Insights into Carbon-Based Aerogels Toward High-Performance Lithium–Sulfur Batteries: A Review of Strategies for Sulfur Incorporation Within Carbon Aerogel Frameworks
Abstract
1. Introduction
2. Preparation of Carbon-Based Aerogels
2.1. Gelation of Precursors

2.2. Drying Processes
2.2.1. Ambient Pressure Drying
2.2.2. Freeze-Drying
2.2.3. Supercritical Drying
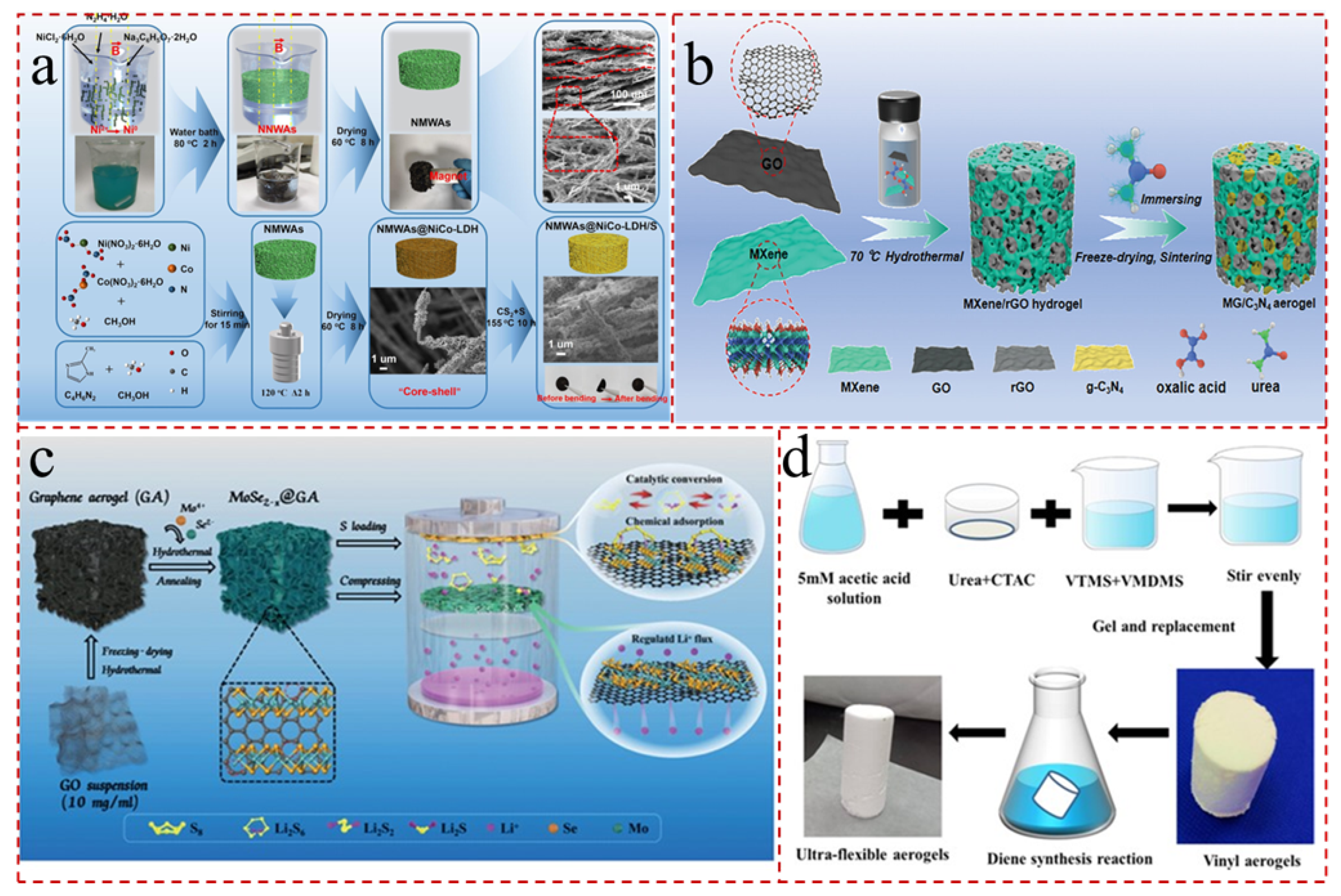
2.3. Carbonization
3. Carbon Nanofiber Aerogels
3.1. Synthesis of CNF Aerogel
3.2. Modified CNF Aerogel
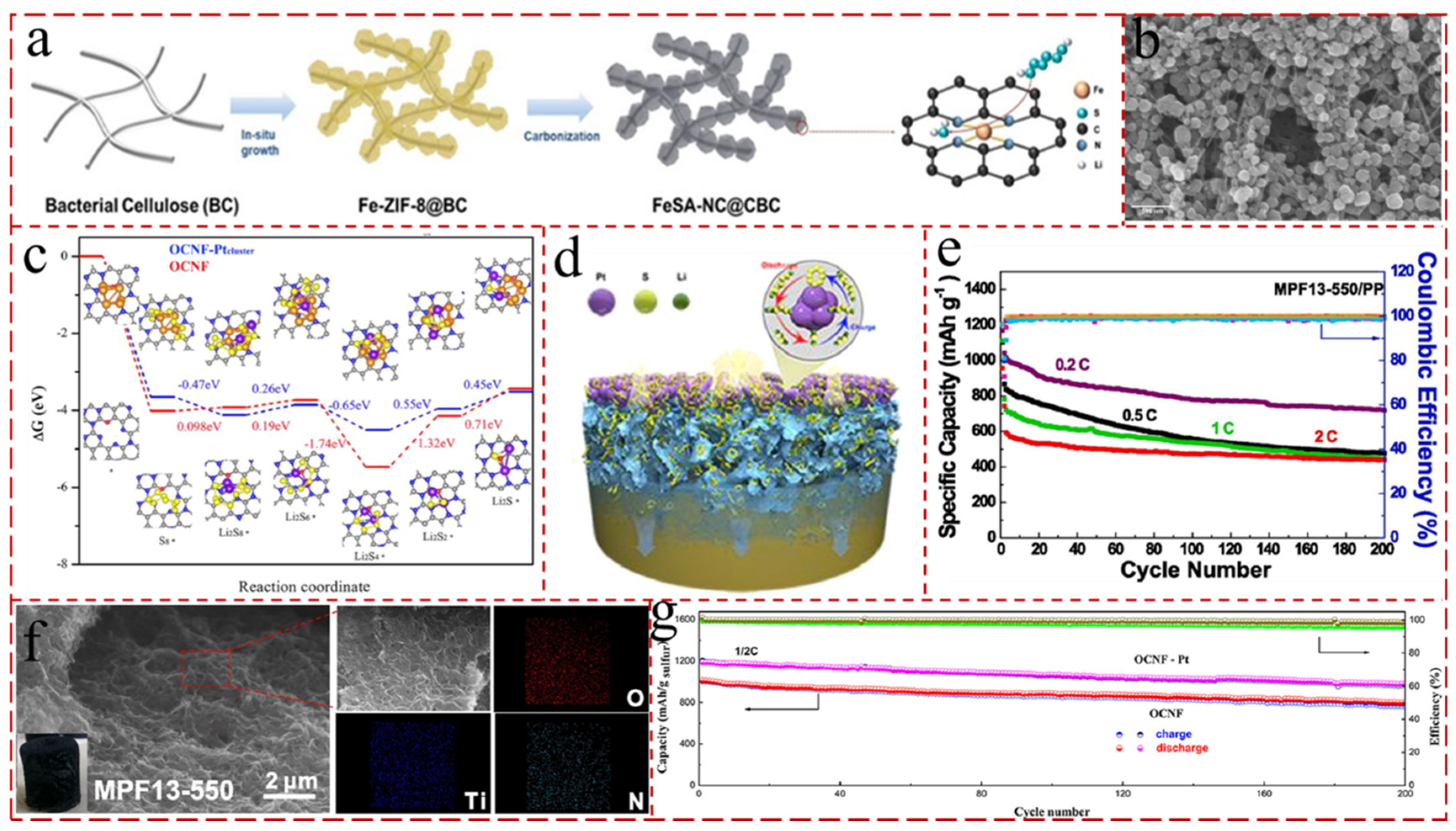
4. Carbon Nanotube Aerogels
4.1. Synthesis and Characterization of CNT Aerogels
4.2. CNT Composite Aerogels for Cathodes
4.3. CNT Composite Aerogel for Interlayer
5. Graphene Aerogels
5.1. Synthesis and Characterization of GAs
5.2. Pure GAs
5.3. Heteroatom-Doped GAs
5.4. Composite GAs
5.4.1. GA with TMOs
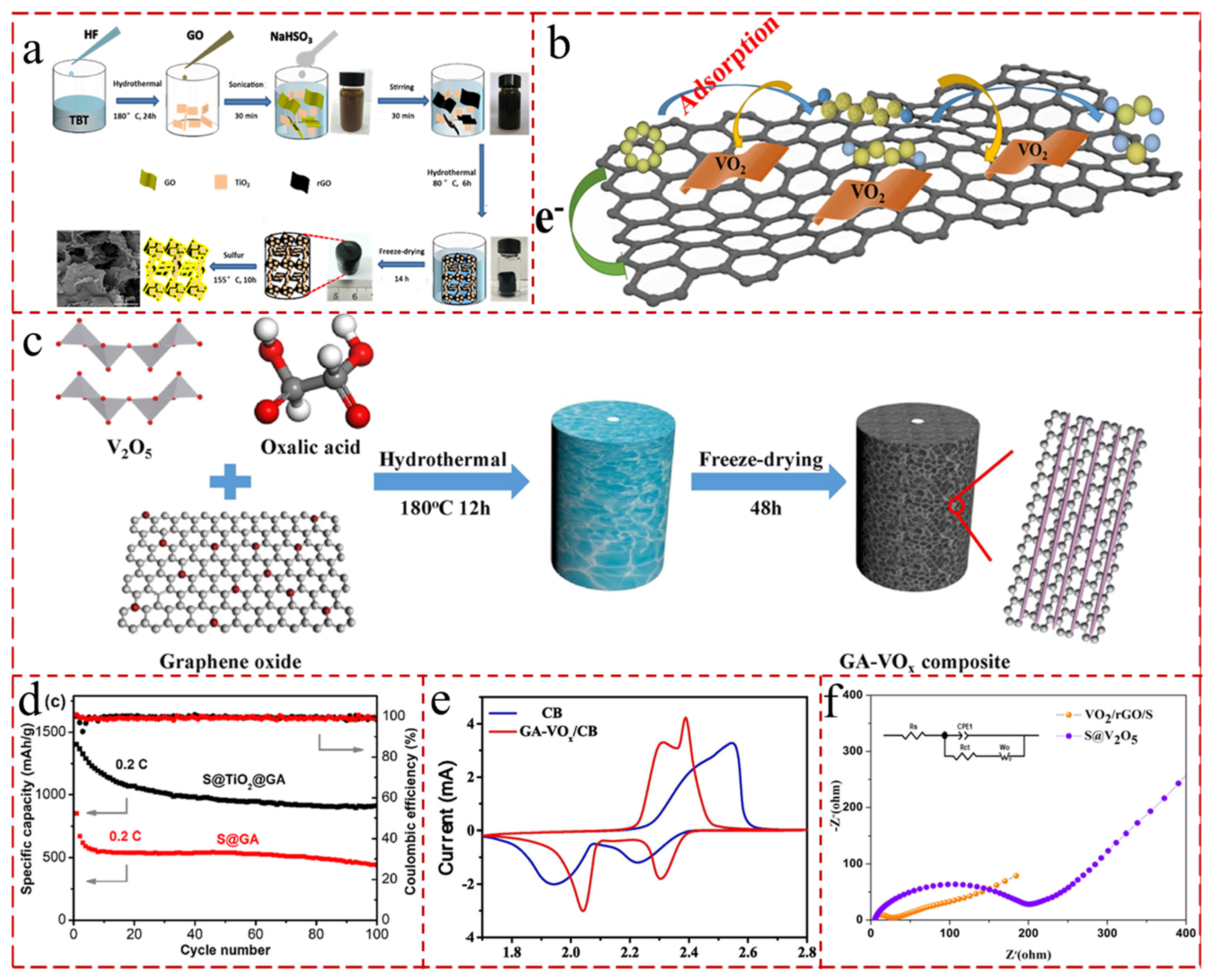
5.4.2. GA with TMSs
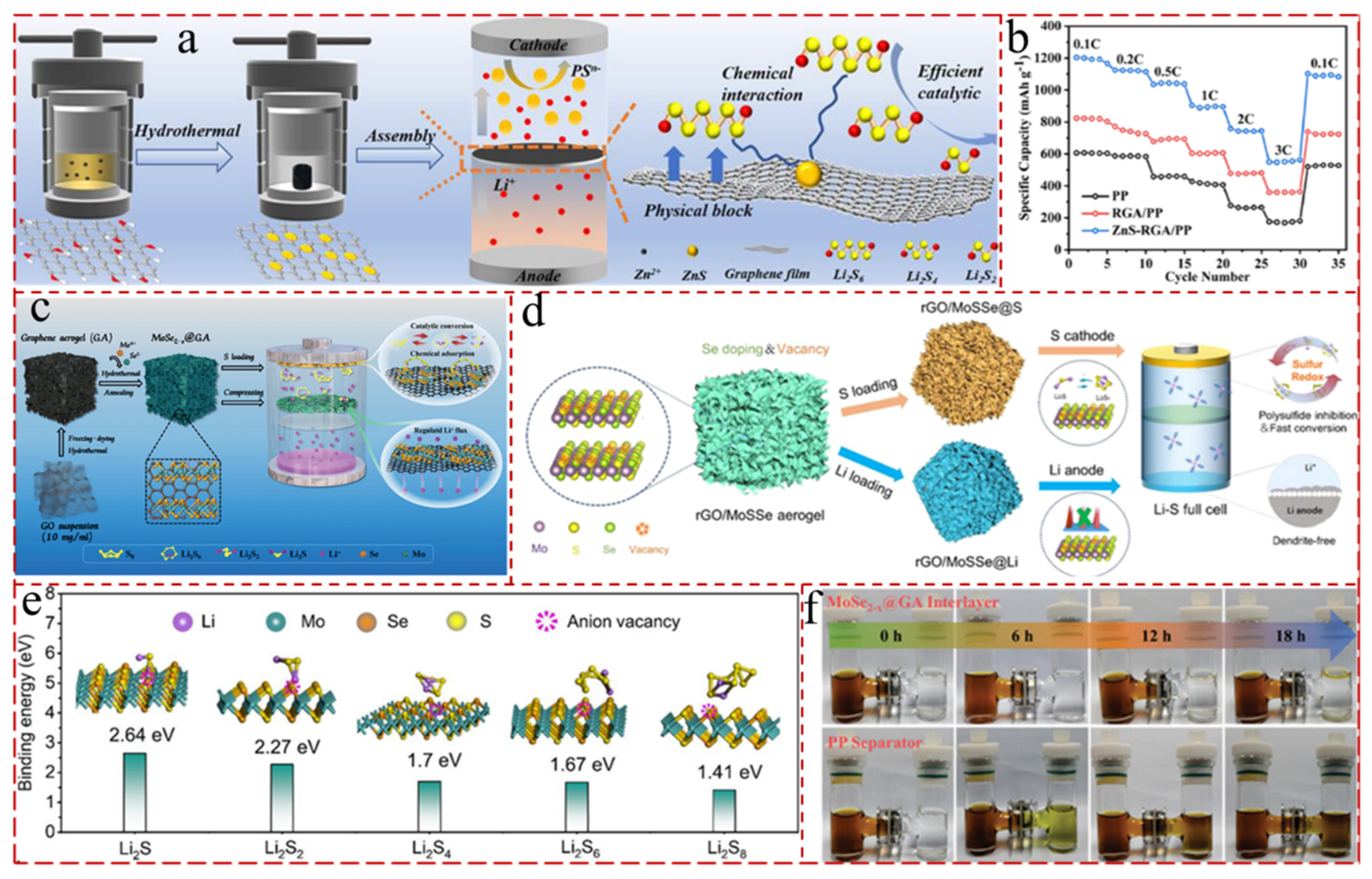
5.4.3. GA with Bimetallic Compound
5.4.4. GAs with Multi-Components
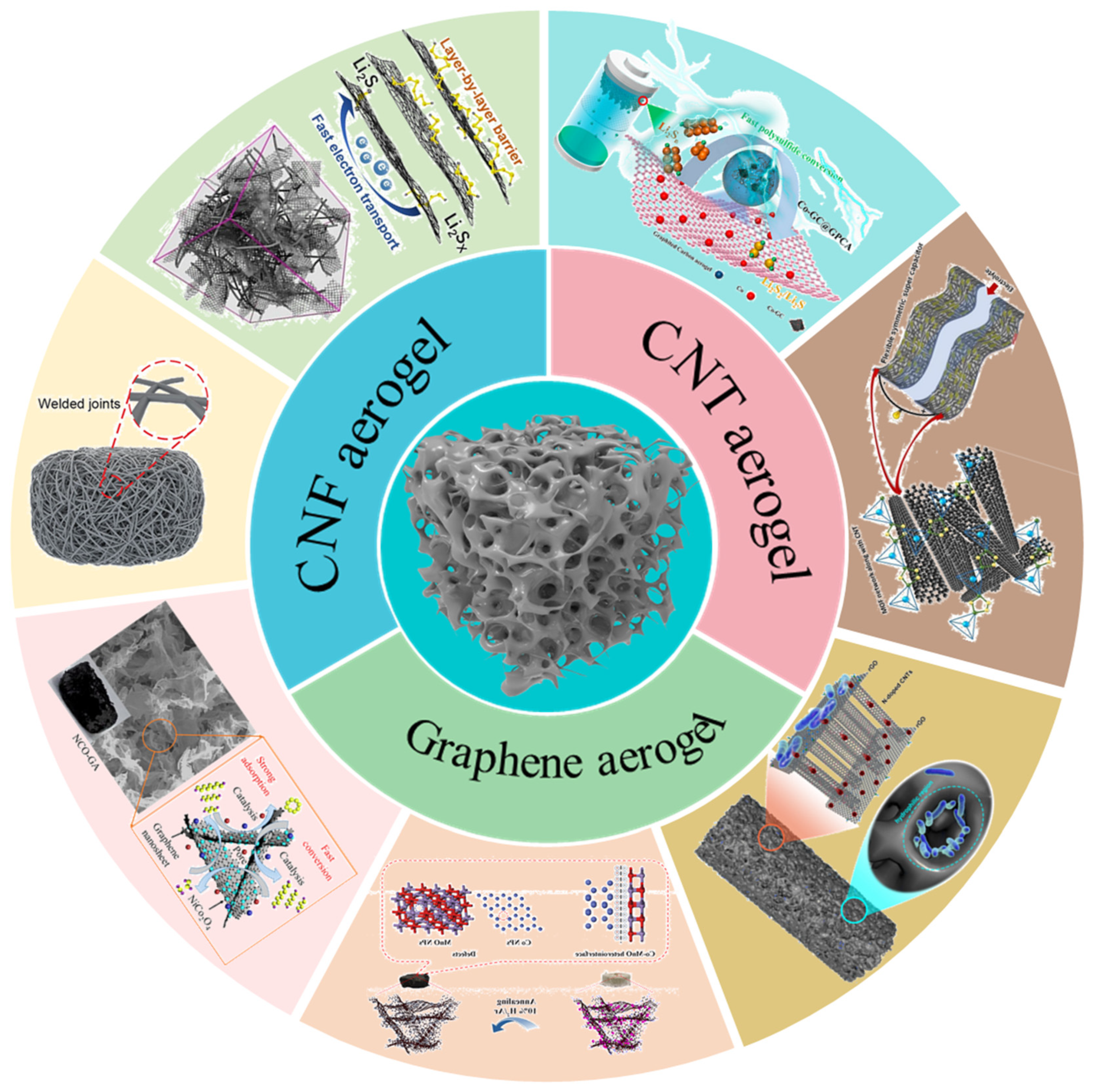
6. Summary and Future Perspectives
- (1)
- Compared to GAs and CNT aerogels, carbon nanofiber aerogels are underdeveloped, which is mainly related to the manufacture technology of carbon nanofibers. Therefore, the development of carbon nanofibres, particularly derived from polymer nanofibers, is highly important. For example, the development of novel spinning needles to increase the yield of polymer nanofibers.
- (2)
- The generally low mechanical properties of aerogels restrict their use in complex environments; so, a comprehensive strategy is needed to enhance their mechanical properties, from molecular design to macroscopic structure. At the molecular level, introducing flexible siloxane segments or constructing a dual-network, cross-linked structure can enhance the material’s inherent toughness effectively. At the mesoscale, a biomimetic hierarchical structure design can optimise stress distribution. At the macroscale, composite designs incorporating carbon fibre frameworks can withstand extreme loads.
- (3)
