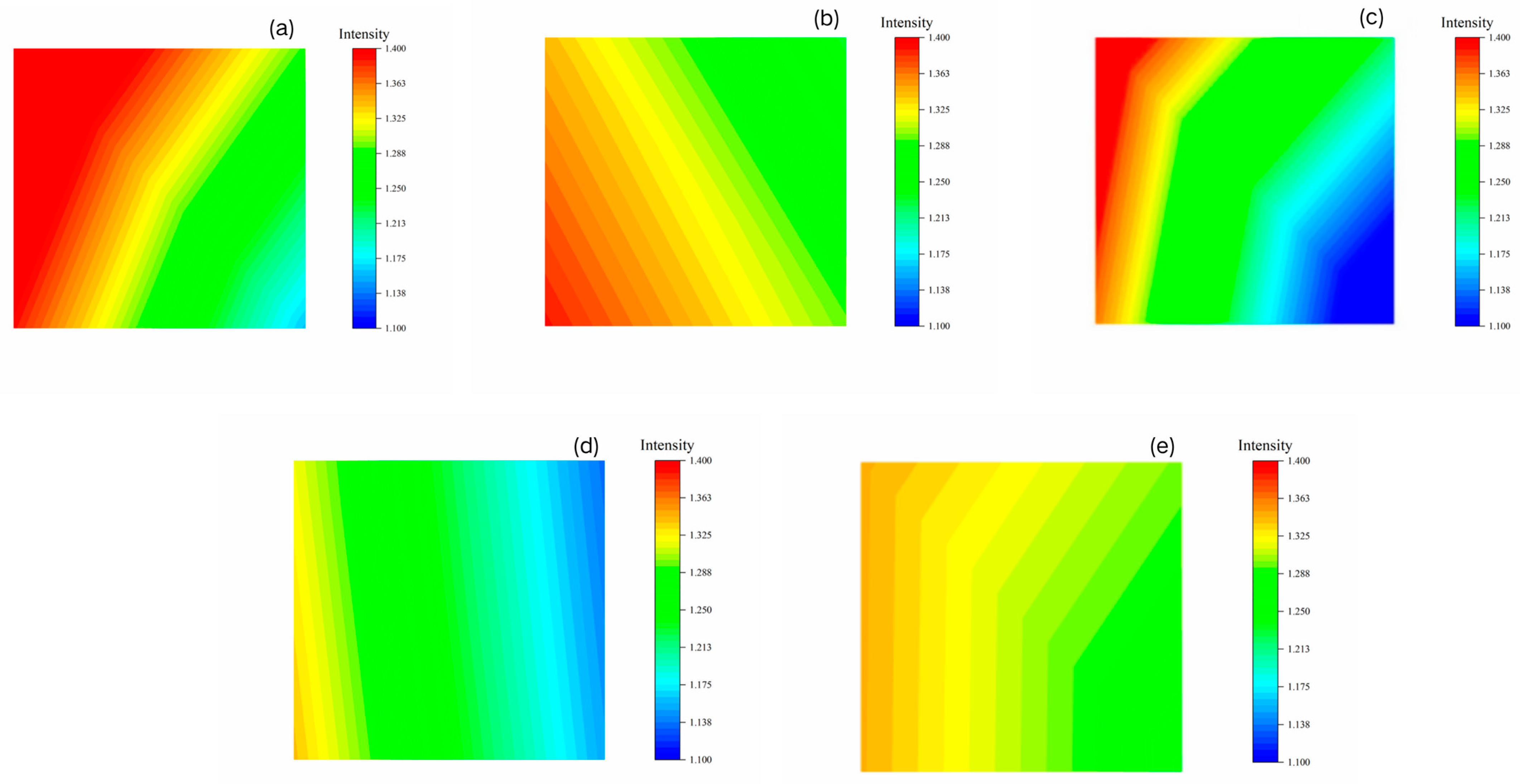
2.2.3. Mapping
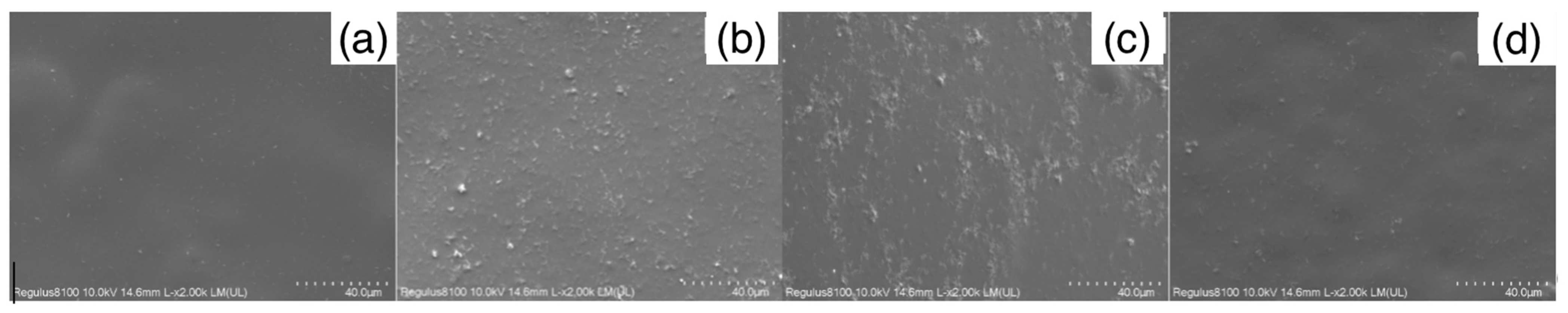
2.2.4. SEM
2.2.5. FT-IR
2.2.6. FTIR Mapping
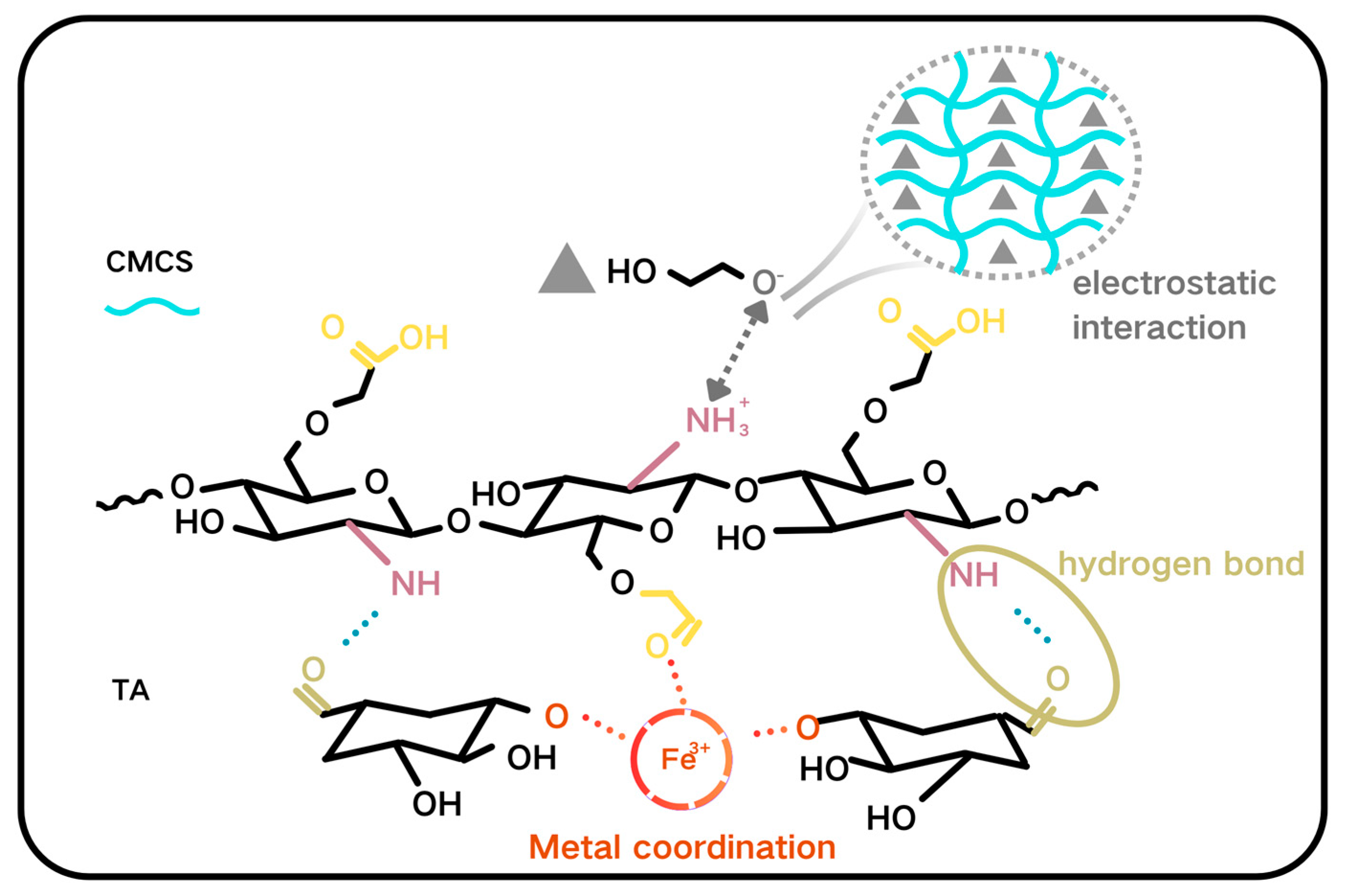
2.3. Cleaning Mechanism
2.4. Field Application Experiment
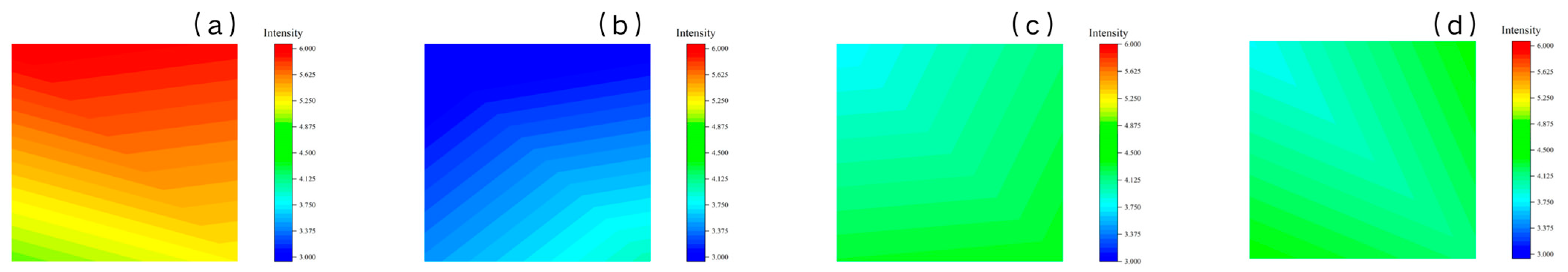
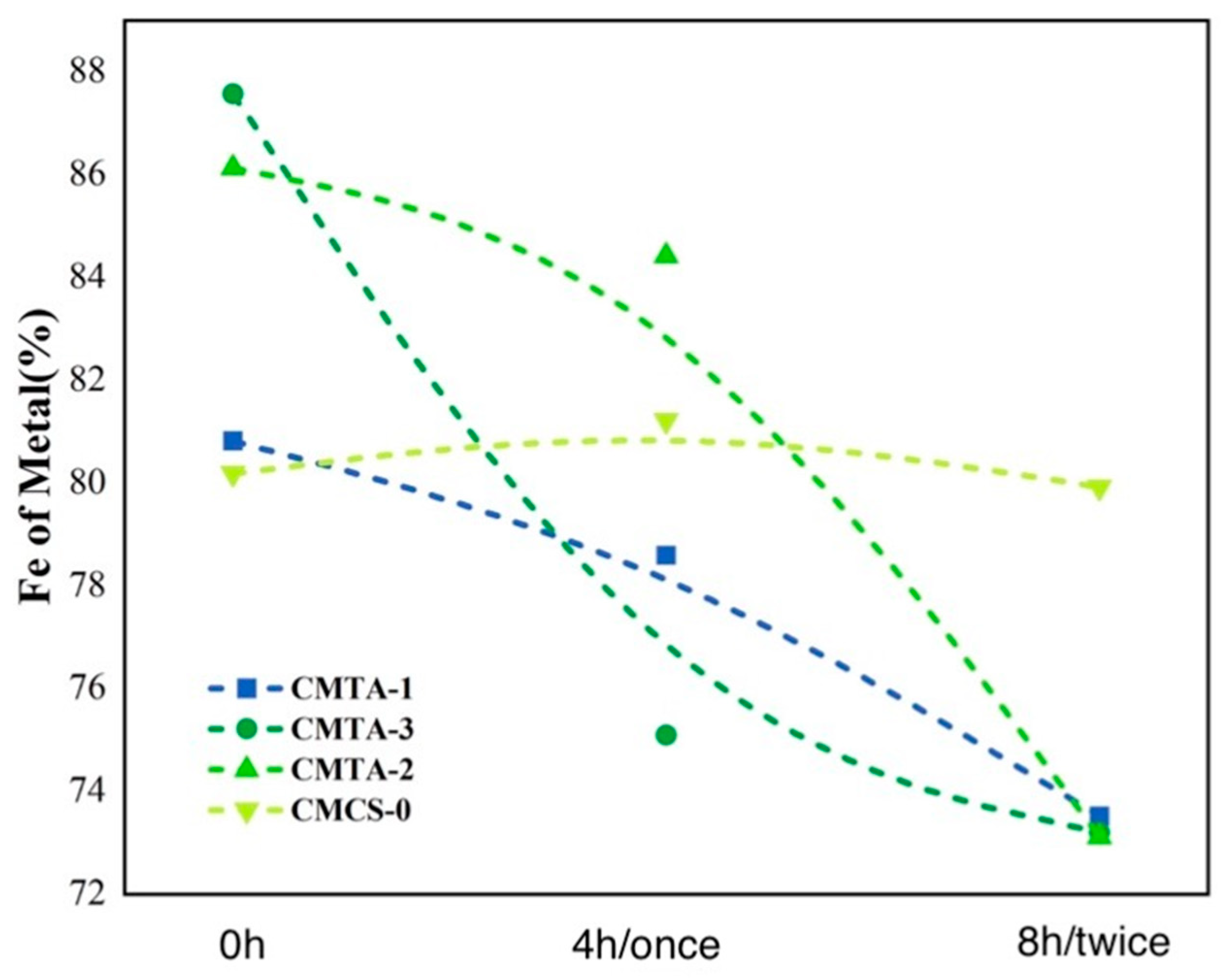
2.4.1. XRF
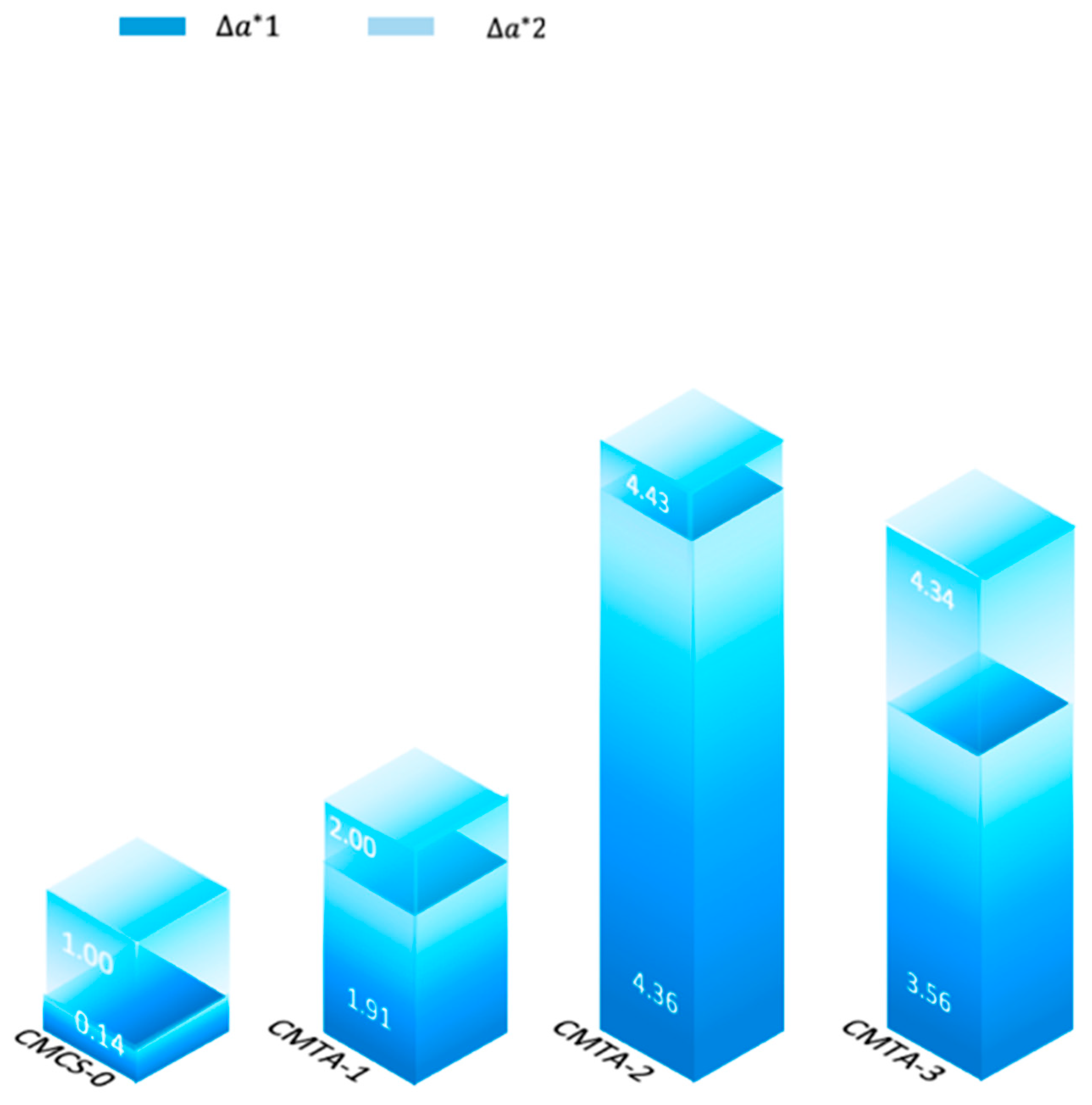
2.4.2. Colour Measurement
3. Conclusions
4. Materials and Methods
4.1. Materials
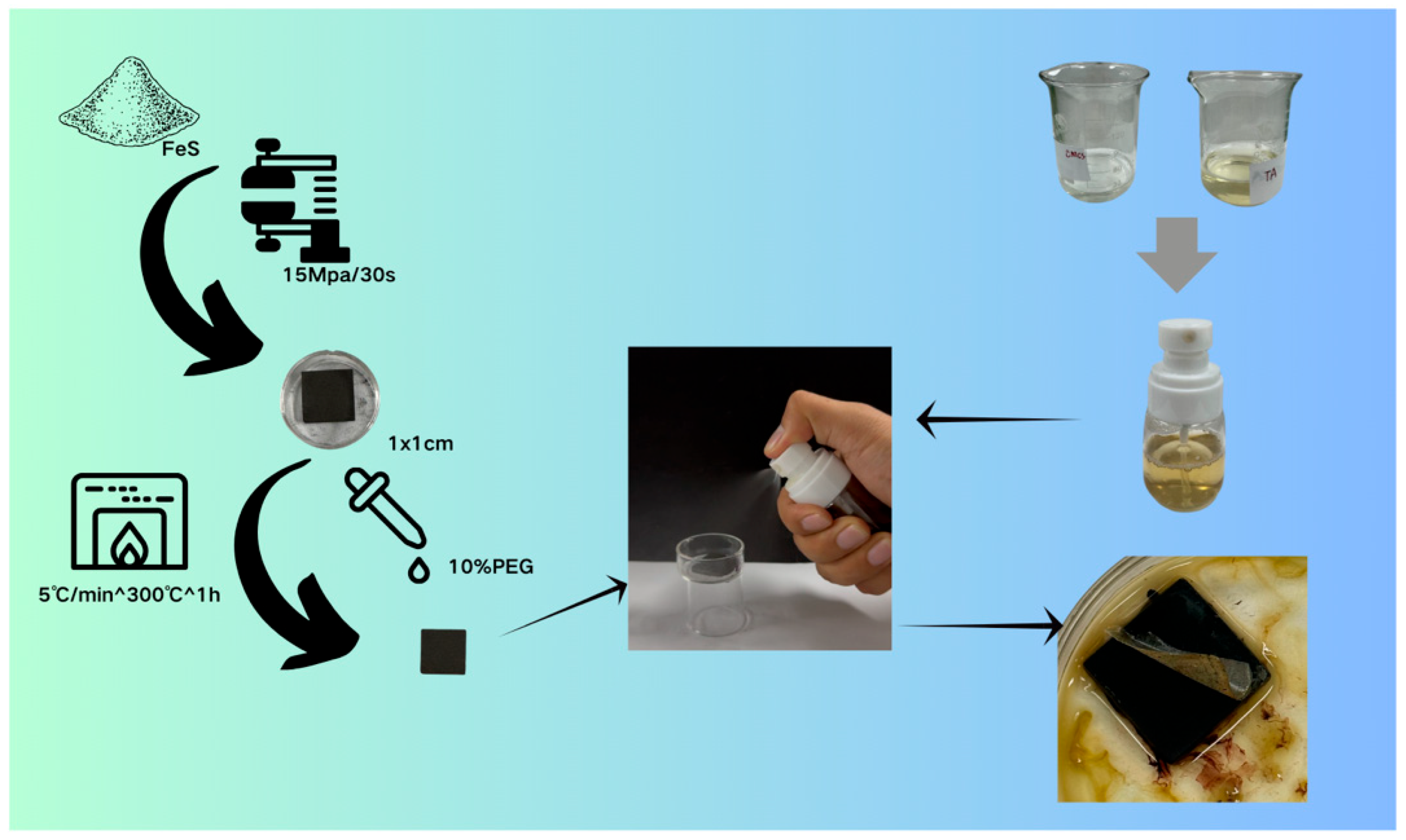
4.1.1. Sample Preparation
4.1.2. Preparation of the Gel Cleaning Solution
4.2. Field Applications for Heritage Conservation
4.3. Characterisation Methods
4.3.1. Viscosity
4.3.2. Fourier Transform Infrared Spectrometer (FTIR)
4.3.3. Three-Dimensional Super Depth-of-Field Microscope
4.3.4. Scanning Electron Microscope (SEM)
4.3.5. X-Ray Fluorescence Spectroscopy
4.3.6. Spectrophotometric Colourimeter
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Du, J.; Huang, X.; Ma, K.; Wang, Y.; Guo, P.; Li, N.; Zhang, Z.; Pan, J. Chemical Properties and Microbial Analysis of Waterlogged Archaeological Wood from the Nanhai No. 1 Shipwreck. Forests 2021, 12, 587. [Google Scholar] [CrossRef]
- Kangfan, W.; Tianshe, Z. The Underwater Archaeological Career Progress of Research in China Showed by “Nanhai I”. In Proceedings of the 2nd International Conference on Contemporary Education, Social Sciences and Ecological Studies (CESSES 2019), Moscow, Russia, 5–6 June 2019. [Google Scholar] [CrossRef]
