Study of the Influence of Selected Carrageenan Fractions on the Physical Properties and Crystal Structure of Mango Sorbet
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties of Sorbet
2.1.1. Osmotic Pressure and Cryoscopic Temperature
2.1.2. Density
2.1.3. Overrun and Melting Time
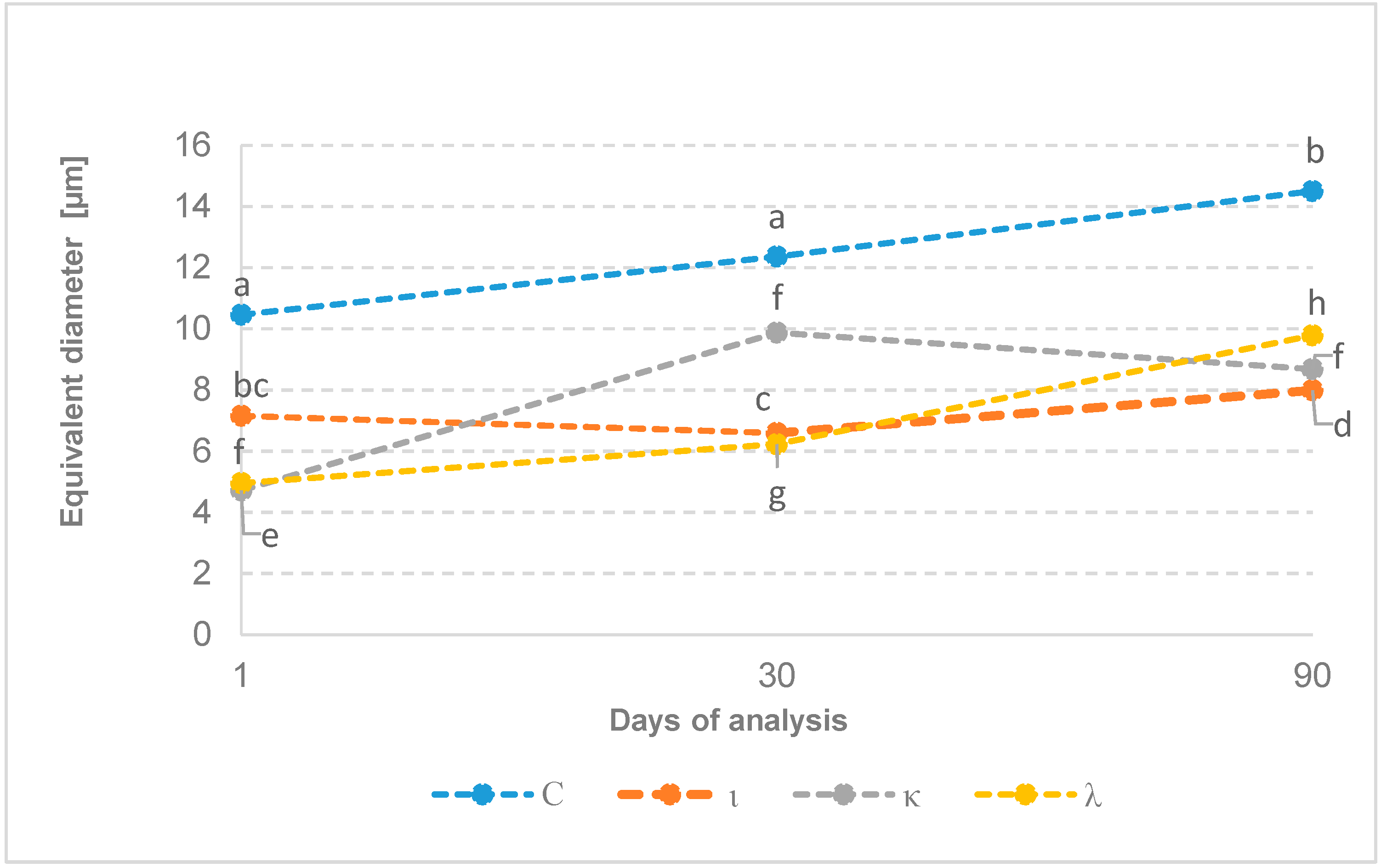
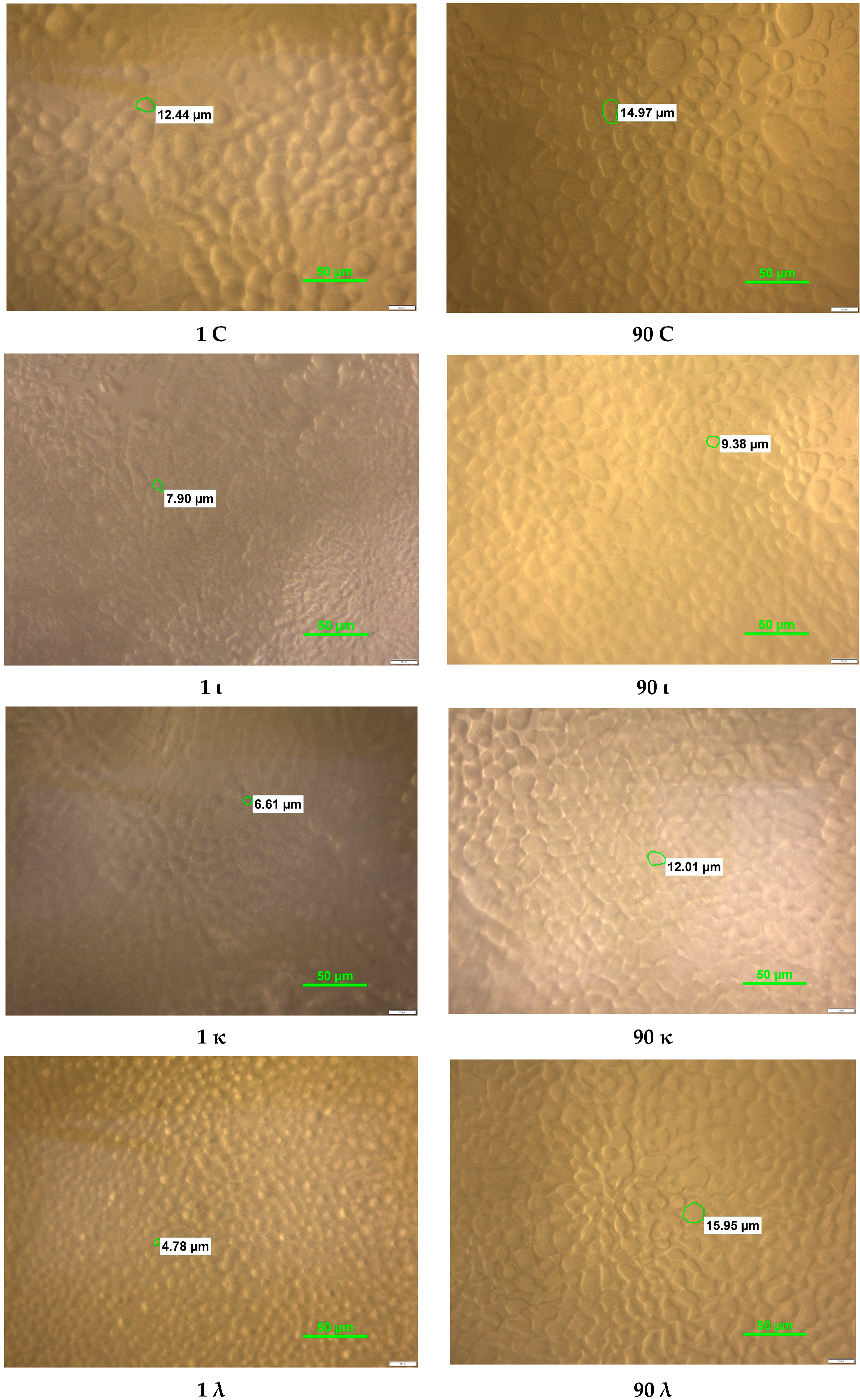
2.2. Crystal Structural Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials and Sorbet Preparation
4.2. Physical Properties’ Analysis
4.2.1. Cryoscopic Temperature and Osmotic Pressure
4.2.2. Density
4.2.3. Overrun and Melting Time
4.3. Crystal Structural Analysis
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamińska-Dwórznicka, A.; Matusiak, M.; Samborska, K.; Witrowa-Rajchert, D.; Gondek, E.; Jakubczyk, E.; Antczak, A. The influence of kappa carrageenan and its hydrolysates on the recrystallization process in sorbet. J. Food Eng. 2015, 167, 162–165. [Google Scholar] [CrossRef]
- Hale, J.; Furch, A.; Gerhäuser, J.; Gaukel, V.; Wefers, D. Ice recrystallization inhibition activity of chemically defined carrageenans. Food Hydrocoll. 2024, 157, 110423. [Google Scholar]
- Kiran-Yildirim, B.; Hale, J.; Wefers, D.; Gaukel, V. Ice recrystallization inhibition of commercial κ-, ι-, and λ-carrageenans. J. Food Eng. 2021, 290, 110269. [Google Scholar]
- Ndoye, F.T.; Alvarez, G. Characterization of ice recrystallization in ice cream during storage using the focused beam reflectance measurement. J. Food Eng. 2015, 148, 24–34. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar]
- Kiran-Yildirim, B.; Gaukel, V. Ice Crystal Growth in Sucrose Solutions Containing Kappa- and Iota-Carrageenans. Chem. Eng. Technol. 2020, 43, 1040–1047. [Google Scholar] [CrossRef]
- Leiter, A.; Mailänder, J.; Wefers, D.; Bunzel, M.; Gaukel, V. Influence of acid hydrolysis and dialysis of κ-carrageenan on its ice recrystallization inhibition activity. J. Food Eng. 2017, 209, 26–35. [Google Scholar]
- Adapa, S.; Schmidt, K.A.; Jeon, I.J.; Herald, T.J.; Flores, R.A. Mechanisms of Ice Crystallization and Recrystallization in Ice Cream: A Review. Food Rev. Int. 2000, 16, 259–271. [Google Scholar] [CrossRef]