- The manufacturing process of most carbon-based aerogels is complex and costly, which makes it difficult to apply in practice, especially in some underpriviledged and distant regions. The development of most aerogel materials has been only limited to the laboratory, and many aerogel devices are too miniaturized for large-scale application. Therefore, aerogels will move towards the direction of being applied in large areas, low cost, and emerging technologies.
- (4)
- Carbon-based aerogels are predominantly employed as sulfur hosts, functional separators, and freestanding interlayers in LSBs. And the utilization of carbon-based aerogels in anodes is still in its infancy. However, it is essential that the meticulous carbon-based aerogel design is adopted to guarantee the decent protection of lithium anodes under high sulphur and current density conditions. One of the main problems with lithium metal anodes is electrolyte consumption and anode corrosion caused by a high E/S ratio. Constructing a thin film of aerogel-based solid electrolyte on the anode is a viable solution to reduce side reactions.
- (5)
- Solid-state LSBs are currently undergoing in a a boom in development to address the severe shuttle effect of LSBs. The low-density solid polymer electrolytes possess high gravimetric energy density, considerable design flexibility, and outstanding contact with electrodes and have attracted extensive attention. One major ambitious goal is the integration of aerogel materials as polymer electrolytes into LSBs. It is indispensable to establish the high ionic conductivity, excellent interfacial compatibility, and wide electrochemical window of the ideal aerogel-based electrolyte with both sulfur cathode and lithium anodes. Therefore, developing aerogel composite electrolytes has become a promising future development.
- (6)
- Many laboratory-scale studies of LSBs are limited to coin cells and lack the performance associated with practical multilayer pouch cell configurations. And few studies demonstrated promising the performance of aerogels in high sulfur loading at the pouch cell level, and there is a lack of data on multilayer pouch cells. Therefore, it is critical to scale up successful aerogel optic technology for high loaded lsbs from the coin cell level to adequately address the challenges and requirements of multilayer pouch cells for commercial prototyping.