- Peining, L. The trade patterns of the South China Sea during the Song period. Asian Archaeol. 2020, 3, 83–93. [Google Scholar] [CrossRef]
- Jiao, P.; Yeqing, H.; Cen, W.; Jing, D.; Yu, W.; Yue, C.; Xinduo, H.; Kaixuan, M.; Zhiguo, Z.; Naisheng, L. Analysis of microbial community and biodeterioration of maritime cultural relics (ironware, porcelain, axes, hull wood) from the Nanhai No. 1 shipwreck. Ann. Microbiol. 2023, 73, 8. [Google Scholar] [CrossRef]
- Ke-jia, H.; Jing, D.; Jian, Z.; Nai-sheng, L.; Yue, C.; Yuan-yuan, W. Mapping Analysis by mu-X-Ray Fluorescence for Waterlogged Archaeological Wood From” Nanhai No. 1” Shipwreck. Spectrosc. Spectr. Anal. 2021, 41, 2930–2933. [Google Scholar]
- Hongying, Z.; Dawa, S.; Zhiguo, Z.; Qinglin, M. Characterization of degradation and iron deposits of the wood of Nanhai I shipwreck. Herit. Sci. 2022, 10, 202. [Google Scholar] [CrossRef]
- Mathilde, M.; Magdalena, A.-B.; Charlène, P.; Emilie, C.; Elodie, G.; Céline, R.; Edith, J. Characterization of model samples simulating degradation processes induced by iron and sulfur species on waterlogged wood. Microchem. J. 2020, 155, 104756. [Google Scholar] [CrossRef]
- Magdalena, B.; Callum, A.S.H. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Aslı Gökçe, K.; Namık, K.; Donna, C.A. Analyses of Sulfur and Iron in Waterlogged Archaeological Wood: The Case of Polyethylene-Glycol-Treated Yenikapı 12 Shipwreck. Forests 2023, 14, 530. [Google Scholar] [CrossRef]
