Preparation and Enhanced Oil Recovery Mechanisms of Janus-SiO2-Reinforced Polymer Gel Microspheres
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Janus-SiO2 Nanoparticles
2.1.1. Chemical Structure Analysis
2.1.2. Particle Size Distribution and Microscopic Morphology
2.1.3. Interface Activity and Wettability
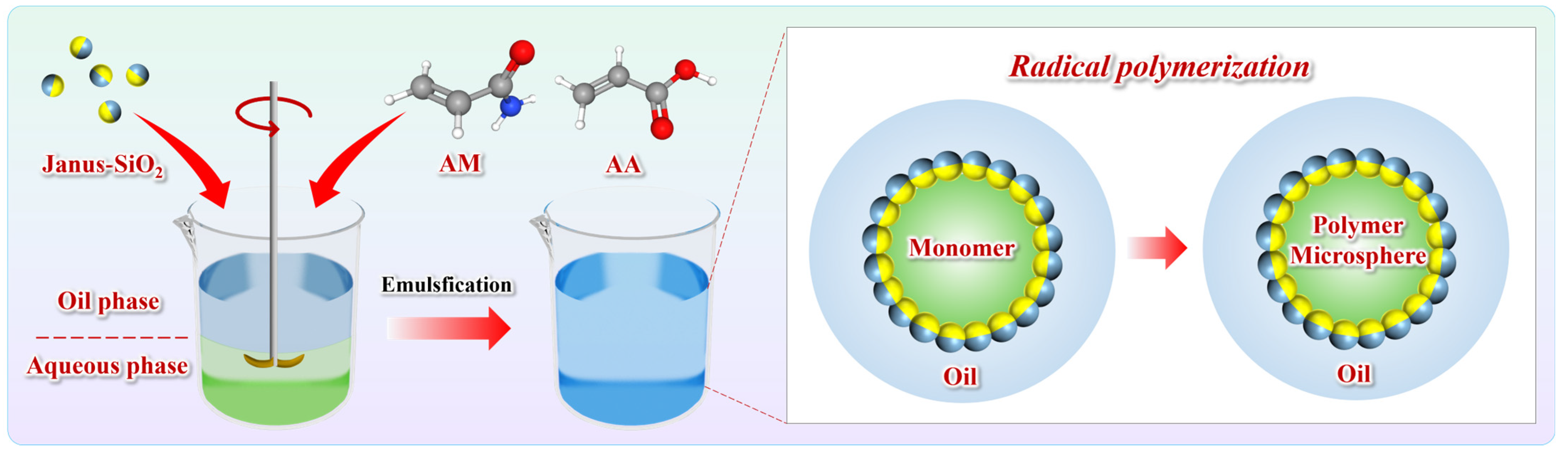
2.2. Performances of Polymer Gel Microspheres
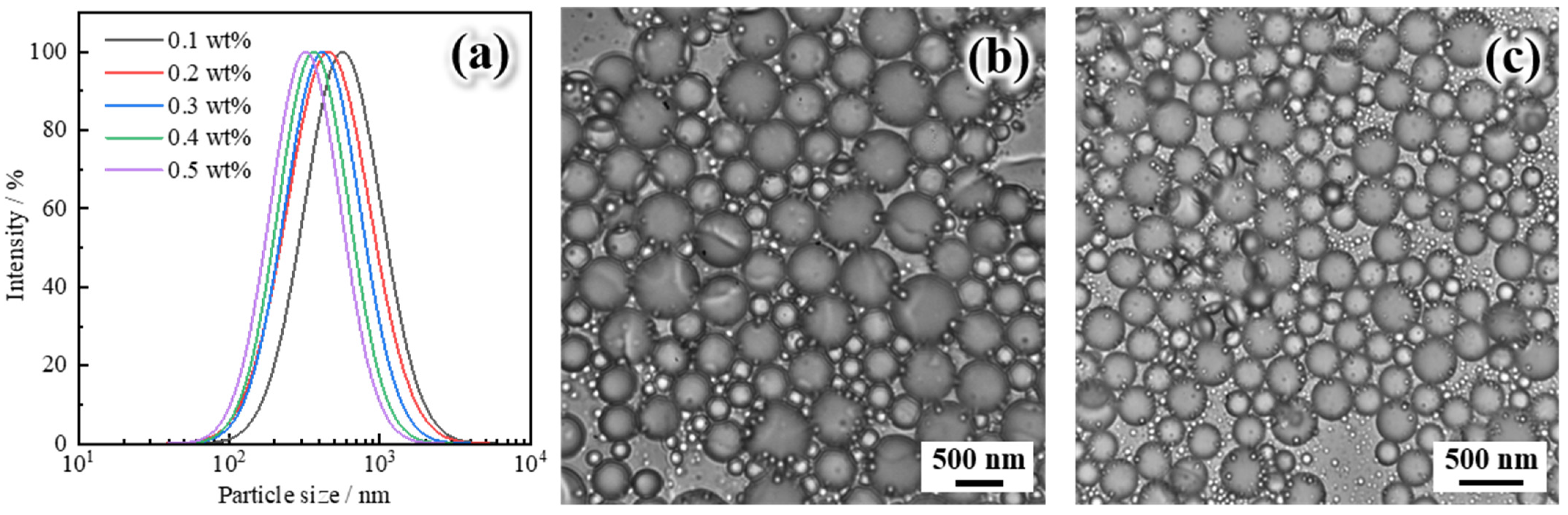
2.2.1. Particle Size Distribution and Microscopic Morphology
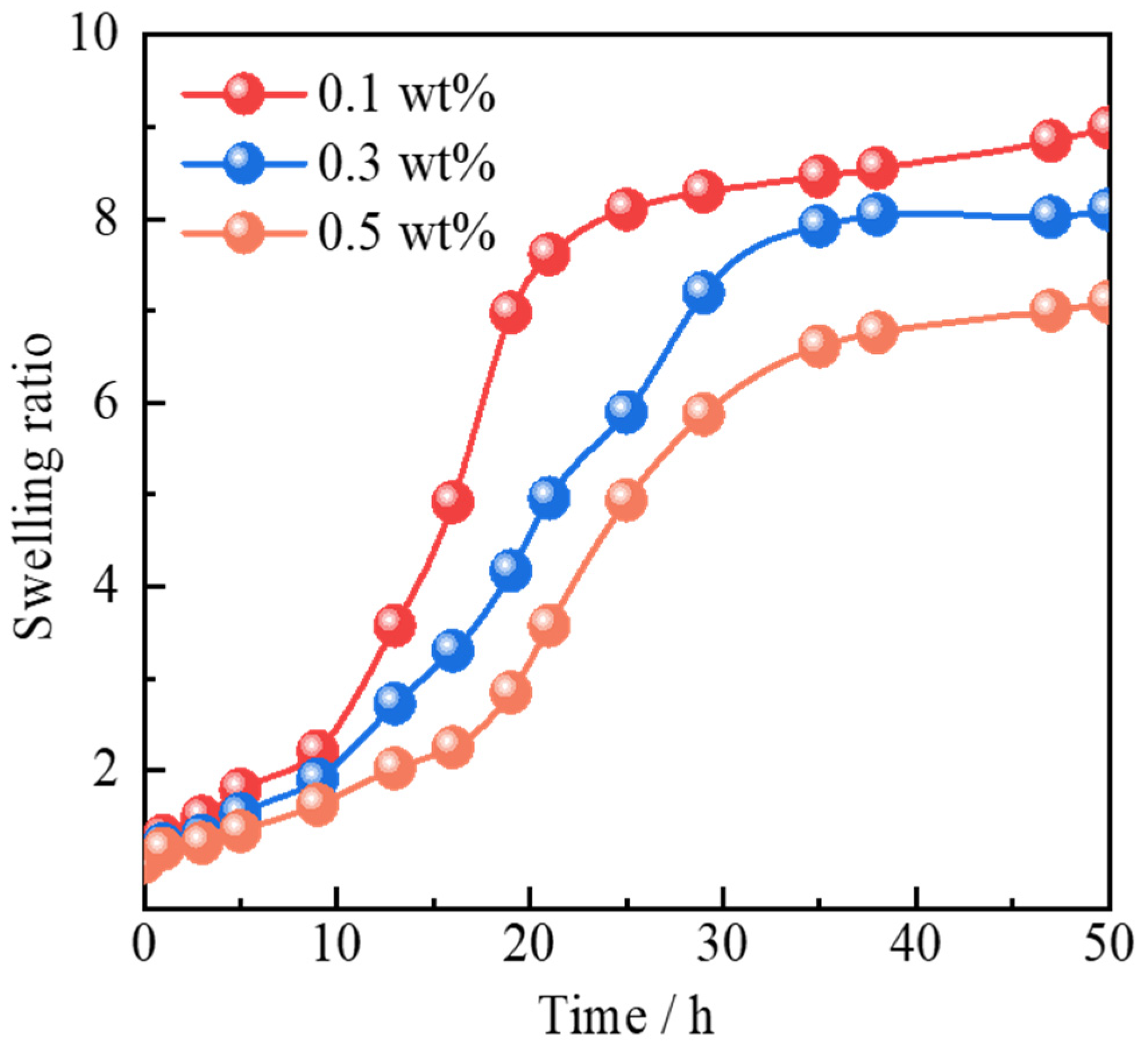
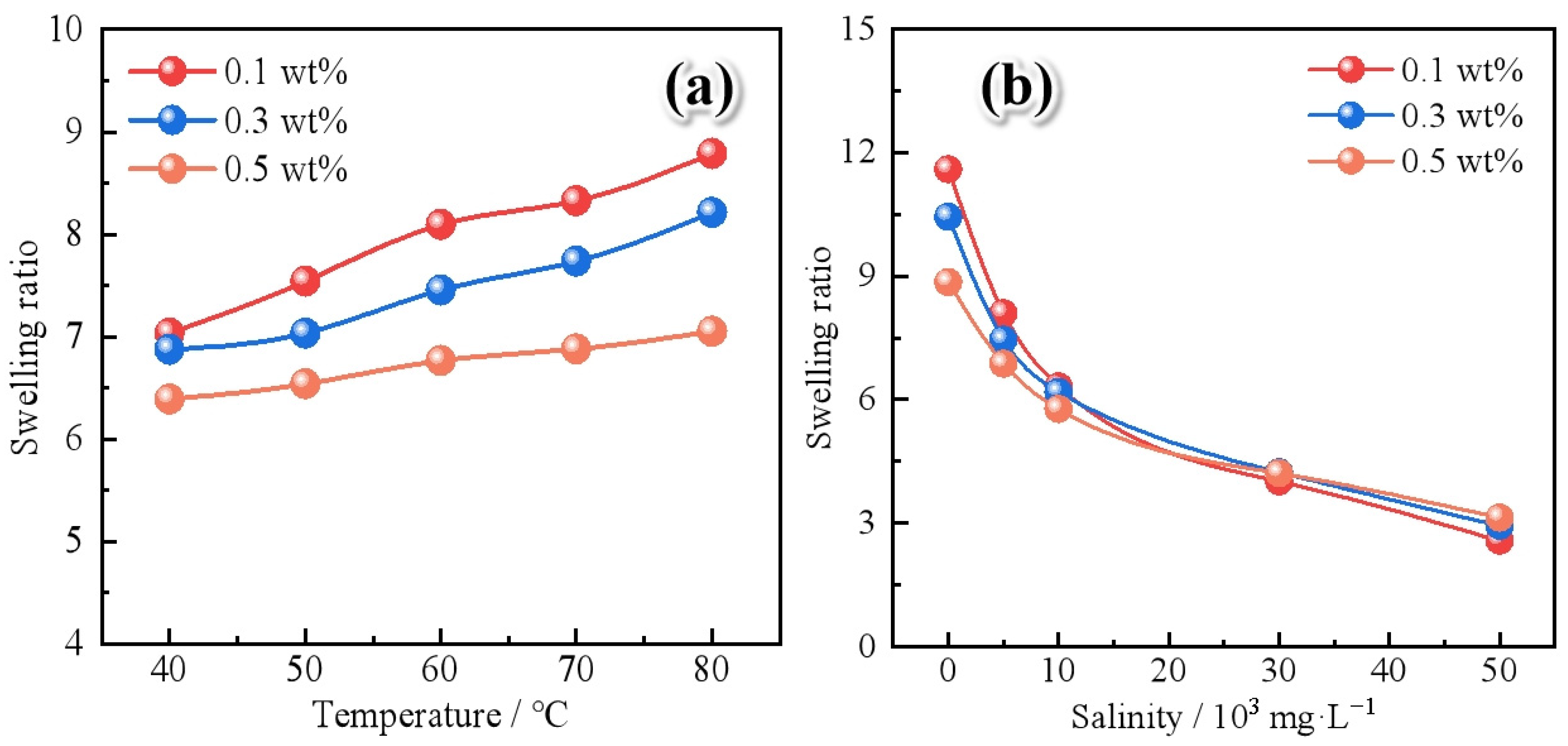
