The Effect of Light on the Germination of Raphanus sativus Seeds and the Use of Sprout Extracts in the Development of a Dermatocosmetic Gel
Abstract
1. Introduction
2. Results and Discussion
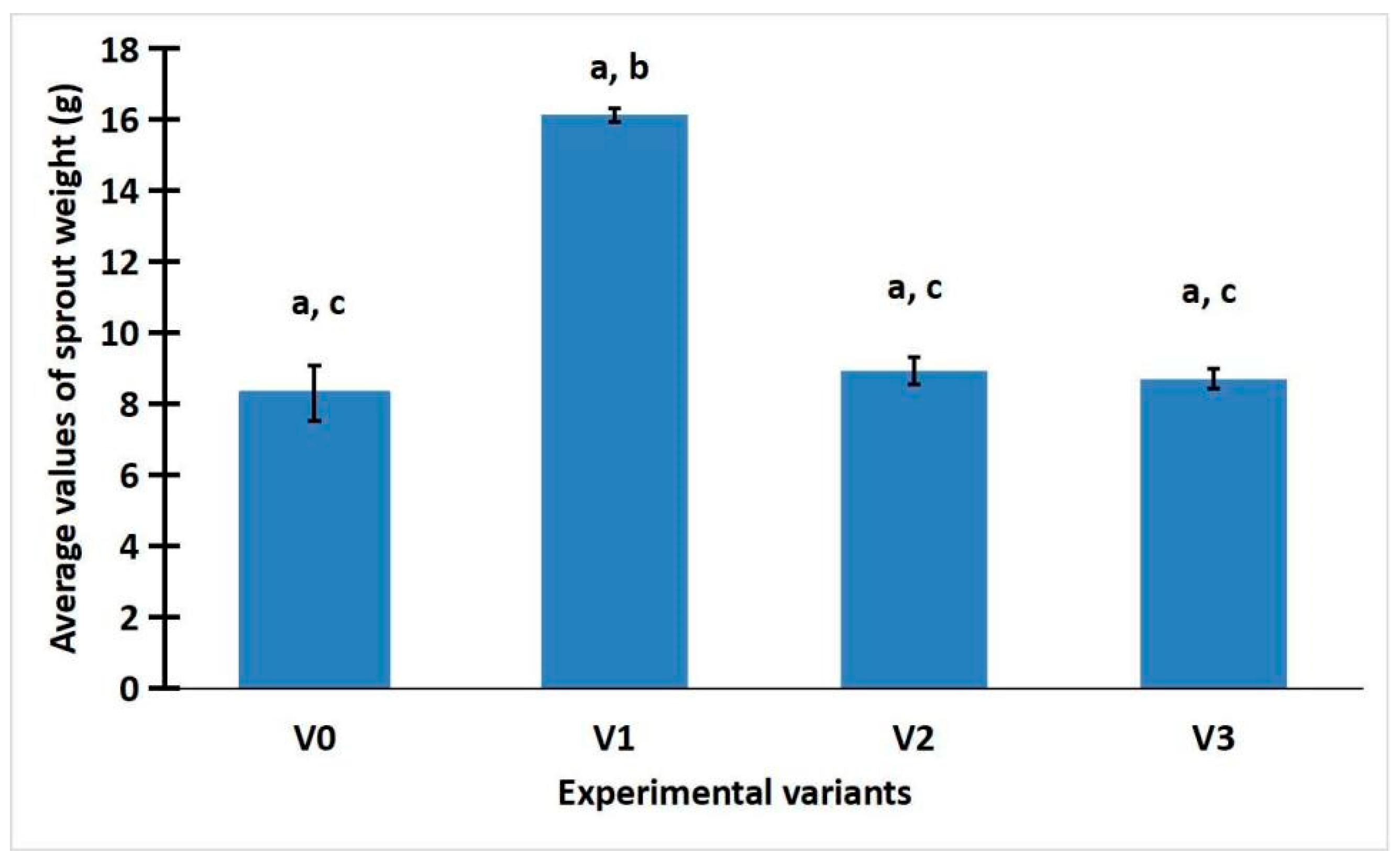
2.1. The Effect of Light on the Development of R. sativus
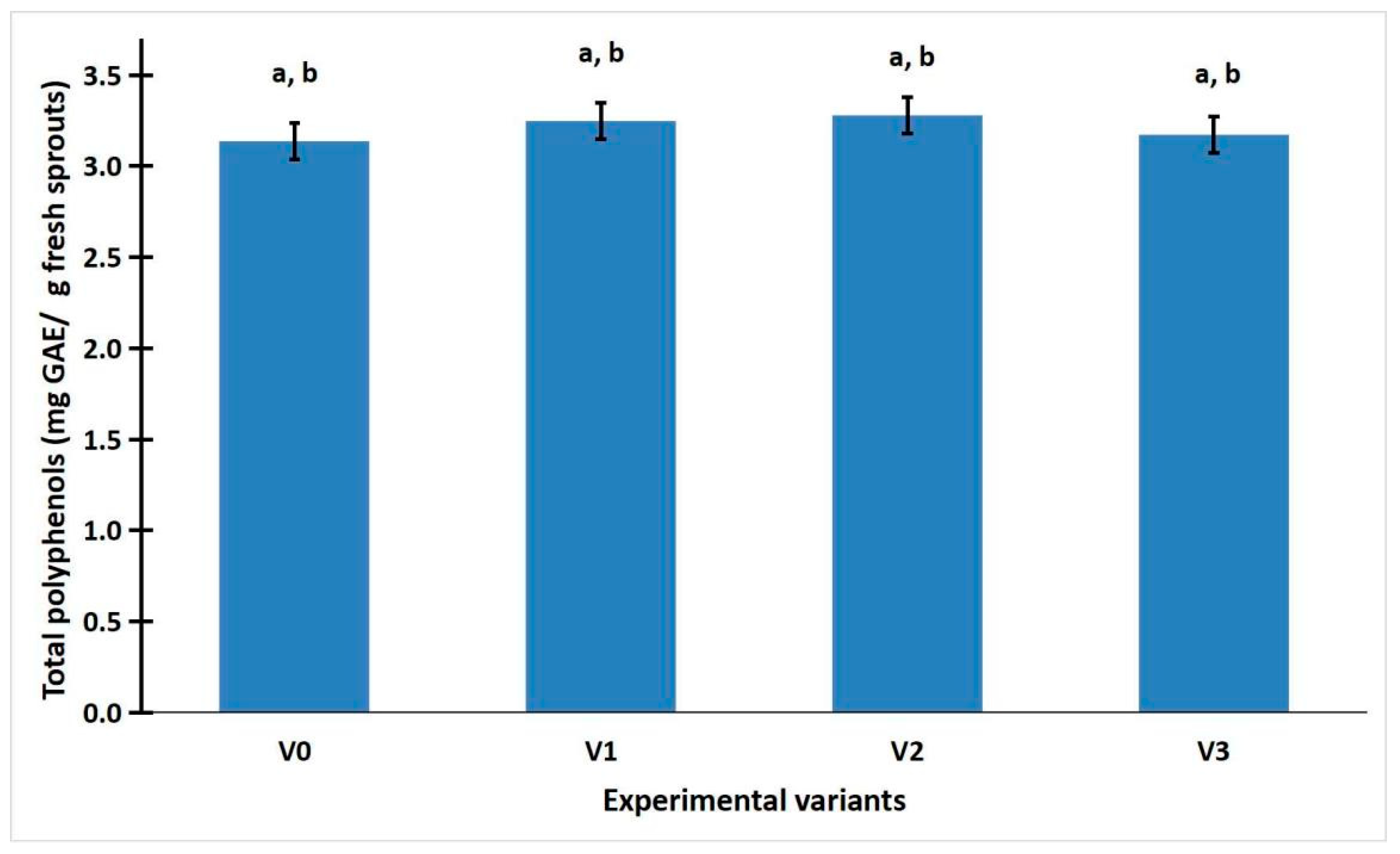
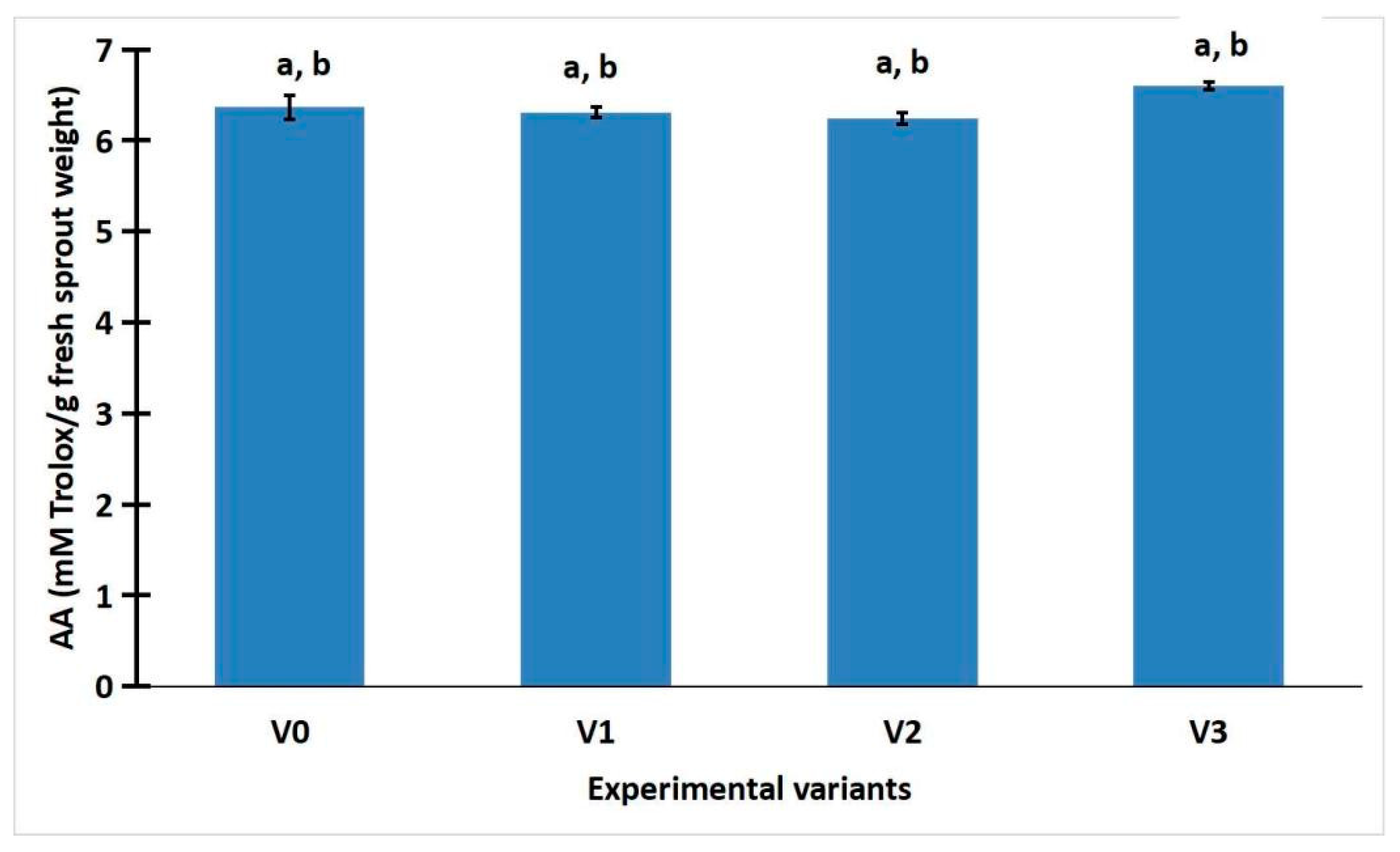
2.2. The Polyphenol Content and Antioxidant Activity
2.3. Statistic Analysis of Weight, Total Phenolic Content, and Antioxidant Activity of R. sativus Sprouts
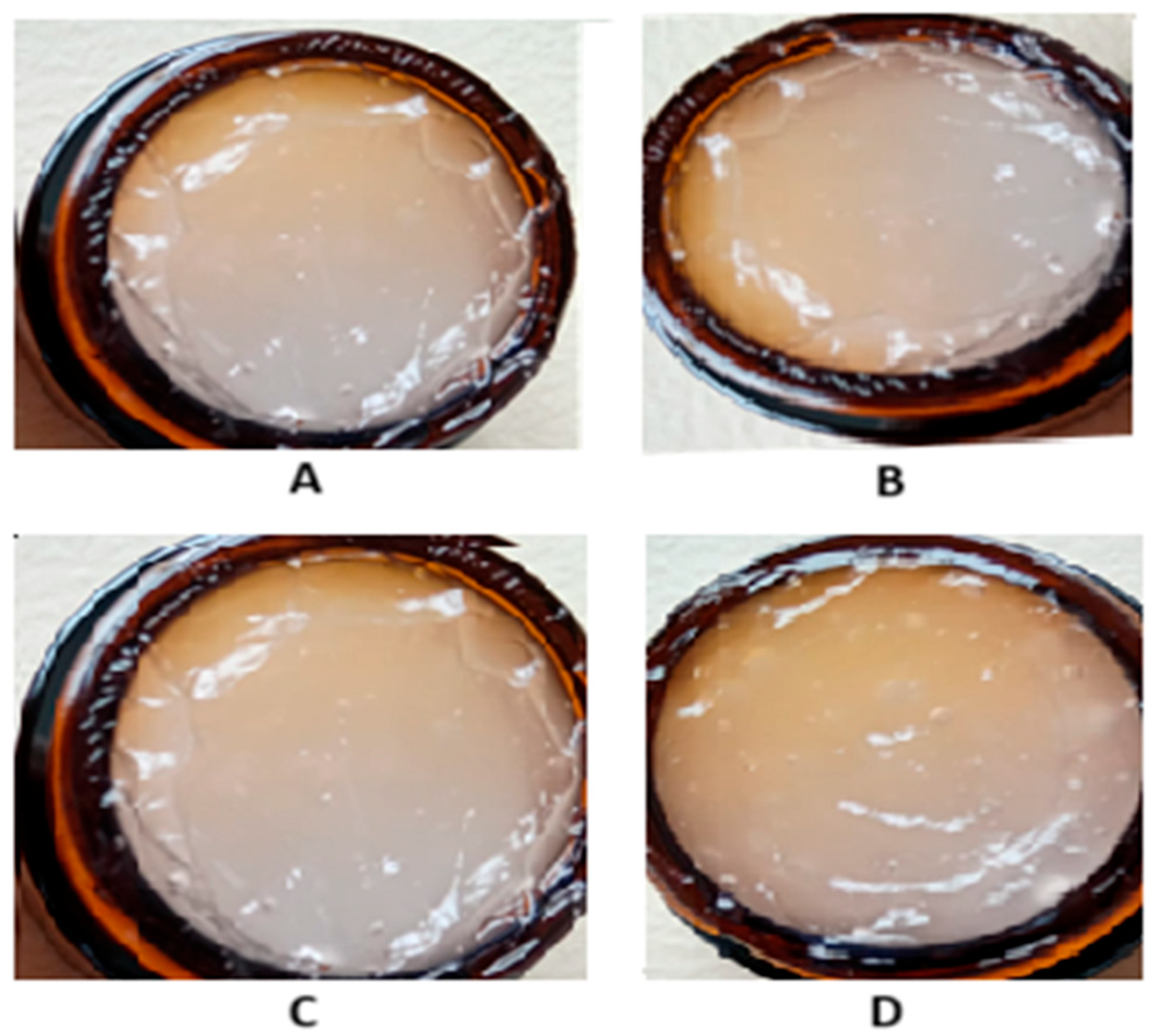
2.4. Characteristics of Dermatocosmetic Products Containing R. sativus Sprouts Extract
2.5. Antioxidant Activity of the Gel Formulations
2.6. Discussions
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Experimental Design
4.3. Determination of the Total Polyphenol Content of the R. sativus Sprouts Extracts
4.4. Determination of the Antioxidant Activity of Radish Sprouts Extracts
4.5. Formulation of Dermatocosmetic Products Containing R. sativus Sprouts Extract
4.6. Characterization of Dermatocosmetic Formulations
4.6.1. Visual Inspection
4.6.2. pH Measurement
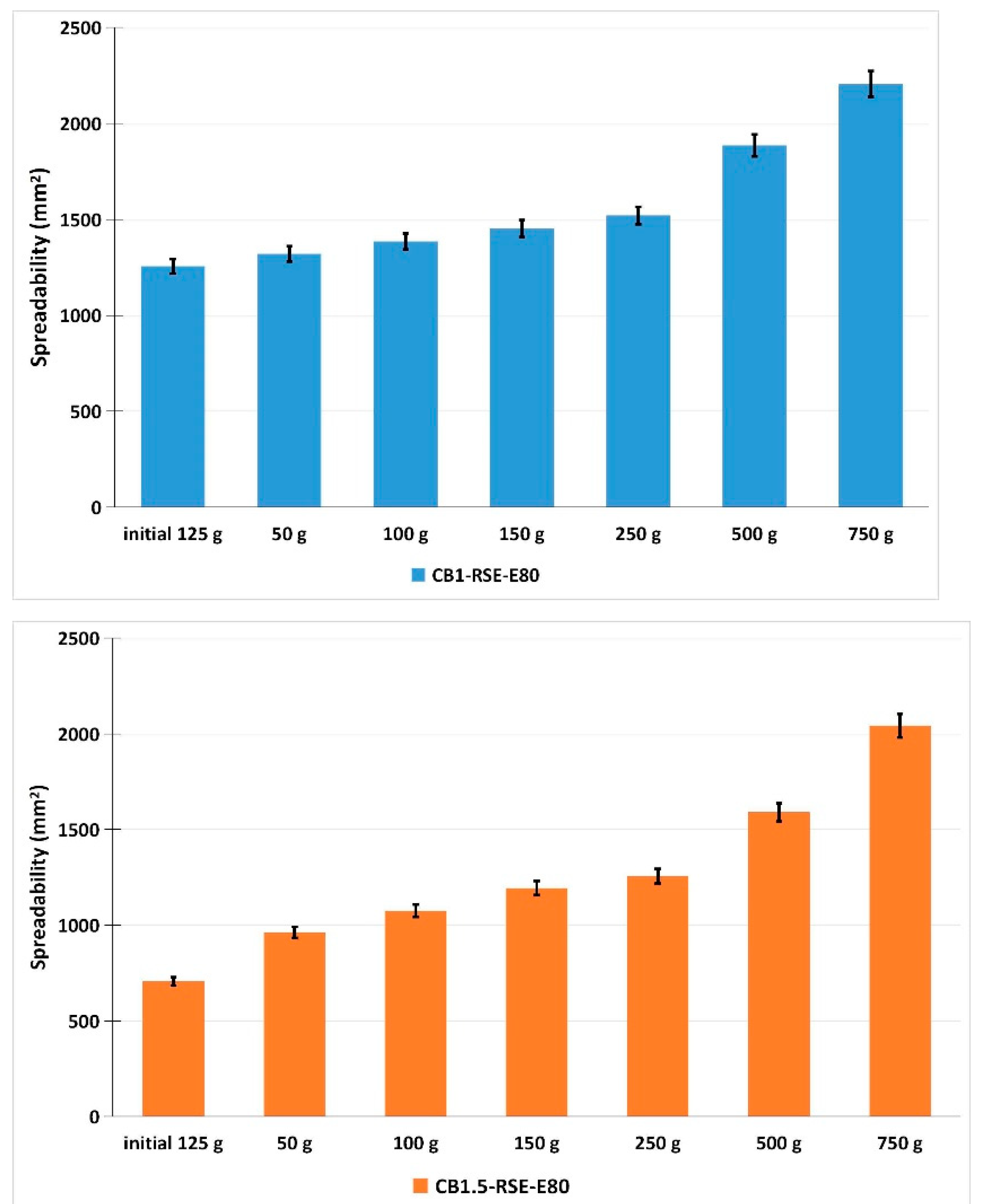
4.6.3. Spreadability Assessment