- (7)
- Advanced characterization techniques, such as XRD, Raman spectroscopy, TEM, and in situ XAS, etc., should be employed to monitor and visualize the transformation of intermediates and the concentration of soluble polysulphides at different stages of the LiPS conversion process in real time, providing direct data to gain a deeper understanding of the electrochemical reaction pathways. Meanwhile, machine learning can accelerate the discovery of functional carbon-based aerogel materials by recommending experimental conditions that quickly achieve the target performance when screening adsorbents and catalysts.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Li, Y.; Zhang, L. Designing principles of advanced sulfur cathodes toward practical lithium-sulfur batteries. SusMat 2022, 2, 34–64. [Google Scholar] [CrossRef]
- Cha, E.; Patel, M.; Bhoyate, S.; Prasad, V.; Choi, W. Nanoengineering to achieve high efficiency practical lithium–sulfur batteries. Nanoscale Horiz. 2020, 5, 808–831. [Google Scholar] [CrossRef]
- Shao, Q.; Zhu, S.; Chen, J. A review on lithium-sulfur batteries: Challenge, development, and perspective. Nano Res. 2023, 16, 8097–8138. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Q.; Bak, S.-M.; Hwang, S.; Du, Y.; Wang, H. Identification and Catalysis of the Potential-Limiting Step in Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2023, 145, 7390–7396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, N.; Ju, J.; Wang, G.; Wei, L.; Gao, H.; Cheng, B.; Kang, W. Flower-like heterostructured MoP–MoS2 hierarchical nanoreactor enabling effective anchoring for LiPS and enhanced kinetics for high performance Li–S batteries. J. Membr. Sci. 2022, 642, 120003. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.-B.; Yan, C.; Huang, J.-Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef]
- He, M.; Zhang, X.; Li, H.; Jin, X.; Teng, L.; Liu, Q.; Liu, W. Comparative study on the sulfation of spent lithium-ion battery under different sulfur inputs: Extraction efficiency, SO2 emission and mechanism. J. Environ. Chem. Eng. 2023, 11, 111099. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Z.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.; Yang, J.; Dou, S.; Wang, N. Advances in High Sulfur Loading Cathodes for Practical Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Jia, S.; Zhao, Q.; Zheng, Q.; Ma, Y.; Ma, T.; Li, X. Recent advances in inhibiting shuttle effect of polysulfide in lithium-sulfur batteries. J. Energy Storage 2023, 72, 108372. [Google Scholar] [CrossRef]
- Wang, B.; Chen, K.; Liang, J.; Yu, Z.; Wang, D.-W.; Fang, R. Lithium cation-doped tungsten oxide as a bidirectional nanocatalyst for lithium-sulfur batteries with high areal capacity. J. Energy Chem. 2024, 98, 406–413. [Google Scholar] [CrossRef]
- Qi, S.; Li, C.; Chen, G.; Zhao, M. Single-atom catalysts supported on graphene/electride heterostructures for the enhanced sulfur reduction reaction in lithium-sulfur batteries. J. Energy Chem. 2024, 97, 738–746. [Google Scholar] [CrossRef]
- Lin, Y.; Li, L.; Tan, L.; Li, Y.; Ren, X.; Zhang, P.; He, C.; Sun, L. Accelerating lithium-sulfur battery reaction kinetics and inducing 3D deposition of Li2S using interactions between Fe3Se4 and lithium polysulfides. J. Energy Chem. 2024, 95, 540–553. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Liu, X.; Yan, Z.; Liu, W.; Deng, N.; Wei, L.; Cheng, B.; Kang, W. Cobalt-doping of molybdenum phosphide nanofibers for trapping-diffusion-conversion of lithium polysulfides towards high-rate and long-life lithium-sulfur batteries. J. Colloid Interface Sci. 2022, 628, 247–258. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, L.-C.; Wang, D.-W.; Li, L.; Pe, S.; Gentle, I.R.; Li, F.; Cheng, H.-M. Fibrous Hybrid ofGraphene and Sulfur Nanocrystals for High-Performance Lithium Sulfur Batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.-Y.; Peng, H.-J.; Cheng, X.-B.; Zhu, L.; Huang, J.-Q.; Zhu, W.; Zhang, Q. Scaled-up fabrication of porous-graphene-modified separators for high-capacity lithium–sulfur batteries. Energy Storage Mater. 2017, 7, 56–63. [Google Scholar] [CrossRef]
- Deng, B.; Liu, Z.; Peng, H. Toward Mass Production of CVD Graphene Films. Adv. Mater. 2018, 31, 1800996. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yao, Y.; Yuan, M.; Chen, G.; Wang, Y.; Rao, L.; Li, S.; Kara, U.I.; Dupont, R.L.; Zhang, C.; et al. Functional nanoporous graphene superlattice. Nat. Commun. 2024, 15, 1295. [Google Scholar] [CrossRef]
- Marongiu, M.; Ha, T.; Gil-Guerrero, S.; Garg, K.; Mandado, M.; Melle-Franco, M.; Diez-Perez, I.; Mateo-Alonso, A. Molecular Graphene Nanoribbon Junctions. J. Am. Chem. Soc. 2024, 146, 3963–3973. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Ma, Z.; Xu, X.; Xu, J.; Chen, Y. Construction of Fe3C@N-doped graphene layers yolk-shelled nanoparticles on the graphene sheets for high-efficient electromagnetic wave absorption. Carbon 2024, 229, 119448. [Google Scholar] [CrossRef]
- Lin, Z.; Shen, C.; Xia, Y.; Ma, R.; He, J.; Zhu, B.; Liu, Y.; Xu, Z.; Gao, W.; Gao, C. Multifunctional layered structure graphene aerogel with customizable shape by ion diffusion-directed assembly. Carbon 2025, 238, 120265. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Y.; Yan, Y.; Cheng, Z.; Ma, G.; Zhang, K.; Huang, X. Combined experiment and simulation on pore structure of graphene aerogel for microwave absorption and thermal insulation. Compos. Part B Eng. 2025, 298, 112397. [Google Scholar] [CrossRef]
- Yue, J.; Qin, M.; Yu, H.; He, Q.; Feng, W. Superelastic Graphene-Based Composite Aerogel for Thermal and Electromagnetic Protection in Extreme Temperature Environments. Adv. Funct. Mater. 2025, 2508319. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Kadam, V.; Jagtap, C.; Rednam, U.; Lakal, N.; Al-Asbahi, B.A. Fast synthesis of Co3O4-MXene nanocomposites via microwave assistance for energy storage applications. Diam. Relat. Mater. 2025, 154, 112191. [Google Scholar] [CrossRef]
- BhaskaraRao, B.V.; Pabba, D.P.; Aepuru, R.; Akbari-Fakhrabadi, A.; Lokhande, P.; Udayabhaskar, R.; Rosales-Vera, M.; Espinoza-González, R. Fe3O4 nanoparticles intercalated reduced graphene oxide nanosheets for supercapacitor and lithium-ion battery anode performance. J. Mater. Sci. Mater. Electron. 2023, 34, 1910. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Y.; Pei, Z.; Luan, D.; Wang, X.; Lou, X.W. Bimetal-Organic Framework Nanoboxes Enable Accelerated Redox Kinetics and Polysulfide Trapping for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2023, 62, e202305828. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Z.; Wu, J.; Liu, C.; Peng, Y.; Fan, Y.; Chang, J.; Zheng, Z.; Huang, W.; Chen, G.; et al. Cationic surfactant for lithium-sulfur batteries enables efficient use of sulfur and limits lithium dendrite formation. Cell Rep. Phys. Sci. 2023, 4, 101658. [Google Scholar] [CrossRef]
- Shan, X.; Guo, Z.; Zhang, C.; Wang, W.; Zhao, L. Nickel aerogel @ ultra-thin NiCo-LDH nanosheets integrated freestanding film as a collaborative adsorption and accelerated conversion cathode to improve the rate performance of lithium sulfur batteries. Chem. Eng. J. 2024, 488, 151105. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Cheng, X.-B.; Zhu, J.; Lu, B.; Wu, Y. Strategies toward High-Loading Lithium–Sulfur Batteries. ACS Energy Lett. 2022, 8, 116–150. [Google Scholar] [CrossRef]
- Weret, M.A.; Su, W.N.; Hwang, B.J. Strategies towards High Performance Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200059. [Google Scholar] [CrossRef]
- Fei, Y.; Li, Z.; Li, P.; Zhang, X.; Xu, Z.; Deng, W.; Zhang, H.; Li, G. Dual-Functional Metal–Organic Framework Freestanding Aerogel Boosts Sulfur Reduction Reaction for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 53833–53842. [Google Scholar] [CrossRef]
- Wang, J.; Yi, S.; Liu, J.; Sun, S.; Liu, Y.; Yang, D.; Xi, K.; Gao, G.; Abdelkader, A.; Yan, W.; et al. Suppressing the Shuttle Effect and Dendrite Growth in Lithium–Sulfur Batteries. ACS Nano 2020, 14, 9819–9831. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Feng, S.; Zhao, M.; Zhao, C.X.; Chen, X.; Li, B.Q.; Huang, J.Q.; Zhang, Q. Surface Gelation on Disulfide Electrocatalysts in Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2021, 61, e202114671. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, Y.; Wang, G.; Deng, N.; Wei, L.; Yang, Q.; Cheng, B.; Kang, W. YF3/CoF3 co-doped 1D carbon nanofibers with dual functions of lithium polysulfudes adsorption and efficient catalytic activity as a cathode for high-performance Li-S batteries. J. Colloid Interface Sci. 2022, 607, 922–932. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. A Perspective toward Practical Lithium–Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Wang, X.; Deng, N.; Liu, Y.; Wei, L.; Wang, H.; Li, Y.; Cheng, B.; Kang, W. Porous and heterostructured molybdenum-based phosphide and oxide nanobelts assisted by the structural engineering to enhance polysulfide anchoring and conversion for lithium–sulfur batteries. Chem. Eng. J. 2022, 450, 138191. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, B.; Li, X.; He, X.; Sun, Z.; Zhang, W.; Gao, Z.; Zhang, L.; Chen, X.; Chen, Z.; et al. A high-energy-density long-cycle lithium–sulfur battery enabled by 3D graphene architecture. Carbon Energy 2024, 6, e599. [Google Scholar] [CrossRef]
- Zhai, S.; Ye, Z.; Liu, R.; Xu, H.; Li, C.; Liu, W.; Wang, X.; Mei, T. Defect Strategy-Regulated MoSe2-Modified Self-Supporting Graphene Aerogels Serving as Both Cathode and Interlayer of Lithium-Sulfur Batteries. Adv. Funct. Mater. 2023, 34, 2314379. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Hu, Z.; Yu, J.; Wang, Y. Mesoporous MXene nanosheets/CNF composite aerogels for electromagnetic wave absorption and multifunctional response. Chem. Eng. J. 2024, 502, 157770. [Google Scholar] [CrossRef]
