Agar–Agar Gels Carrying Curative and Preventive Agents Against Helminths: An In Vitro Compatibility Evaluation
Abstract
1. Introduction
2. Results and Discussion
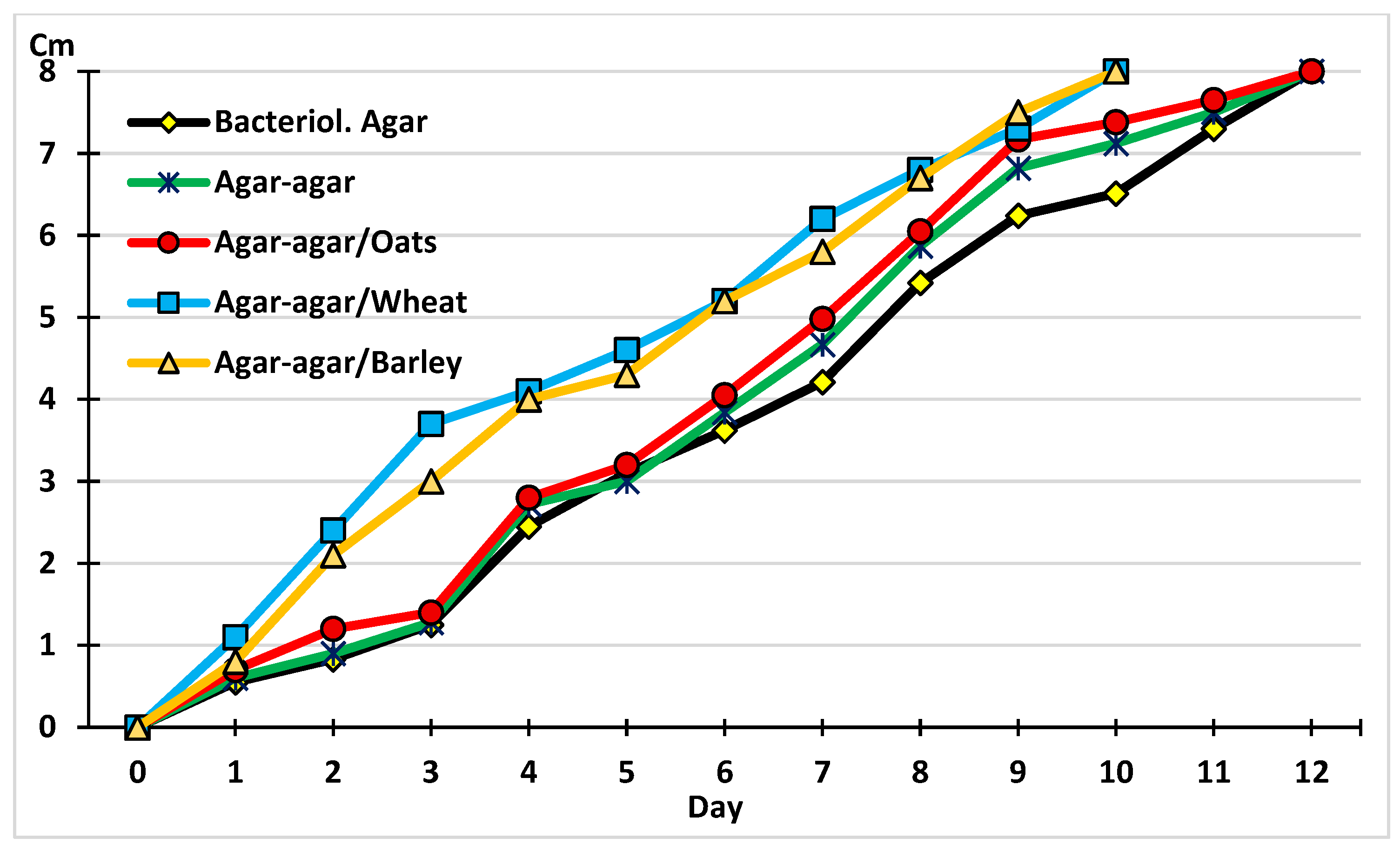
2.1. The Development of M. circinelloides in Different Culture Media
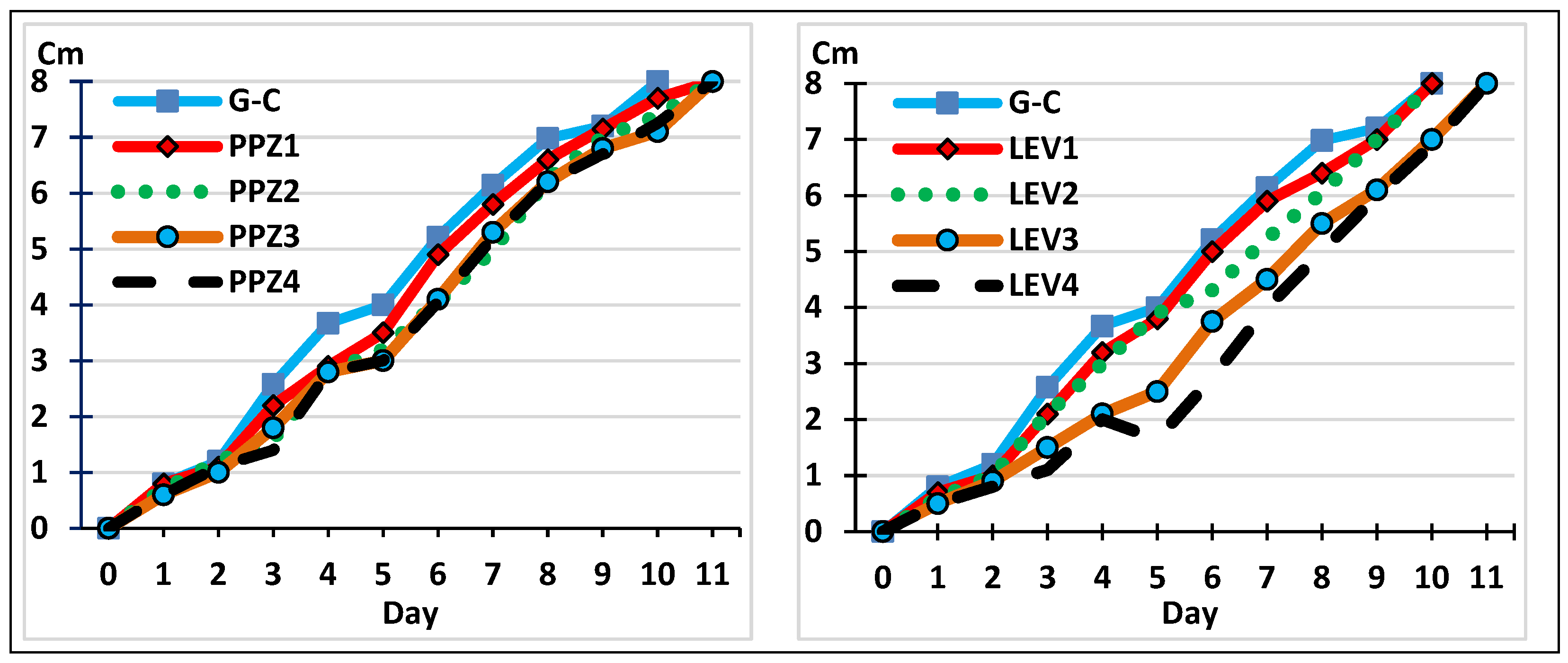
2.2. The Development of M. circinelloides in the Presence of Anthelmintics