4.6.4. Texture Analysis
4.6.5. Antioxidant Activity
4.6.6. Stability
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guti’érrez, R.M.P.; Perez, R.L. Raphanus sativus (Radish): Their Chemistry and Biology Review. Sci. World J. 2004, 4, 811–837. [Google Scholar] [CrossRef]
- Beevi, S.S.; Mangamoori, L.N.; Gowda, B.B. Polyphenolics profile and antioxidant properties of Raphanus sativus L. Nat. Prod. Res. 2012, 26, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.; Narasu, M.L.; Gowda, B.B. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum. Nutr. 2010, 65, 8–17. [Google Scholar]
- Mohammed, G.J.; Hameed, I.H. Pharmacological activities: Hepatoprotective, Cardio protective, Anti-cancer and anti-microbial activity of (Raphanus raphanistrum subsp. sativus): A review. Indian J. Public Health Res. Dev. 2018, 9, 212–217. [Google Scholar] [CrossRef]
- Arro, J.; Labate, J.A. Genetic variation in a radish (Raphanus sativus L.) geodiversity collection. Genet. Resour. Crop Evol. 2022, 69, 163–171. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.), leaves and roots. J. Funct. Foods. 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Khan, R.S.; Khan, S.S.; Siddique, R. Radish (Raphanus sativus): Potential antioxidant role of bioactive compounds extracted from radish leaves—A review. Pak. J. Med. Health Sci. 2022, 16, 2. [Google Scholar] [CrossRef]
