Abstract
Thermosensitive hydrogels undergo reversible sol-gel phase transitions in response to changes in temperature. Owing to their excellent biocompatibility, mild reaction conditions, and controllable gelation properties, these hydrogels represent a promising class of biomaterials suitable for minimally invasive treatment systems in diverse biomedical applications. This review systematically summarizes the gelation mechanisms of thermosensitive hydrogels and optimization strategies to enhance their performance for broader application requirements. In particular, we highlight recent advances in injectable thermosensitive hydrogels as a carrier within stem cells, bioactive substances, and drug delivery for treating various tissue defects and diseases involving bone, cartilage, and other tissues. Furthermore, we propose challenges and directions for the future development of thermosensitive hydrogels. These insights provide new ideas for researchers to explore novel thermosensitive hydrogels for tissue repair and disease treatment.
1. Introduction
Diseases, trauma, inflammation, and other factors can lead to damage in various tissues of the human body, strongly impacting patients’ daily lives and mental health. Currently, transplantation and pharmacological treatments are commonly used in clinical settings to address tissue damage [1,2]. However, transplantation faces challenges such as a high risk of infection and immunogenicity [3]. Traditional drug delivery methods, including oral and intravenous administration, often suffer from limitations such as short drug half-life, insufficient drug accumulation at target sites, and significant systemic side effects [4]. With continuous advancements in biomedical engineering, innovative delivery platforms—such as hydrogels [5,6], microneedles [7,8], and liposomes [9]—have been developed to achieve spatiotemporally controlled substance release, improve therapeutic precision, and promote functional tissue repair.
Among these platforms, hydrogels are widely used and highly regarded due to their structural similarity to natural tissues, excellent biocompatibility, and biodegradability [10]. However, traditional hydrogels often form irreversible crosslinked polymer networks [11], making in situ injection into small or delicate sites challenging. The dynamic nature of damaged tissues further limits the utility of these materials as they often fail to adapt rapidly to physiological changes [11], leading to suboptimal patient outcomes. Recent advances in stimuli-responsive hydrogels address these limitations by enabling precise reactions to environmental factors—including pH fluctuations, light exposure, temperature variations, mechanical stress, and electromagnetic fields—demonstrating significant potential for tissue regeneration [12].
Thermosensitive polymers, in particular, respond rapidly to localized temperature changes. Thermosensitive hydrogels, a subclass of smart hydrogels, have gained widespread attention due to their sensitivity to temperature and tunable phase transition behavior. These hydrogels permit in situ gelation, thereby enhancing site-specific delivery. The sol–gel transition triggered by external or internal temperature changes facilitates minimally invasive and targeted therapies [13]. Furthermore, since temperature is a benign stimulus, gelation can occur without the use of harmful solvents, which simplifies the preparation process [14]. Importantly, the thermosensitive hydrogel gradually degrades in vivo, eliminating the need for surgical removal and improving patient convenience.
Thermosensitive hydrogels offer distinct advantages as carriers for localized therapeutic delivery [15,16], making them a prominent focus in tissue engineering research. Despite their widespread exploration, systematic reviews on their role in repairing tissue defects remain scarce. This review examines the gelation mechanisms underlying thermosensitive hydrogels and evaluates current optimization approaches. It further analyzes recent advances in delivering stem cells, drugs, and bioactive molecules via injectable thermosensitive hydrogels across bone, cartilage, and other tissues, providing critical perspectives for future hydrogel design.
2. Phase Transition and Gelation Mechanisms of Thermosensitive Hydrogel
The advancement of biomaterials science and tissue engineering has stimulated extensive research into thermoresponsive hydrogels. These smart polymer networks exhibit volume phase transition behavior upon thermal stimulation, with the corresponding temperature referred to as the volume phase transition temperature [17]. Based on their phase transition temperature, thermosensitive hydrogels can be classified into two categories: those with a lower critical solution temperature (LCST) and those with an upper critical solution temperature (UCST) (Figure 1). The LCST marks the temperature at which a material transitions from one phase to another upon heating. Heating above this threshold induces molecular precipitation and a subsequent sol–gel transition [18,19]. Conversely, the UCST follows an inverse pattern, with polymers gelling at lower temperatures and dissolving when heated beyond this point [20]. Although UCST-type thermoresponsive polymers, such as agarose and cold glue, exist, their limited responsiveness at physiological temperatures restricts their biomedical utility [21]. By contrast, LCST-type polymers show greater promise for biomedical applications and have consequently attracted more research attention [22].
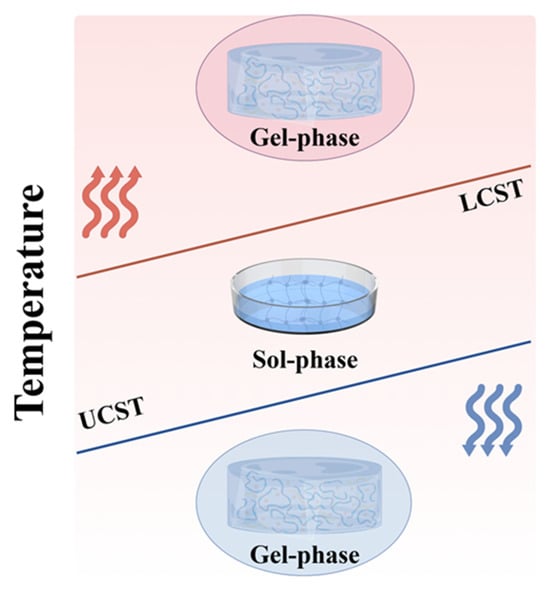
Figure 1.
Diagram of the LCST and UCST phase transitions of thermosensitive hydrogels (The arrows indicate heating and cooling.).
Thermodynamically speaking, the contraction of polymers to form gels is attributed to the interplay between enthalpy, entropy, and temperature. At high temperatures, the increase in entropy of water molecules dominates, leading to a decrease in free energy (ΔG = ΔH − ΔS < 0) and resulting in phase separation. Conversely, at low temperatures, the exothermic effect (ΔH < 0) compensates for the cost of entropy reduction (ΔG = ΔH − TΔS < 0), leading to phase separation [23]. Specifically, LCST-type hydrogels are predominantly driven by entropy, while UCST-type hydrogels are primarily driven by enthalpy. This difference arises from the temperature dependence of polymer-solvent interactions and the competing roles of enthalpy and entropy in determining the free energy [24].
Thermosensitive hydrogels exhibit remarkable molecular diversity in their structural features and gelation mechanisms. Among them, N-isopropylacrylamide (NIPAm) stands out as the most representative thermosensitive monomer. Its polymer, poly(N-isopropylacrylamide) (PNIPAm), possesses a distinctive amphiphilic molecular architecture comprising hydrophilic amide groups and hydrophobic isopropyl side chains [25]. The temperature-responsive behavior originates from dynamic interactions between functional groups and solvent molecules. At temperatures below the LCST, hydrogen bonding between amide groups and water molecules maintains chain expansion, while heating above the LCST triggers chain collapse and subsequent hydrophobic aggregation through hydrogen bond dissociation and hydrophobic interactions (Figure 2A) [26,27]. In addition to NIPAm, poly(2-dimethylaminoethyl methacrylate) serves as a typical thermosensitive monomer. The tertiary amine groups in its side chains undergo dynamic hydrophilic–hydrophobic transitions in response to temperature variations, thereby inducing phase separation [28]. Similarly, 2-(2-methoxyethoxy)ethyl methacrylate (MEO2MA) and oligo(ethylene glycol) methacrylate (OEGMA) are common thermosensitive monomers. The thermoresponsive behavior of MEO2MA originates from the dissociation and reconstruction of dynamic hydrogen-bonding networks between ether oxygen atoms in its side chains and water molecules upon temperature changes [29], whereas OEGMA achieves thermosensitivity through hydrogen bond interactions among ether oxygen atoms, methylene groups in the polymer chains, and water molecules [30]. Furthermore, when the OEGMA monomer undergoes polymerization, it can undergo copolymerization with other monomers, thereby acquiring the ability to control the LCST [31].
Other important thermosensitive systems include chitosan (CS), which requires polyol salts, such as β-sodium glycerophosphate (β-GP), to achieve thermosensitivity [32]. Under physiological pH conditions (pH = 7–7.4), polyol molecules form a protective hydrophobic layer through hydrogen bonding at low temperatures, maintaining the solution state. When the temperature increases to 37 °C, thermodynamic forces disrupt this hydrophobic layer, leading to a sol–gel transition via enhanced hydrophobic interactions (Figure 2B) [33,34]. Pluronic F127 (F127) copolymers, with their poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) sequence, undergo dehydration-driven micellization of the hydrophobic PPO blocks above the critical micellar temperature. Further heating beyond the critical gelation temperature induces intermicellar entanglement, leading to a sharp increase in viscosity and the formation of a gel network (Figure 2C) [35]. Notably, F127 self-assembles into a face-centered cubic ordered structure at 37 °C [36]. Cellulose derivatives, such as methylcellulose (MC), derive their thermosensitivity from β-1,4-glycosidic bonded glucose backbones [22]. Heating disrupts hydrogen bonds, allowing methoxy groups to crosslink through strong hydrophobic interactions, which promotes the self-assembly of random-coiled molecular chains into persistent fibrils approximately 14 nm in diameter, ultimately forming a stable three-dimensional hydrogel network (Figure 2D) [37]. The poly (D, L-lactide-co-glycolide)-b-poly (ethyleneglycol)-b-poly (D, L-lactide-co-glycolide) triblock copolymer system, abbreviated as PLGA-PEG-PLGA, exhibits temperature responsiveness through dehydration of the PEG segment upon heating, which enhances hydrophobicity and promotes micelle aggregation and three-dimensional network formation(Figure 2E) [38,39].
The common feature of these systems lies in the thermally induced phase transition driven by physical or chemical interactions such as dynamic reorganization of hydrogen-bond networks, hydrophobic interactions, and solute solvent interactions. Currently, some characterization techniques, such as dynamic light scattering, scanning electron microscopy, Fourier transform infrared spectroscopy, and Raman spectroscopy, provide multi-scale insights into the gel mechanisms and structure performance relationships of these complex systems, laying the foundation for the development of new thermosensitive hydrogels and the exploration of the gelation mechanisms [40,41].
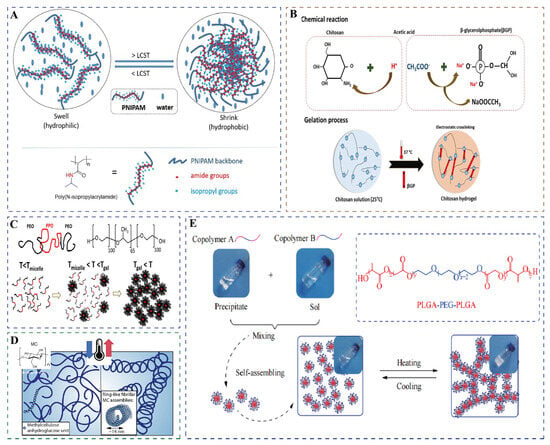

Figure 2.
Schematic diagram of gelation of common thermosensitive hydrogels. (A) PNIPAm. Used with permission of the Royal Society of Chemistry, from Thermoresponsive polymers and their biomedical application in tissue engineering—a review, Falko Doberenz(s), 4, 8 and 2020 of copyright [26]. (B) CS/β-GP. Reprinted from International Journal of Biological Macromolecules, 164, Nourollah Rezaei(s), Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates, 855–862, copyright (2020), with permission from Elsevier [33]. (C) F127 (T represents temperature). Reprinted with permission from [35]. Copyright 2021, American Chemical Society. (D) Methylcellulose (The arrows indicate heating and cooling.). Adapted with permission from [37]. Copyright 2018, American Chemical Society. (E) PLGA-PEG-PLGA. Reprinted with permission from [38].
3. Optimization Strategies of Thermosensitive Hydrogels
Thermosensitive hydrogels respond intrinsically to temperature changes, yet both natural and synthetic variants suffer from inherent drawbacks, including poor stability, inadequate mechanical properties, and uncontrolled release profiles. To address these challenges, optimization strategies—including modifications in crosslinking methods, internal encapsulation, three-dimensional printing, and multi-stimuli responsiveness—have been employed to enhance the performance of thermosensitive hydrogels and expand their applications in tissue engineering (Figure 3).
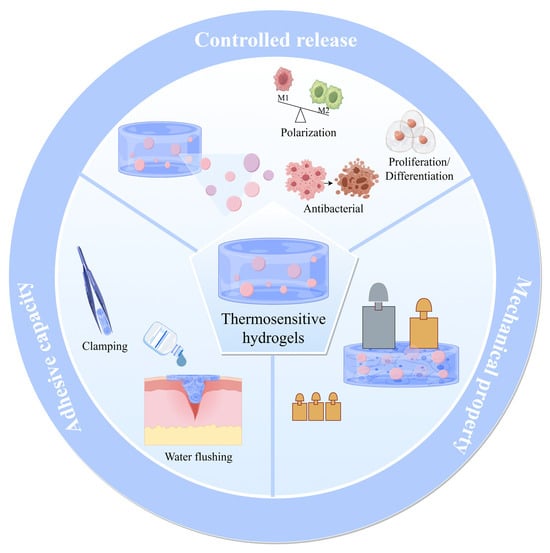
Figure 3.
The optimized superior performance of thermosensitive hydrogels.
3.1. Crosslinking Method
Physical crosslinking is a fundamental approach in the preparation of hydrogels. Physical crosslinking involves the formation of three-dimensional networks through reversible, non-covalent interactions between polymer chains, including but not limited to hydrophobic associations, electrostatic forces, hydrogen bonding, van der Waals interactions, and physical chain entanglements during gelation [42,43]. Thermosensitive hydrogels formed through physical crosslinking can undergo temperature-induced phase transformation without the need for coupling agents or organic solvents, offering excellent biological safety. However, the thermosensitive hydrogels prepared solely through physical crosslinking often exhibit limited functional properties [44] (including low mechanical strength, weak adhesion, burst release of encapsulated substances, slow gelation kinetics, etc.) and fail to achieve the material diversity required for broader biomedical applications. Therefore, selecting an appropriate crosslinking method is a crucial strategy to further enhance the performance and functionality of thermosensitive hydrogels.