2.2.2. Swelling Behavior
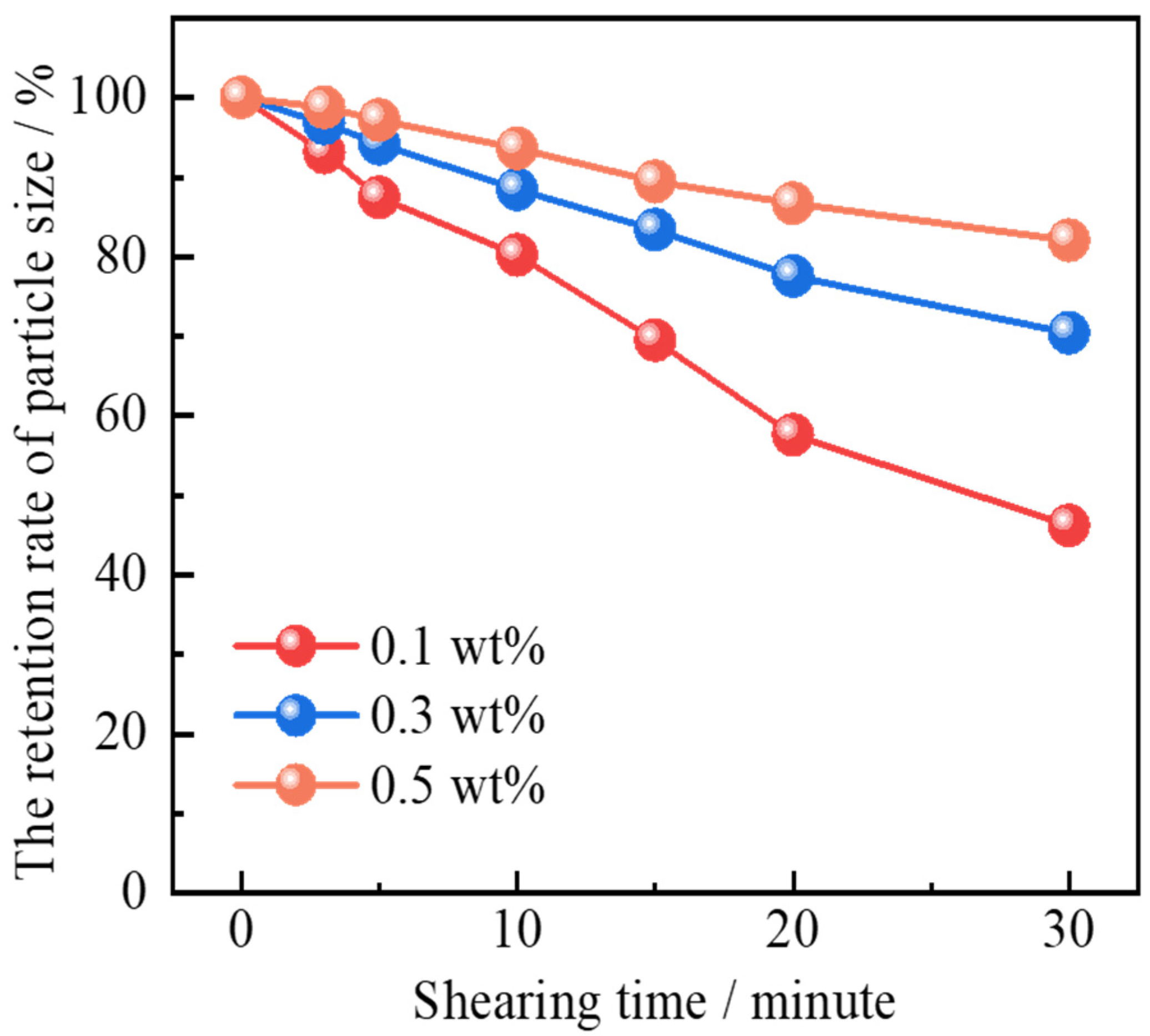
2.2.3. Shearing Resistance
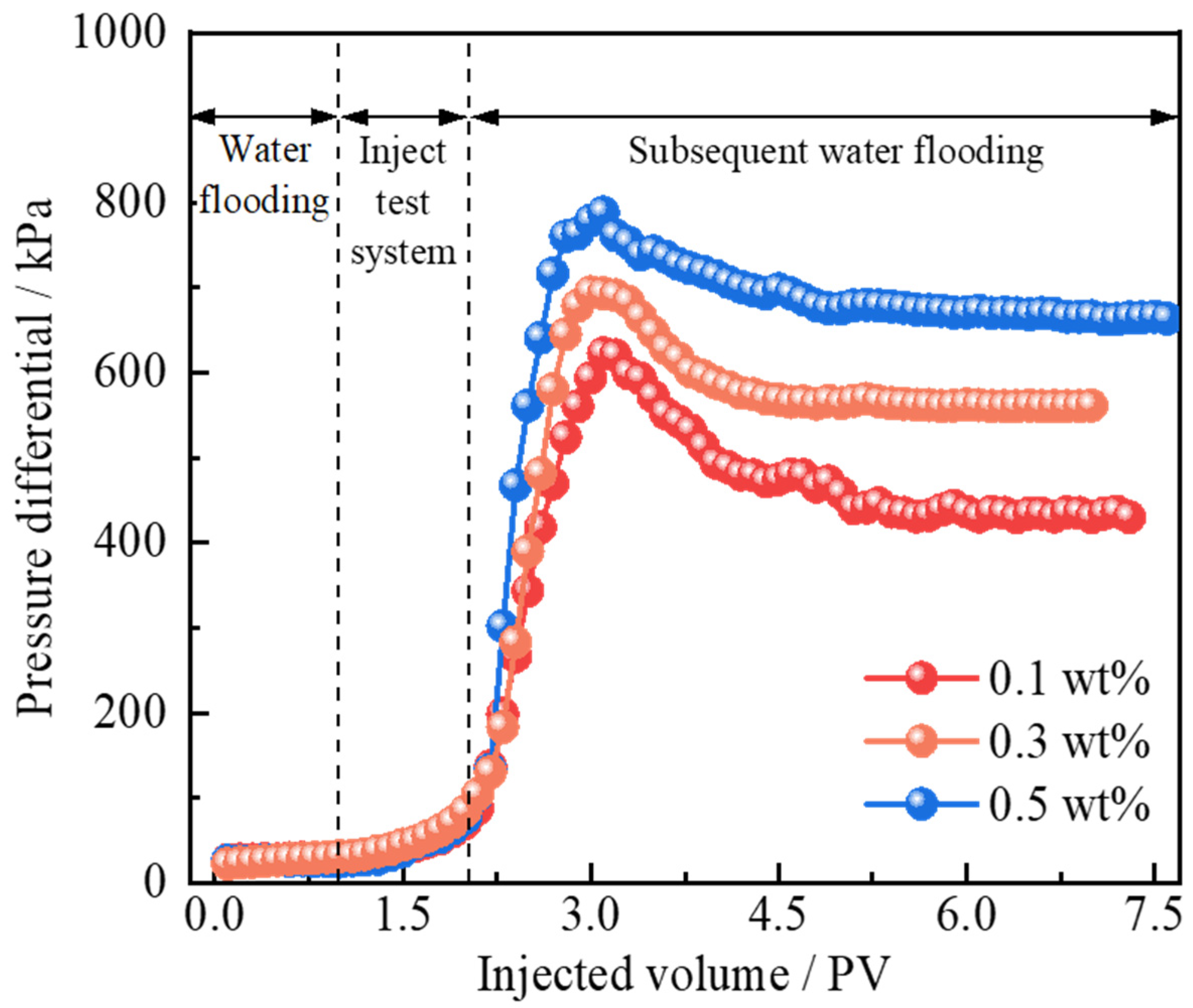
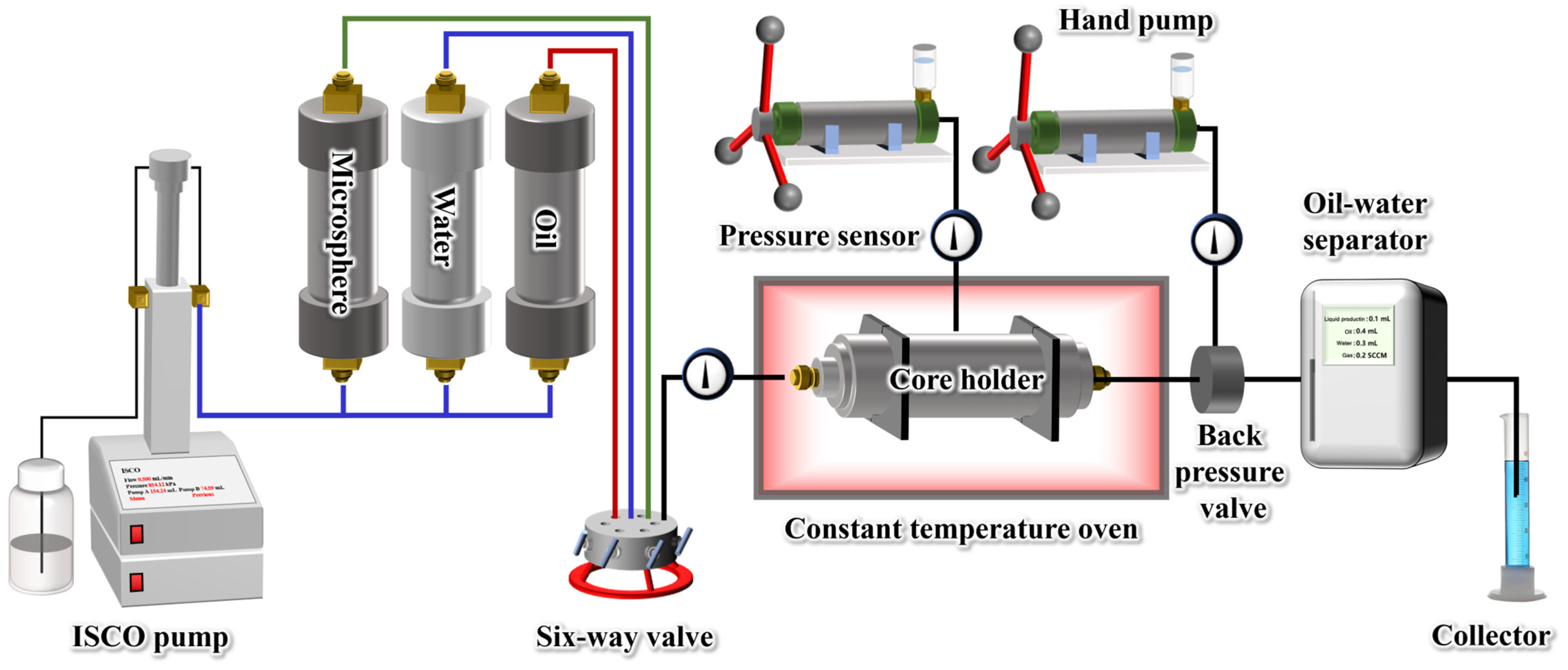
2.3. Injection and Plugging Capacity
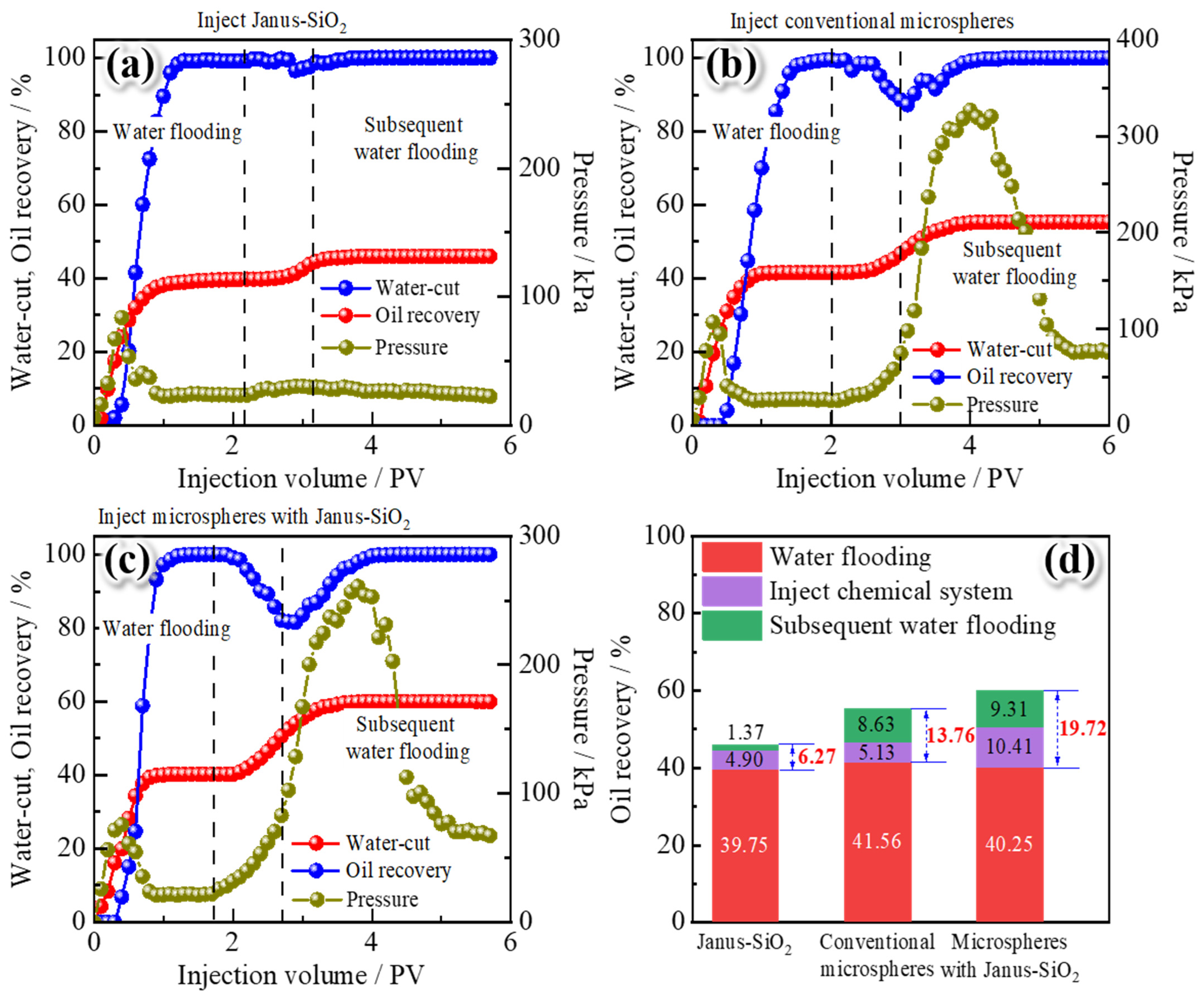
2.4. The Potential for Enhanced Oil Recovery (EOR)
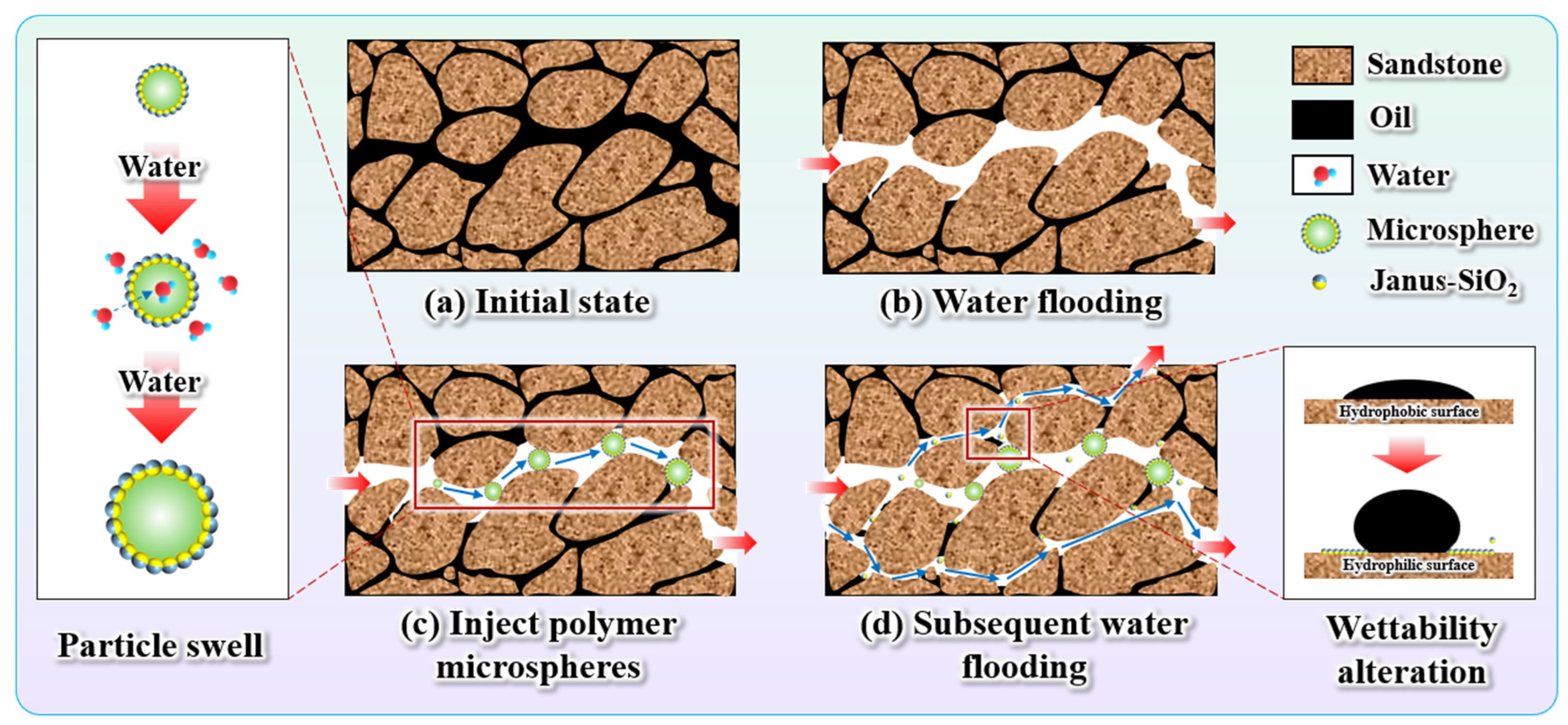
2.5. The EOR Mechanism
3. Conclusions
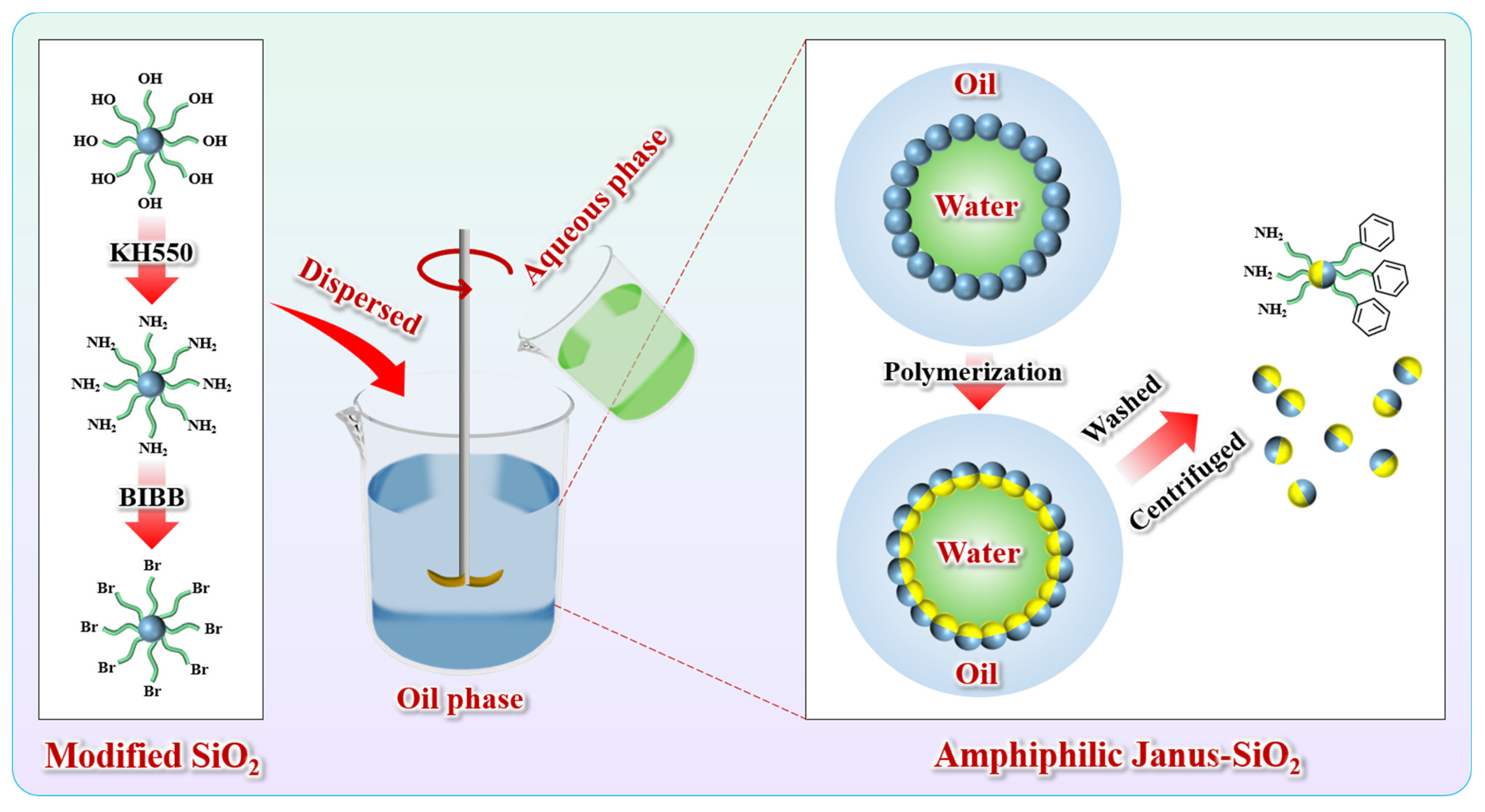
- Amphiphilic Janus-SiO2 nanoparticles with controlled asymmetric wettability were successfully synthesized via a Pickering