- Goyeneche, R.; Rodrigues, C.R.; Quispe-Fuentes, I.; Pellegrini, M.C.; Cumino, A.; Di Scala, K. Radish leaves extracts as functional ingredients: Evaluation of bioactive compounds and health-promoting capacities. Waste Biomass Valorization 2024, 16, 1003–1014. [Google Scholar] [CrossRef]
- Borș, M.D.; Semeniuc, C.A.; Socaci, S.; Vârva, L.; Moldovan, O.; Vlaic, R.; Tofană, M. Total Phenolic Content and Antioxidant Capacity of Radish as Influenced by the Variety and Vegetative Stage. Bull. UASVM Food Sci. Technol. 2015, 72, 2344. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.; Glisic, M.; Franco, O.; Minder, B.; Bussler, W.; Metzger, B.; Kera, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and Diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef]
- Kajszczak, D.; Sosnowska, D.; Fraszczak, B.; Podsedek, A. Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves. Molecules 2024, 29, 5689. [Google Scholar] [CrossRef] [PubMed]
- Sham, T.T.; Yuen, A.C.; Ng, Y.F.; Chan, C.O.; Mok, D.K.; Chan, S.W. A review of the phytochemistry and pharmacological activities of raphani semen. Evid. Based Complement. Alternat. Med. 2013, 2013, 636194. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, Y. Comparative analysis of phytochemicals and antioxidant activities in seeds and sprouts of different varieties of radish (Raphanus sativus L.): TOPSIS-entropy weight method. Front. Plant Sci., Sec. Plant Nutr. 2025, 16, 1531570. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, J.; Tian, J.; Li, N.; Jia, L.; Shen, W.; Cui, J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Choi, I.L.; Wang, L.; Lee, J.H.; Han, S.J.; Ko, Y.W.; Kim, Y.; Kang, H.M. Effect of LED and QD-LED (Quantum Dot) treatments on production and quality of red radish (Raphanus sativus L.) sprout. J Bio-Env Con 2019, 28, 265–272. [Google Scholar] [CrossRef]
- Chen, C.; Kim, R.H.; Hwang, K.T.; Kim, J. Chemical compounds and bioactivities of the extracts from radish (Raphanus sativus) sprouts exposed to red and blue light-emitting diodes during cultivation. Eur. Food Res. Technol. 2023, 249, 1551–1562. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Livadariu, O.; Raiciu, D.; Maximilian, C.; Căpitanu, E. Studies regarding treatments of LED-s emitted light on sprouting hemp (Cannabis sativa L.). Rom. Biotechnol. Lett. 2019, 24, 485–490. [Google Scholar] [CrossRef]