- Yu, H.; Yao, J.; Lv, J.; Zhang, R.; Wang, Z.; Song, X.; Wei, X.; Zhou, J. Low-temperature carbonization MOF/CNF aerogel for high-performance microwave absorption and thermal camouflage. Carbon 2025, 243, 120485. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Zhou, L.; Li, H.; Meng, Q.; Jing, L.; Tian, Z.; Hou, C. 2D N-doped graphene/CoFe MOFs heterostructure functionalized CNF aerogels impart highly efficient photocatalytic oxidation of gaseous VOCs. J. Environ. Chem. Eng. 2024, 12, 112225. [Google Scholar] [CrossRef]
- Ma, M.; Liao, Y.; Lin, H.; Shao, W.; Tao, W.; Chen, S.; Shi, Y.; He, H.; Zhu, Y.; Wang, X. Double-layer of CNF/rGO film and CNF/rGO/FeCo-LDO aerogel structured composites for efficient electromagnetic interference shielding. Carbon 2024, 220, 118863. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Xu, X.; Zhu, Y.; Liu, Y.; Yuan, J.; Men, X. Enhancement on the thermal and tribological behaviors of polyurethane/epoxy-based interpenetrating network composites by orientationally aligned CNF/MXene/WPU aerogels. Compos. Part A Appl. Sci. Manuf. 2024, 187, 108477. [Google Scholar] [CrossRef]
- Gueye, A.B.; Thomas, S. Review: A critical analysis of recent advancements on carbon-based materials for lithium–sulfur batteries. J. Mater. Sci. 2025, 60, 7797–7825. [Google Scholar] [CrossRef]
- Hu, W.; Liu, H.; Fan, X.; Tian, X.; Pang, L. Nitrogen-Doped Porous Nanofiber Aerogel-Encapsulated Staphylo-Ni3S2 Accelerating Polysulfide Conversion for Efficient Li–S Batteries. ACS Appl. Mater. Interfaces 2025, 17, 6304–6314. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Gao, B.; Fan, Z.; Wang, M.; Lan, X.; Fu, H. Research on vanadium-chromium oxide Lithium-sulfur battery cathode materials derived from metal-organic frameworks on reduced graphene oxide aerogels. J. Energy Storage 2025, 125, 117001. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, L.; Pan, Y.; Deng, Y.; Zhou, Y.; Cheng, X.; Zhang, H. Coral-like interconnected carbon aerogel modified separator for advanced lithium-sulfur batteries. Electrochim. Acta 2020, 354, 136637. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Sun, H.; Lai, C.; Zhao, X.; Peng, H.; Liu, T. A hybrid carbon aerogel with both aligned and interconnected pores as interlayer for high-performance lithium–sulfur batteries. Nano Res. 2016, 9, 3735–3746. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liao, Y.; Li, X.; Li, W. Sulfur loaded in micropore-rich carbon aerogel as cathode of lithium-sulfur battery with improved cyclic stability. J. Power Sources 2016, 334, 23–30. [Google Scholar] [CrossRef]
- Ji, L.; Wang, X.; Jia, Y.; Qin, X.; Sui, Y.; Yan, H.; Niu, Z.; Liu, J.; Zhang, Y. Oxygen and nitrogen tailoring carbon fiber aerogel with platinum electrocatalysis interfaced lithium/sulfur (Li/S) batteries. Chin. Chem. Lett. 2023, 34, 107123. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Sun, Q.; Liang, X.; Gu, P.; Huang, J.; Zu, G. Bioinspired Gradient Stretchable Aerogels for Ultrabroad-Range-Response Pressure-Sensitive Wearable Electronics and High-Efficient Separators. Angew. Chem. Int. Ed. 2022, 62, e202213952. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Y.G.; Zhan, L.; Liang, X.Y.; Wu, G.P.; Ling, L.C. Monolithic carbon aerogels from sol-gel polymerization of phenolic resoles and methylolated melamine. Carbon 2003, 41, 1660–1663. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R. Synthesis of organic and carbon aerogels from phenol–furfural by two-step polymerization. Microporous Mesoporous Mater. 2006, 96, 115–120. [Google Scholar] [CrossRef]
- Zubyk, H.; Mykhailiv, O.; Papathanassiou, A.N.; Sulikowski, B.; Zambrzycka-Szelewa, E.; Bratychak, M.; Plonska-Brzezinska, M.E. A phenol-formaldehyde polymeric network to generate organic aerogels: Synthesis, physicochemical characteristics and potential applications. J. Mater. Chem. A 2018, 6, 845–852. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Mikami, M.; Okazaki, M. Porous structure of organic and carbon aerogels synthesized by sol-gel polycondensation of resorcinol with formaldehyde. Carbon 1997, 35, 791–796. [Google Scholar] [CrossRef]
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in Tailoring Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Crane, M.J.; Lim, M.B.; Zhou, X.; Pauzauskie, P.J. Rapid synthesis of transition metal dichalcogenide–carbon aerogel composites for supercapacitor electrodes. Microsyst. Nanoeng. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Zhang, H.M.; Feng, J.Z.; Li, L.J.; Jiang, Y.G.; Feng, J. Controlling the microstructure of resorcinol-furfural aerogels and derived carbon aerogels via the salt templating approach. RSC Adv. 2019, 9, 5967–5977. [Google Scholar] [CrossRef]
- Wang, L.; Men, J.; Feng, J.; Jiang, Y.; Li, L.; Hu, Y.; Feng, J. Extrusion 3D printing of carbon nanotube-assembled carbon aerogel nanocomposites with high electrical conductivity. Nano Mater. Sci. 2023, 6, 312–319. [Google Scholar] [CrossRef]
- Gao, H.; Zuo, S.; Wang, S.; Xu, F.; Yang, M.; Hu, X. Graphitic crystallite nanomaterials enable the simple and ultrafast synthesis of resorcinol-formaldehyde carbon aerogel monoliths. Carbon 2022, 194, 220–229. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zou, R.; Huang, Y.; Lai, H.; Chen, Z.; Peng, X. Cellulose nanofiber-derived carbon aerogel for advanced room-temperature sodium–sulfur batteries. Carbon Energy 2022, 5, e203. [Google Scholar] [CrossRef]
- Shi, H.; Cao, J.; Sun, W.; Lu, G.; Lan, H.; Xu, L.; Ghazi, Z.A.; Fan, D.; Mao, Z.; Han, D.; et al. Ultrasmall, Amorphous V2O3 Intimately Anchored on a Carbon Nanofiber Aerogel Toward High-Rate Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 18812–18823. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Wang, X.; Xu, T.; Zhang, M.; Wang, Y.; Zhu, L.; Tong, S.; Zhou, X.; Li, J.; et al. Highly reversible and rapid charge transfer Zn-MnO2 battery by MnO2 nanosheet arrays anchored nanocellulose-based carbon aerogel. Adv. Compos. Hybrid Mater. 2024, 7, 90. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, X.; Ning, Y.; Jiang, X.; Yu, R.; Zhang, X. 3D multifunctional porous pine carbon aerogels coupled with highly dispersed CoFe nanoparticles for robust electromagnetic wave response. J. Mater. Sci. Technol. 2024, 192, 6–18. [Google Scholar] [CrossRef]
- Lin, C.; Tang, J.; Wang, S.; Gao, Q.; Liu, Y.; Wu, W.; Wang, X.; Huang, Z.; Yang, L. Fabrication of FeP2/C/CNTs@3D interconnected graphene aerogel composite for lithium-ion battery anodes and the electrochemical performance evaluation using machine learning. J. Alloys Compd. 2024, 996, 174800. [Google Scholar] [CrossRef]
- Sultanov, F.; Tatykayev, B.; Bakenov, Z.; Mentbayeva, A. The role of graphene aerogels in rechargeable batteries. Adv. Colloid Interface Sci. 2024, 331, 103249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, T.; Yu, D.; Wang, W. Fe3O4@CNTs/WPU@CNF aerogel/Ag foam composites with a double-layer structure for absorption-dominated electromagnetic shielding performance. J. Alloys Compd. 2024, 1002, 175170. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Lu, Z.; Cheng, R.; Wang, N.; Li, Y. Construction of multi-dimensional NiCo/C/CNT/rGO aerogel by MOF derivative for efficient microwave absorption. Carbon 2023, 205, 411–421. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Yalcintas, E.P.; Haghniaz, R.; de Barros, N.R.; Mecwan, M.; Nasiri, R.; Davoodi, E.; Nasrollahi, F.; Erdem, A.; Kang, H.; et al. Aerogel-Based Biomaterials for Biomedical Applications: From Fabrication Methods to Disease-Targeting Applications. Adv. Sci. 2023, 10, 2204681. [Google Scholar] [CrossRef]
- Cao, J.; Sun, G.; Wang, P.; Meng, C. Microstructured CNTs/Cellulose Aerogel for a Highly Sensitive Pressure Sensor. ACS Appl. Mater. Interfaces 2024, 16, 54652–54662. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Lv, T.; Liu, Y.; Qi, Y.; Dong, K.; Cao, S.; Chen, T. Designing free-standing 3D lamellar/pillared RGO/CNTs aerogels with ultra-high conductivity and compressive strength for elastic energy devices. J. Mater. Chem. A 2023, 11, 14187–14194. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, S.; All Amin Newton, M.; Cai, J.; Feng, H.; Xin, B.; Xing, W. Ultra-light, ultra-resilient and ultra-flexible, multifunctional composite carbon nanofiber aerogel for physiological signal monitoring and hazard warning in extreme environments. Chem. Eng. J. 2024, 494, 153017. [Google Scholar] [CrossRef]
- Chen, H.; Fu, G.; Xu, X.; Xu, X.; Ju, W.; Ma, Z.; Wang, X.; Lei, W. NiO quantum dot-modified high specific surface carbon aerogel materials as an advanced host for lithium-sulfur batteries. Electrochim. Acta 2023, 467, 143087. [Google Scholar] [CrossRef]
- Yu, C.; Lin, D.; Guo, J.; Zhuang, K.; Yao, Y.; Zhang, X.; Jiang, X. Ultralight Three-Layer Gradient-Structured MXene/Reduced Graphene Oxide Composite Aerogels with Broadband Microwave Absorption and Dynamic Infrared Camouflage. Small 2024, 20, 2401755. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, F.; Mentbayeva, A.; Kalybekkyzy, S.; Zhaisanova, A.; Myung, S.-T.; Bakenov, Z. Advances of graphene-based aerogels and their modifications in lithium-sulfur batteries. Carbon 2023, 201, 679–702. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, Z.; Gao, X.; Hong, H.; Nie, C.; Jiang, Y.; Jiang, H.; Zhang, J.; Cao, X.; Zhuang, Q.; et al. Rationally incorporated SnO2/SnS2 nanoparticles on sulfur-doped graphene aerogels for high-performance lithium/sodium-ion batteries. J. Energy Storage 2023, 65, 107344. [Google Scholar] [CrossRef]
- Hou, M.; Chen, K.; Zhang, G.; Liang, X.; Liu, X.; Xing, S. 3D conductive molecular framework derived MnO2/N, P co-doped carbon as sulfur hosts for high-performance lithium-sulfur batteries. J. Energy Storage 2023, 72, 108339. [Google Scholar] [CrossRef]