emulsion template method. FTIR and SEM characterization confirmed the successful surface modification with amino and bromo groups, with the average particle size increasing from 23.8 nm to 32.9 nm while maintaining excellent dispersibility.
- The Janus-SiO2 nanoparticles demonstrated outstanding interfacial activity, effectively reducing the oil–water interfacial tension to 0.095 mN/m and altering the rock surface wettability from oil-wet to strongly water-wet, thereby significantly enhancing oil stripping efficiency.
- Polymer gel microspheres were prepared by reversed-phase emulsion polymerization using Janus-SiO2 nanoparticles as emulsifiers. When the concentration range of nanoparticles was 0.1–0.5 wt%, the particle size range of polymer gel microspheres was 316.4–562.7 nm.
- Polymer gel microspheres reinforced by Janus-SiO2 nanoparticles exhibit swelling behavior in response to temperature and salinity. Polymer gel microspheres prepared with a high concentration of Janus-SiO2 nanoparticles can ensure the moderate swelling capacity of the particles under high-temperature and high-salinity conditions. At the same time, it can also improve the mechanical strength and shear resistance of the microspheres.
- Core displacement experiments demonstrated the dual synergistic effects of the polymer gel microspheres reinforced by Janus-SiO2 nanoparticles. On one hand, microspheres can effectively plug high-permeability zones (the plugging rate of the system with 0.5 wt% nanoparticles can reach 96.32%) and improve sweep volume, while Janus-SiO2 nanoparticles can further improve oil displacement efficiency. These combined effects have increased the oil recovery by 19.72%, which is more than 5.96% higher than that of the conventional microsphere system.
- The expansion behavior of polymer gel microspheres is still difficult to control. Determining how to optimize the initial particle size of microspheres according to reservoir physical parameters (such as temperature, salinity, pore throat characteristics, etc.) to achieve the balance between injectivity and expansion plugging capacity is the key direction of future research. In addition, field tests are needed to verify the long-term stability of the system in a dynamic reservoir environment.