- Raiciu, A.D.; Livadariu, O.; Șerbănică, C.P. The assesment of the influence induced by LED-s irradiation on basil sprouts (Ocimum basilicum L.). Rom. Biotechnol. Lett. 2020, 25, 1334–1339. [Google Scholar]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Folta, K.M. Environmentally modified organisms—Expanding genetic potential with light. Crit. Rev. Plant Sci. 2014, 33, 486–508. [Google Scholar] [CrossRef]
- Han, J.H.; Moon, H.K.; Chung, S.K.; Kang, W.W. Comparison of physiological activities of radish bud (Raphanus sativus L.) according to extraction solvent and sprouting period. J. Korean Soc. Food Sci. Nutr. 2015, 44, 549–556. [Google Scholar] [CrossRef]
- Onder, F.C.; Dŏgrular, N.; Guzduzalp, E. A comparative study of three Brassicaceae vegetables grown in Canakkale: Determination of total phenolic content and antioxidant activity of pulp and juice samples of radish (Raphanus sativus L.), cabbage (Brassica oleracea L. var capitata L.) and cauliflower (Brassica oleracea L.). Canakkale Onsekiz Mart Univ. Fen Bilim. Enst. Derg. 2020, 6, 30–38. [Google Scholar]
- Li, Y.; Guo, H.; Lv, L.; Liu, J.; Luo, H. Effects of red and blue light on the growth, photosynthesis, and antioxidant metabolism of soybean sprouts. Plant Physiol. Biochem. 2021, 166, 275–284. [Google Scholar]
- Zhang, J.; Chen, L.; Yu, S.; Li, Y. Red light-mediated enhancement of phenylpropanoid metabolism in lettuce seedlings is linked to phytochrome B signaling. Plant Sci. 2022, 316, 111146. [Google Scholar]
- Borbély, P.; Gasperl, A.; Pálmai, T.; Ahres, M.; Asghar, M.A.; Galiba, G.; Müller, M.; Kocsy, G. Light Intensity- and Spectrum-Dependent Redox Regulation of Plant Metabolism. Antioxidants 2022, 11, 1311. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Ma, H. White LED light enhances reactive oxygen species signaling and antioxidant responses in young tomato plants. Front. Plant Sci. 2023, 14, 1160547. [Google Scholar]
- Li, X.; He, K. The role of NAC transcription factors in plant abiotic stress tolerance. Plant Mol. Biol. 2017, 95, 1–12. [Google Scholar]
- Chen, Y.; Liu, X.; Wei, Q. Reactive oxygen species as signaling molecules in plant photomorphogenesis and stress responses. Int. J. Mol. Sci. 2021, 22, 4147. [Google Scholar]