Chemical crosslinking relies on crosslinking agents to mediate irreversible covalent bonding (e.g., through addition or condensation reactions), resulting in a permanent hydrogel network with chemically bonded polymer chains [45,46]. Compared with thermosensitive hydrogels formed through physical crosslinking, those synthesized via chemical crosslinking exhibit substantially greater mechanical robustness and enhanced structural stability. Furthermore, reactions between chemical bonds and chemical groups can further improve the material’s adhesive properties [47]. However, residual crosslinking agents such as formaldehyde and glutaraldehyde may exert adverse effects on biological tissues. In contrast, plant-derived extracts—including genipin [48], proanthocyanidins [49], and tea polyphenols [50]—serve as natural crosslinkers that are devoid of toxicity and side effects, rendering them highly favored by researchers. Representative examples of widely utilized chemically crosslinked thermosensitive hydrogels include polyether F127 diacrylate (F127DA) [51] and polyethylene glycol diacrylate [52], which have become benchmark materials in this field due to their tunable network properties.
Metal coordination crosslinking is a novel strategy for constructing thermosensitive hydrogels, relying on the dynamic interactions between metal ions and polymer coordination groups. By modulating the strength and density of coordination bonds in response to temperature changes, these hydrogels achieve reversible contraction or swelling of the gel network [53,54]. The core mechanism involves the formation of dynamic coordination bonds between metal ions (such as Cu2+, Zn2+, and Al3+) and coordination groups like carboxylic acid, catechol, and amino groups on the polymer chains [53,55,56]. As the temperature increases, the bond energy of these coordination interactions weakens or dissociates, reducing the number of crosslinking points, relaxing the gel network, and promoting water absorption and swelling. Conversely, the coordination bonds re-form when the temperature decreases, leading the network to contract and expel water. For instance, the coordination system between Fe3+ and catechol groups (mimicking the structure of mussel adhesive proteins) dissociates above body temperature, imparting the material with significant thermosensitive volume phase transition characteristics [57,58]. Additionally, the strength of the coordination bonds can be precisely controlled by adjusting the metal valence state, ligand type, and environmental pH, allowing for the customization of the gel’s response temperature and mechanical properties. Some metal ions can pose a threat to tissue safety, so reducing their toxicity through controlled concentrations or biological methods, such as transformation and adsorption, remains essential. Beyond tissue engineering, temperature-sensitive hydrogels with metal coordination crosslinking also function effectively in wearable devices [59,60].
Photothermal synergistic crosslinking is achieved by copolymerizing monomers containing thermosensitive groups with those containing photosensitive groups or by introducing photosensitive groups into the terminal groups of polymers through chemical methods. Local polymerization is then initiated via ultraviolet (UV) illumination, forming a temperature-responsive network [61,62]. In recent years, upconversion nanoparticles have emerged as an effective means of converting external near-infrared light into internal light, demonstrating the potential of near-infrared light to replace UV in triggering drug release [63,64]. This innovation provides new insights for the development of photothermal synergistic hydrogels. Numerous experiments have introduced polydopamine nanoparticles, gold nanorods, or nano-liposomes into thermally responsive hydrogels. Upon exposure to near-infrared light, these hydrogels alter their local crosslink density, thereby enhancing spatiotemporal precision and synergistic thermotherapeutic effects and enabling the controlled release of internal gel contents [65,66]. The above-mentioned crosslinking methods are summarized in Table 1.

Table 1.
Comparison of different crosslinking methods of thermosensitive hydrogel.
3.2. Internal Encapsulation
Although thermosensitive hydrogels are highly diverse, conventional thermosensitive hydrogels cannot promote tissue healing. Considering that these hydrogels consist of three-dimensional polymer networks with high water content, they provide a moist microenvironment that enhances biocompatibility. Furthermore, their gradual degradation after implantation and these characteristics make them particularly suitable as carrier materials.
To fulfill the three essential components of tissue engineering, researchers typically incorporate seed cells and growth factors (GFs) into thermosensitive hydrogels [67]. Seed cells may include autologous, allogeneic, stem, or genetically modified cells [68]. The thermosensitive hydrogel provides a microenvironment similar to the natural extracellular matrix (ECM), which facilitates cell encapsulation and survival, enabling the delivery of exogenous cells to the injury site and promoting tissue repair. GFs are bioactive molecules that regulate cellular behaviors by binding to cell surface receptors, activating signaling pathways, and modulating cell proliferation and differentiation [69]. As the thermosensitive hydrogel degrades, the encapsulated GFs are gradually released, promoting tissue healing and regeneration. Consequently, cells and GFs represent the most common internal cargoes within thermosensitive hydrogels.
Drugs with antibacterial or anti-inflammatory effects are also frequently loaded into thermosensitive hydrogels. This is attributed to tissue damage, where bacteria and inflammation often play a role, hindering the subsequent tissue repair and regeneration. By loading drugs onto hydrogels and achieving sustained release at the defect site, the effects of antibacterial, anti-inflammatory, and pro-regenerative impact, such as promoting cell proliferation and differentiation, are particularly significant. In this regard, thermosensitive hydrogels offer distinct advantages. Because they contain hydrophilic polymers and adapt well to the shape of the loaded drug, drug incorporation is relatively easy. Water-soluble drugs can be mixed with high-molecular-weight aqueous solutions to prepare hydrophilic drug-loaded gels, which enable the diffusion of drugs from the matrix and achieve in situ release functionality [70]. However, for some hydrophobic drugs, the direct use of gel encapsulation results in a poor utilization rate. By combining drugs with nanomaterials, a stable suspension can be formed in thermosensitive hydrogels, enabling the controlled release of small doses and addressing the issue of poor water solubility [71]. Notably, liposomes exhibit low cytotoxicity and immunogenicity, serving as an effective delivery platform for both hydrophobic and hydrophilic molecules while protecting their biological activity from degradation [72]. Combining drug-loaded nanomaterials or liposomes with thermosensitive hydrogels facilitates controlled drug release, presenting a potential strategy for targeted delivery to inflammatory tissue injury sites.
Moreover, studies have demonstrated that incorporating materials such as hydroxyapatite, calcium carbonate, and decellularized bone matrix into thermosensitive hydrogels can significantly enhance their mechanical properties. The calcium, phosphorus, and other bioactive components released from these materials can meet the functional demands of the deficient areas, promote the healing of bone tissues, and enhance bone strength [73,74].
3.3. Three-Dimensional Printing Technique
Three-dimensional (3D) printing, also known as additive manufacturing, is a layer-by-layer fabrication technology widely applied across multiple disciplines. 3D bioprinting, an advanced technique that involves the spatially controlled deposition of living cells, biomaterials, pharmaceuticals, GFs, and genes layer by layer, has rapidly evolved in recent years and demonstrated extensive utility in various biomedical applications [75]. However, identifying suitable bioinks remains a significant challenge in 3D bioprinting, reflecting the delicate balance between reproducible additive manufacturing and biological requirements [76].
Thermosensitive hydrogels exhibit intrinsic properties that render them promising candidates for bioinks. However, their precursor solutions often exhibit poor rheological characteristics, including low viscosity and shear resistance, which compromise the shape fidelity of printed constructs. This limitation can be mitigated by incorporating nanocellulose or nanoclay into the hydrogel matrix. During printing, the sol state of the hydrogel is extruded and undergoes instant gelation upon exposure to physiological temperatures. This occurs because nanocellulose bridges micelles through intermolecular hydrogen bonds and hydrophobic interactions, enabling physical crosslinking [77]. This approach ensures high shape fidelity and enables controlled drug release, thereby facilitating personalized therapeutic applications. In addition, the challenge of low-viscosity hydrogels being difficult to shape can be addressed through the freeform reversible embedding of suspended hydrogels (FRESH) method. This approach utilizes gelatin particles to prepare a supporting bath, allowing the printed hydrogel structure to maintain its position without collapsing [78]. It is particularly suitable for constructing multifunctional hydrogels, such as human cardiac capillaries [79]. The prepared thermosensitive hydrogel is used as the bioink and printed via the FRESH method. Upon heating to 37 °C, the gelatin supporting bath melts, yielding the final material. This technique eliminates the need for secondary crosslinking, preserves bioactivity, simplifies the fabrication process, and lays the foundation for large-scale production and clinical translation [80].
Two-photon polymerization (2PP), one of the most precise 3D printing technologies, enables the fabrication of high-resolution materials in conjunction with computer-aided design tools. Compared with the conventional 3D printing UV polymerization method, 2PP does not require toxic reagents or UV exposure and can rapidly produce flexible, high-precision structures with complex geometries [81,82]. Thermosensitive hydrogel microrobots fabricated via 2PP exhibit superior swelling properties, facilitating efficient drug release [83]. In addition, grafting thermosensitive P (NIPAMx-co-NtBAMy) side chains enabled the formulation of a functional bioink. This composition forms a weak gel during printing, shielding cells from shear-induced damage. Subsequent hydrophobic interactions enhance mechanical stability, facilitating cell migration and spheroid formation while establishing an optimal growth microenvironment [84]. Overall, the thermosensitive hydrogel produced by 3D printing has excellent mechanical properties and the ability to control drug release.
3.4. Multiple Stimulus Response
The human body maintains a constant core temperature, yet microenvironmental variations at tissue injury sites remain clinically unpredictable. Thermosensitive hydrogels that integrate temperature responsiveness with additional stimulus-triggered mechanisms show enhanced drug release profiles and therapeutic efficacy. In cancer therapy, the characteristic acidic tumor microenvironment enables pH-responsive drug release through the protonation of chitosan’s amino groups [85]. Magnetic responsiveness can be achieved by incorporating Fe3O4 nanoparticles into PNIPAm-based hydrogels, thereby combining magnetic targeting with thermally controlled drug delivery for precision oncology applications [86]. For cutaneous wound repair, in situ-forming CS gels functionalized with gallic acid modulate anti-inflammatory and regenerative drug release through reactive oxygen species (ROS) scavenging mechanisms [87]. Such ROS-responsive thermosensitive hydrogels have also proven effective in other tissue regeneration contexts [88,89]. When combined with previously described photothermal crosslinking techniques, these systems successfully integrate multiple stimuli-responsive modalities—including light, pH, ROS, and magnetism—demonstrating both practical utility and translational potential across diverse clinical applications.
4. Application of Thermosensitive Hydrogel in Tissue Repair and Treatment
Tissue engineering integrates multidisciplinary approaches to create biological substitutes that safely and effectively repair or replace damaged human tissues. While some tissue-engineered products have achieved clinical success, persistent challenges include optimizing material biocompatibility, cellular stability, and functional tissue integration. Thermosensitive hydrogels provide a versatile platform for delivering stem cells, pharmaceuticals, and bioactive components, including exosomes, peptides, and metal ions. These hydrogels combine intelligent stimuli-responsiveness with biocompatibility, injectability, and controlled release properties, substantially improving therapeutic outcomes in both drug delivery and in situ tissue regeneration applications. Their unique characteristics make them particularly suitable for addressing diverse tissue defects (Figure 4).
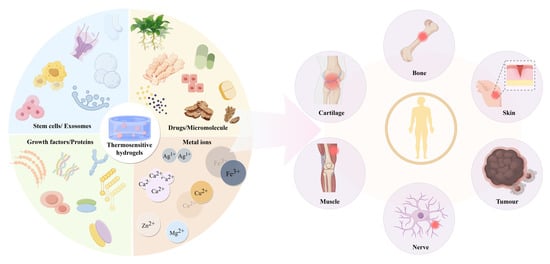
Figure 4.
Thermosensitive hydrogel carriers and their application as delivery systems in tissue repair and treatment.
4.1. Application of Thermosensitive Hydrogel in Bone Tissues
In the clinical treatment of bone defects, autologous, allogeneic, and xenogeneic bone transplantation are commonly used methods; however, their development is limited by issues such as insufficient donor sites and immunogenicity [2,90]. Due to its excellent properties, thermosensitive hydrogel-based delivery systems are widely used in bone tissue repair.
Stem cells, present in embryos and fetuses as undifferentiated cells, possess self-replication capabilities and are multipotent, meaning they can differentiate into various types of functional cells [91]. Thermosensitive hydrogels achieve tissue repair and regeneration by delivering stem cells. Guo et al. [92] prepared mesenchymal stem cell (MSC)–endothelial cell (EC) mixed spheroids using an AggreWell™400 24-well plate, then uniformly dispersed them in F127DA to create a composite bone repair material. The thermosensitive hydrogel increased in viscosity when injected into rat tooth extraction sockets, ensuring stable retention within the alveolar cavity. Subsequent photopolymerization enhanced material stability, while the encapsulated spheroids demonstrated active 3D cellular migration, exhibiting both pro-angiogenic and osteogenic potential during the early stages. With the advancement of the research field, extracellular vesicles (EVs) produced by stem cells, a bioactive substance, have emerged as a viable option for treating bone defects [93]. Ming et al. [94] investigated a hydrogel made of F127, CS, and crosslinked hyaluronic acid (c-HA) modified with calcium peroxide (CPO) and ascorbic acid (AsA) and loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles (BMSCs-EVs). The crosslinking and modification processes yielded a gel with more uniform and compact pores, providing effective channels for sustained oxygen and EV release. This PCc/CPO/AsA/sEVs hydrogel material significantly promoted the repair and regeneration of alveolar bone defects. Wu et al. [95] isolated EVs from BMSCs and mixed them with CS/β-GP hydrogel. Their study demonstrated that hydrogel degradation was accompanied by sustained release of BMSC-derived EVs, wherein EV-encapsulated miR-21 promoted angiogenesis through targeting the SPRY2 gene. The osteogenic efficacy of this hydrogel was shown to be significant in critical-sized calvarial defects.