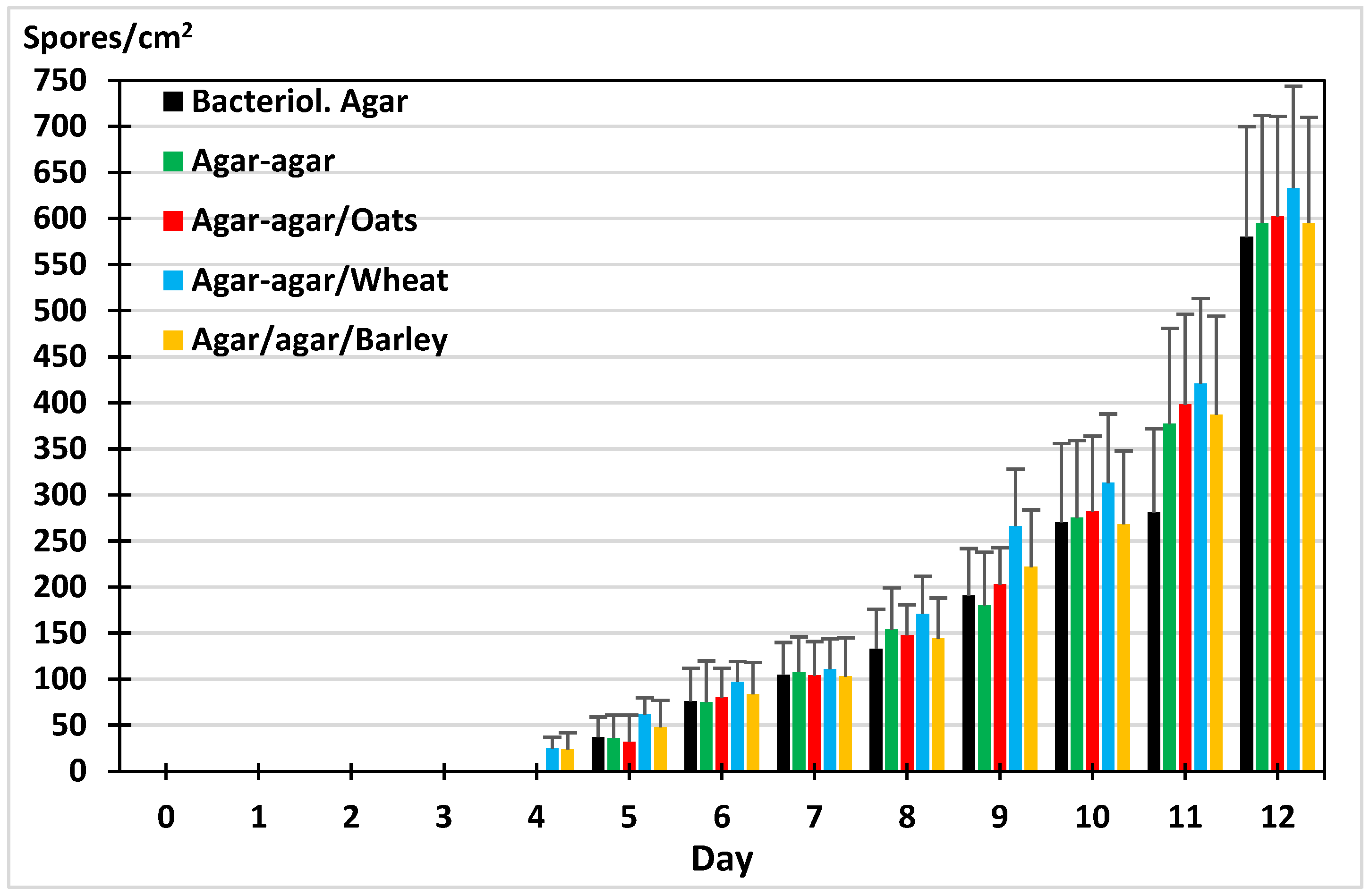
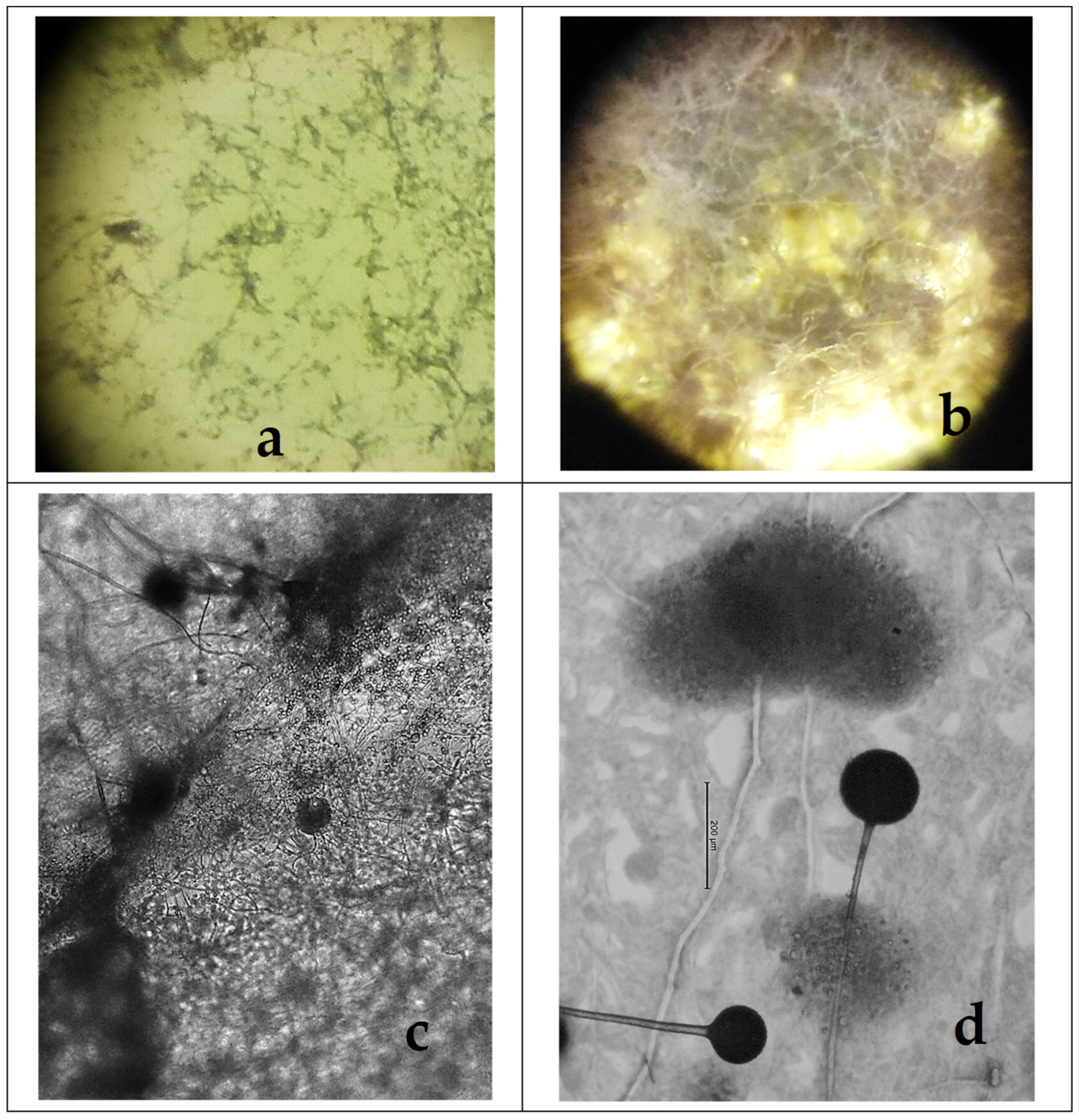
2.3. Sporogenesis of M. circinelloides in Different Media
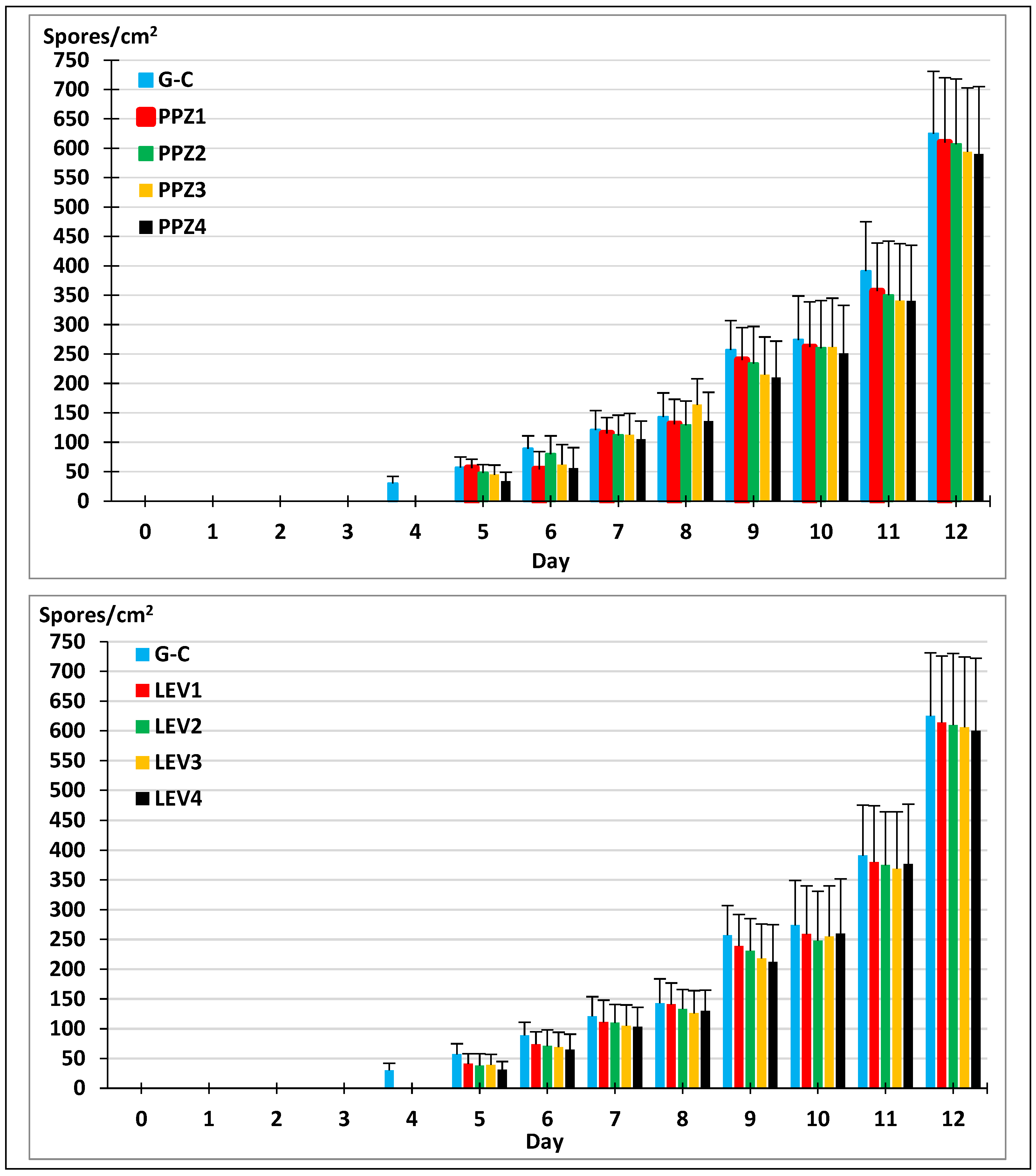
2.4. Sporogenesis of M. circinelloides in the Presence of Anthelmintics
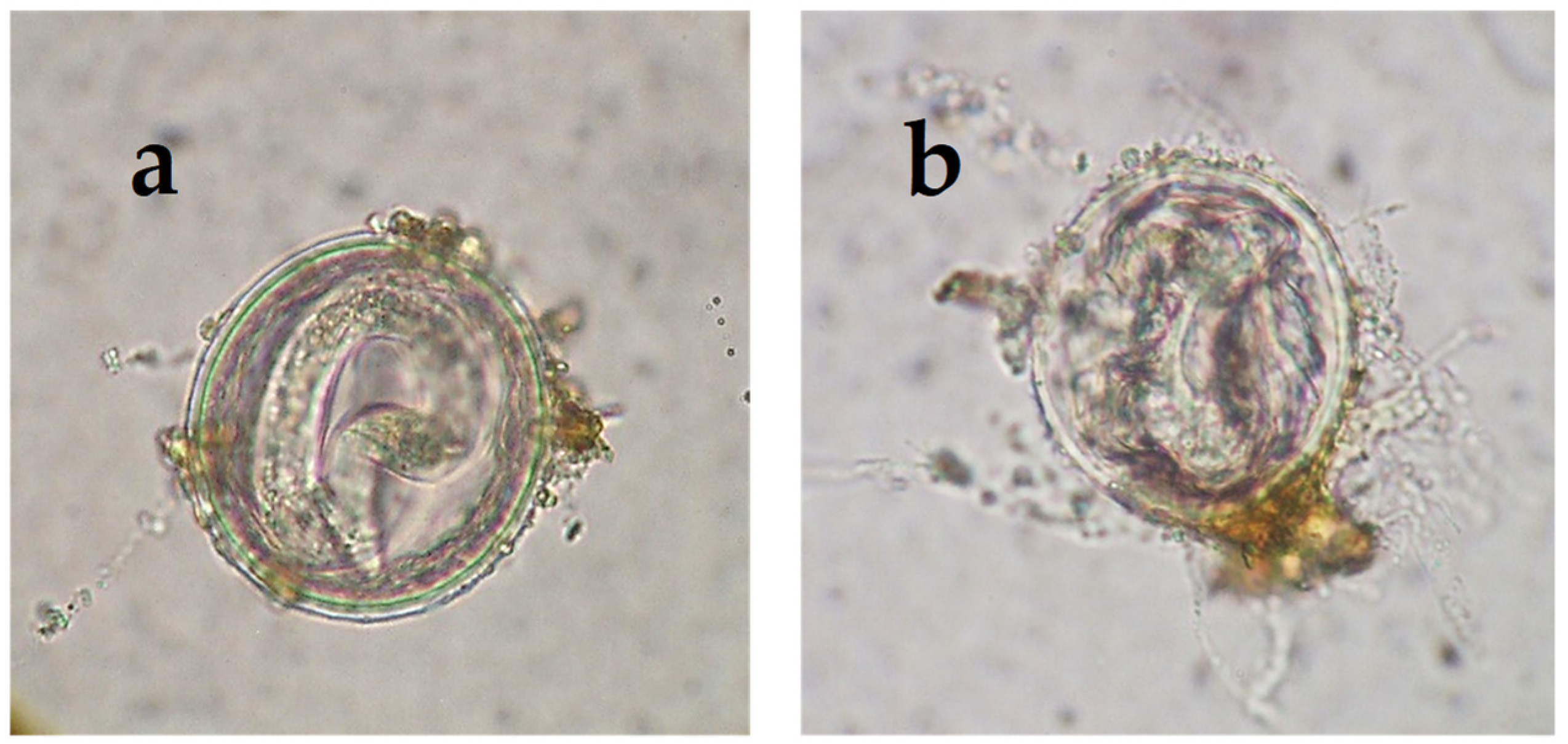
2.5. The Analysis of Possible Applications