4. Materials and Methods
4.1. Materials
4.2. Preparation of Amphiphilic Janus-SiO2 Nanoparticles
4.2.1. Amination of SiO2 Nanoparticles
4.2.2. Bromination of SiO2 Nanoparticles
4.2.3. Preparation of Amphiphilic Janus-SiO2 Nanoparticles
4.3. Preparation of Janus-SiO2-Reinforced Polymer Gel Microspheres
4.4. Characterization of Janus-SiO2 Nanoparticles
4.4.1. Fourier-Transform Infrared Spectroscopy (FTIR) Characterization
4.4.2. Scanning Electron Microscopy (SEM) Characterization
4.4.3. Dynamic Light Scattering (DLS) Analysis
4.4.4. Oil–Water Interfacial Tension Measurement
4.4.5. Wettability Measurement
4.5. Swelling Behavior Analysis
4.6. Conformance Control Experiment
4.7. Oil Displacement Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arain, A.H.; Negash, B.M.; Farooqi, A.S.; Alshareef, R.S. Improving Oil Recovery through Low Salinity Waterflooding and Nanoparticles: A Mini Review. Energy Fuels 2024, 38, 17109–17127. [Google Scholar] [CrossRef]
- Zou, C.; Liu, Y.; Dai, C.; Yuan, X.; Wang, H.; Huang, Y.; Xu, Z.; Yang, N. Salt-Induced Dual Network Gel-Strengthened Foam for Gas Channeling Control in Fractured-Cavity Carbonate Reservoirs. Geoenergy Sci. Eng. 2025, 253, 213975. [Google Scholar] [CrossRef]
- Hu, W.; Wei, Y.; Bao, J. Development of the Theory and Technology for Low Permeability Reservoirs in China. Pet. Explor. Dev. 2018, 45, 646–656. [Google Scholar] [CrossRef]
- Shangguan, Y.; Zhang, Y.; Xiong, W. The Effect of Physical Property Change on the Water Flooding Development in Changqing Oilfield Jurassic Low Permeability Reservoir. Petroleum 2015, 1, 300–306. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, C.; Zhou, D.; Li, H.; Gao, M.; Zhao, G.; Dai, C. Novel Chemical Flooding System Based on Dispersed Particle Gel Coupling In-Depth Profile Control and High Efficient Oil Displacement. Energy Fuels 2019, 33, 3123–3132. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Dou, H.; Yang, Y. Further Understanding on Fluid Flow through Multi-Porous Media in Low-Permeability Reservoirs. Pet. Explor. Dev. 2012, 39, 674–682. [Google Scholar] [CrossRef]
- Dai, C.; Liu, Y.; Zou, C.; You, Q.; Yang, S.; Zhao, M.; Zhao, G.; Wu, Y.; Sun, Y. Investigation on Matching Relationship between Dispersed Particle Gel (DPG) and Reservoir Pore-Throats for in-Depth Profile Control. Fuel 2017, 207, 109–120. [Google Scholar] [CrossRef]
- Bai, B.; Leng, J.; Wei, M. A Comprehensive Review of In-Situ Polymer Gel Simulation for Conformance Control. Pet. Sci. 2022, 19, 189–202. [Google Scholar] [CrossRef]
- Abdulbaki, M.; Huh, C.; Sepehrnoori, K.; Delshad, M.; Varavei, A. A Critical Review on Use of Polymer Microgels for Conformance Control Purposes. J. Pet. Sci. Eng. 2014, 122, 741–753. [Google Scholar] [CrossRef]
- Liu, J.; Almakimi, A.; Wei, M.; Bai, B.; Ali Hussein, I. A Comprehensive Review of Experimental Evaluation Methods and Results of Polymer Micro/Nanogels for Enhanced Oil Recovery and Reduced Water Production. Fuel 2022, 324, 124664. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Wang, X.; Wang, Y.; Hu, W.; Duan, M.; Fang, S. Preparation and Profile Control Evaluation of Core-Shell Structure and Surface-Hydrophobic Modified Polyacrylamide Microsphere. J. Appl. Polym. Sci. 2022, 139, e53110. [Google Scholar] [CrossRef]
- Shagymgereyeva, S.; Sarsenbekuly, B.; Kang, W.; Yang, H.; Turtabayev, S. Advances of polymer gel microspheres and Its Applications for Enhanced Oil Recovery. Colloids Surf. B Biointerfaces 2024, 233, 113622. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pu, W.; Chen, D.; Zhang, J.; Zhou, F.; Zhang, R.; Gu, S. Degradable Cross-Linked Polymeric Microsphere for Enhanced Oil Recovery Applications. RSC Adv. 2015, 5, 62752–62762. [Google Scholar] [CrossRef]
- Cheng, L.; Xie, Y.; Chen, J.; Wang, X.; Luo, Z.; Chen, G. Multi-Scale Visualization Study of Water and Polymer Microsphere Flooding through Horizontal Wells in Low-Permeability Oil Reservoir. Energies 2024, 17, 4597. [Google Scholar] [CrossRef]
- Pu, J.; Bai, B.; Geng, J.; Zhang, N.; Schuman, T. Dual Crosslinked Poly(Acrylamide-Co-N-Vinylpyrrolidone) Microspheres with Re-Crosslinking Ability for Fossil Energy Recovery. Geoenergy Sci. Eng. 2023, 224, 211604. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wu, T.; Zhao, Y.; Zhou, D.; Liu, S. Pore-Scale Investigation on the Plugging Behavior of Submicron-Sized Microspheres for Heterogeneous Porous Media with Higher Permeability. Geofluids 2020, 2020, 8869760. [Google Scholar] [CrossRef]
- Zhao, W.; Ni, J.; Hou, G.; Jia, Y.; Diwu, P.; Yuan, X.; Liu, T.; Hou, J. Research on Optimal Blocking Limits and Injection Parameter Optimization of Polymer Microsphere Conformance Control. ACS Omega 2021, 6, 8297–8307. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of Deep Profile Control and Oil Displacement Technologies with Nanoscale polymer gel microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Guo, J.; Xu, F.; Li, Z.; Li, M. Study on Plugging Performance of Cross-Linked polymer gel microspheres with Reservoir Pores. J. Pet. Sci. Eng. 2013, 105, 70–75. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Wang, A. A Novel N-Succinylchitosan-Graft-Polyacrylamide/Attapulgite Composite Hydrogel Prepared through Inverse Suspension Polymerization. Macromol. Mater. Eng. 2007, 292, 962–969. [Google Scholar] [CrossRef]
- Ji, Y.; Kang, W.; Meng, L.; Hu, L.; Yang, H. Study of the Solution Behavior of β-Cyclodextrin Amphiphilic Polymer Inclusion Complex and the Stability of Its O/W Emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 117–124. [Google Scholar] [CrossRef]
- Zareie, C.; Bahramian, A.R.; Sefti, M.V.; Salehi, M.B. Network-Gel Strength Relationship and Performance Improvement of Polyacrylamide Hydrogel Using Nano-Silica; with Regards to Application in Oil Wells Conditions. J. Mol. Liq. 2019, 278, 512–520. [Google Scholar] [CrossRef]
- Ji, J.; Zeng, C.; Ke, Y.; Pei, Y. Preparation of Poly(Acrylamide-Co-Acrylic Acid)/Silica Nanocomposite Microspheres and Their Performance as a Plugging Material for Deep Profile Control. J. Appl. Polym. Sci. 2017, 134, 45502. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear Resistance Performance of Low Elastic polymer gel microspheres Used for Conformance Control Treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Cao, B.; Xie, K.; Lu, X.; Cao, W.; He, X.; Xiao, Z.; Zhang, Y.; Wang, X.; Su, C. Effect and Mechanism of Combined Operation of Profile Modification and Water Shutoff with In-Depth Displacement in High-Heterogeneity Oil Reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127673. [Google Scholar] [CrossRef]