During the delivery process, GFs are prone to being degraded into proteins by enzymes [96]. However, the hydrogel absorbs water and expands, forming a hydrophilic environment that prevents the inactivation of GFs [97]. This enables the precise release at the damaged site. Lv et al. [98] developed a dual-mode hydrogel designated as CSP-LB. This composite material consists of a CS/silk fibroin(SF) hydrogel loaded with platelet-derived growth factor-BB (PDGF-BB) as the matrix, incorporated with bone morphogenetic protein-2 (BMP-2)-functionalized two-dimensional layered double hydroxide (MgFe-LDH) nanosheets. The addition of nanosheets significantly improved the mechanical strength of the CSP-LB hydrogel, while simultaneously enhancing its thermosensitivity and reducing the gelation temperature. The weak electrostatic attraction between PDGF-BB and CS promoted rapid release, whereas the strong interaction between BMP-2 and LDH resulted in sustained release, maintaining prolonged growth factor activity during treatment. The hydrogel demonstrated superior osteogenic properties in critical-sized calvarial defects (Figure 5). Basic fibroblast growth factor (bFGF) induces BMSC differentiation into neuronal cells while enhancing vascular endothelial growth factor (VEGF) expression [99,100], although its therapeutic potential is constrained by rapid metabolic clearance and instability. Chu et al. [101] overcame these limitations by grafting NIPAm onto sodium alginate chains through PNIPAm-based free radical copolymerization, then integrating bFGF/dexamethasone (Dex)-loaded liposomes prepared via thin-lipid film hydration. The resulting thermosensitive bFGF/Dex lipo-gel combines manufacturing scalability with biocompatibility. Within this system, hydrophilic bFGF is encapsulated in the liposomal aqueous phase, while hydrophobic Dex is incorporated into the lipid bilayer, resulting in sequential release kinetics. This dual-compartment architecture sustained prolonged bFGF elution alongside controlled Dex delivery. In cranial defect models, the lipo-gel significantly enhanced angiogenesis, neural regeneration, and osseous repair.
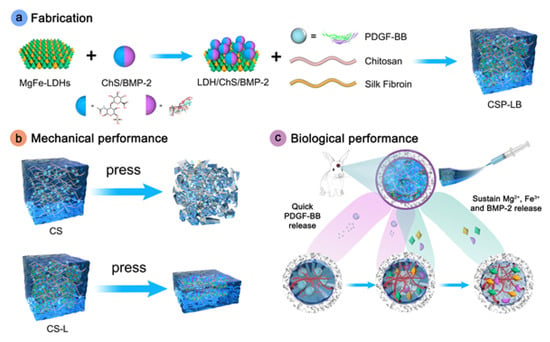
Figure 5.
Preparation and functional schematic diagram of CSP-LB hydrogel. Reprinted with permission from [98].
Bacteria and inflammatory microenvironments are common etiological factors of bone defects, making infection control particularly crucial. Thermosensitive hydrogels with strong adhesive properties play a pivotal role in the repair of inflammatory bone defects. These systems are typically administered via injection, filling, or surgical implantation to achieve localized drug release within the defect area, thereby exerting anti-inflammatory, antibacterial, and osteogenic effects. FDA-approved F127 thermosensitive hydrogel serves as an effective carrier for anti-inflammatory and antibacterial agents [102], although its limited bone adhesion restricts clinical utility. Almoshari et al. [103] addressed this limitation by developing a pyrophosphorylated Pluronic F127 composite incorporating BIO, a glycogen synthase kinase 3 beta inhibitor with dual anti-inflammatory and osteogenic activity, which demonstrated stable bone adhesion. The micellar encapsulation system enhanced the solubility of hydrophobic BIO, while enabling controlled drug release through a combination of surface erosion and diffusion mechanisms. In vivo studies confirmed that the PF-127-BIO hydrogel promoted the expression of β-catenin-positive cells, reduced inflammatory cell infiltration, and protected the alveolar bone and periodontal ligament structures. Similarly, to achieve the three interrelated objectives of antibacterial action, anti-inflammatory effects, and periodontal regeneration in periodontitis treatment strategies, Yang et al. [104] developed a thermosensitive and adhesive SFD/CS/ZIF-8@QCT hydrogel. This material was prepared by mixing dopamine-modified SF (SFD) with CS/β-GP and encapsulating quercetin (QCT)-modified zeolitic imidazolate framework-8 (ZIF-8). Studies demonstrated that after 24 h of gelation at physiological temperature, the free amino groups in CS and SFD underwent covalent double-crosslinking under the action of genipin, further enhancing the material’s adhesive properties. This modification provided optimal conditions for prolonged retention within periodontal pockets, resistance to gingival crevicular fluid, and sustained release of QCT and Zn2+ from ZIF-8. The SFD/CS/ZIF-8@QCT system meets the criteria for antibacterial, anti-inflammatory, and osteogenic activity. It exhibits excellent repair outcomes in inflammatory alveolar bone defects and holds great potential for clinical application (Figure 6).
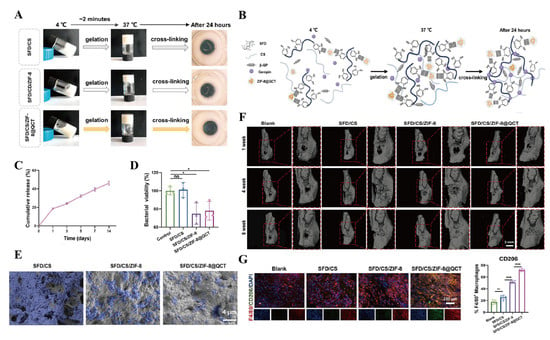
Figure 6.
SFD/CS/ZIF-8@QCT hydrogel for bone tissue engineering. (A) Optical photograph of SFD/CS/ZIF-8@QCT after gelation for 24 h. (B) Molecular crosslinking diagram of SFD/CS/ZIF-8@QCT. (C) The sustained-release effect of QCT. (D) Quantitative analysis of the viability of P. gingivalis after 24 h of treatment. (E) SEM images of P. gingivalis after 48 h of treatment. (F) The 3D reconstructed images of inflammatory alveolar socket repair.(The red squares refers to the local magnification of alveolar bone defects implanted with different composite hydrogels.) (G) CD206 immunofluorescence staining and quantitative analysis. Data are presented as mean ± SD. * p < 0.05, ** p < 0.01, and *** p < 0.001; ns, not significant. Reproduced with permission from Ref. [104], Elsevier.
Bone defects that occur in patients with diabetes due to the influence of a high-glucose environment are often difficult to repair. Currently, the conventional treatment of diabetic bone defects requires a multidisciplinary approach, including systemic and local infection control, blood glucose regulation, and promotion of local bone growth [105]. However, the delivery of bioactive substances through thermosensitive hydrogels provides a new strategy for treating diabetic bone defects. A bioactive compound called Coenzyme Q10 (CoQ10), used to improve alveolar sockets, can protect cells from damage and promote osteoblast proliferation and differentiation [106]. Ghanem’s team [107] developed a clinically viable CoQ10/collagen thermosensitive hydrogel by dispersing CoQ10 into an F127 solution and mixing it with a neutralized collagen solution (pH 7.4). The researchers recruited 18 patients with type II diabetes mellitus who were scheduled for single or multiple tooth extractions and implanted the composite material post-extraction. Histological and RT-qPCR analyses confirmed enhanced osteogenic activity in the CoQ10-treated group. The controlled CoQ10 release also markedly reduced post-operative pain and inflammation in these patients. The successful development and clinical implementation of this material broadens therapeutic approaches for diabetic bone defect repair. Surface area and diffusion rate are the main factors affecting the release of protein materials from hydrogels, and generally, the release rate is relatively fast. However, incorporating proteins into a crystalline matrix can further slow the release rate. Sheng et al. [108] adopted an innovative approach: they loaded bone morphogenetic protein-4 (BMP-4) onto graphene oxide (GO) and encapsulated it using the porcine small intestinal submucosa (SIS) as an ECM material, thus preparing a GB@SIS hydrogel with temperature-sensitive properties. The presence of GO and the external hydrogel introduces an additional step for BMP-4 release, thereby achieving its slow and uniform release. BMP-4 synergizes with ECM components in SIS to effectively suppress the NLRP3 signaling pathway and upregulate the expression levels of adhesion-related proteins. Through these mechanisms, the hydrogel demonstrates remarkable efficacy in modulating immune responses and promoting multifunctional osteogenesis, providing robust support for the repair of diabetic bone defects. The application scope and structural design of thermosensitive hydrogels in bone tissues are shown in Table 2.

Table 2.
Thermosensitive hydrogels for bone tissues.
4.2. Application of Thermosensitive Hydrogels in Cartilage Tissues
Trauma, functional degeneration and inflammation are all causes of cartilage damage. Among them, inflammatory factors are the most common. Osteoarthritis (OA) is characterized by subchondral bone remodelling, synovial inflammation, and cartilage degeneration, making it one of the most prevalent chronic degenerative joint diseases [119]. Thermosensitive hydrogels exhibit significant potential in the treatment of OA. When the thermosensitive hydrogel delivery system is locally injected into the joint cavity, it gels at physiological temperatures to achieve material retention, thereby prolonging the drug release time, enhancing drug targeting, and enabling the repair and regeneration of the joint cartilage area [120].
Radiosynoviorthesis refers to the intra-articular administration of radiopharmaceutical agents to alleviate synovial inflammation and reduce cartilage damage [121]. However, potential leakage of radioactive isotopes remains a challenge in current practice. Liu et al. [122] developed a thermosensitive hydrogel as a novel radiopharmaceutical delivery system (177Lu/AMP@CG). The system utilized self-assembled nanoparticles composed of adenosine monophosphate (AMP) ligands and the isotope-labeled metal ion 177Lu3+, which were incorporated into a CS/β-GP hydrogel to achieve prolonged intra-articular retention post-injection. The nanoparticles exhibited sustained-release characteristics, maintaining 86% of their radioactivity after 16 days in the joint space. This 177Lu/AMP@CG hydrogel reduced radioactive leakage risks while simultaneously delivering anti-inflammatory and chondroprotective effects, markedly enhancing OA treatment outcomes. N-acetyl-D-glucosamine (GlcNAc) is a nutritional supplement that can activate chondrocytes. Chang et al. [123] prepared a continuous delivery of GlcNAc based on F127, providing a promising strategy for enhancing cartilage repair. This hydrogel system prolonged GlcNAc release, improving its therapeutic effects in cartilage injury models. In rats with articular cartilage damage, intra-articular injection of GlcNAc hydrogel significantly improved joint function, as evidenced by increased step length, reduced foot angle, and delayed fall time. The hydrogel promoted cartilage regeneration by upregulating Sox9 and collagen II, while reducing chondrocyte apoptosis by approximately 50% through the suppression of cleaved caspase 3 and caspase 8. This hydrogel is easy to prepare, safe, and reliable and is expected to be further utilized in the clinical treatment of cartilage defects.
Thermosensitive hydrogels that achieve stem cell recruitment by releasing bioactive substances represent an innovative strategy for promoting tissue regeneration, especially in challenging environments such as articular cartilage defects. Yuan et al. [124] established a dual-drug delivery system, HSDKN, containing stromal cell-derived factor-1α-like polypeptide (SDFP) and kartogenin (KGN). This material first loads KGN into the hydrophobic cavity of aldehyde-modified β-cyclodextrin (OCD) through the interaction between the host and guest. Then, the two are fixed by the Schiff base reaction between the aldehyde in OCD and the amino groups in the hydroxypropyl chitosan hydrogel (HPCH). Subsequently, SDFP is physically mixed into the gel to prepare the final drug-loaded gel. The release of the loaded drugs synergistically promotes stem cell recruitment and cartilage differentiation. SDFP was critical in early-stage MSCs homing, addressing the limited endogenous cell recruitment in cartilage injuries. Meanwhile, KGN promoted chondrogenic differentiation, synergistically improving cartilage repair. In vivo studies confirmed the ability to accelerate cartilage regeneration in rat models (Figure 7). This dual-drug HPCH system offered a promising strategy for cartilage repair by overcoming key challenges in stem cell recruitment and differentiation.
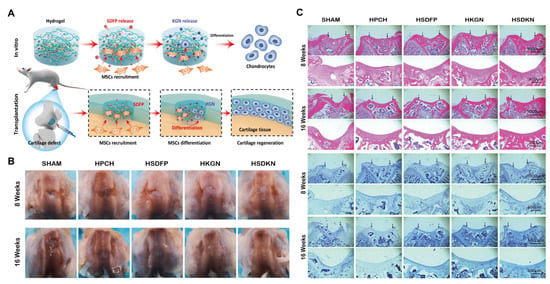
Figure 7.
HSDKN hydrogel for cartilage tissue engineering. (A) Schematic diagram of HSDKN hydrogel promoting stem cell recruitment and cartilage differentiation through bioactive substances release. (B) Macroscopic diagram of knee cartilage healing. (C) Histological evaluation of knee cartilage healing-H&E staining and toluidine blue staining (Black arrows indicate the defect area.) (HSDFP, HPCH mix with 2 μg/mL SDFP; HKGN, HPCH/OCD-KGN 200; HSDKN, HPCH/OCD-KGN 200 mix with μg/mL SDFP;). Reproduced with permission from Ref. [124], Elsevier.
Rheumatoid arthritis (RA) is a chronic autoimmune disorder driven by dysregulated immune responses. These responses induce persistent synovial inflammation, which clinically presents as joint stiffness, pain, and swelling, ultimately leading to progressive cartilage and bone destruction [125,126]. Toll-like receptor 4 (TLR4)-mediated signaling plays a central role in RA’s immune pathogenesis [127]. Targeted delivery of receptor inhibitors through advanced systems may offer therapeutic benefits for RA. The Lee team [128] developed cyclic phage-display-derived inhibitory peptides (CP) as TLR4 antagonists, which were subsequently conjugated to hyaluronic acid (HA-CP) and incorporated into methoxy polyethylene glycol-b-poly(ε-caprolactone)-ran-poly(lactide) (PC) hydrogel to form the composite material PC + (HA-CP). Compared with the physically blended PC + (HA + CP) material, the chemically conjugated formulation demonstrated prolonged in vivo retention time and enhanced CP persistence. In preclinical arthritis models, this system demonstrated significant anti-inflammatory effects, as evidenced by the marked suppression of pro-inflammatory mediators and the enhanced restoration of the cartilage ECM. These findings establish a robust methodological framework for the targeted intra-articular delivery of therapeutic peptides, addressing critical challenges in pharmacology and expanding the therapeutic landscape for degenerative joint disorders. The scope and organization of thermosensitive hydrogels in cartilage tissues are shown in Table 3.