- Flores, A.; Goff, H. Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. J. Dairy Sci. 1999, 82, 1399–1407. [Google Scholar]
- Regand, A.; Goff, H.D. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 2003, 17, 95–102. [Google Scholar]
- Goff, H.D.; Hartel, R.W. Ice cream structure. In Ice Cream; Springer: Cham, Switzerland, 2013; pp. 313–352. [Google Scholar]
- Soukoulis, C.; Chandrinos, I.; Tzia, C. Study of the functionality of selected hydrocolloids and their blends with κ-carrageenan on storage quality of vanilla ice cream. LWT-Food Sci. Technol. 2008, 41, 1816–1827. [Google Scholar]
- Kamińska-Dwórznicka, A.; Janczewska-Dupczyk, A.; Kot, A.; Laba, S.; Samborska, K. The impact of iota- and kappa-carrageenan addition on freezing process and ice crystals structure of strawberry sorbet frozen by various methods. J. Food Sci. 2020, 85, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, S.; Brooks, M.; Campbell, R.; Philp, K.; Trius, A. The use of carrageenan in food. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 229–243. [Google Scholar]
- Kot, A.; Kamińska-Dwórznicka, A.; Antczak, A.; Jakubczyk, E.; Matwijczuk, A. Effect of ι-carrageenan and its acidic and enzymatic hydrolysates on ice crystal structure changes in model sucrose solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128744. [Google Scholar] [CrossRef]
- Góral, M.; Kozłowicz, K.; Pankiewicz, U.; Góral, D.; Kluza, F.; Wójtowicz, A. Impact of stabilizers on the freezing process, and physicochemical and organoleptic properties of coconut milk-based ice cream. Lwt 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Łaba, S.; Jakubczyk, E. The effects of selected stabilizers addition on physical properties and changes in crystal structure of whey ice cream. Lwt 2022, 154, 112841. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Mitra, H.; Franklin, M.E.E.; Ghoroi, C.; Ambrose, R.P.K.; Battula, S.N. Physicochemical, thermal, and flow properties of ice cream powder as influenced by moisture content. J. Food Process. Preserv. 2021, 45, e15106. [Google Scholar]
- Kamińska-Dwórznicka, A.; Kot, A. The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing. Processes 2024, 12, 1957. [Google Scholar] [CrossRef]