- Shen, D. Iron Sulfide of Marine Archaeological Wood; China Science Publishing: Beijing, China, 2020. [Google Scholar]
- Liu, Z.; Fu, T.; Hu, C.; Shen, D.; Macchioni, N.; Sozzi, L.; Chen, Y.; Liu, J.; Tian, X.; Ge, Q. Microbial community analysis and biodeterioration of waterlogged archaeological wood from the Nanhai No. 1 shipwreck during storage. Sci. Rep. 2018, 8, 7170. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of Fe (II) sulphides in waterlogged archaeological wood. Stud. Conserv. 2013, 58, 297–307. [Google Scholar] [CrossRef]
- Xunming, G.; Jian, Z.; Jiahui, L.; Lihua, F.; Dong, Z. How do water and acid in marine archaeological wood affect its mechanical properties? J. Cult. Herit. 2024, 70, 431–440. [Google Scholar] [CrossRef]
- Caire, J.; Bouh, A.; Guilminot, E. Numerical modelling of electrophoresis applied to restoration of archaeological organic materials. In Proceedings of the COMSOL Conference, Hannover, Germany, 4–6 November 2008; pp. 4–6. [Google Scholar]
- Monachon, M.; Pelé-Meziani, C.; Ganesan, S.; De Weck, S.; Moll-Dau, F.; Schramm, J.; Schmidt-Ott, K.; Joseph, E. Assessing the versatility of bioextraction to preserve waterlogged wood. Forests 2023, 14, 1656. [Google Scholar] [CrossRef]
- Yishu, W.; Zijun, Z.; Jianqun, L.; Qinglin, M.; Linxu, C. A new bio-oxidation method for removing iron deposits from waterlogged wood of Nanhai I shipwreck, Guangdong, China. Eng. Microbiol. 2024, 4, 100107. [Google Scholar] [CrossRef]