Table 3.
Thermosensitive hydrogels for cartilage tissues.
4.3. Application of Thermosensitive Hydrogels in Other Tissues
4.3.1. Skin Injuries
The skin, as the largest organ of the human body, is crucial for maintaining physiological homeostasis and protecting internal organs from external insults. It serves as the primary barrier against external pathogens [139]. Furthermore, skin injuries are often accompanied by inflammatory responses, which further complicate the repair process. The highly adhesive thermosensitive hydrogel delivery system is commonly used for skin defect repair, and the administration methods are diverse (such as injection, application, spraying, or dressing) [140].
Crocin-1 (CRO-1), an active bioactive compound extracted from Crocus sativus L., exhibits potent anti-inflammatory and antioxidant properties. Lv et al. [141] prepared a CRO solution and incorporated it into hydroxybutyl chitosan (HBC) solution, followed by water bath heating to form CRO-HBC hydrogel. This material initially exhibited a burst release of CRO-1 to regulate ROS levels at the wound site, followed by sustained release to prolong the therapeutic efficacy in full-thickness burn wounds. Additionally, the combination of therapeutic agents and metal ions offers synergistic benefits for treating chronic, non-healing wounds, such as those associated with diabetes. A MC/carboxymethyl chitosan(CMC)-based thermosensitive hydrogel incorporating metal–organic framework ZIF-8 loaded with curcumin (Cur) and Zn2+ has been reported [142]. This hydrogel formed a protective gel barrier at temperatures ≥28 °C and exhibited controlled release of Cur in response to the wound’s acidic environment (pH 5.2). The experimental results demonstrated enhanced diabetic wound healing through a coordinated therapeutic cascade that reduced oxidative stress and modulated immune responses by switching macrophage phenotypes.
In skin wound repair, sprayable gelation provides an efficient and practical therapeutic approach. Pan et al. [143] designed a photothermal hydrogel system by integrating ILGA nano-hybrids—liposome-encapsulated imipenem modified with a gold shell and lipopolysaccharide aptamer—into a PLGA-PEG-PLGA hydrogel matrix. The composite material forms a sprayable gel that solidifies rapidly under NIR-II laser irradiation, exhibiting hemostatic, antibacterial, and anti-infective effects with promising clinical utility.
4.3.2. Tumor
Malignant tumors, as a major global public health challenge, exhibit limited therapeutic efficacy with conventional combination therapies in clinical practice [144]. The core challenge lies in achieving an optimal spatiotemporal distribution of free drug molecules in vivo and precisely coordinating the co-delivery and controlled release of multiple drugs at targeted sites [145]. In recent years, thermosensitive hydrogels have been combined with nanomaterials. Through intratumoral injection, subcutaneous injection, and perfusion, the materials are implanted, effectively achieving controlled drug release and bringing new hope to tumor treatment [146].
Li et al. [147] innovatively developed a thermosensitive hydrogel/nanogel composite system (Gel/REG + NG/LY). This system was formed by incorporating both transforming growth factor β inhibitor LY3200882 (LY) encapsulated in nanogels and free regorafenib (REG) into a methoxy poly(ethylene glycol)-block-poly(l-alanine) hydrogel, thereby establishing a foundation for sequential drug release. Studies demonstrated that this material preferentially releases REG to suppress tumor proliferation while upregulating ROS levels in the tumor microenvironment, subsequently triggering the responsive release of LY to block immune evasion pathways. This cascade release strategy remodels the tumor immune microenvironment by modulating macrophage polarization and T-cell infiltration, thereby significantly inhibiting both primary tumor progression and distant metastasis. Furthermore, certain tumors, such as glioblastoma (GBM), exhibit poor responsiveness to postoperative radiotherapy and chemotherapy. Kang et al. [148] used poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) hydrogel as the base and loaded doxorubicin (Dox) and T7 peptide through the nanoprecipitation method and grafting techniques. They also added water-rich ferromagnetic iron oxide nanotubes to the gel to prepare a hydrogel nanocomposite. Following administration at the tumor resection site, this system rapidly forms a deep-seated drug depot, and under mild hyperthermia, its unique magnetic field-responsive properties enable controlled deep-tissue drug penetration. This hydrogel nanocomposite has improved the survival rate of the GBM model, providing an innovative solution for removing deep-infiltrating tumor cells after surgery (Figure 8). Low-grade non-muscle-invasive bladder cancer (LG-NMIBC) is characterized by the absence of tumor cell invasion into the deep layers of the bladder wall. The current standard treatment, transurethral resection of the bladder tumor, carries risks including bladder injury, postoperative hemorrhage, and frequent recurrence. Stover et al. [149] evaluated UGN-102, a thermosensitive PLGA-PEG-PLGA hydrogel encapsulating mitomycin, administered via intravesical instillation as a non-surgical alternative. This sustained-release system enhanced local drug exposure while minimizing systemic effects. Follow-up assessments revealed no abdominal distension or flatulence, with a 65% complete response rate at three months and no observed recurrence. As an effective, low-toxicity, non-surgical first-line therapy, UGN-102 gel therapy provides a novel therapeutic option for cancer treatment.
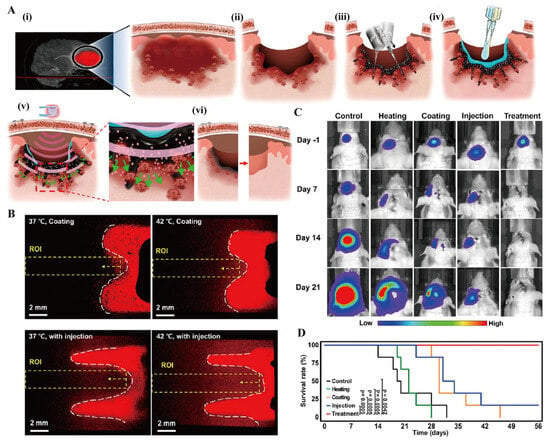
Figure 8.
Hydrogel nanocomposite removes deeply infiltrating tumor cells. (A) Schematic diagram of the GBM treatment procedure using a hydrogel nanocomposite. (i) Detection of GBM, (ii) surgical removal, (iii) injection material, (iv) bioglue coating reduces drug leakage, (v) periodic magnetic activation under mild hyperthermia, and (vi) biodegradation.(Green arrows indicate the diffusion of micelles and drugs from hydrogel nanocomposite into the postoperative margin in the deep brain region.) (B) Fluorescence observation of the diffusion of Dox after nanocomposite coating and injection(red) onto the surface of artificial tissues(black) at different temperatures. (C) The results of luminescence imaging of the GBM volume after treatment (Control, injection of PBS; Heating, injection of the material without drug but with mild hyperthermia; Coating, coating of the material without mild hyperthermia; Injection, injection of the material without mild hyperthermia; and Treatment, injection of the material with mild hyperthermia. All groups underwent surgical resection of GBM). (D) Kaplan–Meier survival analysis for each group (by log-rank test). Reprinted with permission from [148]. Copyright 2023, American Chemical Society.
4.3.3. Tendon–Bone Interface
The tendon–bone interface is a crucial junction between muscle and bone, maintaining the body’s motor function. The postoperative inflammatory environment after traditional surgery often leads to delayed remodeling of the tendon–bone interface [150]. Through metal coordination crosslinking, thermosensitive hydrogels capable of sustained metal ion release offer a promising strategy for accelerating tendon–bone interface repair. Li et al. [151] used F127 and dopamine-modified HA as the base, incorporating magnesium-procyanidin coordinated metal polyphenol nanoparticles (Mg-PC). Crosslinking Mg-PC through catechol coordination bonds within the hydrogel further enhanced the material’s mechanical strength, adhesiveness, and sustained ion release. Studies demonstrated that the release of procyanidins promotes M2 macrophage polarization. Additionally, magnesium ions facilitate cartilage matrix production through the TRPM7 channel protein. The therapeutic effect of this material was verified by injecting it into the shoulder tendon tear site of rats, providing a practical approach to accelerate the recovery of the tendon–bone interface after reconstructive surgery (Figure 9).
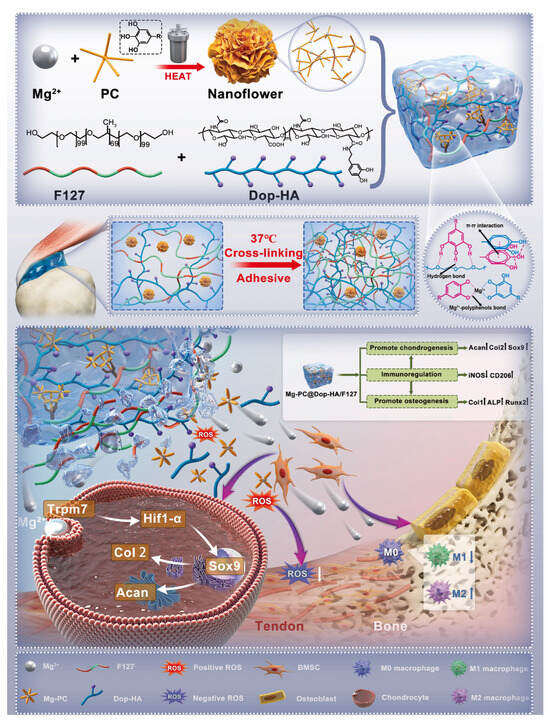
Figure 9.
Schematic diagram of Mg2+ crosslinking mode and mechanism of action of Mg-PC@Dop-HA/F127 hydrogel. Reproduced with permission from Ref. [151], Elsevier.
4.3.4. Muscle
Peripheral artery disease is characterized by its most severe manifestation, limb ischemia, which leads to widespread skeletal muscle damage and alterations in homeostasis [152]. Bioactive substances such as GFs can promote vascular regeneration, but the efficacy of systemic administration is limited [153]. The thermosensitive hydrogel delivery system is usually administered by injection or implantation, which is more convenient and practical. Niu et al. [154] employed benzoyl peroxide as an initiator to polymerize PNIPAm, 2-hydroxyethyl methacrylate radically, and acrylated 4-(hydroxymethyl)-phenylboronic acid pinacol ester, forming an injectable thermosensitive hydrogel carrier. This carrier encapsulates VEGF and the Notch ligand Delta-like 4 (DII4). During gel degradation, the gradual release of encapsulated VEGFR2, Notch-1, and Erk 1/2 activated relevant signaling pathways, promoting robust microvascular formation in ischemic limbs. In a clinical mouse model of critical limb ischemia, this approach significantly promoted the proliferation of muscle cells, effectively increased muscle fiber diameter, and facilitated muscle tissue regeneration, demonstrating great potential for clinical application (Figure 10).
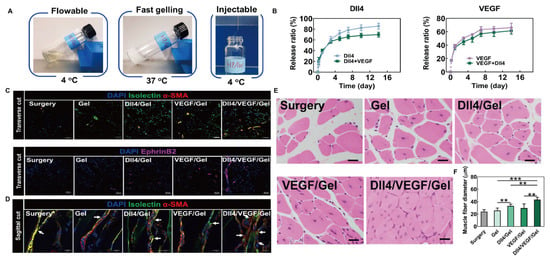
Figure 10.
DII4/VEGF/Gel hydrogel for muscle tissue engineering. (A) Schematic diagram of DII4/VEGF/Gel gelation and injection. (B) The in vitro release rates of DII4 and VEGF. (C) The transverse cut of ischemic hind limb tissues was stained with isolectin, α-SMA, and EphrinB2, to observe the formation of blood vessels and small arteries 14 days after surgery (isolectin, α-SMA, and EphrinB2 are the markers of endothelial cells, vascular smooth muscle cells, and arterial endothelial cells, respectively). Scale bar = 50 μm. (D) The sagittal cut of ischemic hind limb tissues was stained with isolectin, α-SMA to observe the number of vessel branches 14 days after surgery (White arrows indicate the vessel branching.). Scale bar = 40 μm. (E) H&E staining of ischemic limb tissue samples at 14 days. Scale bar = 20 μm. (F) Quantitative analysis of muscle fiber diameter. ** p < 0.01, *** p < 0.001. Reprinted from Acta Biomaterialia, 143, Hong Niu(s), Delivery of VEGF and delta-like 4 to synergistically regenerate capillaries and arterioles in ischemic limbs, 15., Copyright (2022), with permission from Elsevier [154].
4.3.5. Nerve
Spinal cord injury (SCI), a severe central nervous system pathology, induces primary neuronal death and subsequent tissue cavitation [155]. The thermosensitive hydrogel’s neural tissue-matching elastic modulus prevents nerve compression while facilitating drug delivery through injection or nasal administration. This combination of mechanical compatibility and sustained release properties offers a minimally invasive therapeutic approach for SCI.
The induction of stem cell differentiation into neurons holds great promise for treating SCI. Albashari et al. [156] engineered a thermosensitive injectable hydrogel based on F127, denoted as PF-OMSF@JK. This hydrogel achieved controlled and sustained release of H2S by incorporating octylsilane-functionalized mesoporous silica nanoparticles with the micromolecule H2S donor, JK. This biomimetic system demonstrated optimal mechanical compatibility with neural tissue and maintained excellent viability of encapsulated dental pulp stem cells (DPSCs). The continuous release of H2S modulates the expression of genes related to neuronal differentiation, thereby inducing the differentiation of DPSCs into neurons. Essentially, it achieves a driving effect by controlling local inflammation, which is conducive to attenuating neuroinflammation and repairing neural tissue in rat SCI models.
Similarly, Wang et al. [157] developed a hydrogel by combining GNA12/GNA13-overexpressing exosomes from engineered macrophages with CS/β-GP, creating an intranasally deliverable material. The hydrogel forms a sustained-release depot at the nasal mucosa, thereby avoiding hepatic and renal clearance and bypassing the blood brain barrier. These exosomes induce macrophage reprogramming toward anti-inflammatory M2c phenotypes. G12G13MExos@Hydrogel significantly reduced neuronal apoptosis while enhancing V2a interneuron differentiation from neural stem cells. This approach achieved effective spinal cord injury repair through a clinically feasible administration method. The scope and organization of thermosensitive hydrogels in other tissues are shown in Table 4.