- Rebber, M.; Trommler, M.; Lokteva, I.; Ehteram, S.; Schropp, A.; König, S.; Fröba, M.; Koziej, D. Additive-Free, Gelled Nanoinks as a 3D Printing Toolbox for Hierarchically Structured Bulk Aerogels. Adv. Funct. Mater. 2022, 32, 2112914. [Google Scholar] [CrossRef]
- Tang, S.; Ma, M.; Zhang, X.; Zhao, X.; Fan, J.; Zhu, P.; Shi, K.; Zhou, J. Covalent Cross-Links Enable the Formation of Ambient-Dried Biomass Aerogels through the Activation of a Triazine Derivative for Energy Storage and Generation. Adv. Funct. Mater. 2022, 32, 2205417. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; Yang, J. Highly compressible and hydrophobic anisotropic cellulose-based aerogel fabricated by bidirectional freeze-drying for selective oil absorption. J. Mater. Sci. 2022, 57, 13097–13108. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, R.; Zhang, H.; Guo, M.; Zhang, D.; Gao, C.; Gao, F.; Chen, X.; Terrones, M.; Wang, Y. Ambient-drying to construct unidirectional cellulose nanofibers/carbon nanotubes aerogel with ultra-lightweight, robust, and superior microwave absorption performance. Carbon 2023, 212, 118150. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Li, X.; Zhang, J.; Nawaz, H.; Xu, Y.; Kong, F.; Xu, F. Anisotropic cellulose nanofibril aerogels fabricated by directional stabilization and ambient drying for efficient solar evaporation. Chem. Eng. J. 2023, 453, 139844. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Feng, Q.; Chen, C.; Xu, Z. Preparation of antifouling and highly hydrophobic cellulose nanofibers/alginate aerogels by bidirectional freeze-drying for water-oil separation in the ocean environment. J. Hazard. Mater. 2023, 441, 129965. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Ding, R.; Su, P.G.; Zeng, F.R.; Jia, X.X.; Hu, Z.Y.; Wang, Y.Z.; Zhao, H.B. Biomimetic Ambient-Pressure-Dried Aerogels with Oriented Microstructures for Enhanced Electromagnetic Shielding. Adv. Funct. Mater. 2025, 35, 2414683. [Google Scholar] [CrossRef]
- Shi, C.; Huang, J.; Tang, Y.; Cen, Z.; Wang, Z.; Liu, S.; Fu, R. A hierarchical porous carbon aerogel embedded with small-sized TiO2 nanoparticles for high-performance Li–S batteries. Carbon 2023, 202, 59–65. [Google Scholar] [CrossRef]
- Zhao, S.; Hao, Q.; Qian, X.; Jin, L.; Li, B.; Xu, H. Application of Y-Zn-MOF derived Y2O3/ZnO@C in modification of lithium-sulfur battery separator. J. Energy Storage 2024, 101, 113833. [Google Scholar] [CrossRef]
- Cao, L.; Wang, C.; Huang, Y. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, L.; Chen, J.; Qian, X.; Hao, Q.; Zhao, S.; Li, B.; Pang, S.; Shen, X. Ketjen Black@Ce-MOF derived KB@CeO2-C as separator coating for lithium sulfur batteries. J. Energy Storage 2024, 78, 110006. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhang, C.; Yu, F. Cascade phase change based on hydrate salt/carbon hybrid aerogel for intelligent battery thermal management. J. Energy Storage 2022, 45, 103771. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Fu, Z.; Qian, X.; Cheng, J.; Hao, Q.; Zhang, K. ZIF-8/ZIF-67 derived ZnS@Co-N-C hollow core-shell composite and its application in lithium-sulfur battery. Sustain. Mater. Technol. 2023, 35, e00571. [Google Scholar] [CrossRef]
- Taurbekov, A.; Fierro, V.; Kuspanov, Z.; Abdisattar, A.; Atamanova, T.; Kaidar, B.; Mansurov, Z.; Atamanov, M. Nanocellulose and carbon nanotube composites: A universal solution for environmental and energy challenges. J. Environ. Chem. Eng. 2024, 12, 113262. [Google Scholar] [CrossRef]
- Wang, L.; Shen, H.; Zhang, H.; Xu, D.; Zhou, J. Fabrication of anisotropic carbon aerogels from cellulose nanofiber/graphene oxide composites for electromagnetic interference shielding. J. Alloys Compd. 2024, 980, 173505. [Google Scholar] [CrossRef]
- Gong, L.; An, X.; Ma, C.; Wang, R.; Zhou, X.; Liu, C.; Li, N.; Liu, Z.; Li, X. Double cross-linked biomass aerogels with enhanced mechanical strength and flame retardancy for construction thermal insulation. Int. J. Biol. Macromol. 2024, 281, 136304. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Liu, J.; Wang, P.; Wang, C.; Wang, Z.; Chen, M.; Zhu, X.; Fu, B. Integration of N- and P-elements in sodium alginate aerogels for efficient flame retardant and thermal insulating properties. Int. J. Biol. Macromol. 2024, 273, 132643. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, L.; Li, X.; Sun, J.; Ge, D.; Lin, Y.; Li, X.; Liu, G.; Gong, Y.; Zhang, X.; et al. Construction of heterogeneous interfaces modulating dielectric loss in MOF-derived FeCoNi/C confined carbon aerogels as multifunctional microwave absorbers. J. Alloys Compd. 2024, 996, 174764. [Google Scholar] [CrossRef]
- Yang, B.; Xu, M.; Gao, Y.; Zhu, Q.; Xu, B. Interfacial Engineering and Coupling of MXene/Reduced Graphene Oxide/C3N4 Aerogel with Optimized d-Band Center as a Free-Standing Sulfur Carrier for High-Performance Li-S Batteries. Small Methods 2024, 8, 2301102. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Caminero-Huertas, S.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Effects of freeze-drying conditions on aerogel properties. J. Mater. Sci. 2016, 51, 8977–8985. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Effect of freeze-drying parameters on the microstructure and thermal insulating properties of nanofibrillated cellulose aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 475–485. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Politowicz, A.L.; Chen, E.; Huang, H.X.; Turng, L.S. Highly compressible ultra-light anisotropic cellulose/graphene aerogel fabricated by bidirectional freeze drying for selective oil absorption. Carbon 2018, 132, 199–209. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.Y.; Xue, T.T.; Yang, F.; Fan, W.; Liu, T.X. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Luo, Y.; Jiang, Y.; Zhang, G.; Feng, J. Versatile Thermal-Solidifying Direct-Write Assembly towards Heat-Resistant 3D-Printed Ceramic Aerogels for Thermal Insulation. Small Methods 2022, 6, 2200045. [Google Scholar] [CrossRef] [PubMed]
- Camani, P.H.; Gonçalo, M.G.M.; Barbosa, R.F.S.; Rosa, D.S. Comprehensive insight of crosslinking agent concentration influence on starch-based aerogels porous structure. J. Appl. Polym. Sci. 2021, 138, 50863. [Google Scholar] [CrossRef]
- Ren, L.; Dong, Y.; Dong, L.; Liu, T.; Tan, W. Carnauba Wax-Assisted Preparation of Eco-Friendly Aerogels by the Ambient Pressure Drying Method and their Applications for Photothermal Evaporation and Dye Wastewater Treatment. Adv. Mater. Technol. 2023, 8, 2202078. [Google Scholar] [CrossRef]
- Du, C.; Chen, Y.; He, S.; Ruan, C.; Liu, X.; He, C.; Jin, X.; Chen, Q.; Ma, Y.; Chen, G. Insight into ultra-flexible & robust silica aerogels based on diene synthesis reaction: Preparation and oil/water separation. Appl. Surf. Sci. 2022, 606, 154902. [Google Scholar] [CrossRef]
- Lyu, S.; Chang, H.; Zhang, L.; Wang, S.; Li, S.; Lu, Y.; Li, S. High specific surface area MXene/SWCNT/cellulose nanofiber aerogel film as an electrode for flexible supercapacitors. Compos. Part B Eng. 2023, 264, 110888. [Google Scholar] [CrossRef]
- Tao, M.; Chen, X.; Lin, H.; Jin, Y.; Shan, P.; Zhao, D.; Gao, M.; Liang, Z.; Yang, Y. Clarifying the Temperature-Dependent Lithium Deposition/Stripping Process and the Evolution of Inactive Li in Lithium Metal Batteries. ACS Nano 2023, 17, 24104–24114. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Zhao, J.; Zhang, Z.; Luo, Z.; Guo, Y.; Zhang, H.; Wang, Y.; Bai, R.; Zhao, D.; et al. Mechanically Interlocked Aerogels with Densely Rotaxanated Backbones. J. Am. Chem. Soc. 2022, 144, 11434–11443. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Guo, P.; Pang, S.; Hu, C.; Zhao, R.; Tang, S.; Cheng, H.-M. Tailoring microstructures of carbon fiber reinforced carbon aerogel-like matrix composites by carbonization to modulate their mechanical properties and thermal conductivities. Carbon 2022, 196, 807–818. [Google Scholar] [CrossRef]
- Hanzawa, Y.; Hatori, H.; Yoshizawa, N.; Yamada, Y. Structural changes in carbon aerogels with high temperature treatment. Carbon 2002, 40, 575–581. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Yavari, M. Effect of synthesis conditions on the structural properties of CNT-doped carbon aerogels. Diam. Relat. Mater. 2023, 136, 110012. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Zhang, H.-B.; Dai, Y.; Liu, Z.; Yu, Z.-Z. Superelastic and multifunctional graphene-based aerogels by interfacial reinforcement with graphitized carbon at high temperatures. Carbon 2018, 132, 95–103. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Zhou, C.; Wang, C. Biomass-derived dendritic-like porous carbon aerogels for supercapacitors. Electrochim. Acta 2016, 210, 897–904. [Google Scholar] [CrossRef]
- Su, X.; Wang, J.; Han, M.; Liu, Y.; Zhang, B.; Huo, S.; Wu, Q.; Liu, Y.; Xu, H.-X. Broadband electromagnetic wave absorption using pure carbon aerogel by synergistically modulating propagation path and carbonization degree. J. Colloid Interface Sci. 2023, 652, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Rajan, B.S.; Basha, K.S.; Madhu, P.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Zhang, B.A.; Kang, F.Y.; Tarascon, J.M.; Kim, J.K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Shojaei, T.R.; Choong, T.S.Y.; Asim, N. Fundamental and recent progress on the strengthening strategies for fabrication of polyacrylonitrile (PAN)-derived electrospun CNFs: Precursors, spinning and collection, and post-treatments. J. Ind. Eng. Chem. 2022, 110, 329–344. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Abdolrazzaghian, E.; Chen, L.; Kim, J.; Kim, J.J. Centrifugally Spun PVA/PVP Based B, N, F Doped Carbon Nanofiber Electrodes for Sodium Ion Batteries. Polymers 2022, 14, 5541. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zhao, H.Y.; Bao, S.; Yin, Y.S.; Zhang, Y.; Lu, J.L. Hollow FeS2 nanospheres encapsulated in N/S co-doped carbon nanofibers as electrode material for electrochemical energy storage. J. Alloys Compd. 2022, 905, 164184. [Google Scholar] [CrossRef]