- Céline, R.; Laure, M.; François, L.; Nicolas, P.; Egle, C.; Marine, C.; Philippe, R.; Loïc, C. Post-treatment Study of Iron/Sulfur-containing Compounds in the Wreck of Lyon Saint-Georges 4 (Second Century ACE). Stud. Conserv. 2019, 65, 28–36. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Extraction of iron compounds from wood from the Vasa. Holzforschung 2006, 60, 678–684. [Google Scholar] [CrossRef]
- Zhiguo, Z.; Naisheng, L.; Xingling, T.; Jie, L.; Dawa, S. Research on the removal of the iron sulfides in the Qing Dynasty marine shipwreck, Ningbo Xiaobaijiao No. 1. Sci. Conserv. Archaeol. 2014, 26, 30–38. [Google Scholar]
- Eric, S.; Robert, B.; Held, B.; Jurgens, J.; Cook, D.; Drews, M.; Hand, S.; Betty, S. An Evaluation of Supercritical Drying and PEG/Freeze Drying of Waterlogged Archaeological Wood; National Park Service, Harpers Ferry Center: Harpers Ferry, WV, USA, 2005. [Google Scholar]
- Lindfors, E.-L.; Lindström, M.; Iversen, T. Polysaccharide degradation in waterlogged oak wood from the ancient warship Vasa. Holzforschung 2008, 62, 57–63. [Google Scholar] [CrossRef]
- Pecoraro, E.; Pelé-Meziani, C.; Macchioni, N.; Lemoine, G.; Guilminot, E.; Shen, D.; Pizzo, B. The removal of iron from waterlogged archaeological wood: Efficacy and effects on the room temperature wood properties. Wood Mater. Sci. Eng. 2023, 18, 672–689. [Google Scholar] [CrossRef]