3. Conclusions
4. Materials and Methods
4.1. Experimental Design
4.1.1. The Development of M. circinelloides in Agar–Agar
4.1.2. The Development of M. circinelloides in Agar–Agar with Added Anthelmintics
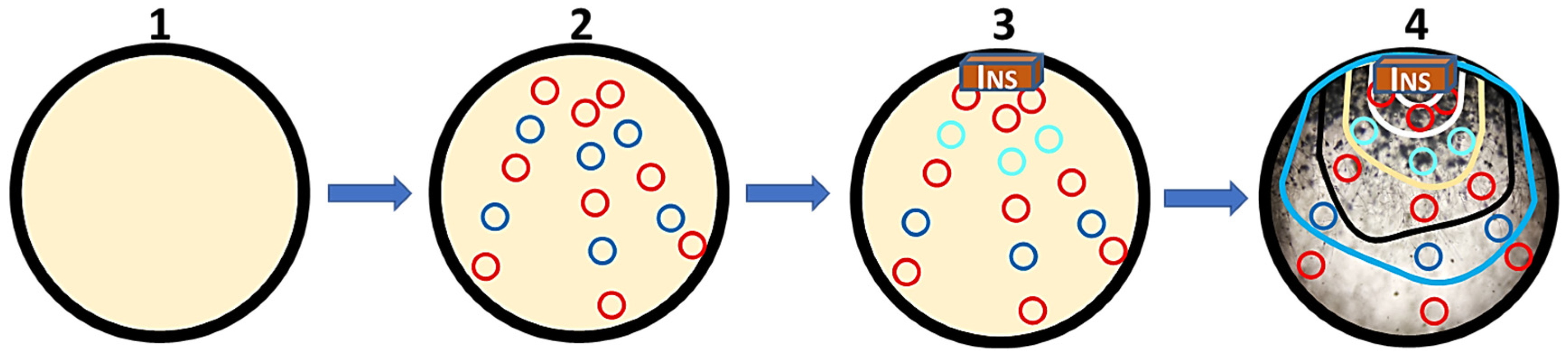
4.1.3. Evaluating the Development of M. circinelloides
4.1.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magill, E.; Demartis, S.; Gavini, E.; Permana, A.D.; Thakur, R.R.S.; Adrianto, M.F.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A.; et al. Solid implantable devices for sustained drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar] [CrossRef] [PubMed]
- Unde, J.S.; Ahirwar, K.; Kumar, A.; Alsheri, S.A.; Wahab, S.; Kesharwani, P.; Shukla, R. Manoeuvring the innovative drug delivery systems for veterinary therapeutics: Present day demand. Eur. Polym. J. 2024, 215, 113244. [Google Scholar] [CrossRef]
- Raber, J.M.; Niekrasz, M.; Linkenhoker, J.; Perdue, K.A. Veterinary Care. In Management of Animal Care and Use Programs in Research, Education, and Testing, 2nd ed.; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; pp. 733–770. [Google Scholar] [CrossRef]
- Global Market Insights. Animal Parasiticides Market—By Product, By Type, By Animal Type, By Drug Class, By End Use & Global Forecast, 2025–2034. Available online: https://www.gminsights.com/industry-analysis/animal-parasiticides-market (accessed on 19 June 2025).