- Khoramian, R.; Kharrat, R.; Golshokooh, S. The Development of Novel Nanofluid for Enhanced Oil Recovery Application. Fuel 2022, 311, 122558. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Jin, S.; Yang, Z.; Dong, Z.; Zhang, J. Combined Flooding Systems with polymer microspheres and Nonionic Surfactant for Enhanced Water Sweep and Oil Displacement Efficiency in Heterogeneous Reservoirs. J. Dispers. Sci. Technol. 2020, 41, 267–276. [Google Scholar] [CrossRef]
- Xu, G.; Chang, J.; Wu, H.; Shao, W.; Li, G.; Hou, J.; Kang, N.; Yang, J. Enhanced Oil Recovery Performance of Surfactant-Enhanced Janus SiO2 Nanofluid for High Temperature and Salinity Reservoir. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130545. [Google Scholar] [CrossRef]
- Tohidi, Z.; Teimouri, A.; Jafari, A.; Gharibshahi, R.; Omidkhah, M.R. Application of Janus Nanoparticles in Enhanced Oil Recovery Processes: Current Status and Future Opportunities. J. Pet. Sci. Eng. 2022, 208, 109602. [Google Scholar] [CrossRef]
- Yin, T.; Yang, Z.; Dong, Z.; Lin, M.; Zhang, J. Physicochemical Properties and Potential Applications of Silica-Based Amphiphilic Janus Nanosheets for Enhanced Oil Recovery. Fuel 2019, 237, 344–351. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, Y.; Xu, G.; Wang, X.; Li, Y.; Zhao, S.; Liu, C.; Wang, X. Study on Interface Regulation Effects of Janus Nanofluid for Enhanced Oil Recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129880. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z.; Yang, M.; Qu, M.; Liu, T.; Hou, J.; et al. Silica-Based Amphiphilic Janus Nanofluid with Improved Interfacial Properties for Enhanced Oil Recovery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124162. [Google Scholar] [CrossRef]
- Colver, P.J.; Colard, C.A.L.; Bon, S.A. Multilayered Nanocomposite Polymer Colloids Using Emulsion Polymerization Stabilized by Solid Particles. J. Am. Chem. Soc. 2008, 130, 16850–16851. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, W.; Meng, H.; Guo, K.; Chen, J. Pickering Emulsion Polymerization: Preparation of Polystyrene/Nano-SiO2 Composite Microspheres with Core-Shell Structure. Powder Technol. 2009, 190, 393–400. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Samarajeewa, S.; Sun, G.; Yang, C.; Wooley, K.L. Orthogonally Dual-Clickable Janus Nanoparticles via a Cyclic Templating Strategy. J. Am. Chem. Soc. 2011, 133, 11046–11049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, H.; Hu, J.; Chi, M.; Fan, J.; Zhang, T.; Xie, W.; Ituen, E.; Sun, S.; Li, C.; Hu, S. Design and Synthesis of Temperature-Responsive Janus Nanoparticles with High Salt Tolerant for Enhanced Heavy Oil Recovery. Geoenergy Sci. Eng. 2025, 244, 213433. [Google Scholar] [CrossRef]
- Zang, L.; Hu, M.; Cao, J.; Cheng, Y.; Li, P.; Guo, J.; Zhang, H. Design of Janus Nanoparticles for Mobility Control in Heterogeneous Reservoirs. ACS Omega 2024, 9, 16536–16546. [Google Scholar] [CrossRef]
- Ling, X.; Phang, I.Y.; Acikgoz, C.; Yilmaz, M.D.; Hempenius, M.A.; Vancso, G.J.; Huskens, J. Janus Particles with Controllable Patchiness and Their Chemical Functionalization and Supramolecular Assembly. Angew. Chem. Int. Ed. 2009, 48, 7677–7682. [Google Scholar] [CrossRef]
- Xu, R.; Liao, L.; Liang, W.; Wang, H.; Zhou, Q.; Liu, W.; Chen, M.; Fang, B.; Wu, D.; Jin, H.; et al. Fast Removing Ligands from Platinum-Based Nanocatalysts by a Square-Wave Potential Strategy. Angew. Chem. Int. Ed. Engl. 2025, 5, e202509746. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Yang, Z.; Yue, D. Preparation and Surface Structure Study of Novel Asymmetric Janus Carbon Dots. Appl. Surf. Sci. 2025, 691, 162655. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Gao, S.; Xiao, X.; Xiang, C.; Tao, J. Formulation and characterization of surfactants with antibacterial and corrosion-inhibiting properties for enhancing shale gas drainage and production. Sci. Rep. 2025, 15, 2376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, M.; Jiang, Z.; Xian, L.; Liu, J.; Chen, J. Development and characterization of a surfactant responsive to redox conditions for gas recovery in foam drainage. Sci. Rep. 2025, 15, 511. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, W.; Zhang, H.; Wang, H.; He, X. The oil/water interfacial behavior of microgels used for enhancing oil recovery: A comparative study on microgel powder and microgel emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127731. [Google Scholar] [CrossRef]
- Liao, Y.; Jin, J.; Du, S.; Ren, Y.; Li, Q. Research on performance evaluation of polymeric surfactant cleaning gel-breaking fluid (GBF) and its enhanced oil recovery (EOR) effect. Polymers 2024, 16, 397. [Google Scholar] [CrossRef]



| No. | Length/mm | Diameter/mm | Permeability/mD | Porosity/% | Nanoparticle Concentration/wt% | Plugging Rate/% |
|---|---|---|---|---|---|---|
| 1 | 98.95 | 25.03 | 43.74 | 14.97 | 0.1 | 93.08 |
| 2 | 99.36 | 25.02 | 45.14 | 15.68 | 0.3 | 94.49 |
| 3 | 98.54 | 25.02 | 47.55 | 16.34 | 0.5 | 96.32 |
| Ions | Na+/K+ | Mg2+ | Ca2+ | Cl− | SO42− | CO32− | HCO3− | Total |
|---|---|---|---|---|---|---|---|---|
| Concentration/mg·L−1 | 4392.0 | 39.2 | 34.8 | 3922.9 | 47.7 | 385.3 | 4358.2 | 13,180.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Liu, B.; Liu, Y.; Xing, L.; Zhang, Y. Preparation and Enhanced Oil Recovery Mechanisms of Janus-SiO2-Reinforced Polymer Gel Microspheres. Gels 2025, 11, 506. https://doi.org/10.3390/gels11070506
Gao F, Liu B, Liu Y, Xing L, Zhang Y. Preparation and Enhanced Oil Recovery Mechanisms of Janus-SiO2-Reinforced Polymer Gel Microspheres. Gels. 2025; 11(7):506. https://doi.org/10.3390/gels11070506
Chicago/Turabian StyleGao, Fei, Baolei Liu, Yuelong Liu, Lei Xing, and Yan Zhang. 2025. "Preparation and Enhanced Oil Recovery Mechanisms of Janus-SiO2-Reinforced Polymer Gel Microspheres" Gels 11, no. 7: 506. https://doi.org/10.3390/gels11070506
APA StyleGao, F., Liu, B., Liu, Y., Xing, L., & Zhang, Y. (2025). Preparation and Enhanced Oil Recovery Mechanisms of Janus-SiO2-Reinforced Polymer Gel Microspheres. Gels, 11(7), 506. https://doi.org/10.3390/gels11070506