- Rapti, S.; Rivers, S.; Pournou, A. Removing iron corrosion products from museum artefacts: Investigating the effectiveness of innovative green chelators. In Proceedings of the SCinTE 2015, Jerusalem, Israel, 27 July 2015. [Google Scholar]
- Pecoraro, E.; Pelé-Meziani, C.; Macchioni, N.; Lemoine, G.; Guilminot, E.; Pizzo, B. Effects of iron removal treatments on the chemical and viscoelastic properties of waterlogged wood. J. Cult. Herit. 2022, 56, 149–158. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, X.; Yang, Z.; Zhou, Y.; Zhang, X.; Wang, Y. Self-shaping microemulsion gels for cultural relic cleaning. Langmuir 2021, 37, 11474–11483. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N, O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef]
- Liefeng, H.; Panpan, Z.; Xin, W.; Xu, C.; Jiejie, Q.; Rupei, T. pH-sensitive carboxymethyl chitosan hydrogels via acid-labile ortho ester linkage for potential biomedical applications. Carbohydr. Polym. 2017, 178, 166–179. [Google Scholar] [CrossRef]
- Andrew, B.; Brian, W.B. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- George, K.B.L.; Herbert, M.S.; Marcelo, H.-L. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Harmon, B.A.; Ahmad, B.R.; Brushmiller, J.G. Photochemical and spectroscopic studies of complexes, of iron(III) with citric acid and other carboxylic acids. Inorganica Chim. Acta 1994, 226, 117–127. [Google Scholar] [CrossRef]