- Xie, L.L.; Bi, J.Q.; Gao, X.C.; Meng, L.J.; Liu, C.; Rong, J.C. Preparation of hollow core-shell structured Ti3C2@Ti2SnC/CNFs with stable electrochemical performance as anode material for lithium ion battery. Ceram. Int. 2023, 49, 23003–23010. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Chen, L.F.; Zhu, Z.; Zhang, W.J.; Yu, S.H. Macroscopic-Scale Template Synthesis of Robust Carbonaceous Nanofiber Hydrogels and Aerogels and Their Applications. Angew. Chem.-Int. Ed. 2012, 51, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Q.; Li, W.; Zheng, Y.; Shi, Q.; Zhou, Z.; Shao, G.; Yang, W.; Chen, D.; Fang, X. Ultralight and robust carbon nanofiber aerogels for advanced energy storage. J. Mater. Chem. A 2021, 9, 900–907. [Google Scholar] [CrossRef]
- Zhao, Z.; Su, Z.; Chen, H.; Yi, S.; Zhang, W.; Niu, B.; Zhang, Y.; Long, D. Renewable biomass-derived carbon-based hosts for lithium–sulfur batteries. Sustain. Energy Fuels 2022, 6, 5211–5242. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- He, Q.; Ning, J.; Chen, H.; Jiang, Z.; Wang, J.; Chen, D.; Zhao, C.; Liu, Z.; Perepichka, I.F.; Meng, H.; et al. Achievements, challenges, and perspectives in the design of polymer binders for advanced lithium-ion batteries. Chem. Soc. Rev. 2024, 53, 7091–7157. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef]
- Lin, X.; Li, W.; Nguyen, V.; Wang, S.; Yang, S.; Ma, L.; Du, Y.; Wang, B.; Fan, Z. Fe-single-atom catalyst nanocages linked by bacterial cellulose-derived carbon nanofiber aerogel for Li-S batteries. Chem. Eng. J. 2023, 477, 146977. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, X.; Tian, M.; Zhang, X.; Qu, L.; Fan, T.; Miao, J. Smart fibers and textiles for emerging clothe-based wearable electronics: Materials, fabrications and applications. J. Mater. Chem. A 2023, 11, 17336–17372. [Google Scholar] [CrossRef]
- Aksoy, S.A.; Yılmaz, D.; Maleki, H.; Rahbar, R.S.; Barani, H. Fabrication and characterization of nanoencapsulated PCM-doped cotton/PAN nanofiber based composite yarns for thermoregulation. J. Energy Storage 2024, 101, 113849. [Google Scholar] [CrossRef]
- Rong, H.; Gao, T.; Zhang, Y.; Liu, X.; Zhang, X.; Yan, M. Carbonized fibers with multi-elemental doping and hollow architecture derived from natural cotton for tunable microwave absorption properties. J. Alloys Compd. 2021, 884, 161084. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, J.; Cai, R.; Fu, J.; Xiang, S.; Zhao, S.; Fu, F.; Diao, H.; Liu, X. A Novel L-Cys@Cu MOF Embedding onto Cotton Fiber Surfaces to Exert Excellent Antiviral and Antibacterial Effects. Adv. Fiber Mater. 2024, 6, 444–457. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, Y.; Guccini, V.; Hahn, M.; Yan, B.; Salazar-Alvarez, G.; Piao, Y. TEMPO-oxidized cellulose nanofibers as versatile additives for highly stable silicon anode in lithium-ion batteries. Electrochim. Acta 2021, 369, 137708. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. Emerging Catalysts to Promote Kinetics of Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Xia, J.; Gao, R.; Yang, Y.; Tao, Z.; Han, Z.; Zhang, S.; Xing, Y.; Yang, P.; Lu, X.; Zhou, G. TinO2n–1/MXene Hierarchical Bifunctional Catalyst Anchored on Graphene Aerogel toward Flexible and High-Energy Li–S Batteries. ACS Nano 2022, 16, 19133–19144. [Google Scholar] [CrossRef]
- Shen, M.; Hu, W.; Duan, C.; Li, J.; Ding, S.; Zhang, L.; Zhu, J.; Ni, Y. Cellulose nanofibers carbon aerogel based single-cobalt-atom catalyst for high-efficiency oxygen reduction and zinc-air battery. J. Colloid Interface Sci. 2023, 629, 778–785. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, C.; Bi, Z.; Xu, X.; Ren, X.; Li, X.; Lu, K.; Yuan, S. Preparation of Highly Pyrrolic-Nitrogen-Doped Carbon Aerogels for Lithium-Sulfur Batteries. ChemElectroChem 2021, 8, 895–902. [Google Scholar] [CrossRef]
- Talebi, N.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Dan, A.K.; Ghanbari, R.; Kahkesh, K.H.; Peixoto, D.; Giram, P.S.; Raza, F.; et al. Natural polymeric nanofibers in transdermal drug delivery. Appl. Mater. Today 2023, 30, 101726. [Google Scholar] [CrossRef]
- Schneider, M.; Rodríguez-Castellón, E.; Guerrero-Pérez, M.O.; Hotza, D.; De Noni, A.; de Fátima Peralta Muniz Moreira, R. Advances in electrospun composite polymer/zeolite and geopolymer nanofibers: A comprehensive review. Sep. Purif. Technol. 2024, 340, 126684. [Google Scholar] [CrossRef]
- Lin, L.-W.; Qi, M.; Bai, Z.-T.; Yan, S.-X.; Sui, Z.-Y.; Han, B.-H.; Liu, Y.-W. Crumpled nitrogen-doped aerogels derived from MXene and pyrrole-formaldehyde as modified separators for stable lithium-sulfur batteries. Appl. Surf. Sci. 2021, 555, 149717. [Google Scholar] [CrossRef]
- Kim, K.H.; Oh, Y.; Islam, M.F. Mechanical and Thermal Management Characteristics of Ultrahigh Surface Area Single-Walled Carbon Nanotube Aerogels. Adv. Funct. Mater. 2013, 23, 377–383. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Karakoti, A.S.; Kumar, A.; Joung, D.; Li, Q.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight Multiwalled Carbon Nanotube Aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zeng, Z.; Gui, X.; Tang, Z.; Zou, M.; Cao, A. Carbon Nanotube Sponges, Aerogels, and Hierarchical Composites: Synthesis, Properties, and Energy Applications. Adv. Energy Mater. 2016, 6, 1600554. [Google Scholar] [CrossRef]
- Fang, Z.H.; Luo, Y.F.; Wu, H.C.; Yan, L.J.; Zhao, F.; Li, Q.Q.; Fan, S.S.; Wang, J.P. Mesoporous carbon nanotube aerogel-sulfur cathodes: A strategy to achieve ultrahigh areal capacity for lithium -sulfur batteries via capillary action. Carbon 2020, 166, 183–192. [Google Scholar] [CrossRef]
- Li, X.; Pu, X.; Han, S.; Liu, M.; Du, C.; Jiang, C.; Huang, X.; Liu, T.; Hu, W. Enhanced performances of Li/polysulfide batteries with 3D reduced graphene oxide/carbon nanotube hybrid aerogel as the polysulfide host. Nano Energy 2016, 30, 193–199. [Google Scholar] [CrossRef]
- Pu, X.; Yang, G.; Yu, C. Liquid-Type Cathode Enabled by 3D Sponge-Like Carbon Nanotubes for High Energy Density and Long Cycling Life of Li-S Batteries. Adv. Mater. 2014, 26, 7456–7461. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Hou, P.-X.; Yin, L.; Liu, C.; Lu, G.Q.; Gentle, I.R.; Cheng, H.-M. A flexible nanostructured sulphur–carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ. Sci. 2012, 5, 8901–8906. [Google Scholar] [CrossRef]
- Bordjiba, T.; Mohamedi, M.; Dao, L.H. New Class of Carbon-Nanotube Aerogel Electrodes for Electrochemical Power Sources. Adv. Mater. 2008, 20, 815–819. [Google Scholar] [CrossRef]
- Kim, K.H.; Vural, M.; Islam, M.F. Single-Walled Carbon Nanotube Aerogel-Based Elastic Conductors. Adv. Mater. 2011, 23, 2865–2869. [Google Scholar] [CrossRef]
- Schiffres, S.N.; Kim, K.H.; Hu, L.; McGaughey, A.J.H.; Islam, M.F.; Malen, J.A. Gas Diffusion, Energy Transport, and Thermal Accommodation in Single-Walled Carbon Nanotube Aerogels. Adv. Funct. Mater. 2012, 22, 5251–5258. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Xu, C.; Zhang, W.; Qin, W.; He, W. Three-Dimensional CNT/Graphene–Li2S Aerogel as Freestanding Cathode for High-Performance Li–S Batteries. ACS Energy Lett. 2016, 1, 820–826. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Zhou, G.; Pan, Z.-Z.; Ma, J.; Nishihara, H.; He, Y.-B.; Kang, F.; Lv, W.; Yang, Q.-H. Lamellar MXene Composite Aerogels with Sandwiched Carbon Nanotubes Enable Stable Lithium–Sulfur Batteries with a High Sulfur Loading. Adv. Funct. Mater. 2021, 31, 2100793. [Google Scholar] [CrossRef]
- Zhu, S.; Sheng, J.; Chen, Y.; Ni, J.; Li, Y. Carbon nanotubes for flexible batteries: Recent progress and future perspective. Natl. Sci. Rev. 2021, 8, nwaa261. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Y.; Zhang, J.; Wang, R.; Gong, Y.; He, B.; Wang, H.; Jin, J. Constructing MXene hydrogels and aerogels for rechargeable supercapacitors and batteries. J. Mater. Chem. C 2023, 11, 2414–2429. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Q.; Bai, Y.; Wang, X.; Liu, X.; Yan, C.; Wang, Y.; Qin, J.; Cheng, P. Toward strong X-band-electromagnetic-wave-absorbing materials: Polyimide/carbon nanotube composite aerogel with radial needle-like porous structure. J. Mater. Chem. A 2022, 10, 25140–25147. [Google Scholar] [CrossRef]
- Shen, Z.; Qin, M.; Xiong, F.; Zou, R.; Zhang, J. Nanocellulose-based composite phase change materials for thermal energy storage: Status and challenges. Energy Environ. Sci. 2023, 16, 830–861. [Google Scholar] [CrossRef]
- Sehrawat, M.; Rani, M.; Sharma, S.; Bharadwaj, S.; Falzon, B.G.; Singh, B.P. Floating catalyst chemical vapour deposition (FCCVD) for direct spinning of CNT aerogel: A review. Carbon 2024, 219, 118747. [Google Scholar] [CrossRef]
- Bai, L.; Ma, J.; Song, H.; Yang, Y.; Zhi, C.; Lee, S.-Y.; Yu, H.; Liu, S.; Li, J.; Yu, M.; et al. Flexible, Electrically Conductive, Nanostructured, Asymmetric Aerogel Films for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 59174–59184. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, N.; Kong, W.; Wu, H.; Wang, K.; Fan, S.; Duan, W.; Wang, J. Multifunctional Interlayer Based on Molybdenum Diphosphide Catalyst and Carbon Nanotube Film for Lithium–Sulfur Batteries. Small 2017, 14, 1702853. [Google Scholar] [CrossRef]
- Cao, J.; Chen, P.; Liu, M.; Yuan, H.; Geng, H.; Xu, L.; Li, Y.; Zhang, C.; Fu, Y.; Song, Y. Vanadium disulfide-coated carbon nanotube film as an interlayer for high-performance lithium-sulfur batteries. J. Energy Storage 2022, 52, 104818. [Google Scholar] [CrossRef]
- Yang, B.; Guo, D.; Lin, P.; Zhou, L.; Li, J.; Fang, G.; Wang, J.; Jin, H.; Chen, X.a.; Wang, S. Hydroxylated Multi-Walled Carbon Nanotubes Covalently Modified with Tris(hydroxypropyl) Phosphine as a Functional Interlayer for Advanced Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2022, 61, e202204327. [Google Scholar] [CrossRef]