Table 4.
Thermosensitive hydrogels for other tissues.
5. Challenge and Prospects
Thermosensitive hydrogels undergo sol–gel transitions in response to temperature variations, offering a tunable and mild response mechanism. Their precise spatiotemporal control enhances the efficiency of drug delivery while facilitating targeted release at pathological sites. These properties position thermosensitive hydrogels as a versatile platform for biomedical applications. Despite the remarkable potential of thermosensitive hydrogels in tissue engineering applications, their performance varies significantly across different tissue repair scenarios. To systematically evaluate their key advantages and major challenges, this review summarizes the core characteristics of thermosensitive hydrogels in common tissue repair and treatment (Table 5). This comparative analysis highlights some common issues in thermosensitive hydrogel materials, namely, stability, biological safety, and degradation rate. For thermosensitive hydrogels, the incorporation of nanomaterials or surface coatings can enhance their structural stability [188], while expanding in vivo studies across multiple species remains crucial to validate material safety.

Table 5.
The advantages and challenges of thermosensitive hydrogels for tissue repair.
Currently, four-dimensional (4D) bioprinting represents a significant advancement in the development of thermosensitive hydrogels. This innovative technique builds upon conventional 3D printing by incorporating materials that respond to environmental stimuli, such as temperature, humidity, or light, thereby facilitating autonomous shape transformation and structural reconfiguration [189]. Thermoresponsive hydrogel-based bioinks serve as key components in 4D printing systems. Nakamura et al. [190] engineered a granular HA ink exhibiting temperature-dependent phase transitions, which permitted controlled sequential actuation in multimaterial constructs. The porous structure of the material undergoes reversible changes in response to temperature variations, while maintaining stable deformation capabilities over repeated heating and cooling cycles, with mechanical properties optimized to the greatest extent. Similarly, Li et al. [191] utilized shear forces during extrusion-based printing to align short carbon fibers within PNIPAm. Upon temperature changes, the hydrogel exhibited differential swelling and shrinkage rates parallel and perpendicular to the carbon fiber orientation. This anisotropic behavior generated internal stresses, allowing flexible modulation of the hydrogel’s deformation pathway. Thermosensitive hydrogels integrated with 4D printing have shown particular promise for tissue engineering applications. Wang et al. [192] developed a CS/CMC crosslinked bioink, where temperature variations triggered the scaffold’s transformation from its printed state into a structure matching corneal curvature. The method resolved uneven pore distribution in traditional hydrogels while optimizing cell loading efficiency. Temperature-induced gelation allowed in situ corneal adaptation without sutures, providing both mechanical support and tunable degradation rates for stable tissue regeneration.
We anticipate broader applications of 4D-printed thermosensitive hydrogels in tissue engineering repair, leveraging their superior shape-morphing capability and structural stability to develop more advantageous clinical treatment strategies. However, further developments necessitate collaborative efforts among researchers in tissue engineering, medicine, and additive manufacturing to optimize formulations, enhance scalability, and promote the continued evolution of thermosensitive hydrogels.
6. Conclusions
This review summarizes recent research progress in thermosensitive hydrogels, elucidating the gelation mechanisms of common thermosensitive hydrogel systems. Multiple optimization strategies are proposed to enhance their adhesion, mechanical strength, and multifunctionality. In the applications of bone, cartilage, and other tissue repair, we have particularly emphasized the mechanism of the thermosensitive hydrogel delivery system in substance delivery and controlled release, as well as its therapeutic effects in practical applications, highlighting its potential for development. Finally, potential development strategies are provided to address the current limitations of thermosensitive hydrogel materials. Looking ahead, the development of smarter and more responsive thermosensitive hydrogels holds great promise for advancing tissue healing and regeneration.
Author Contributions
T.L. and Y.C. equally contributed to this work. Conceptualization, Y.G. and K.H.; writing—original draft preparation, T.L. and Y.C.; visualization, N.L., X.L. and Y.H.; writing—review and editing, Y.G. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shaanxi Science and Technology Development Plan Projects, grant number 2024SF-YBXM-434; Major special project of Qin Chuang Yuan, grant number 23LLRHZDZX0010; General Project of Shaanxi Natural Science Foundation, grant number 2025JC-YBQN-1274; and The Project for Enhancing the Research and Innovation Capacity of Health Sciences in Shaanxi Province, grant number 2025YF-44.
Acknowledgments
The authors sincerely appreciate the financial support provided by the aforementioned funding sources. Some of the figures in this review were drawn by Figdraw2.0 (www.figdraw.com, accessed on 12 July 2025), and we would like to thank the Figdraw platform. We would like to thank Xiangxiang Hu from the Department of Stomatology, The Ohio State University, USA, for his guidance on the language expression of this review.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this review.
References
- Hong, S.-C.; Yoo, S.-Y.; Kim, H.; Lee, J. Chitosan-Based Multifunctional Platforms for Local Delivery of Therapeutics. Mar. Drugs 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Beaman, F.D.; Bancroft, L.W.; Peterson, J.J.; Kransdorf, M.J. Bone Graft Materials and Synthetic Substitutes. Radiol. Clin. N. Am. 2006, 44, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, C.; Yao, M.; Wang, X.; Wang, J.; Zhang, W.; Chen, W.; Lv, H. Advances in Osseointegration of Biomimetic Mineralized Collagen and Inorganic Metal Elements of Natural Bone for Bone Repair. Regen. Biomater. 2023, 10, rbad030. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Saltzman, W.M. Controlled Release for Local Delivery of Drugs: Barriers and Models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef]
- Li, B.-Y.; Lin, T.-Y.; Lai, Y.-J.; Chiu, T.-H.; Yeh, Y.-C. Engineering Multiresponsive Alginate/PNIPAM/Carbon Nanotube Nanocomposite Hydrogels as On-Demand Drug Delivery Platforms. Small 2025, 21, e2407420. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Jiang, Y.; Sun, J.; Zheng, W.; Qian, H.; Sun, J.; Hu, W. Controlled Delivery of MTX from Thermosensitive Hydrogels Using Au Nanorods for Enhanced Therapeutics of Psoriasis via Improving Stratum Corneum Penetration. Small 2025, 21, e2410566. [Google Scholar] [CrossRef]
- Mi, B.; Mu, J.; Ding, X.; Guo, S.; Hua, X. Responsive Microneedles for Diagnostic and Therapeutic Applications of Ocular Diseases. Small Methods 2025, 2402048. [Google Scholar] [CrossRef]
- Liu, T.; Fu, J.; Zheng, Z.; Chen, M.; Wang, W.; Wu, C.; Quan, G.; Pan, X. Active Microneedle Patch Equipped with Spontaneous Bubble Generation for Enhanced Rheumatoid Arthritis Treatment. Theranostics 2025, 15, 3424–3438. [Google Scholar] [CrossRef]
- Wang, C.; Dong, Z.; Zhang, Q.; Guo, M.; Hu, W.; Dong, S.; Jakkree, T.; Lu, Y.; Du, S. Stimulus-Responsive Nano Lipidosome for Enhancing the Anti-Tumour Effect of a Novel Peptide Dermaseptin-PP. IET Nanobiotechnol. 2023, 17, 352–359. [Google Scholar] [CrossRef]
- Guo, W.; Dong, H.; Wang, X. Emerging Roles of Hydrogel in Promoting Periodontal Tissue Regeneration and Repairing Bone Defect. Front. Bioeng. Biotechnol. 2024, 12, 1380528. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent Advances in Conductive Hydrogels: Classifications, Properties, and Applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive Hydrogels for Sustained-Release of Sorafenib and Selenium Nanoparticles for Localized Synergistic Chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.; Quadir, M.A.; Strumia, M.; Haag, R. Functional Dendritic Polymer Architectures as Stimuli-Responsive Nanocarriers. Biochimie 2010, 92, 1242–1251. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel Thermo-Sensitive Hydrogel System with Paclitaxel Nanocrystals: High Drug-Loading, Sustained Drug Release and Extended Local Retention Guaranteeing Better Efficacy and Lower Toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef]
- Shim, W.S.; Kim, J.-H.; Park, H.; Kim, K.; Chan Kwon, I.; Lee, D.S. Biodegradability and Biocompatibility of a pH- and Thermo-Sensitive Hydrogel Formed from a Sulfonamide-Modified Poly(Epsilon-Caprolactone-Co-Lactide)-Poly(Ethylene Glycol)-Poly(Epsilon-Caprolactone-Co-Lactide) Block Copolymer. Biomaterials 2006, 27, 5178–5185. [Google Scholar] [CrossRef]
- Liu, F.; Seuring, J.; Agarwal, S. A Non-Ionic Thermophilic Hydrogel with Positive Thermosensitivity in Water and Electrolyte Solution. Macromol. Chem. Phys. 2014, 215, 1466–1472. [Google Scholar] [CrossRef]
- Crespy, D.; Rossi, R.M. Temperature-responsive Polymers with LCST in the Physiological Range and Their Applications in Textiles. Polym. Int. 2007, 56, 1461–1468. [Google Scholar] [CrossRef]
- Belal, K.; Stoffelbach, F.; Lyskawa, J.; Fumagalli, M.; Hourdet, D.; Marcellan, A.; Smet, L.D.; de la Rosa, V.R.; Cooke, G.; Hoogenboom, R.; et al. Recognition-Mediated Hydrogel Swelling Controlled by Interaction with a Negative Thermoresponsive LCST Polymer. Angew. Chem. Int. Ed. Engl. 2016, 55, 13974–13978. [Google Scholar] [CrossRef]
- Zhao, H.; An, H.; Xi, B.; Yang, Y.; Qin, J.; Wang, Y.; He, Y.; Wang, X. Self-Healing Hydrogels with Both LCST and UCST through Cross-Linking Induced Thermo-Response. Polymers 2019, 11, 490. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive Hydrogels in Biomedical Applications: A Seven-Year Update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-Responsive Polymers and Their Application as Smart Biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Leroux, J.-C. In Situ-Forming Hydrogels—Review of Temperature-Sensitive Systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuo, R. Synthesis of Temperature-Sensitive Poly(N-Isopropylacrylamide) Hydrogel with Improved Surface Property. J. Colloid Interface Sci. 2000, 223, 311–313. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering—a Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Buchecker, T.; Schmid, P.; Grillo, I.; Prévost, S.; Drechsler, M.; Diat, O.; Pfitzner, A.; Bauduin, P. Self-Assembly of Short Chain Poly- N-Isopropylacrylamid Induced by Superchaotropic Keggin Polyoxometalates: From Globules to Sheets. J. Am. Chem. Soc. 2019, 141, 6890–6899. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of Composition of PDMAEMA-b-PAA Block Copolymers on Their pH- and Temperature-Responsive Behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Maeda, Y.; Kubota, T.; Yamauchi, H.; Nakaji, T.; Kitano, H. Hydration Changes of Poly(2-(2-Methoxyethoxy)Ethyl Methacrylate) during Thermosensitive Phase Separation in Water. Langmuir 2007, 23, 11259–11265. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)Ethyl Methacrylate and Oligo(Ethylene Glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Grishkewich, N.; Akhlaghi, S.P.; Zhaoling, Y.; Berry, R.; Tam, K.C. Cellulose Nanocrystal-Poly(Oligo(Ethylene Glycol) Methacrylate) Brushes with Tunable LCSTs. Carbohydr. Polym. 2016, 144, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Schnell, C.N.; Galván, M.V.; Zanuttini, M.A.; Mocchiutti, P. Hydrogels from Xylan/Chitosan Complexes for the Controlled Release of Diclofenac Sodium. Cellulose 2020, 27, 1465–1481. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial Peptides-Loaded Smart Chitosan Hydrogel: Release Behavior and Antibacterial Potential against Antibiotic Resistant Clinical Isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef]
- Chenite, A. Rheological Characterisation of Thermogelling Chitosan/Glycerol-Phosphate Solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Kushan, E.; Senses, E. Thermoresponsive and Injectable Composite Hydrogels of Cellulose Nanocrystals and Pluronic F127. ACS Appl. Bio Mater. 2021, 4, 3507–3517. [Google Scholar] [CrossRef]
