Sodium Alginate Hydrogel Sponges Embedded with M2 Macrophages: An Adoptive Cell Therapy Strategy for Accelerated Diabetic Wound Healing
Abstract
1. Introduction
2. Results and Discussion
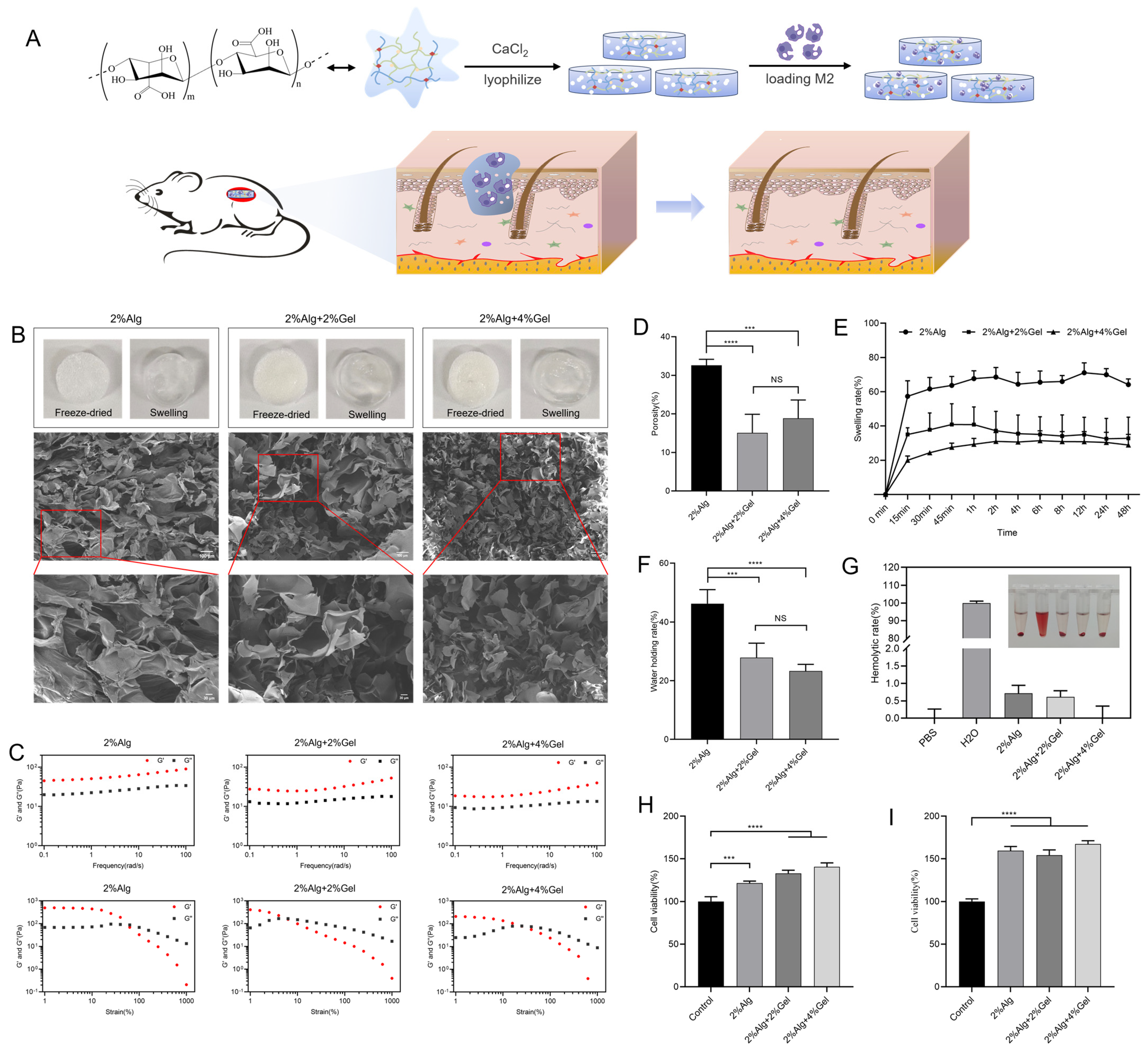