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Strydom, T.; Lavan, R.P.; Torres, S.; Heaney, K. The Economic Impact of Parasitism from Nematodes, Trematodes and Ticks on Beef Cattle Production. Animals 2023, 13, 1599. [Google Scholar] [CrossRef] [PubMed]
- Sünnemann, M.; Barnes, A.D.; Amyntas, A.; Ciobanu, M.; Jochum, M.; Lochner, A.; Potapov, A.M.; Reitz, T.; Rosenbaum, B.; Schädler, M.; et al. Sustainable Land Use Strengthens Microbial and Herbivore Controls in Soil Food Webs in Current and Future Climates. Glob. Change Biol. 2024, 30, e17554. [Google Scholar] [CrossRef]
- Hernández, J.Á.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Sanchís, J.M.; Paz-Silva, A.; Arias, M.S. A combined effort to avoid strongyle infection in horses in an oceanic climate region: Rotational grazing and parasiticidal fungi. Parasites Vectors 2018, 11, 240. [Google Scholar] [CrossRef]
- Canhão-Dias, M.; Paz-Silva, A.; Madeira de Carvalho, L.M. The efficacy of predatory fungi on the control of gastrointestinal parasites in domestic and wild animals-A systematic review. Vet. Parasitol. 2020, 283, 109173. [Google Scholar] [CrossRef]
- Lozano, J.; Cunha, E.; de Carvalho, L.M.; Paz-Silva, A.; Oliveira, M. First insights on the susceptibility of native coccidicidal fungi Mucor circinelloides and Mucor lusitanicus to different avian antiparasitic drugs. BMC Vet. Res. 2024, 20, 63. [Google Scholar] [CrossRef]
- Chan, L.G.; Cohen, J.L.; Ozturk, G.; Hennebelle, M.; Taha, A.Y.; de Moura Bell, J.M.L.N. Bioconversion of cheese whey permeate into fungal oil by Mucor circinelloides. J. Biol. Eng. 2018, 12, 25. [Google Scholar] [CrossRef]
- Fazili, A.B.A.; Shah, A.M.; Zan, X.; Naz, T.; Nosheen, S.; Nazir, Y.; Ullah, S.; Zhang, H.; Song, Y. Mucor circinelloides: A model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb. Cell Fact. 2022, 21, 29. [Google Scholar] [CrossRef]
- de Oliveira Tavela, A.; de Araújo, J.V.; Braga, F.R.; da Silveira, W.F.; Dornelas e Silva, V.H.; Carretta Júnior, M.; Borges, L.A.; Araujo, J.M.; Laércio dos Anjos, B.; Carvalho, G.R.; et al. Coadministration of sodium alginate pellets containing the fungi Duddingtonia flagrans and Monacrosporium thaumasium on cyathostomin infective larvae after passing through the gastrointestinal tract of horses. Res. Vet. Sci. 2013, 94, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.D.S.; Valverde, H.A.; Barbosa, B.B.; Santos, H.A.; de Araújo, J.V. Assessing the applications and efficacy of using helminthophagous fungi to control canine gastrointestinal parasites. Acta Trop. 2024, 254, 107180. [Google Scholar] [CrossRef] [PubMed]
- Paz-Silva, A.; Cazapal-Monteiro, C.; Viña, C.; Palomero, A.M.; Salmo, R.; Hernández, J.Á.; Sánchez-Andrade, R.; Arias, M.S. Gelatin treats containing filamentous fungi to promote sustainable control of helminths among animals. Biol. Control 2023, 179, 105184. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; De Morais, A.; De Morais, R. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Zegbi, S.; Sagües, F.; Iglesias, L.; Guerrero, I.; Saumell, C. Trapping Behaviour of Duddingtonia flagrans against Gastrointestinal Nematodes of Cattle under Year-Round Grazing Conditions. Pathogens 2023, 12, 401. [Google Scholar] [CrossRef]
- Singh, R.K.; Sanyal, P.K.; Patel, N.K.; Sarkar, A.K.; Santra, A.K.; Pal, S.; Mandal, S.C. Fungus-benzimidazole interactions: A prerequisite to deploying egg-parasitic fungi Paecilomyces lilacinus and Verticillium chlamydosporium as biocontrol agents against fascioliasis and amphistomiasis in ruminant livestock. J. Helminthol. 2010, 84, 123–131. [Google Scholar] [CrossRef]
- Vieira, J.N.; Maia Filho, F.S.; Ferreira, G.F.; Mendes, J.F.; Gonçalves, C.L.; Villela, M.M.; Pereira, D.I.B.; Nascente, P.S. In vitro susceptibility of nematophagous fungi to antiparasitic drugs: Interactions and implications for biological control. Braz. J. Biol. 2017, 77, 476–479. [Google Scholar] [CrossRef]
- Arias, M.S.; Suárez, J.; Cazapal-Monteiro, C.F.; Francisco, I.; López-Arellano, M.E.; Piñeiro, P.; Suárez, J.L.; Sánchez-Andrade, R.; Mendoza de Gives, P.; Paz-Silva, A. Trematodes enhance the development of the nematode-trapping fungus Arthrobotrys (Duddingtonia) flagrans. Fungal Biol. 2013, 117, 540–544. [Google Scholar] [CrossRef]
- Hrckova, G.; Velebny, S. Parasitic Helminths of Humans and Animals: Health Impact and Control. In Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases; Springer Briefs in Pharmaceutical Science & Drug Development; Springer: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- AAEP Internal Parasite Control Guidelines. American Association of Equine Practitioners. May 2025. Available online: https://aaep.org/resource/internal-parasite-control-guidelines/ (accessed on 14 February 2025).