- Yan, L.; Luo, N.; Kong, W.; Luo, S.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Duan, W.; Wang, J. Enhanced performance of lithium-sulfur batteries with an ultrathin and lightweight MoS2/carbon nanotube interlayer. J. Power Sources 2018, 389, 169–177. [Google Scholar] [CrossRef]
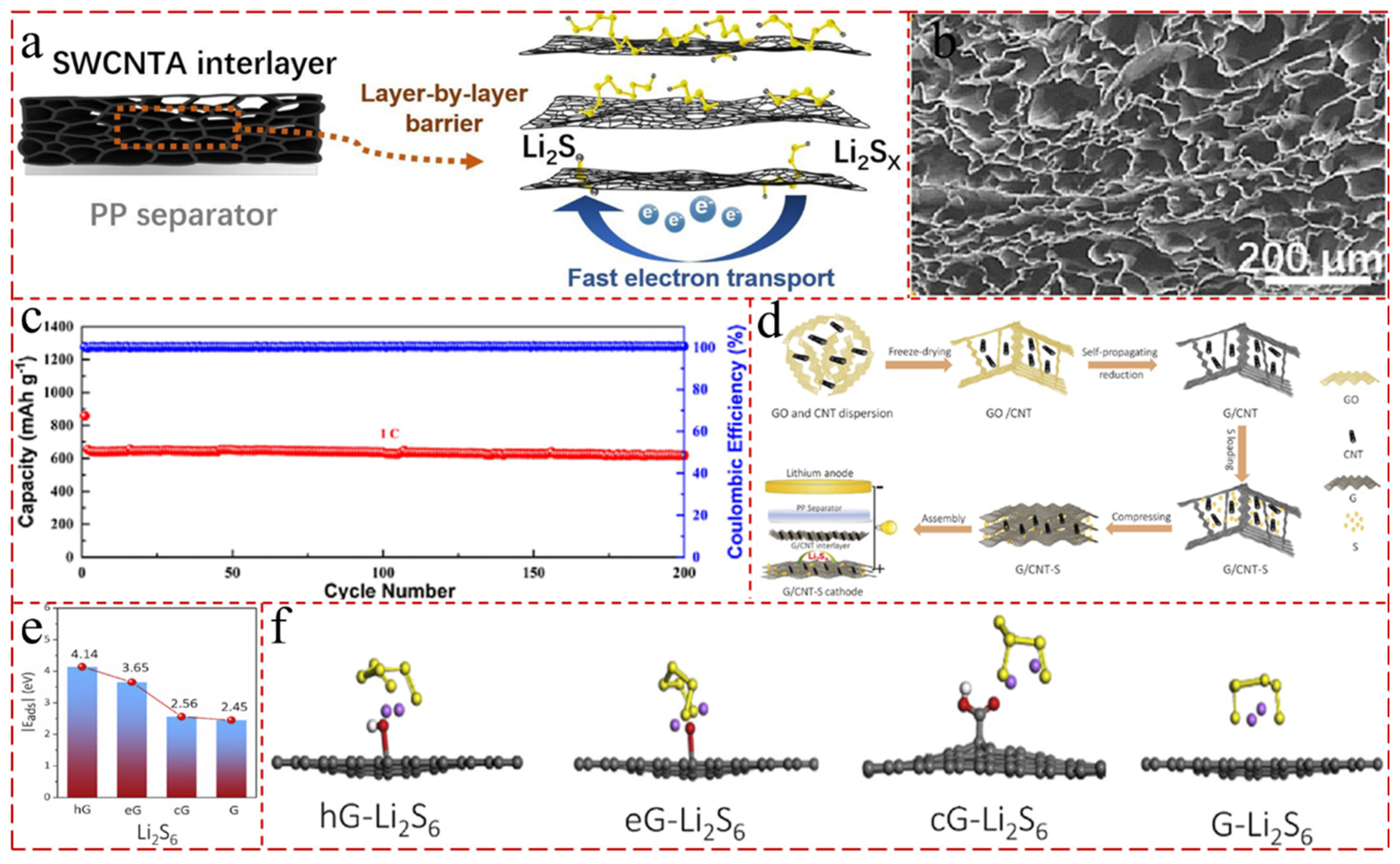
- Li, D.; Wang, W.; Liu, J.; He, M. Hierarchical lamellar single-walled carbon nanotube aerogel interlayers for stable lithium-sulfur batteries with high-sulfur-loading. Chem. Eng. J. 2023, 461, 142031. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, X.; Wu, Z.-S.; Dong, Y.; Lu, P.; Chen, J.; Ren, W.; Cheng, H.-M.; Bao, X. Free-standing integrated cathode derived from 3D graphene/carbon nanotube aerogels serving as binder-free sulfur host and interlayer for ultrahigh volumetric-energy-density lithium sulfur batteries. Nano Energy 2019, 60, 743–751. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.J.; Wei, X.J.; Song, L.X.; Tao, G.; Cao, X.; Wang, D.; Zhou, G.M.; Song, Y.Z. Dual-Functional V2C MXene Assembly in Facilitating Sulfur Evolution Kinetics and Li-Ion Sieving toward Practical Lithium-Sulfur Batteries. Adv. Mater. 2023, 35, 2300771. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Li, L.B.; Shan, Y.H.; Zhou, D.; Cui, W.J.; Zhao, Y.M.Y. Notes in accordions-organized MXene equipped with CeO2 for synergistically adsorbing and catalyzing polysulfides for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 70, 502–510. [Google Scholar] [CrossRef]
- Zhang, C.F.; Cui, L.F.; Abdolhosseinzadeh, S.; Heier, J. Two-dimensional MXenes for lithium-sulfur batteries. Infomation 2020, 2, 613–638. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.Z.; Liu, Y.; Xu, B. Status and Prospects of MXene-Based Lithium-Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100457. [Google Scholar] [CrossRef]
- Yin, F.; Jin, Q.; Zhang, X.-t.; Wu, L.-l. Design of a 3D CNT/Ti3C2Tx aerogel-modified separator for Li–S batteries to eliminate both the shuttle effect and slow redox kinetics of polysulfides. New Carbon Mater. 2022, 37, 724–733. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Ishikawa, R.; Yamazaki, S.; Watanabe, S.; Tsuboi, N. Layer dependency of graphene layers in perovskite/graphene solar cells. Carbon 2021, 172, 597–601. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; khan, A.u. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Kornilov, D.; Penki, T.R.; Cheglakov, A.; Aurbach, D. Li/graphene oxide primary battery system and mechanism. Battery Energy 2022, 1, 20210002. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Zhou, H.; Fu, C.; Wang, G.; Yang, L.; Xu, C.; Chen, Z.; Yang, W.; Kuang, Y. Hydrothermal preparation of nitrogen, boron co-doped curved graphene nanoribbons with high dopant amounts for high-performance lithium sulfur battery cathodes. J. Mater. Chem. A 2017, 5, 7403–7415. [Google Scholar] [CrossRef]
- Li, H.P.; Sun, L.C.; Wang, Z.; Zhang, Y.G.; Tan, T.Z.; Wang, G.K.; Bakenov, Z. Three-Dimensionally Hierarchical Graphene Based Aerogel Encapsulated Sulfur as Cathode for Lithium/Sulfur Batteries. Nanomaterials 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
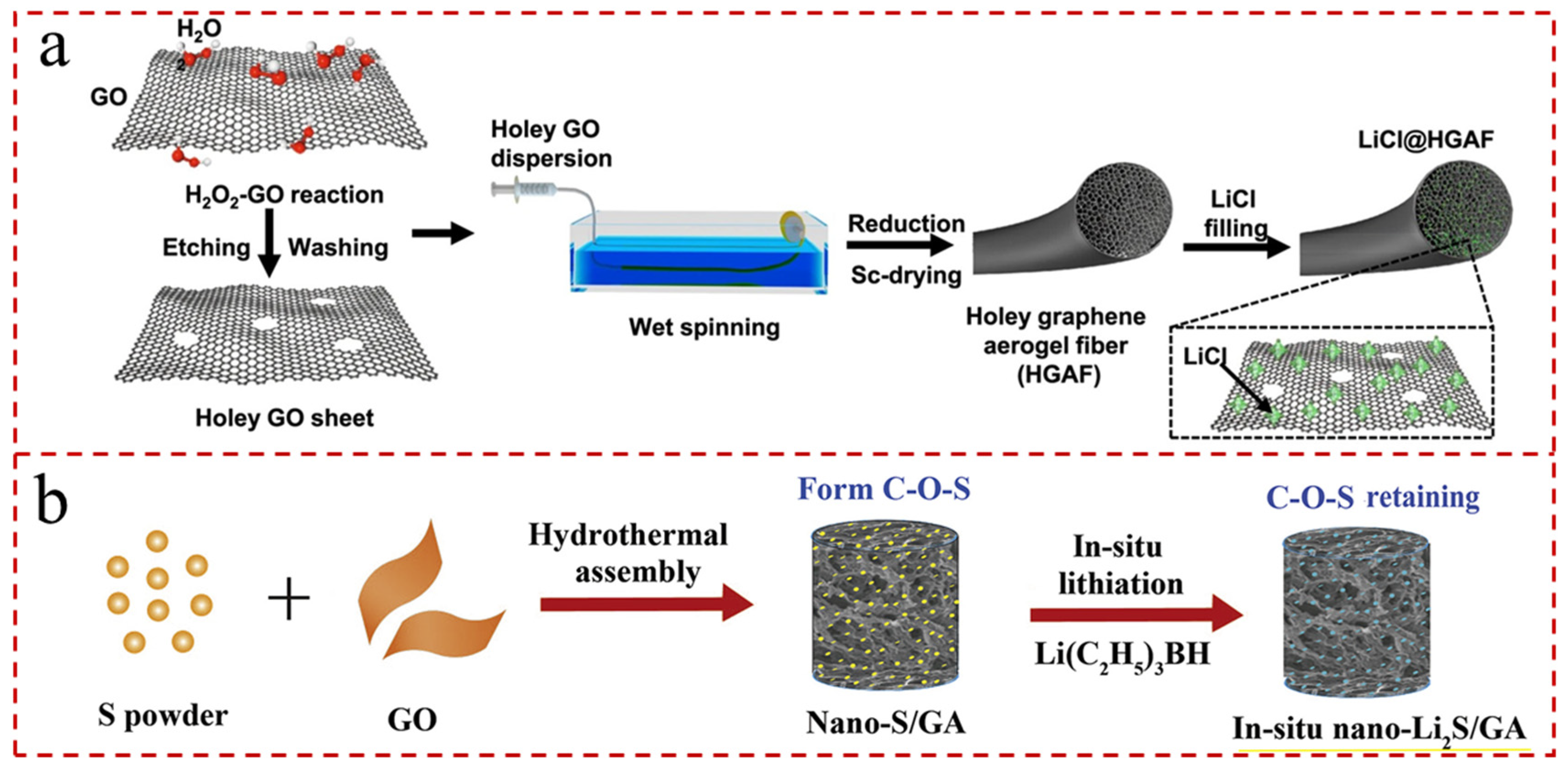
- Hou, Y.; Sheng, Z.; Fu, C.; Kong, J.; Zhang, X. Hygroscopic holey graphene aerogel fibers enable highly efficient moisture capture, heat allocation and microwave absorption. Nat. Commun. 2022, 13, 1227. [Google Scholar] [CrossRef]
- Yin, W.; Qin, M.; Yu, H.; Sun, J.; Feng, W. Hyperelastic Graphene Aerogels Reinforced by In-suit Welding Polyimide Nano Fiber with Leaf Skeleton Structure and Adjustable Thermal Conductivity for Morphology and Temperature Sensing. Adv. Fiber Mater. 2023, 5, 1037–1049. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Chen, L.; Si, J.; Li, W.; Huang, S.; Jiang, Y.; Chen, Z.; Zhao, B. In-situ lithiation synthesis of nano-sized lithium sulfide/graphene aerogel with covalent bond interaction for inhibiting the polysulfides shuttle of Li-S batteries. Electrochim. Acta 2019, 312, 282–290. [Google Scholar] [CrossRef]
- Cavallo, C.; Agostini, M.; Genders, J.P.; Abdelhamid, M.E.; Matic, A. A free-standing reduced graphene oxide aerogel as supporting electrode in a fluorine-free Li2S8 catholyte Li-S battery. J. Power Sources 2019, 416, 111–117. [Google Scholar] [CrossRef]
- Yang, T.; Xia, J.; Piao, Z.; Yang, L.; Zhang, S.; Xing, Y.; Zhou, G. Graphene-Based Materials for Flexible Lithium–Sulfur Batteries. ACS Nano 2021, 15, 13901–13923. [Google Scholar] [CrossRef]
- Zhang, J.; Chou, J.; Luo, X.X.; Yang, Y.M.; Yan, M.Y.; Jia, D.; Zhang, C.H.; Wang, Y.H.; Wang, W.P.; Tan, S.J.; et al. A Fully Amorphous, Dynamic Cross-Linked Polymer Electrolyte for Lithium-Sulfur Batteries Operating at Subzero-Temperatures. Angew. Chem. Int. Ed. 2023, 63, e202316087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Yuan, J.; Song, N.; An, X.; Ma, X.; Feng, J.; Xi, B.; Xiong, S. Binary Sulfiphilic Nickel Boride on Boron-Doped Graphene with Beneficial Interfacial Charge for Accelerated Li–S Dynamics. Small 2023, 19, 2208281. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Ye, Y.; She, J.; Kong, X.; Jin, S.; Peng, Z.; Ji, H. Suppressing Shuttle Effect via Cobalt Phthalocyanine Mediated Dissociation of Lithium Polysulfides for Enhanced Li-S Battery Performance. Adv. Funct. Mater. 2024, 34, 2403888. [Google Scholar] [CrossRef]
- Kang, J.-H.; Chen, J.-S. Using ethylenediamine to prepare three dimensional nitrogen-doped graphene aerogel/sulfur composite for lithium-sulfur batteries. Diam. Relat. Mater. 2018, 88, 222–229. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, Z.; Cai, T.; Han, W.-Q. Effect of Boron-Doping on the Graphene Aerogel Used as Cathode for the Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2015, 7, 25202–25210. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, X.; Cui, Y.; Xiong, D.; Yan, B.; Li, D.; Lushington, A.; Sun, X. Sulfur/Nitrogen Dual-doped Porous Graphene Aerogels Enhancing Anode Performance of Lithium Ion Batteries. Electrochim. Acta 2016, 205, 188–197. [Google Scholar] [CrossRef]
- Liu, H.; Liu, F.; Qu, Z.; Chen, J.; Liu, H.; Tan, Y.; Guo, J.; Yan, Y.; Zhao, S.; Zhao, X.; et al. High sulfur loading and shuttle inhibition of advanced sulfur cathode enabled by graphene network skin and N, P, F-doped mesoporous carbon interfaces for ultra-stable lithium sulfur battery. Nano Res. Energy 2023, 2, e9120049. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, D.; Yang, B.; Zhou, L.; Lin, P.; Wang, J.; Chen, X.a.; Wang, S. Toward High-Performance Lithium–Sulfur Batteries: Efficient Anchoring and Catalytic Conversion of Polysulfides Using P-Doped Carbon Foam. ACS Appl. Mater. Interfaces 2021, 13, 50093–50100. [Google Scholar] [CrossRef]
- Wu, M.; Wei, Z.; Fei, K.; Xiao, W.; Wu, J.; Xu, X.; Zhao, Y.; He, Q. Theoretical Investigation of Nonmetallic Single-Atom Catalysts for Polysulfide Immobilization and Kinetic Enhancement in Lithium–Sulfur Batteries. J. Phys. Chem. C 2024, 128, 6551–6561. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, P.; Wang, J.; Tang, X.; An, T.; Zhou, H.; Zhang, D.; Fan, T. Synergetic pore structure optimization and nitrogen doping of 3D porous graphene for high performance lithium sulfur battery. Carbon 2019, 143, 869–877. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, Y.-S.; Yang, X.-X.; Ren, M.-X.; Wang, Y.-Q.; Lei, B.-Y.; Zhao, D.-L. Sulfur encapsulated in nitrogen-doped graphene aerogel as a cathode material for high performance lithium-sulfur batteries. Int. J. Hydrog. Energy 2021, 46, 7642–7652. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Sui, Z.-Y.; Liu, S.; Wang, H.-Y.; Han, B.-H. Nanostructured porous carbons derived from nitrogen-doped graphene nanoribbon aerogels for lithium–sulfur batteries. J. Colloid Interface Sci. 2019, 541, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, A.; Xiang, Y.; Niu, J. Biomass-based porous carbon/graphene self-assembled composite aerogels for high-rate performance supercapacitor. J. Clean. Prod. 2021, 315, 128110. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, R.; Cao, Y.; Cai, Z.; Zhang, Z.; Huang, Y. Intelligent Off/On Switchable Microwave Absorption Performance of Reduced Graphene Oxide/VO2 Composite Aerogel. Adv. Funct. Mater. 2022, 32, 2205160. [Google Scholar] [CrossRef]
- Jin, S.; Feng, Y.; Jia, J.; Zhao, F.; Wu, Z.; Long, P.; Li, F.; Yu, H.; Yang, C.; Liu, Q.; et al. Three-Dimensional N-Doped Carbon Nanotube/Graphene Composite Aerogel Anode to Develop High-Power Microbial Fuel Cell. Energy Environ. Mater. 2022, 6, e12373. [Google Scholar] [CrossRef]
- Kong, D.; He, L.; Li, H.; Zhang, F.; Song, Z. Preparation and characterization of graphene oxide/chitosan composite aerogel with high adsorption performance for Cr(VI) by a new crosslinking route. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126832. [Google Scholar] [CrossRef]
- He, Z.; Zhang, W.; Zhang, J.; Xie, J.; Su, F.; Li, Y.; Yao, D.; Wang, Y.; Zheng, Y. Enhancing the electromagnetic interference shielding of epoxy resin composites with hierarchically structured MXene/graphene aerogel. Compos. Part B Eng. 2024, 274, 111230. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Xue, J.; He, J.; Zhao, R.; Chen, Z.; Sun, Z.; Liu, H.K.; Dou, S.X. ZIF-67/ZIF-8 and its Derivatives for Lithium Sulfur Batteries. Adv. Funct. Mater. 2024, 35, 2414671. [Google Scholar] [CrossRef]