- Tang, L.; Gong, J.; Li, J.; Fang, S.; Wang, Y.; Zhou, H. Synergistic effect between tannic acid-Fe3+ and chitosan hydrogel-coated covalent organic framework: Endowing better nanofiltration performance and stability. Sep. Purif. Technol. 2023, 323, 124469. [Google Scholar] [CrossRef]
- Üçer, A.; Uyanik, A.; Aygün, Ş. Adsorption of Cu (II), Cd (II), Zn (II), Mn (II) and Fe (III) ions by tannic acid immobilised activated carbon. Sep. Purif. Technol. 2006, 47, 113–118. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, C.; Tian, T.; Batur, S.; Lv, J.; Xie, Q.; Kong, L.; Yang, C.; Zhang, Z. In Situ Ultrafast Self-gelling Coacervate Powder with Antibacterial, Antioxidant, and Robust Wet Adhesion Properties for Hemostasis and Wound Healing. Adv. Funct. Mater. 2025. early view. [Google Scholar] [CrossRef]
- Wu, Z.-c.; Tao, T.-x.; Wang, X.-Q. The IR spectra of complexes of fiber containing amidoxime groups with Fe (III), Co (II), Ni (II), Cd (II) and Hg (II). Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2004, 24, 440–443. [Google Scholar] [PubMed]
- Lin, Q.-q.; Huang, Y.-x.; Zhang, Y.-h.; Rao, F.; Yu, W.-j. Measurement of surface roughness of Salix psammophila scrimber using stylus profilometer and extended depth-of-field 3D microscope. J. For. Eng. 2021, 6, 40–48. [Google Scholar] [CrossRef]