2.1. Characterization of Hydrogel Sponges
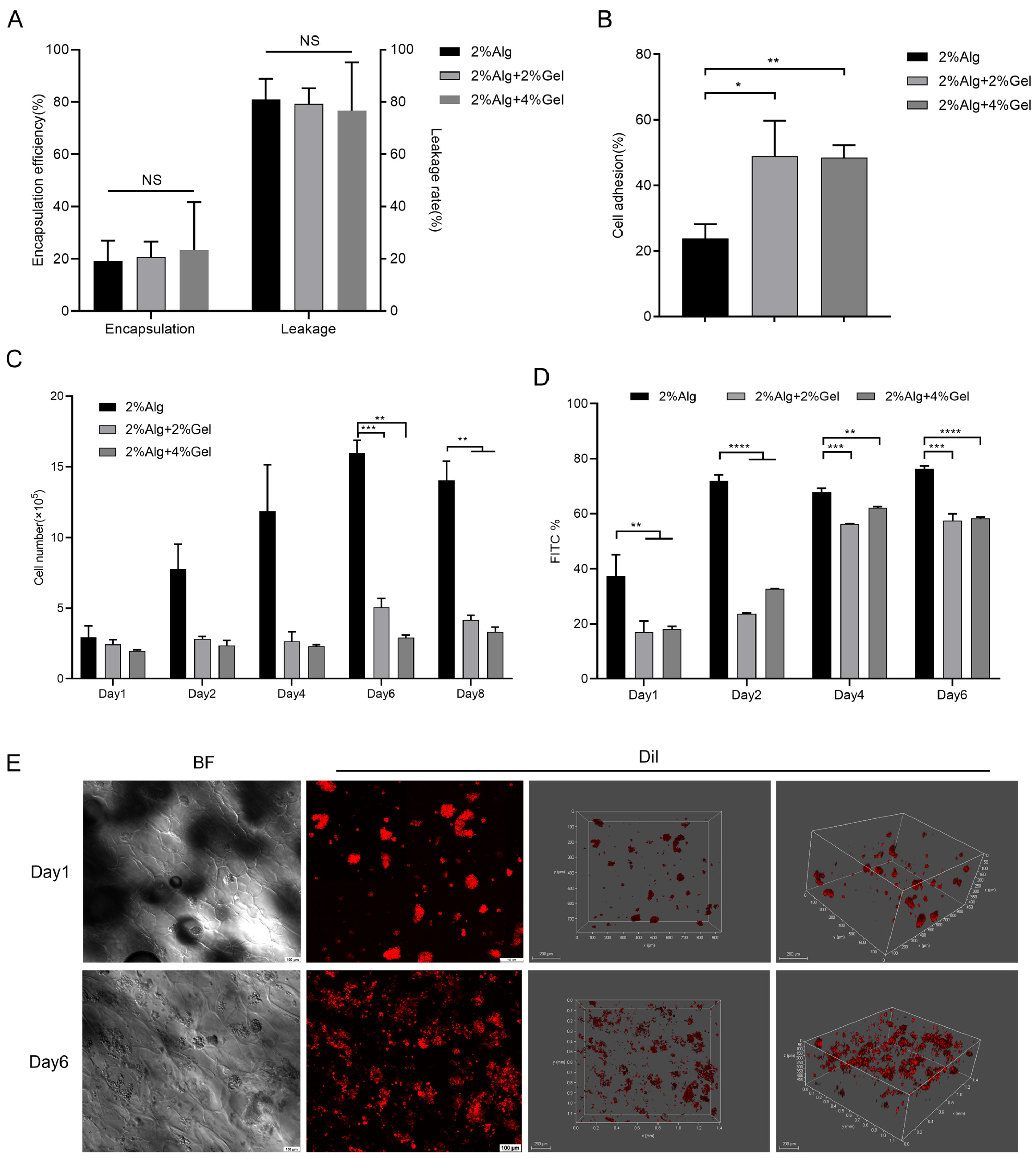
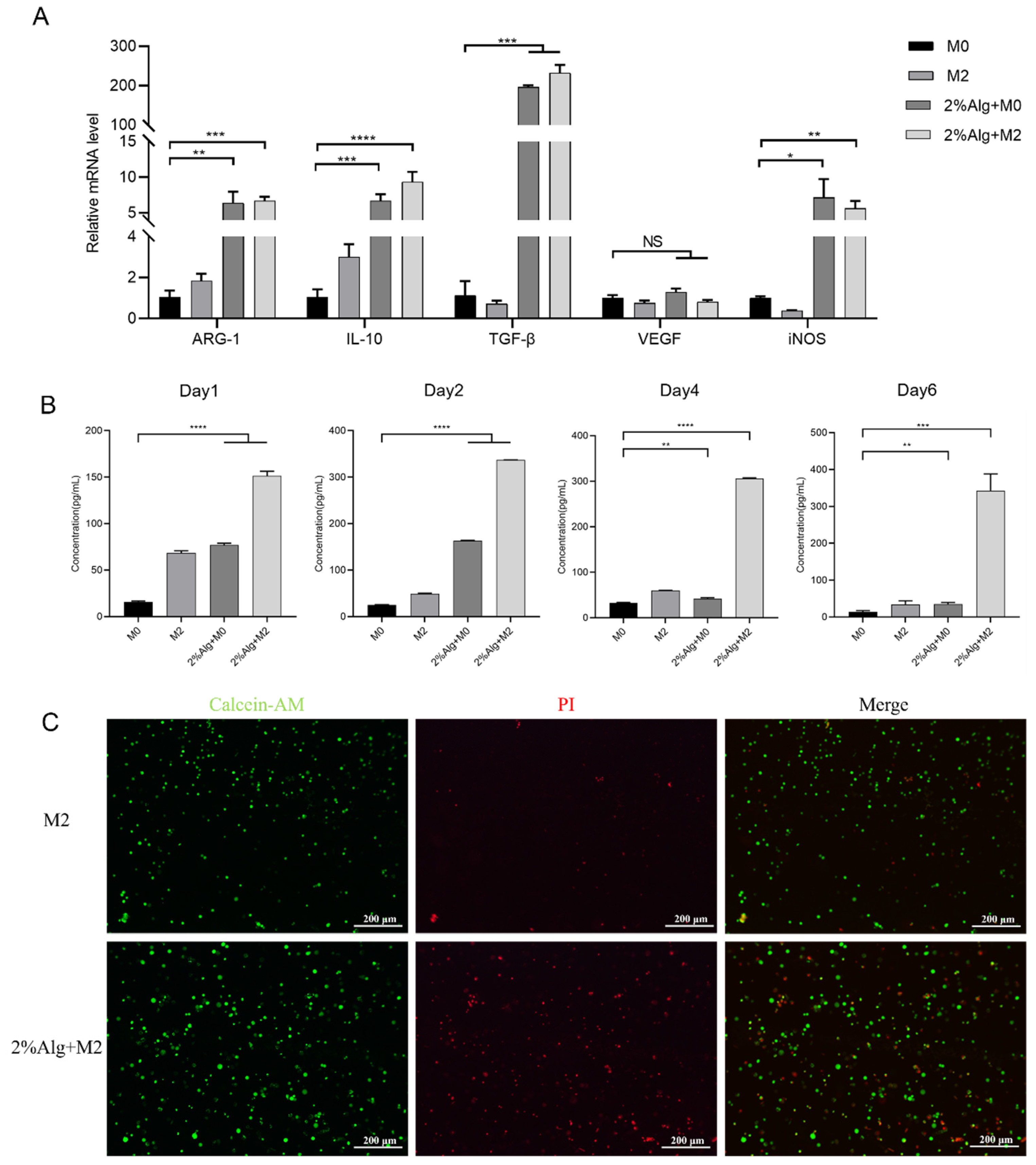