- Huang, Y.; Xie, W.; Zhang, J. The NADPH oxidase–MAPK–NAC transcription factor signaling pathway regulates light-induced antioxidant responses in Arabidopsis. Plant Mol. Biol. 2020, 104, 217–232. [Google Scholar]
- Silva, P.; Freitas, F.; Santos, J. Optimizing LED lighting to tailor phytochemical profiles and biomass in microgreens for cosmetic applications. Ind. Crops Prod. 2022, 180, 114756. [Google Scholar]
- Gómez, C.; Navarro, M.M.; Lara, I. Limitations and perspectives of antioxidant assays in plant extracts: A review. Food Chem. 2023, 404, 134534. [Google Scholar]
- Lee, K.; Park, S.; Oh, M. Advances in comprehensive antioxidant activity evaluation of plant extracts: Integrating multiple assay systems. Antioxidants 2024, 13, 354. [Google Scholar]
- Zahid, M.; Javed, M.L.T.; Shabbir, R.; Afzal, A.; Rehan, Z.A.; Siuta, D.; Mehmood, M.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Raiciu, D.; Livadariu, O.; Maximilian, C.; Bira, A. The evaluation of the effect of LED-s irradiation on wheat sprouts (Triticum aestivum L.). Rom. Biotechnol. Lett. 2020, 25, 1615–1620. [Google Scholar] [CrossRef]
- Romanian Pharmacopoeia, X ed.; Editura Medicala: Bucuresti, Romania, 1993; Volume 1, pp. 334–335, 1063–1064.