- White, J.M.; Calabrese, M.A. Impact of Small Molecule and Reverse Poloxamer Addition on the Micellization and Gelation Mechanisms of Poloxamer Hydrogels. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 638, 128246. [Google Scholar] [CrossRef]
- Hynninen, V.; Hietala, S.; McKee, J.R.; Murtomäki, L.; Rojas, O.J.; Ikkala, O. Nonappa Inverse Thermoreversible Mechanical Stiffening and Birefringence in a Methylcellulose/Cellulose Nanocrystal Hydrogel. Biomacromolecules 2018, 19, 2795–2804. [Google Scholar] [CrossRef]
- Xu, W.-K.; Tang, J.-Y.; Yuan, Z.; Cai, C.-Y.; Chen, X.-B.; Cui, S.-Q.; Liu, P.; Yu, L.; Cai, K.-Y.; Ding, J.-D. Accelerated Cutaneous Wound Healing Using an Injectable Teicoplanin-Loaded PLGA-PEG-PLGA Thermogel Dressing. Chin. J. Polym. Sci. 2019, 37, 548–559. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Haim-Zada, M.; Goldstein, I.A.; Goldberg, A.H.; Haber, T.; Berlin, J.M.; Domb, A.J. Effect of PLGA Block Molecular Weight on Gelling Temperature of PLGA-PEG-PLGA Thermoresponsive Copolymers. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 35–39. [Google Scholar] [CrossRef]
- Ma, L.; Shi, T.; Zhang, Z.; Liu, X.; Wang, H. Wettability of HPMC/PEG/CS Thermosensitive Porous Hydrogels. Gels 2023, 9, 667. [Google Scholar] [CrossRef]
- Kara, M.; Kocaaga, N.; Akgul, B.; Abamor, E.S.; Erdogmus, A.; Topuzogullari, M.; Acar, S. Micelles of Poly[Oligo(Ethylene Glycol) Methacrylate] as Delivery Vehicles for Zinc Phthalocyanine Photosensitizers. Nanotechnology 2024, 35, 475602. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.-K.; Wang, X.-P.; Guo, R.-H.; Zhong, M.; Xie, X.-M. Highly Stretchable and Super Tough Nanocomposite Physical Hydrogels Facilitated by the Coupling of Intermolecular Hydrogen Bonds and Analogous Chemical Crosslinking of Nanoparticles. J. Mater. Chem. B 2015, 3, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, R.; Xu, S.; Liu, H.; Hu, Y. Thermoresponsive Behavior and Rheology of SiO–Hyaluronic Acid/Poly(N-Isopropylacrylamide) (NaHA/PNIPAm) Core–Shell Structured Microparticles. J. Chem. Technol. Biotechnol. 2015, 90, 407–414. [Google Scholar] [CrossRef]
- Yan, X.; Huang, H.; Bakry, A.M.; Wu, W.; Liu, X.; Liu, F. Advances in Enhancing the Mechanical Properties of Biopolymer Hydrogels via Multi-Strategic Approaches. Int. J. Biol. Macromol. 2024, 272, 132583. [Google Scholar] [CrossRef]
- Hao, J.; Weiss, R.A. Mechanical Behavior of Hybrid Hydrogels Composed of a Physical and a Chemical Network. Polymer 2013, 54, 2174–2182. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and Interactions in Covalently and Ionically Crosslinked Chitosan Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Calder, D.; Fathi, A.; Oveissi, F.; Maleknia, S.; Abrams, T.; Wang, Y.; Maitz, J.; Tsai, K.H.-Y.; Maitz, P.; Chrzanowski, W.; et al. Thermoresponsive and Injectable Hydrogel for Tissue Agnostic Regeneration. Adv. Healthc. Mater. 2022, 11, 2201714. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; He, X.; Tian, N.; Ding, P.; Guo, W.; Okoro, O.V.; Sun, Y.; Jiang, G.; Liu, Z.; et al. Fabrication and Properties of Hydrogel Dressings Based on Genipin Crosslinked Chondroitin Sulfate and Chitosan. Polymers 2024, 16, 2876. [Google Scholar] [CrossRef]
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of Glutaraldehyde and Procyanidin Cross-Linked Scaffolds for Soft Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 263–273. [Google Scholar] [CrossRef]
- Ahammed, S.; Easdani, M.; Liu, F.; Zhong, F. Encapsulation of Tea Polyphenol in Zein through Complex Coacervation Technique to Control the Release of the Phenolic Compound from Gelatin-Zein Composite Film. Polymers 2023, 15, 2882. [Google Scholar] [CrossRef]
- Deng, Q.-S.; Gao, Y.; Rui, B.-Y.; Li, X.-R.; Liu, P.-L.; Han, Z.-Y.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; et al. Double-Network Hydrogel Enhanced by SS31-Loaded Mesoporous Polydopamine Nanoparticles: Symphonic Collaboration of near-Infrared Photothermal Antibacterial Effect and Mitochondrial Maintenance for Full-Thickness Wound Healing in Diabetes Mellitus. Bioact. Mater. 2023, 27, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, W.; Wu, J.; Huang, J.; Wang, W.; Peng, W.; Chen, J.; Bo, R.; Liu, M.; Li, J. Polyvinyl Alcohol/Chitosan Hydrogel Based on Deep Eutectic Solvent for Promoting Methicillin-Resistant Staphylococcus Aureus-Infected Wound Healing. Int. J. Biol. Macromol. 2025, 297, 139916. [Google Scholar] [CrossRef]
- Wu, Q.; Fu, Y.; Yang, W.; Liu, S. A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine. Gels 2022, 8, 570. [Google Scholar] [CrossRef]
- Nordin, N.; Zairul Azman, Z.A.; Adnan, N.A.; Majid, S.R. On the Dual Crosslinking for Functionality Enhancement of Poly (Acrylamide-Co-Acrylic Acid)/Chitosan-Aluminum (III) Ions and Its Characterization and Sensory Hydrogel Fibers. Int. J. Biol. Macromol. 2024, 274, 133383. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, C.; Lu, S.; Abushammala, H.; Gao, D.; Xu, P.; Niu, D.; Yang, W.; Ma, P. Skin-Inspired Polysaccharide-Based Hydrogels with Tailored Properties for Information Transmission Application. Int. J. Biol. Macromol. 2025, 306, 141354. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Du, H.; Zhao, Q.; Wang, S.; Liu, T.; Bi, S.; Zhang, Q.; An, M.; Wen, S. A Dynamically Cross-Linked Catechol-Grafted Chitosan/Gelatin Hydrogel Dressing Synergised with Photothermal Therapy and Baicalin Reduces Wound Infection and Accelerates Wound Healing. Int. J. Biol. Macromol. 2024, 273, 132802. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Wu, M.; Wang, X.; Zhao, Y.; Zhong, S.; Gao, Y.; Cui, X. Fe3+-Induced Coordination Cross-Linking Gallic Acid-Carboxymethyl Cellulose Self-Healing Hydrogel. Int. J. Biol. Macromol. 2024, 267, 131626. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Fu, Y.; Wang, N.N.; Zhan, S.Q.; Sheng, L.; Yang, H.Y.; Liu, C. Healable and Transparent Ionic Conductive Hydrogels Based on PNATF as Multiple-Signal Sensors. ACS Appl. Polym. Mater. 2025, 7, 2529–2540. [Google Scholar] [CrossRef]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive Ionic Conductive Alginate/Gelatin Organohydrogels with Tunable Functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar] [CrossRef]
- Torchio, A.; Boffito, M.; Laurano, R.; Cassino, C.; Lavella, M.; Ciardelli, G. Double-Crosslinkable Poly(Urethane)-Based Hydrogels Relying on Supramolecular Interactions and Light-Initiated Polymerization: Promising Tools for Advanced Applications in Drug Delivery. J. Mater. Chem. B 2024, 12, 8389–8407. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, Q.; Yang, Y.; Du, C.; Nie, Z. Dual Light-Responsive Shape Transformations of a Nanocomposite Hydrogel Sheet Enabled by in Situ Etching Shaped Plasmonic Nanoparticles. RSC Adv. 2024, 14, 34804–34810. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zeng, B.; Yang, W. Light Responsive Hydrogels for Controlled Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart Wound Dressing for Infection Monitoring and NIR-Triggered Antibacterial Treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, Q.; Zhang, Q.; Li, K.; Shi, X.; Liang, F.; Han, D. Temperature/near-Infrared Light-Responsive Conductive Hydrogels for Controlled Drug Release and Real-Time Monitoring. Nanoscale 2020, 12, 8679–8686. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-Responsive Liposomal Nanoformulations in Cancer Therapy: Pre-Clinical & Clinical Approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Yang, M.; Liu, L. MHC II Gene Knockout in Tissue Engineering May Prevent Immune Rejection of Transplants. Med. Hypotheses 2008, 70, 798–801. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2019, 7, 469. [Google Scholar] [CrossRef]
- Li, C.; Nyaruaba, R.; Zhao, X.; Xue, H.; Li, Y.; Yang, H.; Wei, H. Thermosensitive Hydrogel Wound Dressing Loaded with Bacteriophage Lysin LysP53. Viruses 2022, 14, 1956. [Google Scholar] [CrossRef]
- Koland, M.; Narayanan Vadakkepushpakath, A.; John, A.; Tharamelveliyil Rajendran, A.; Raghunath, I. Thermosensitive In Situ Gels for Joint Disorders: Pharmaceutical Considerations in Intra-Articular Delivery. Gels 2022, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Kansız, S.; Elçin, Y.M. Advanced Liposome and Polymersome-Based Drug Delivery Systems: Considerations for Physicochemical Properties, Targeting Strategies and Stimuli-Sensitive Approaches. Adv. Colloid Interface Sci. 2023, 317, 102930. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lin, T.; Khalaf, A.T.; Zhang, Y.; He, H.; Yang, L.; Yan, S.; Zhu, J.; Shi, Z. The Preparation and Application of Calcium Phosphate Biomedical Composites in Filling of Weight-Bearing Bone Defects. Sci. Rep. 2021, 11, 4283. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, J.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Peng, C.; Liu, H.; Wu, D. Functionalized Decellularized Bone Matrix Promotes Bone Regeneration by Releasing Osteogenic Peptides. ACS Biomater. Sci. Eng. 2023, 9, 4953–4968. [Google Scholar] [CrossRef]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Tashman, J.W.; Shiwarski, D.J.; Palchesko, R.N.; Feinberg, A.W. FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459. [Google Scholar] [CrossRef]
- Khaled Wassif, R.; Daihom, B.A.; Maniruzzaman, M. FRESH 3D Printing of Zoledronic Acid-Loaded Chitosan/Alginate/Hydroxyapatite Composite Thermosensitive Hydrogel for Promoting Bone Regeneration. Int. J. Pharm. 2024, 667, 124898. [Google Scholar] [CrossRef]
- Koskela, J.E.; Turunen, S.; Ylä-Outinen, L.; Narkilahti, S.; Kellomäki, M. Two-Photon Microfabrication of Poly(Ethylene Glycol) Diacrylate and a Novel Biodegradable Photopolymer—Comparison of Processability for Biomedical Applications. Polym. Adv. Technol. 2012, 23, 992–1001. [Google Scholar] [CrossRef]
- Fu, H.; Yu, B. 3D Micro/Nano Hydrogel Structures Fabricated by Two-Photon Polymerization for Biomedical Applications. Front. Bioeng. Biotechnol. 2024, 12, 1339450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, M.; Zhao, H.; Wang, X. 3D-Printed PNAGA Thermosensitive Hydrogelbased Microrobots: An Effective Cancer Therapy by Temperature-Triggered Drug Release. Int. J. Bioprint. 2023, 9, 709. [Google Scholar] [CrossRef]
- Saravanou, S.F.; Ioannidis, K.; Dimopoulos, A.; Paxinou, A.; Kounelaki, F.; Varsami, S.M.; Tsitsilianis, C.; Papantoniou, I.; Pasparakis, G. Dually Crosslinked Injectable Alginate-Based Graft Copolymer Thermoresponsive Hydrogels as 3D Printing Bioinks for Cell Spheroid Growth and Release. Carbohydr. Polym. 2023, 312, 120790. [Google Scholar] [CrossRef]
- Mansha, S.; Sajjad, A.; Zarbab, A.; Afzal, T.; Kanwal, Z.; Iqbal, M.J.; Raza, M.A.; Ali, S. Development of pH-Responsive, Thermosensitive, Antibacterial, and Anticancer CS/PVA/Graphene Blended Hydrogels for Controlled Drug Delivery. Gels 2024, 10, 205. [Google Scholar] [CrossRef]
- Liu, G.; Hu, D.; Chen, M.; Wang, C.; Wu, L. Multifunctional PNIPAM/Fe3O4-ZnS Hybrid Hollow Spheres: Synthesis, Characterization, and Properties. J. Colloid Interface Sci. 2013, 397, 73–79. [Google Scholar] [CrossRef]
- Kang, W.; Fu, S.; Li, W.; Wu, Y.; Li, H.; Wang, J. Design and Characterization of a ROS-Responsive Antibacterial Composite Hydrogel for Advanced Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2025, 294, 139349. [Google Scholar] [CrossRef]
- Fan, K.; Yang, D.; Zhu, X.; Zheng, L.; Han, Y.; Lin, J.; Xiang, Z.; Guo, Y.; Tao, K.; Li, J.; et al. High-Efficiency Antioxidant ROS-Responsive Thermosensitive Hydrogel Encapsulated Fenofibrate for the Treatment of Corneal Neovascularization. J. Control. Release 2025, 382, 113650. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.; Zheng, B.; Wu, P.; He, X.; Zhao, Y.; Zhong, Z.; Zhang, X.; Guan, J.; Wang, H.; et al. Lubricating and Dual-Responsive Injectable Hydrogels Formulated From ZIF-8 Facilitate Osteoarthritis Treatment by Remodeling the Microenvironment. Small 2025, 21, 2407885. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Pikos, M.A.; Nakamura, T.; Imafuji, T.; Zhang, Y.; Shinohara, Y.; Sculean, A.; Shirakata, Y. The Development of Non-Resorbable Bone Allografts: Biological Background and Clinical Perspectives. Periodontology 2000 2024, 94, 161–179. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zheng, H.; Guo, Y.; Heng, B.C.; Yang, Y.; Yao, W.; Jiang, S. A Three-Dimensional Actively Spreading Bone Repair Material Based on Cell Spheroids Can Facilitate the Preservation of Tooth Extraction Sockets. Front. Bioeng. Biotechnol. 2023, 11, 1161192. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic Roles of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- Ming, L.; Qu, Y.; Wang, Z.; Dong, L.; Li, Y.; Liu, F.; Wang, Q.; Zhang, D.; Li, Z.; Zhou, Z.; et al. Small Extracellular Vesicles Laden Oxygen-Releasing Thermosensitive Hydrogel for Enhanced Antibacterial Therapy against Anaerobe-Induced Periodontitis Alveolar Bone Defect. ACS Biomater. Sci. Eng. 2024, 10, 932–945. [Google Scholar] [CrossRef]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21. Front. Bioeng. Biotechnol. 2021, 9, 829136. [Google Scholar] [CrossRef]