- Yan, S.; Liu, F.; Ou, Y.; Zhou, H.-Y.; Lu, Y.; Hou, W.; Cao, Q.; Liu, H.; Zhou, P.; Liu, K. Asymmetric Trihalogenated Aromatic Lithium Salt Induced Lithium Halide Rich Interface for Stable Cycling of All-Solid-State Lithium Batteries. ACS Nano 2023, 17, 19398–19409. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Zhang, S.; Liang, G.; Kang, F.; Chen, G.; Li, B. Fe3O4-Decorated Porous Graphene Interlayer for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26264–26273. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L.; Wu, X.; Tian, D.; Cheng, J.; Qiu, Y.; Wu, H.; Guan, B.; Zhang, N.; Sun, K.; et al. Hierarchical mesoporous SnO2 nanosheets on carbon cloth toward enhancing the polysulfides redox for lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 19613–19618. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Wang, M.; Tan, S.; Kan, S.; Wu, Y.; Sang, S.; Liu, K.; Liu, H. In-situ assembly of TiO2 with high exposure of (001) facets on three-dimensional porous graphene aerogel for lithium-sulfur battery. J. Energy Chem. 2020, 49, 316–322. [Google Scholar] [CrossRef]
- Abdulrazzaq, S.J. 3D interconnected N-doped graphene architecture encapsulated with oxygen-deficient TiO2 nanotube array: Synergism of oxygen vacancy and carbon materials on enhanced sulfur conversion and catalytic activity of TiO2 nanotube array in Li–S batteries. Mol. Syst. Des. Eng. 2024, 9, 158–170. [Google Scholar] [CrossRef]
- Song, Z.; Lu, X.; Hu, Q.; Lin, D.; Zheng, Q. Construction of reduced graphene oxide wrapped yolk–shell vanadium dioxide sphere hybrid host for high-performance lithium–sulfur batteries. Dalton Trans. 2020, 49, 14921–14930. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, X.; Kang, Q.; Kong, Z.; Wang, Y.; Zhan, L. Vanadium oxide nanorods embed in porous graphene aerogel as high-efficiency polysulfide-trapping-conversion mediator for high performance lithium-sulfur batteries. Chem. Eng. J. 2020, 393, 124570. [Google Scholar] [CrossRef]
- Chen, B.; Wei, J.; Li, X.; Ji, Y.; Liang, D.; Chen, T. Vanadium dioxide plates reduced graphene oxide as sulfur cathodes for efficient polysulfides trap in long-life lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 629, 1003–1011. [Google Scholar] [CrossRef]
- Raulo, A.; Jalilvand, G. Advances in fibrous materials for high-capacity lithium sulfur batteries. Nano Energy 2024, 122, 109265. [Google Scholar] [CrossRef]
- Dou, Y.; Guo, J.; Shao, J.; Duan, J.; Liang, H.; Cheng, X.; He, Y.; Liu, J. Bi-Functional Materials for Sulfur Cathode and Lithium Metal Anode of Lithium–Sulfur Batteries: Status and Challenges. Adv. Sci. 2024, 11, 2101670. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. High-Performance Lithium-Sulfur Batteries with a Self-Supported, 3D Li2S-Doped Graphene Aerogel Cathodes. Adv. Energy Mater. 2016, 6, 1501355. [Google Scholar] [CrossRef]
- Tang, H.; You, L.; Liu, J.; Wang, S.; Wang, P.; Feng, C.; Guo, Z. Integrated Polypyrrole@Sulfur@Graphene Aerogel 3D Architecture via Advanced Vapor Polymerization for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 18448–18455. [Google Scholar] [CrossRef]
- Luo, L.; Chung, S.H.; Manthiram, A. A three-dimensional self-assembled SnS2-nano-dots@graphene hybrid aerogel as an efficient polysulfide reservoir for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 7659–7667. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, Y.; Al-Tahan, M.A.; Zhang, Y.; Wei, R.; Ma, Y.; Yang, C.; Zhang, J. Insights into the sandwich-like ultrathin Ni-doped MoS2/rGO hybrid as effective sulfur hosts with excellent adsorption and electrocatalysis effects for lithium-sulfur batteries. J. Energy Chem. 2021, 60, 85–94. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Jiang, X.; Zhang, Y.; Wang, X.; Zhang, S. Multi-functional ZnS quantum Dots/Graphene aerogel modified separator for high performance lithium-sulfur batteries. Electrochim. Acta 2022, 422, 140496. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Q.; Li, Q.; Zhang, J.; Ming, J. Electrolyte Issues in Lithium–Sulfur Batteries: Development, Prospect, and Challenges. Energy Fuels 2021, 35, 10405–10427. [Google Scholar] [CrossRef]
- Hou, Y.; Ren, Y.; Zhang, S.; Wang, K.; Yu, F.; Zhu, T. 3D S@MoS2@reduced graphene oxide aerogels cathode for high-rate lithium-sulfur batteries. J. Alloys Compd. 2021, 852, 157011. [Google Scholar] [CrossRef]
- Jung, J.; Cho, H.; Kim, I.; Kim, S.; Jo, W.; Kim, H.-T. Dual Functionalities of Rb Cation in Lean Electrolyte Lithium Sulfur Batteries. Energy Storage Mater. 2023, 63, 103040. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Z.H.; Chen, X.; Zhang, Q. A review on theoretical models for lithium–sulfur battery cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Li, H.; Gao, R.; Chen, B.; Zhou, C.; Shao, F.; Wei, H.; Han, Z.; Hu, N.; Zhou, G. Vacancy-Rich MoSSe with Sulfiphilicity–Lithiophilicity Dual Function for Kinetics-Enhanced and Dendrite-Free Li-S Batteries. Nano Lett. 2022, 22, 4999–5008. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Y.; Chen, C.; Li, B.; Li, W.; Lu, H.; Yu, L.; Zheng, S.; Wang, B. Low-temperature Li–S battery enabled by CoFe bimetallic catalysts. J. Mater. Chem. A 2022, 10, 8378–8389. [Google Scholar] [CrossRef]
- Jin, Y.; Deng, N.; Li, Y.; Wang, H.; Zhang, M.; Kang, W.; Cheng, B. Advanced preparation and application of bimetallic materials in lithium-sulfur batteries: A review. J. Energy Chem. 2024, 88, 469–512. [Google Scholar] [CrossRef]
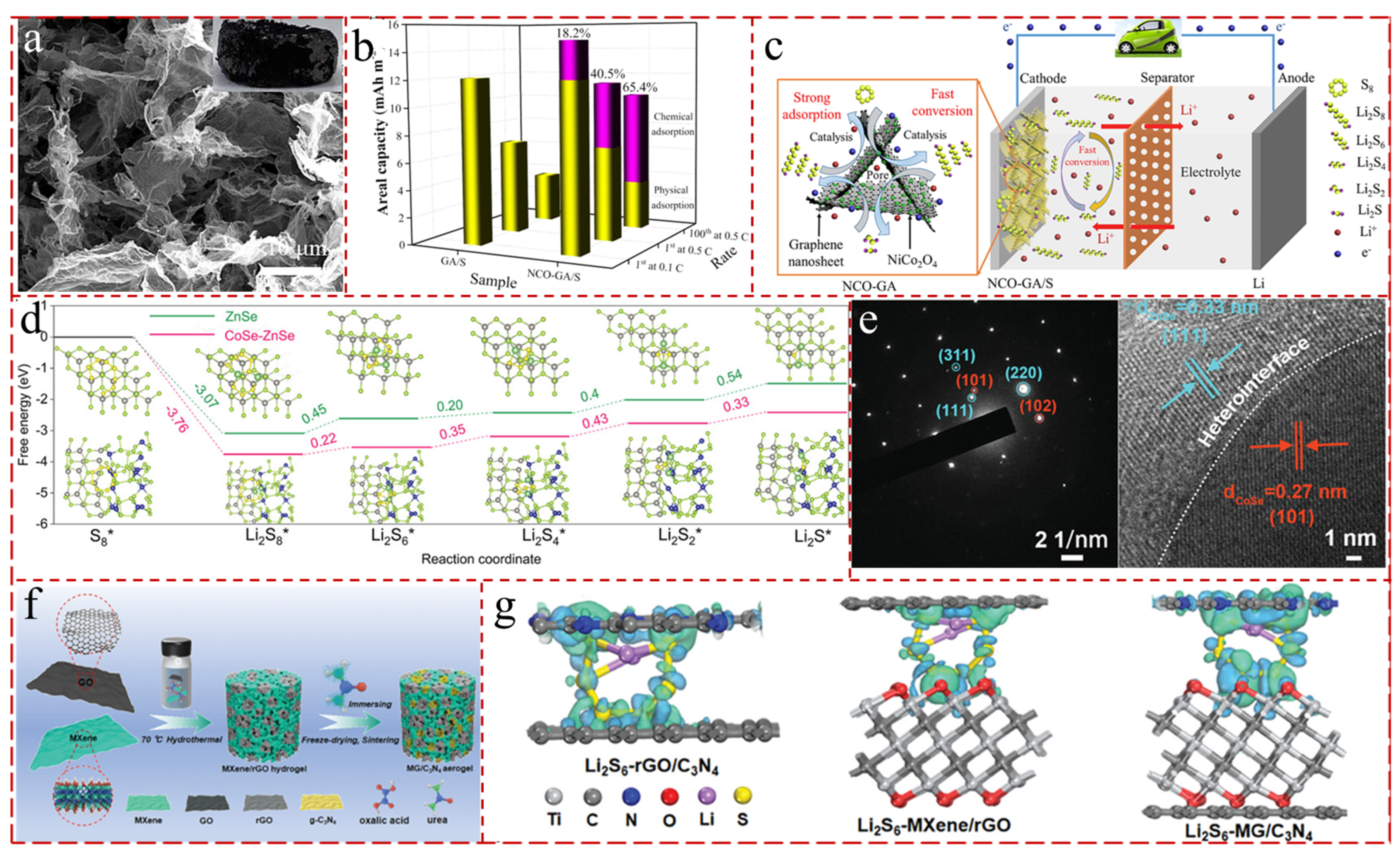
- Tian, X.; Zhou, Y.; Zhang, B.; Selabi, N.B.S.; Wang, G. Monodisperse polar NiCo2O4 nanoparticles decorated porous graphene aerogel for high-performance lithium sulfur battery. J. Energy Chem. 2022, 74, 239–251. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Yang, T.; Li, L.; Wu, F.; Chen, R. Engineering Catalytic CoSe–ZnSe Heterojunctions Anchored on Graphene Aerogels for Bidirectional Sulfur Conversion Reactions. Adv. Sci. 2022, 9, 2103456. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, Y.; Sun, X.; Batool, S.; Li, T. Nanopolyhedron Co–C/Cores triggered carbon nanotube in-situ growth inside carbon aerogel shells for fast and long-lasting lithium–sulfur batteries. J. Power Sources 2022, 520, 230913. [Google Scholar] [CrossRef]
- Yang, W.-H.; Ni, Z.-C.; You, D.; Hou, J.-Y.; Deng, B.-N.; Huang, R.-W.; Sun, S.-G.; Zhao, J.-B.; Li, X.; Zhang, Y.-Y.; et al. Multifunctional sulfur-immobilizing GO/MXene aerogels for highly-stable and long-cycle-life lithium–sulfur batteries. Rare Met. 2023, 42, 2577–2591. [Google Scholar] [CrossRef]



| Electrodes | Sulfur Loading (%/mg cm−2) | Initial Capacity (mAh g−1) | Cycling Number | Final Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| FeSA-NC@CBC | 81.2/5 | 1006.2 (1 C) | 840 (2 C) | 799.8 with 79.5% retention after 500 cycles at 0.05 C | [127] |
| Pt/S/OCNF | 77.5/- | 1188.1 (0.5 C) | 813.2 (2 C) | 982.3 with 82.6% retention after 200 cycles at 0.5 C | [50] |
| S@WCNTAs | 63.33/8.02 | 1018 (0.5 C) | 609 (1 C) | a capacity of 559 after 60 cycles at 0.1 C | [95] |
| CNT@Li2S8 | 43/5 | 1388.2 (0.1 C) | 1268.9 (2 C) | 899.9 with 64.8% retention after 60 cycles at 1 C | [78] |
| MXene/CNT/MXene | -/7 | 712 (0.5 C) | - | 570 with 80% retention after 800 cycles at 0.5 C | [110] |
| 3D CNT/Ti3C2Tx | 70/- | 1329.7 (0.5 C) | 1043.2 (2 C) | a capacity retention of 64% after 800 cycles at 0.5 C | [111] |
| NFC/CNT | 83.3/2.3 | 1143 (0.1 C) | 1675 (1 C) | 704.9 with 85% retention after 100 cycles at 1 C | [112] |
| N-GA/S | 75.5/- | 1210.7 (0.1 C) | 610 (3 C) | 724 with 89% retention after 100 cycles at 0.7 C | [138] |
| FeP/rGO/CNTs -S | 75/3.5 | 1271.6 (0.1 C) | 613.1 (3 C) | 1038.4 with 81% retention after 100 cycles at 0.1 C | [133] |
| FeP/rGO/CNTs | 60/9.6 | 1312.3 (0.1) | 647 (2 C) | - | [141] |
| S@TiO2@GA | 55.2/- | 1404 (0.2 C) | - | - | [203] |
| GA-VOx | 80/2.6 | 1057 (0.05 C) | 442 (2 C) | 734 with 69% retention after 140 cycles at 0.2 C | [152] |
| ZnS-RGA/PP | 66/3.1 | 1211 (0.1 C) | 794 (2 C) | 865 with 71.4% retention after 100 cycles at 0.2 C | [157] |
| NCO-GA/S | 80.4/- | 1241.1 (0.1 C) | 435.7 (5 C) | a capacity retention of 68.5% after 200 cycles at 0.5 C | [163] |
| CoSe-ZnSe@GA | 66.2/7.7 | 1654 (0.1 C) | 808 (3 C) | a capacity retention of 88.8% after 108 cycles at 0.2 C | [166] |
| GM | 53/2 | 1255.62 (0.2 C) | 974.62 (2 C) | 615.7 with 51% retention after 450 cycles at 0.1 C | [134] |
| MG/C3N4 | -/4.92 | 1315.6 (0.2 C) | 1167.4 (2 C) | a capacity retention of 97.5% after 100 cycles at 0.2 C | [167] |
| MoSSe/r-GO aerogel | -/6.5 | 938.8 (0.5 C) | - | 637.3 with 66% retention after 1000 cycles | [172] |
| MoSe2−x@GA/S | -/4.8 | 1256.9 (0.2 C) | 931.7 (2 C) | a capacity retention of 76% after 1000 cycles at 1 C | [173] |
| S/Co-GC@GPCA | 63.33/2.03 | 939.9 (0.1 C) | 439.1 (2 C) | 677.3 with 72.1% retention after 300 cycles at 0.1 C | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Liu, D.; Zhao, Y.; Yang, D.; Zhang, L.; Sun, F.; Wang, X. Insights into Carbon-Based Aerogels Toward High-Performance Lithium–Sulfur Batteries: A Review of Strategies for Sulfur Incorporation Within Carbon Aerogel Frameworks. Gels 2025, 11, 516. https://doi.org/10.3390/gels11070516
Gao Y, Liu D, Zhao Y, Yang D, Zhang L, Sun F, Wang X. Insights into Carbon-Based Aerogels Toward High-Performance Lithium–Sulfur Batteries: A Review of Strategies for Sulfur Incorporation Within Carbon Aerogel Frameworks. Gels. 2025; 11(7):516. https://doi.org/10.3390/gels11070516
Chicago/Turabian StyleGao, Yue, Dun Liu, Yi Zhao, Dongdi Yang, Lugang Zhang, Fei Sun, and Xiaoxiao Wang. 2025. "Insights into Carbon-Based Aerogels Toward High-Performance Lithium–Sulfur Batteries: A Review of Strategies for Sulfur Incorporation Within Carbon Aerogel Frameworks" Gels 11, no. 7: 516. https://doi.org/10.3390/gels11070516
APA StyleGao, Y., Liu, D., Zhao, Y., Yang, D., Zhang, L., Sun, F., & Wang, X. (2025). Insights into Carbon-Based Aerogels Toward High-Performance Lithium–Sulfur Batteries: A Review of Strategies for Sulfur Incorporation Within Carbon Aerogel Frameworks. Gels, 11(7), 516. https://doi.org/10.3390/gels11070516