- Sanchz-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Agric. Food Chem. 1998, 76, 270–276. [Google Scholar] [CrossRef]
| Formulation Component | CB1.5-RSE-E80 | CB1.5-RSE-E80 |
|---|---|---|
| Carbopol 940 | 1% | 1.5% |
| Glycerin | 8% | 8% |
| RSE-E80 | 2.5% | 2.5% |
| Peppermint essential oil | 0.3% | 0.3% |
| Sodium hydroxide | q.s | q.s. |
| Purified water | Up to 100% | Up to 100% |
| Characteristics | CB1-RSE-E80 | CB1.5-RSE-E80 |
|---|---|---|
| Organoleptic evaluation—after 24 h | Appearance: Homogeneous Color: White Smell: Aromatic, specific | Appearance: Homogeneous Color: White Smell: Aromatic, specific |
| pH—after 24 h | 5.05 ± 0.03 | 4.85 ± 0.05 |
| Organoleptic evaluation—after 30 days, room temperature | Appearance: Homogeneous Color: White Smell: Aromatic, specific No signs of phase separation, sedimentation, or color/texture alteration, | Appearance: Homogeneous Color: White Smell: Aromatic, specific No signs of phase separation, sedimentation, or color/texture alteration, |
| pH—after 30 days, room temperature | 5.15 ± 0.04 | 4.95 ± 0.03 |
| Texture | Firmness: 0.824 N Cohesiveness: 0.529 N Springiness: 0.681 mm | Firmness: 0.986 N Cohesiveness: 0.542 N Springiness: 0.737 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremia, M.C.; Pavaloiu, R.D.; Livadariu, O.; Raiciu, A.D.; Sha’at, F.; Bubueanu, C.; Miu, D.M. The Effect of Light on the Germination of Raphanus sativus Seeds and the Use of Sprout Extracts in the Development of a Dermatocosmetic Gel. Gels 2025, 11, 515. https://doi.org/10.3390/gels11070515
Eremia MC, Pavaloiu RD, Livadariu O, Raiciu AD, Sha’at F, Bubueanu C, Miu DM. The Effect of Light on the Germination of Raphanus sativus Seeds and the Use of Sprout Extracts in the Development of a Dermatocosmetic Gel. Gels. 2025; 11(7):515. https://doi.org/10.3390/gels11070515
Chicago/Turabian StyleEremia, Mihaela Carmen, Ramona Daniela Pavaloiu, Oana Livadariu, Anca Daniela Raiciu, Fawzia Sha’at, Corina Bubueanu, and Dana Maria Miu. 2025. "The Effect of Light on the Germination of Raphanus sativus Seeds and the Use of Sprout Extracts in the Development of a Dermatocosmetic Gel" Gels 11, no. 7: 515. https://doi.org/10.3390/gels11070515
APA StyleEremia, M. C., Pavaloiu, R. D., Livadariu, O., Raiciu, A. D., Sha’at, F., Bubueanu, C., & Miu, D. M. (2025). The Effect of Light on the Germination of Raphanus sativus Seeds and the Use of Sprout Extracts in the Development of a Dermatocosmetic Gel. Gels, 11(7), 515. https://doi.org/10.3390/gels11070515