- Bhargava, R.; Wall, B.G.; Koenig, J.L. Comparison of the FT-IR Mapping and Imaging Techniques Applied to Polymeric Systems. Appl. Spectrosc. 2000, 54, 470–479. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Jin, S.-G.; Kim, I.-Y.; Pei, J.; Wen, M.; Jung, T.-Y.; Moon, K.-S.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly (ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf. B Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef]

| Thickness Removal (mm) | Roughness Change (mm) (ΔR = Before-After) | |
|---|---|---|
| CMCS | 0.49 | +0.02 |
| CMTA-1 | 1.02 | +0.15 |
| CMTA-2 | 1.01 | +0.37 |
| CMTA-3 | 0.82 | +0.17 |
| TA | 0.79 | −0.20 |
| Number/Mass (%) | C | O | Fe | S | Fe/S Atomic Ratio | ||
|---|---|---|---|---|---|---|---|
| CMCS | 1 | uncleaned | 4.00 | 1.97 | 17.31 | 9.41 | 1.84 |
| 2 | cleaned | 1.93 | 2.99 | 44.46 | 20.54 | 2.16 | |
| CMTA-1 | 1 | uncleaned | 8.08 | 7.22 | 17.23 | 2.02 | 8.53 |
| 2 | cleaned | 3.28 | 5.81 | 32.31 | 5.70 | 5.67 | |
| CMTA-2 | 1 | uncleaned | 6.37 | 6.77 | 19.00 | 3.46 | 5.49 |
| 2 | cleaned | 2.61 | 6.43 | 29.93 | 10.10 | 2.96 | |
| CMTA-3 | 1 | uncleaned | 7.31 | 6.28 | 8.67 | 1.68 | 5.16 |
| 2 | cleaned | 2.95 | 3.47 | 23.44 | 8.72 | 2.69 | |
| TA | 1 | uncleaned | 10.00 | 7.79 | 19.45 | 6.13 | 3.17 |
| 2 | cleaned | 4.87 | 7.55 | 34.26 | 12.78 | 2.68 | |
| CMCS | CMTA-1 | CMTA-2 | CMTA-3 | TA | |
|---|---|---|---|---|---|
| 1.100–1.175 | 0.4% | 0% | 19.99% | 17.13% | 0% |
| 1.175–1.325 | 48.19% | 56.44% | 60.11% | 79.86% | 71.79% |
| 1.325–1.400 | 51.41% | 43.56% | 19.90% | 3.01% | 28.21% |
| Sample | Uncleaning | Once | Twice | |
|---|---|---|---|---|