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de Gives, P. Soil-Borne Nematodes: Impact in Agriculture and Livestock and Sustainable Strategies of Prevention and Control with Special Reference to the Use of Nematode Natural Enemies. Pathogens 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, J.V. Advances in the Control of the Helminthosis in Domestic Animals. Pathogens 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.M.; Sobral, S.A.; Senna, C.C.; Junior, O.F.; Moreira, T.F.; Tobias, F.L.; de Freitas Soares, F.E.; Geniêr, H.L.A.; Vilela, V.L.R.; Lima, J.A.C.; et al. Combined use of ivermectin, dimethyl sulfoxide, mineral oil and nematophagous fungi to control Rhabditis spp. Vet. Parasitol. 2019, 275, 108924. [Google Scholar] [CrossRef]
- Jumaidin, R. Agar Based Composite as a New Alternative Biopolymer. In Composites from the Aquatic Environment. Composites Science and Technology; Sapuan, S.M., Imran, A., Eds.; Springer: Singapore, 2023; pp. 67–82. [Google Scholar] [CrossRef]
| Active Principle | Formulation | Target |
|---|---|---|
| Piperazine | Pills, powder | Roundworms |
| Pinworms | ||
| Hookworms | ||
| Levamisole | Liquid, powder | Trichostrongylids |
| Hookworms | ||
| Roundworms | ||
| Albendazole | Liquid, paste | Trematodes |
| Trichostrongylids | ||
| Hookworms | ||
| Roundworms | ||
| Ivermectin | Liquid, paste, injectable, poured on | Trichostrongylids |
| Hookworms | ||
| Roundworms | ||
| Whipworms | ||
| Ectoparasites |
| Group | Anthelmintic | Quantity Added/Plate (mg) | Corresponding Dosage | |
|---|---|---|---|---|
| Dogs (kg) | Pigs, Cattle, and Horses (kg) | |||
| PPZ1 | Piperazine | 546 | 2.5 | 5 |
| PPZ2 | 1102.5 | 5.5 | 10 | |
| PPZ3 | 2205 | 11 | 20 | |
| PPZ4 | 5495 | 27 | 50 | |
| LEV1 | Levamisole | 37.5 | 3.7 | 5 |
| LEV2 | 75 | 7.5 | 10 | |
| LEV3 | 150 | 15 | 20 | |
| LEV4 | 375 | 30 | 50 | |
| Group | Name | Piperacina Syva® (g Added/Plate) | Piperazine (mg/Plate) *1 | Panvermin® (g Added/Plate) | Levamisole (mg/Plate) *2 |
|---|---|---|---|---|---|
| G-C | G-C | - | - | - | - |
| G-PPZ | PPZ1 | 1.56 | 546 | ||
| PPZ2 | 3.15 | 1102.5 | |||
| PPZ3 | 6.3 | 2205 | |||
| PPZ4 | 15.7 | 5495 | |||
| G-LEV | LEV1 | 0.25 | 37.5 | ||
| LEV2 | 0.5 | 75 | |||
| LEV3 | 1 | 150 | |||
| LEV4 | 2.5 | 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubiría, I.; Abreu, I.; Boso, D.; Pérez, G.; Cazapal, C.; Sánchez-Andrade, R.; Arias, M.S.; Paz-Silva, A.; Hernández, J.Á.; Camiña, M. Agar–Agar Gels Carrying Curative and Preventive Agents Against Helminths: An In Vitro Compatibility Evaluation. Gels 2025, 11, 542. https://doi.org/10.3390/gels11070542
Zubiría I, Abreu I, Boso D, Pérez G, Cazapal C, Sánchez-Andrade R, Arias MS, Paz-Silva A, Hernández JÁ, Camiña M. Agar–Agar Gels Carrying Curative and Preventive Agents Against Helminths: An In Vitro Compatibility Evaluation. Gels. 2025; 11(7):542. https://doi.org/10.3390/gels11070542
Chicago/Turabian StyleZubiría, Izaro, Inês Abreu, David Boso, Gustavo Pérez, Cristiana Cazapal, Rita Sánchez-Andrade, María Sol Arias, Adolfo Paz-Silva, José Ángel Hernández, and Mercedes Camiña. 2025. "Agar–Agar Gels Carrying Curative and Preventive Agents Against Helminths: An In Vitro Compatibility Evaluation" Gels 11, no. 7: 542. https://doi.org/10.3390/gels11070542
APA StyleZubiría, I., Abreu, I., Boso, D., Pérez, G., Cazapal, C., Sánchez-Andrade, R., Arias, M. S., Paz-Silva, A., Hernández, J. Á., & Camiña, M. (2025). Agar–Agar Gels Carrying Curative and Preventive Agents Against Helminths: An In Vitro Compatibility Evaluation. Gels, 11(7), 542. https://doi.org/10.3390/gels11070542