- Romulo, A.; Meindrawan, B.; Marpietylie. Effect of Dairy and Non-Dairy Ingredients on the Physical Characteristic of Ice Cream: Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012145. [Google Scholar]
- Adapa, S.; Dingeldein, H.; Schmidt, K.; Herald, T. Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers. J. Dairy Sci. 2000, 83, 2224–2229. [Google Scholar]
- Petkova, T.; Doykina, P.; Alexieva, I.; Mihaylova, D.; Popova, A. Characterization of Fruit Sorbet Matrices with Added Value from Zizyphus jujuba and Stevia rebaudiana. Foods 2022, 11, 2748. [Google Scholar] [CrossRef]
- Palka, A.; Wilczyńska, A. Storage quality changes in craft and industrial blueberry, strawberry, raspberry and passion fruit-mango sorbets. Foods 2023, 12, 2733. [Google Scholar] [CrossRef] [PubMed]
- Minhas, K.S.; Sidhu, J.S.; Mudahar, G.S.; Singh, A. Flow behavior characteristics of ice cream mix made with buffalo milk and various stabilizers. Plant Foods Hum. Nutr. 2002, 57, 25–40. [Google Scholar] [PubMed]
- Abbas Syed, Q. Effects of different ingredients on texture of ice cream. J. Nutr. Health Food Eng. 2018, 8, 422–435. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Song, Z.; Chen, X. A review of natural polysaccharides for food cryoprotection: Ice crystals inhibition and cryo-stabilization. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100291. [Google Scholar] [CrossRef]
- Seo, C.W.; Oh, N.S. Functional application of Maillard conjugate derived from a κ-carrageenan/milk protein isolate mixture as a stabilizer in ice cream. Lwt 2022, 161, 113406. [Google Scholar] [CrossRef]
- Lomolino, G.; Zannoni, S.; Zabara, A.; Da Lio, M.; De Iseppi, A. Ice recrystallisation and melting in ice cream with different proteins levels and subjected to thermal fluctuation. Int. Dairy J. 2020, 100, 104557. [Google Scholar]
- Kamińska-Dwórznicka, A.; Kot, A.; Jakubczyk, E.; Buniowska-Olejnik, M.; Nowacka, M. Effect of ultrasound-assisted freezing on the crystal structure of mango sorbet. Crystals 2023, 13, 396. [Google Scholar] [CrossRef]
- Dłużewska, E.; Gazda, B.; Leszczyński, K. Wpływ wybranych hydrokoloidów polisacharydowych na jakość koncentratów lodów owocowych. Acta Sci. Pol. Technol. Aliment. 2003, 2, 97–107. [Google Scholar]