| CMCS-0 | L * | 31.11 | 30.95 | 30.09 |
| a * | 7 | 7.14 | 6 | |
| b * | 14.68 | 14.97 | 11.1 | |
| CMTA-1 | L * | 31.23 | 30.2 | 31.94 |
| a * | 6.8 | 4.89 | 4.8 | |
| b * | 14.1 | 11.04 | 10.71 | |
| CMTA-2 | L * | 30.11 | 30.98 | 32.19 |
| a * | 8.16 | 3.8 | 3.73 | |
| b * | 15.01 | 10.3 | 10.03 | |
| CMTA-3 | L * | 30.44 | 30.95 | 31.3 |
| a * | 8.51 | 4.95 | 4.17 | |
| b * | 15.39 | 11.51 | 11.03 |
| Code | CMCS/H2O (g/mL) | TA/H2O (g/mL) |
|---|---|---|
| CMCS | 0.2/10 | / |
| CMTA-1 | 0.2/10 | 0.05/5 |
| CMTA-2 | 0.4/10 | 0.05/5 |
| CMTA-3 | 0.6/10 | 0.05/5 |
| TA | / | 0.15/15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, J.; Wang, R.; Du, J.; Li, N.; Han, X. In Situ Gel-Forming System for the Removal of Ferruginous Deposits on Nanhai I Shipwreck. Gels 2025, 11, 543. https://doi.org/10.3390/gels11070543
Zha J, Wang R, Du J, Li N, Han X. In Situ Gel-Forming System for the Removal of Ferruginous Deposits on Nanhai I Shipwreck. Gels. 2025; 11(7):543. https://doi.org/10.3390/gels11070543
Chicago/Turabian StyleZha, Jianrui, Ruyi Wang, Jing Du, Naisheng Li, and Xiangna Han. 2025. "In Situ Gel-Forming System for the Removal of Ferruginous Deposits on Nanhai I Shipwreck" Gels 11, no. 7: 543. https://doi.org/10.3390/gels11070543
APA StyleZha, J., Wang, R., Du, J., Li, N., & Han, X. (2025). In Situ Gel-Forming System for the Removal of Ferruginous Deposits on Nanhai I Shipwreck. Gels, 11(7), 543. https://doi.org/10.3390/gels11070543