- Tessmar, J.K.; Göpferich, A.M. Matrices and Scaffolds for Protein Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 274–291. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Alsberg, E. Spatial Regulation of Controlled Bioactive Factor Delivery for Bone Tissue Engineering. Adv. Drug Deliv. Rev. 2015, 84, 45–67. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, T.; Bian, Y.; Wang, G.; Wu, Z.; Li, H.; Liu, X.; Yang, S.; Tan, C.; Liang, R.; et al. A MgFe-LDH Nanosheet-Incorporated Smart Thermo-Responsive Hydrogel with Controllable Growth Factor Releasing Capability for Bone Regeneration. Adv. Mater. 2023, 35, e2206545. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Sun, H.; Wang, F.; Wang, Y.; Zhang, Z.; Teng, W.; Ye, Y.; Huang, D.; Zhang, W.; et al. Spatiotemporal Regulation of Angiogenesis/Osteogenesis Emulating Natural Bone Healing Cascade for Vascularized Bone Formation. J. Nanobiotechnol. 2021, 19, 420. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, T.-J.; Tan, M.-H.; Xu, X.-H.; Huang, Y.-F.; Peng, L.-H. Smart Graphene-Based Hydrogel Promotes Recruitment and Neural-like Differentiation of Bone Marrow Derived Mesenchymal Stem Cells in Rat Skin. Biomater. Sci. 2021, 9, 2146–2161. [Google Scholar] [CrossRef]
- Chu, C.; Qiu, J.; Zhao, Q.; Xun, X.; Wang, H.; Yuan, R.; Xu, X. Injectable Dual Drug-Loaded Thermosensitive Liposome-Hydrogel Composite Scaffold for Vascularised and Innervated Bone Regeneration. Colloids Surf. B Biointerfaces 2025, 245, 114203. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y.; Ren, R.; Zhang, H.; Jia, Z.; Wei, X.; Chen, N.; Li, G.; Ryu, S.; Lele, S.M.; Reinhardt, R.A.; et al. GSK3 Inhibitor-Loaded Osteotropic Pluronic Hydrogel Effectively Mitigates Periodontal Tissue Damage Associated with Experimental Periodontitis. Biomaterials 2020, 261, 120293. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, Y.; Ji, C.; Zhu, H.; Lao, A.; Zhao, R.; Hu, Y.; Zhou, Y.; Zhou, J.; Lin, K.; et al. A Five-in-One Novel MOF-Modified Injectable Hydrogel with Thermo-Sensitive and Adhesive Properties for Promoting Alveolar Bone Repair in Periodontitis: Antibacterial, Hemostasis, Immune Reprogramming, pro-Osteo-/Angiogenesis and Recruitment. Bioact. Mater. 2024, 41, 239–256. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone Fragility in Diabetes: Novel Concepts and Clinical Implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, Tissue Uptake, Metabolism and Pharmacokinetics. Free. Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Ghanem, M.; Heikal, L.; Abdel Fattah, H.; El Ashwah, A.; Fliefel, R. The Effect of Coenzyme Q10/Collagen Hydrogel on Bone Regeneration in Extraction Socket Prior to Implant Placement in Type II Diabetic Patients: A Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 3059. [Google Scholar] [CrossRef]
- Sheng, N.; Xing, F.; Zhang, Q.-Y.; Tan, J.; Nie, R.; Huang, K.; Li, H.-X.; Jiang, Y.-L.; Tan, B.; Xiang, Z.; et al. A Pleiotropic SIS-Based Hydrogel with Immunomodulation via NLRP3 Inflammasome Inhibition for Diabetic Bone Regeneration. Chem. Eng. J. 2024, 480, 147985. [Google Scholar] [CrossRef]
- Moradi, L.; Witek, L.; Vivekanand Nayak, V.; Cabrera Pereira, A.; Kim, E.; Good, J.; Liu, C.-J. Injectable Hydrogel for Sustained Delivery of Progranulin Derivative Atsttrin in Treating Diabetic Fracture Healing. Biomaterials 2023, 301, 122289. [Google Scholar] [CrossRef]
- Gu, Z.; Qiu, C.; Chen, L.; Wang, X. Injectable Thermosensitive Hydrogel Loading Erythropoietin and FK506 Alleviates Gingival Inflammation and Promotes Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1323554. [Google Scholar] [CrossRef]
- Barik, D.; Shyamal, S.; Das, K.; Jena, S.; Dash, M. Glycoprotein Injectable Hydrogels Promote Accelerated Bone Regeneration through Angiogenesis and Innervation. Adv. Healthc. Mater. 2023, 12, e2301959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Zhang, D.; Li, J.; Yue, X.; Kong, W.; Gu, X.; Jiao, Z.; Yang, C. Thermosensitive Hydrogel Loaded with Concentrated Growth Factors Promote Bone Repair in Segmental Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 1039117. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Zhang, C.; Fei, Y.; Wang, Y.; Zhong, Z.; Peng, C.; Li, M.; Gui, S.; Guo, J. Active Targeting Microemulsion-Based Thermosensitive Hydrogel against Periodontitis by Reconstructing Th17/Treg Homeostasis via Regulating ROS-Macrophages Polarization Cascade. Int. J. Pharm. 2024, 659, 124263. [Google Scholar] [CrossRef]
- Deng, J.; Pan, J.; Han, X.; Yu, L.; Chen, J.; Zhang, W.; Zhu, L.; Huang, W.; Liu, S.; You, Z.; et al. PDGFBB-Modified Stem Cells from Apical Papilla and Thermosensitive Hydrogel Scaffolds Induced Bone Regeneration. Chem. Biol. Interact. 2020, 316, 108931. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Wu, T.; Shi, K.; Bei, Z.; Wang, M.; Chu, B.; Xu, K.; Pan, M.; Li, Y.; et al. An Injectable Thermosensitive Hydrogel Containing Resveratrol and Dexamethasone-Loaded Carbonated Hydroxyapatite Microspheres for the Regeneration of Osteoporotic Bone Defects. Small Methods 2023, 316, 2300843. [Google Scholar] [CrossRef]
- Wang, H.; Chang, X.; Ma, Q.; Sun, B.; Li, H.; Zhou, J.; Hu, Y.; Yang, X.; Li, J.; Chen, X.; et al. Bioinspired Drug-Delivery System Emulating the Natural Bone Healing Cascade for Diabetic Periodontal Bone Regeneration. Bioact. Mater. 2023, 21, 324–339. [Google Scholar] [CrossRef]
- Li, J.; Leung, S.S.Y.; Chung, Y.L.; Chow, S.K.H.; Alt, V.; Rupp, M.; Brochhausen, C.; Chui, C.S.; Ip, M.; Cheung, W.-H.; et al. Hydrogel Delivery of DNase I and Liposomal Vancomycin to Eradicate Fracture-Related Methicillin-Resistant Staphylococcus Aureus Infection and Support Osteoporotic Fracture Healing. Acta Biomater. 2023, 164, 223–239. [Google Scholar] [CrossRef]
- Zhou, H.; He, Z.; Cao, Y.; Chu, L.; Liang, B.; Yu, K.; Deng, Z. An Injectable Magnesium-Loaded Hydrogel Releases Hydrogen to Promote Osteoporotic Bone Repair via ROS Scavenging and Immunomodulation. Theranostics 2024, 14, 3739–3759. [Google Scholar] [CrossRef]
- Heir, S.; Nerhus, T.K.; Røtterud, J.H.; Løken, S.; Ekeland, A.; Engebretsen, L.; Arøen, A. Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, W.; Liu, D.; Xiao, Y.; Chen, H.; Chen, J.; Li, W.; Cai, H.; Li, W.; Cai, B.; et al. Development of Brucine-Loaded Microsphere/Thermally Responsive Hydrogel Combination System for Intra-Articular Administration. J. Control. Release 2012, 162, 628–635. [Google Scholar] [CrossRef]
- Kampen, W.U.; Boddenberg-Pätzold, B.; Fischer, M.; Gabriel, M.; Klett, R.; Konijnenberg, M.; Kresnik, E.; Lellouche, H.; Paycha, F.; Terslev, L.; et al. The EANM Guideline for Radiosynoviorthesis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhou, M.; Luo, Z.; Hao, L.; Hsu, J.C.; Cai, W.; Zhou, W.; Hu, S. A 177Lu-Nucleotide Coordination Polymer-Incorporated Thermosensitive Hydrogel with Anti-Inflammatory and Chondroprotective Capabilities for Osteoarthritis Treatment. Biomaterials 2025, 317, 123098. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, Y.; Liu, J.; Chen, X.; Ma, X.; Hu, Y.; Tian, H.; Wang, X.; Mu, C. Glucosamine-Loaded Injectable Hydrogel Promotes Autophagy and Inhibits Apoptosis after Cartilage Injury. Heliyon 2023, 9, e19879. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wan, J.; Yang, Y.; Huang, L.; Zhou, C.; Su, J.; Hua, S.; Pu, H.; Zou, Y.; Zhu, H.; et al. Thermosensitive Hydrogel for Cartilage Regeneration via Synergistic Delivery of SDF-1α like Polypeptides and Kartogenin. Carbohydr. Polym. 2023, 304, 120492. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. The Immunology of Rheumatoid Arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving Concepts of Rheumatoid Arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Joosten, L.A.B.; Abdollahi-Roodsaz, S.; Dinarello, C.A.; O’Neill, L.; Netea, M.G. Toll-like Receptors and Chronic Inflammation in Rheumatic Diseases: New Developments. Nat. Rev. Rheumatol. 2016, 12, 344–357. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Kim, M.S. Intra-Articular Hydrogel Formulation Prolongs the in Vivo Stability of Toll-like Receptor Antagonistic Peptides for Rheumatoid Arthritis Treatment. J. Control. Release 2024, 372, 467–481. [Google Scholar] [CrossRef]
- Shaygani, H.; Shamloo, A.; Akbarnataj, K.; Maleki, S. In Vitro and in Vivo Investigation of Chitosan/Silk Fibroin Injectable Interpenetrating Network Hydrogel with Microspheres for Cartilage Regeneration. Int. J. Biol. Macromol. 2024, 270, 132126. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Lv, Z.; Sun, Z.; Cheng, C.; Tan, G.; Wang, M.; Liu, A.; Sun, H.; Guo, H.; et al. Articular Fibrocartilage-Targeted Therapy by Microtubule Stabilization. Sci. Adv. 2022, 8, eabn8420. [Google Scholar] [CrossRef]
- Sang, X.; Zhao, X.; Yan, L.; Jin, X.; Wang, X.; Wang, J.; Yin, Z.; Zhang, Y.; Meng, Z. Thermosensitive Hydrogel Loaded with Primary Chondrocyte-Derived Exosomes Promotes Cartilage Repair by Regulating Macrophage Polarization in Osteoarthritis. Tissue Eng. Regen. Med. 2022, 19, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chen, J.; Qian, D.; Gao, P.; Qin, T.; Jiang, T.; Yi, J.; Xu, T.; Huang, Y.; et al. Exosomes Derived from Platelet-Rich Plasma Administration in Site Mediate Cartilage Protection in Subtalar Osteoarthritis. J. Nanobiotechnol. 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- García-Sobrino, R.; Casado-Losada, I.; Caltagirone, C.; García-Crespo, A.; García, C.; Rodríguez-Hernández, J.; Reinecke, H.; Gallardo, A.; Elvira, C.; Martínez-Campos, E. Osteoblastic Cell Sheet Engineering Using P(VCL-HEMA)-Based Thermosensitive Hydrogels Doped with pVCL@Icariin Nanoparticles Obtained with Supercritical CO2-SAS. Pharmaceutics 2024, 16, 1063. [Google Scholar] [CrossRef]
- Jia, S.; Liu, W.; Zhang, M.; Wang, L.; Ren, C.; Feng, C.; Zhang, T.; Lv, H.; Hou, Z.; Zou, W.; et al. Insufficient Mechanical Loading Downregulates Piezo1 in Chondrocytes and Impairs Fracture Healing Through ApoE-Induced Senescence. Adv. Sci. 2024, 11, e2400502. [Google Scholar] [CrossRef]
- Zou, S.; Xu, G.; Zheng, Z.; Chen, T.; Huang, Y. Repair of Osteochondral Defect with Acellular Cartilage Matrix and Thermosensitive Hydrogel Scaffold. Tissue Eng. Part A 2024, 31, 1015–1025. [Google Scholar] [CrossRef]
- García-Couce, J.; Schomann, T.; Chung, C.K.; Que, I.; Jorquera-Cordero, C.; Fuentes, G.; Almirall, A.; Chan, A.; Cruz, L.J. Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis. Gels 2022, 8, 488. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.; Gao, T.; Zhao, G.; Huang, G.; Shi, J.; Chen, J.; Song, J.; Xia, J.; Ma, X. Engineered Exosomes with ATF5-Modified mRNA Loaded in Injectable Thermogels Alleviate Osteoarthritis by Targeting the Mitochondrial Unfolded Protein Response. ACS Appl. Mater. Interfaces 2024, 16, 21383–21399. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Blebea, N.-M.; Pușcașu, C.; Vlad, R.-A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. [Google Scholar] [CrossRef]