- Dłużewska, E.; Grodzka, K.; Mosiewska, M. Effect of some hydrocolloids on the quality of health beneficial soy ice. Acta Sci. Pol. Technol. Aliment. 2005, 4, 71–78. [Google Scholar]
| Variant | Osmotic Pressure [mOSM/kg] | Cryoscopic Temperature [°C] |
|---|---|---|
| C | 1617.2 ± 106.5 a | −2.991 ± 0.188 a |
| ι | 1449.5 ± 159.6 a | −2.693 ± 0.297 a |
| κ | 1566.3 ± 93.3 a | −2.910 ± 0.173 a |
| λ | 1651.8 ± 56.3 a | −3.069 ± 0.104 a |
| Variant | Density [g/cm3] |
|---|---|
| C | 1.168 ± 0.013 a |
| ι | 1.162 ± 0.020 a |
| κ | 1.177 ± 0.024 a |
| λ | 1.159 ± 0.019 a |
| Variant | Overrun [%] | Melting Time [min] |
|---|---|---|
| C | 30.59 ± 0.32 a | 17.45 ± 0.75 a |
| ι | 58.72 ± 2.13 b | 19.45 ± 0.25 ab |
| κ | 49.16 ± 8.14 ab | 19.43 ± 0.32 ab |
| λ | 35.15 ± 2.04 ab | 21.14 ± 0.18 b |
| Variant | Equivalent Diameter [µm] |
|---|---|
| 1 C | 10.46 ± 1.67 a |
| 1 ι | 7.16 ± 1.15 bc |
| 1 κ | 4.72 ± 1.06 e |
| 1 λ | 4.96 ± 2.00 f |
| 30 C | 12.36 ± 3.12 a |
| 30 ι | 6.59 ± 1.12 c |
| 30 κ | 9.87 ± 2.14 f |
| 30 λ | 6.23 ± 2.07 g |
| 90 C | 14.50 ± 3.02 b |
| 90 ι | 7.99 ± 0.83 d |
| 90 κ | 8.71 ± 2.14 f |
| 90 λ | 9.79 ± 1.04 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska-Dwórznicka, A.; Gondek, E.; Jakubczyk, E. Study of the Influence of Selected Carrageenan Fractions on the Physical Properties and Crystal Structure of Mango Sorbet. Gels 2025, 11, 531. https://doi.org/10.3390/gels11070531
Kamińska-Dwórznicka A, Gondek E, Jakubczyk E. Study of the Influence of Selected Carrageenan Fractions on the Physical Properties and Crystal Structure of Mango Sorbet. Gels. 2025; 11(7):531. https://doi.org/10.3390/gels11070531
Chicago/Turabian StyleKamińska-Dwórznicka, Anna, Ewa Gondek, and Ewa Jakubczyk. 2025. "Study of the Influence of Selected Carrageenan Fractions on the Physical Properties and Crystal Structure of Mango Sorbet" Gels 11, no. 7: 531. https://doi.org/10.3390/gels11070531
APA StyleKamińska-Dwórznicka, A., Gondek, E., & Jakubczyk, E. (2025). Study of the Influence of Selected Carrageenan Fractions on the Physical Properties and Crystal Structure of Mango Sorbet. Gels, 11(7), 531. https://doi.org/10.3390/gels11070531