- Lv, X.; Li, H.; Chen, Y.; Wang, Y.; Chi, J.; Wang, S.; Yang, Y.; Han, B.; Jiang, Z. Crocin-1 Laden Thermosensitive Chitosan-Based Hydrogel with Smart Anti-Inflammatory Performance for Severe Full-Thickness Burn Wound Therapeutics. Carbohydr. Polym. 2024, 345, 122603. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Song, H.; Liu, X.; Zhang, H.; Jiang, J.; Liu, H.; Zhuo, R.; Cheng, G.; Fang, J.; et al. Injectable Nanocomposite Hydrogel for Accelerating Diabetic Wound Healing Through Inflammatory Microenvironment Regulation. Int. J. Nanomed. 2025, 20, 1679–1696. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wu, B.; Nie, C.; Luo, T.; Song, Z.; Lv, J.; Tan, Y.; Liu, C.; Zhong, M.; Liao, T.; et al. NIR-II Responsive Nanohybrids Incorporating Thermosensitive Hydrogel as Sprayable Dressing for Multidrug-Resistant-Bacteria Infected Wound Management. ACS Nano 2023, 17, 11253–11267. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kwon, M.; Jung, H.; Nam, G.-H.; Kim, I.-S. The Right Timing, Right Combination, Right Sequence, and Right Delivery for Cancer Immunotherapy. J. Control. Release 2021, 331, 321–334. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Yang, J.; Wang, J.; Wang, J.; Zhu, G.; Li, D.; Ding, J.; Sun, T. A Tumor Microenvironments-Adapted Polypeptide Hydrogel/Nanogel Composite Boosts Antitumor Molecularly Targeted Inhibition and Immunoactivation. Adv. Mater. 2022, 34, e2200449. [Google Scholar] [CrossRef]
- Kang, T.; Cha, G.D.; Park, O.K.; Cho, H.R.; Kim, M.; Lee, J.; Kim, D.; Lee, B.; Chu, J.; Koo, S.; et al. Penetrative and Sustained Drug Delivery Using Injectable Hydrogel Nanocomposites for Postsurgical Brain Tumor Treatment. ACS Nano 2023, 17, 5435–5447. [Google Scholar] [CrossRef]
- Stover, A.M.; Basak, R.; Mueller, D.; Lipman, R.; Teal, R.; Hilton, A.; Giannone, K.; Waheed, M.; Smith, A.B. Minimal Patient-Reported Side Effects for a Chemoablative Gel (UGN-102) Used as Frontline Treatment in Adults with Nonmuscle-Invasive Bladder Cancer. J. Urol. 2022, 208, 580–588. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic Strategies for Tendon/Ligament-to-Bone Interface Regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef]
- Li, J.; Ke, H.; Lei, X.; Zhang, J.; Wen, Z.; Xiao, Z.; Chen, H.; Yao, J.; Wang, X.; Wei, Z.; et al. Controlled-Release Hydrogel Loaded with Magnesium-Based Nanoflowers Synergize Immunomodulation and Cartilage Regeneration in Tendon-Bone Healing. Bioact. Mater. 2024, 36, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Eberhardt, R.T. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg. 2016, 151, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhang, X.; Liu, Q.; Zhu, M.; Huang, X. Targeted Delivery of Nanomedicines for Promoting Vascular Regeneration in Ischemic Diseases. Theranostics 2022, 12, 6223–6241. [Google Scholar] [CrossRef]
- Niu, H.; Gao, N.; Dang, Y.; Guan, Y.; Guan, J. Delivery of VEGF and Delta-like 4 to Synergistically Regenerate Capillaries and Arterioles in Ischemic Limbs. Acta Biomater. 2022, 143, 295–309. [Google Scholar] [CrossRef]
- Ropper, A.E.; Ropper, A.H. Acute Spinal Cord Compression. N. Engl. J. Med. 2017, 376, 1358–1369. [Google Scholar] [CrossRef]
- Albashari, A.A.; He, Y.; Luo, Y.; Duan, X.; Ali, J.; Li, M.; Fu, D.; Xiang, Y.; Peng, Y.; Li, S.; et al. Local Spinal Cord Injury Treatment Using a Dental Pulp Stem Cell Encapsulated H2S Releasing Multifunctional Injectable Hydrogel. Adv. Healthc. Mater. 2024, 13, e2302286. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Li, P.; Peng, Z.; Zhang, Y.; Liu, Y.; Li, M.; Gong, X.; Liu, D.; Xu, E.; et al. Nasal Delivery of Engineered Exosomes via a Thermo-Sensitive Hydrogel Depot Reprograms Glial Cells for Spinal Cord Repair. Adv. Sci. 2025, e04486. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Liu, Y.; Li, Y.; Jiang, M.; Li, Y.; Meng, Z.; Zhao, Z.; Liu, Z.; Liu, J.; et al. Injectable and Thermosensitive Hydrogel with Platelet-Rich Plasma for Enhanced Biotherapy of Skin Wound Healing. Adv. Healthc. Mater. 2024, 13, e2303930. [Google Scholar] [CrossRef]
- Zhou, J.; Li, T.; Zhang, M.; Han, B.; Xia, T.; Ni, S.; Liu, Z.; Chen, Z.; Tian, X. Thermosensitive Black Phosphorus Hydrogel Loaded with Silver Sulfadiazine Promotes Skin Wound Healing. J. Nanobiotechnol. 2023, 21, 330. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Yun, H.-L.; Zhang, W.; Ren, L.; Li, W.-W.; Han, C. Effect of Adipose-Derived Stem Cells Exosomes Cross-Linked Chitosan-Aβ-Glycerophosphate Thermosensitive Hydrogel on Deep Burn Wounds. World J. Stem Cells 2025, 17, 102091. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Q.; Liu, W.; Zhang, S.; Wang, N.; Chai, G.; Wang, Y.; Sun, S.; Zheng, R.; Zhao, Y.; et al. A Poloxamer 407/Chitosan-Based Thermosensitive Hydrogel Dressing for Diabetic Wound Healing via Oxygen Production and Dihydromyricetin Release. Int. J. Biol. Macromol. 2024, 263, 130256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Dong, P.; Wang, J.; Wang, L.; Zhao, A.; Qu, G.; Li, H.; Maheshika Gunarathne, K.D.; Zhang, W.; et al. Thermosensitive-Based Synergistic Antibacterial Effects of Novel LL37@ZPF-2 Loaded Poloxamer Hydrogel for Infected Skin Wound Healing. Int. J. Pharm. 2025, 670, 125210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.; Wang, J.; Zhang, R.; Wu, M.; Zhong, S.; He, W.; Cui, X. An Injectable Thermosensitive Hydrogel with Antibacterial and Antioxidation Properties for Accelerating Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 46053–46065. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Wang, L.; Zhang, M.; He, C.; Yang, X.; Pang, Y.; Chen, H.; Cheng, F. TNF-R1 Cellular Nanovesicles Loaded on the Thermosensitive F-127 Hydrogel Enhance the Repair of Scalded Skin. ACS Biomater. Sci. Eng. 2023, 9, 5843–5854. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Ding, J.; Pan, X.; Yang, L.; Guo, C.; Wang, Y.; Gruber, R.; Nan, K.; Li, L. Nerve Growth Factor Loaded Hypotonic Eye Drops for Corneal Nerve Repair. J. Control. Release 2025, 380, 71–84. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.-Y.; Lee, H.S.; Yoo, Y.; Shin, H.; Kim, H.; Min, D.S.; Bae, J.-S.; Seo, Y.-K. Thermosensitive Hydrogel Harboring CD146/IGF-1 Nanoparticles for Skeletal-Muscle Regeneration. ACS Appl. Bio Mater. 2021, 4, 7070–7080. [Google Scholar] [CrossRef]
- Huang, C.; Teng, J.; Liu, W.; Wang, J.; Liu, A. Modulation of Macrophages by a Phillyrin-Loaded Thermosensitive Hydrogel Promotes Skin Wound Healing in Mice. Cytokine 2024, 177, 156556. [Google Scholar] [CrossRef]
- Liu, L.; Yao, S.; Mao, X.; Fang, Z.; Yang, C.; Zhang, Y. Thermosensitive Hydrogel Coupled with Sodium Ascorbyl Phosphate Promotes Human Umbilical Cord-Derived Mesenchymal Stem Cell-Mediated Skin Wound Healing in Mice. Sci. Rep. 2023, 13, 11909. [Google Scholar] [CrossRef]
- Kang, L.; Fang, E.; Gu, M.; Guan, Y.; Wu, D.; Zhang, X.; Yu, W.; Wang, J.; Zeng, Z.; Xu, S.; et al. An Injectable Thermosensitive Pluronic F127 Loaded-Nanohydroxyapatite / Polydopamine for Promoting Sciatic Nerve Repair after Crush Injury. Colloids Surf. B Biointerfaces 2025, 245, 114324. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, W.; Shan, J.; He, J.; Qian, H.; Chen, X.; Wang, X. Thermosensitive Hydrogel Loaded with Nickel-Copper Bimetallic Hollow Nanospheres with SOD and CAT Enzymatic-Like Activity Promotes Acute Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 50677–50691. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, Q.; Li, Y.; Hu, F.; Yu, J.; Liao, X.; Xing, Z.; He, Y.; Ye, Q. Application of Thermosensitive-Hydrogel Combined with Dental Pulp Stem Cells on the Injured Fallopian Tube Mucosa in an Animal Model. Front. Bioeng. Biotechnol. 2022, 10, 1062646. [Google Scholar] [CrossRef] [PubMed]
- Sangboonruang, S.; Semakul, N.; Manokruang, K.; Khammata, N.; Jantakee, K.; Mai-Ngam, K.; Charoenla, S.; Khamnoi, P.; Saengsawang, K.; Wattananandkul, U.; et al. Multifunctional Poloxamer-Based Thermo-Responsive Hydrogel Loaded with Human Lactoferricin Niosomes: In Vitro Study on Anti-Bacterial Activity, Accelerate Wound Healing, and Anti-Inflammation. Int. J. Pharm. X 2024, 8, 100291. [Google Scholar] [CrossRef] [PubMed]
- Sendon-Lago, J.; Rio, L.G.-D.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sang, Y.; Yu, W.; Zhang, F.; Wang, X. Antibacterial Actions of Ag Nanoparticles Synthesized from Cimicifuga Dahurica (Turcz.) Maxim. and Their Application in Constructing a Hydrogel Spray for Healing Skin Wounds. Food Chem. 2023, 418, 135981. [Google Scholar] [CrossRef]
- Fan, R.; Sun, W.; Zhang, T.; Wang, R.; Tian, Y.; Zhang, H.; Li, J.; Zheng, A.; Song, S. Paclitaxel-Nanocrystals-Loaded Network Thermosensitive Hydrogel for Localised Postsurgical Recurrent of Breast Cancer after Surgical Resection. Biomed. Pharmacother. 2022, 150, 113017. [Google Scholar] [CrossRef]
- Feng, T.; Wu, H.; Ma, W.; Wang, Z.; Wang, C.; Wang, Y.; Wang, S.; Zhang, M.; Hao, L. An Injectable Thermosensitive Hydrogel with a Self-Assembled Peptide Coupled with an Antimicrobial Peptide for Enhanced Wound Healing. J. Mater. Chem. B 2022, 10, 6143–6157. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Addisu, K.D.; Gebrie, H.T.; Darge, H.F.; Wu, T.-Y.; Hong, Z.-X.; Tsai, H.-C. Multifunctional Thermosensitive Hydrogel Based on Alginate and P(NIPAM-Co-HEMIN) Composites for Accelerated Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 241, 124540. [Google Scholar] [CrossRef]
- Zhao, G.; Lu, G.; Fan, H.; Wei, L.; Yu, Q.; Li, M.; Li, H.; Yu, N.; Wang, S.; Lu, M. Herbal Products-Powered Thermosensitive Hydrogel with Phototherapy and Microenvironment Reconstruction for Accelerating Multidrug-Resistant Bacteria-Infected Wound Healing. Adv. Healthc. Mater. 2024, 13, e2400049. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Luo, H.; Li, X.; Gao, T.; Wu, Q.; Zeng, D. A Thermosensitive Chitin Hydrogel with Mild Photothermal-Chemotherapy for Facilitating Multidrug-Resistant Bacteria Infected Wound Healing. Int. J. Biol. Macromol. 2025, 293, 139428. [Google Scholar] [CrossRef]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical Strategy to Construct Anti-Osteosarcoma Bone Substitutes by Loading Cisplatin into 3D-Printed Titanium Alloy Implants Using a Thermosensitive Hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Hu, F.; Xu, J.; Ye, J.; Liu, S.; Wang, L.; Zhuo, M.; Ran, B.; Zhang, H.; et al. Novel Thermosensitive Hydrogel Promotes Spinal Cord Repair by Regulating Mitochondrial Function. ACS Appl. Mater. Interfaces 2022, 14, 25155–25172. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Lu, R.; Li, P.; Liao, X.; Tan, Y.; Zhang, S. Inflammation-Reducing Thermosensitive Hydrogel with Photothermal Conversion for Skin Cancer Therapy. J. Control. Release 2025, 378, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pei, B.; Li, Z.; Ou, X.L.; Sun, T.; Zhu, Z. PLGA-PEG-PLGA Hydrogel with NEP1-40 Promotes the Functional Recovery of Brachial Plexus Root Avulsion in Adult Rats. PeerJ 2021, 9, e12269. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Zhang, A.; Tan, X.; Li, S.; Luo, L.; Wang, S.; Wei, G.; Zhang, Z.; Huo, J. Combination of Lipopolysaccharide and Polygalacturonic Acid Exerts Antitumor Activity and Augments Anti-PD-L1 Immunotherapy. Int. J. Biol. Macromol. 2024, 281, 136390. [Google Scholar] [CrossRef]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Wang, B.-X.; Zhang, Y.; Yao, Q.; Cheng, D.-H.; Lu, Y.-H. Wound Microenvironment Self-Adjusting Hydrogels with Thermo-Sensitivity for Promoting Diabetic Wound Healing. Gels 2023, 9, 987. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Yao, Y.; Deng, Y.; Liu, Z.; Ding, J.; Wang, W.; Chen, H.; Nan, K.; Li, L. Injectable Drug-Loaded Thermosensitive Hydrogel Delivery System for Protecting Retina Ganglion Cells in Traumatic Optic Neuropathy. Regen. Biomater. 2024, 11, rbae124. [Google Scholar] [CrossRef]
- Sutar, P.; Bakuru, V.R.; Yadav, P.; Laha, S.; Kalidindi, S.B.; Maji, T.K. Nanocomposite Hydrogel of Pd@ZIF-8 and Laponite® : Size-Selective Hydrogenation Catalyst under Mild Conditions. Chemistry 2021, 27, 3268–3272. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Z.; Huang, X.; Du, L.; Li, W.; Gao, P.; Chen, Z.; Zhang, J.; Guo, Z.; Li, Z.; et al. Advances in 3D and 4D Printing of Gel-Based Foods: Mechanisms, Applications, and Future Directions. Gels 2025, 11, 94. [Google Scholar] [CrossRef]
- Nakamura, K.; Di Caprio, N.; Burdick, J.A. Engineered Shape-Morphing Transitions in Hydrogels Through Suspension Bath Printing of Temperature-Responsive Granular Hydrogel Inks. Adv. Mater. 2024, 36, 2410661. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Chen, G.; Zheng, J.; Wang, W.; Ren, J.; Zhu, C.; Yang, Y.; Cong, Y.; Fu, J. 4D Printing of Biomimetic Anisotropic Self-Sensing Hydrogel Actuators. Chem. Eng. J. 2023, 473, 145444. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Wang, X.; Shang, Z.; Liu, Y.; Pan, N.; Sun, X.; Xu, W. Limbal Stem Cells Carried by a Four-Dimensional -Printed Chitosan-Based Scaffold for Corneal Epithelium Injury in Diabetic Rabbits. Front. Physiol. 2024, 15, 1285850. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).