2.2. In Vitro Evaluation of the Hydrogel Sponges
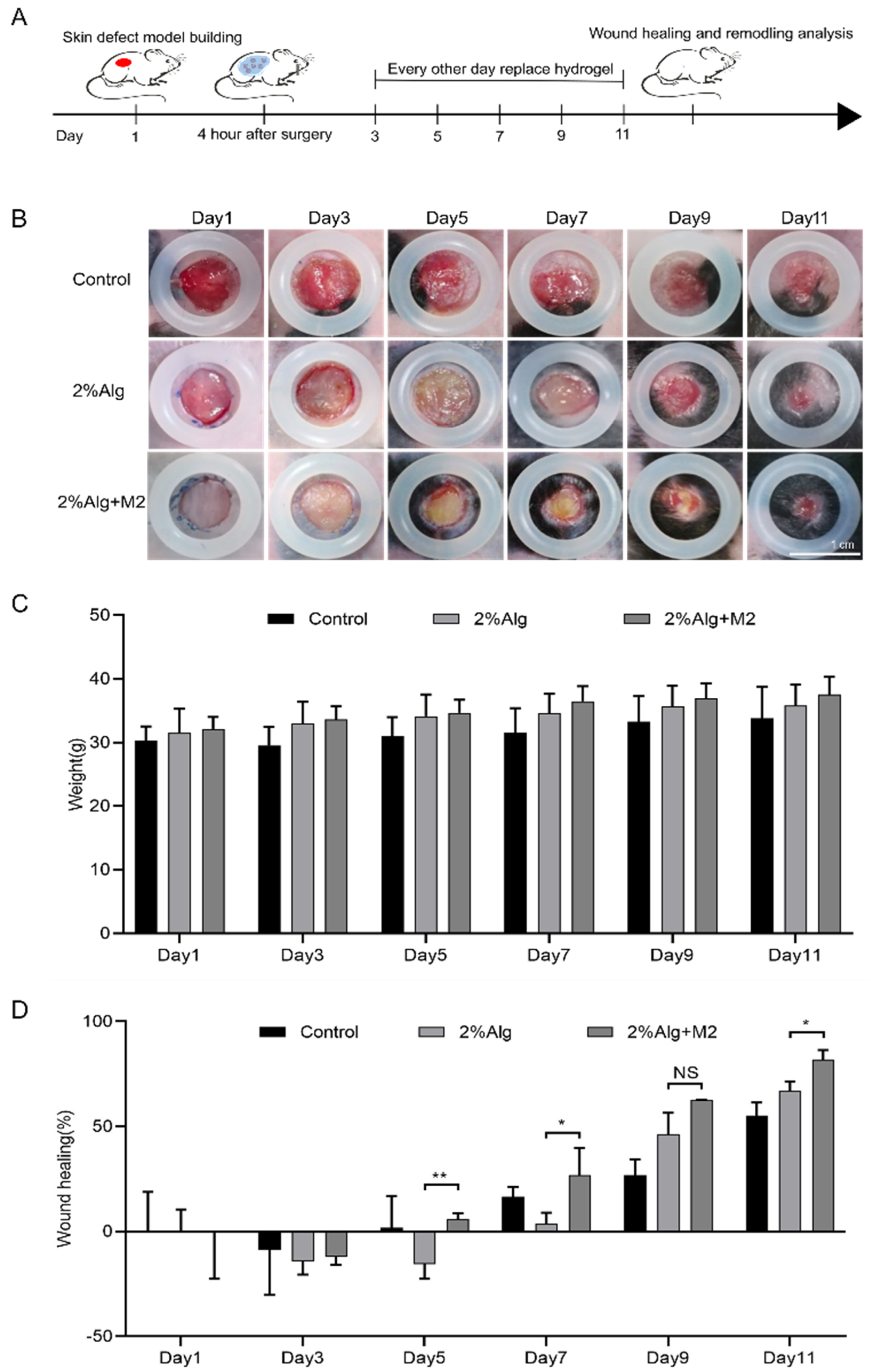
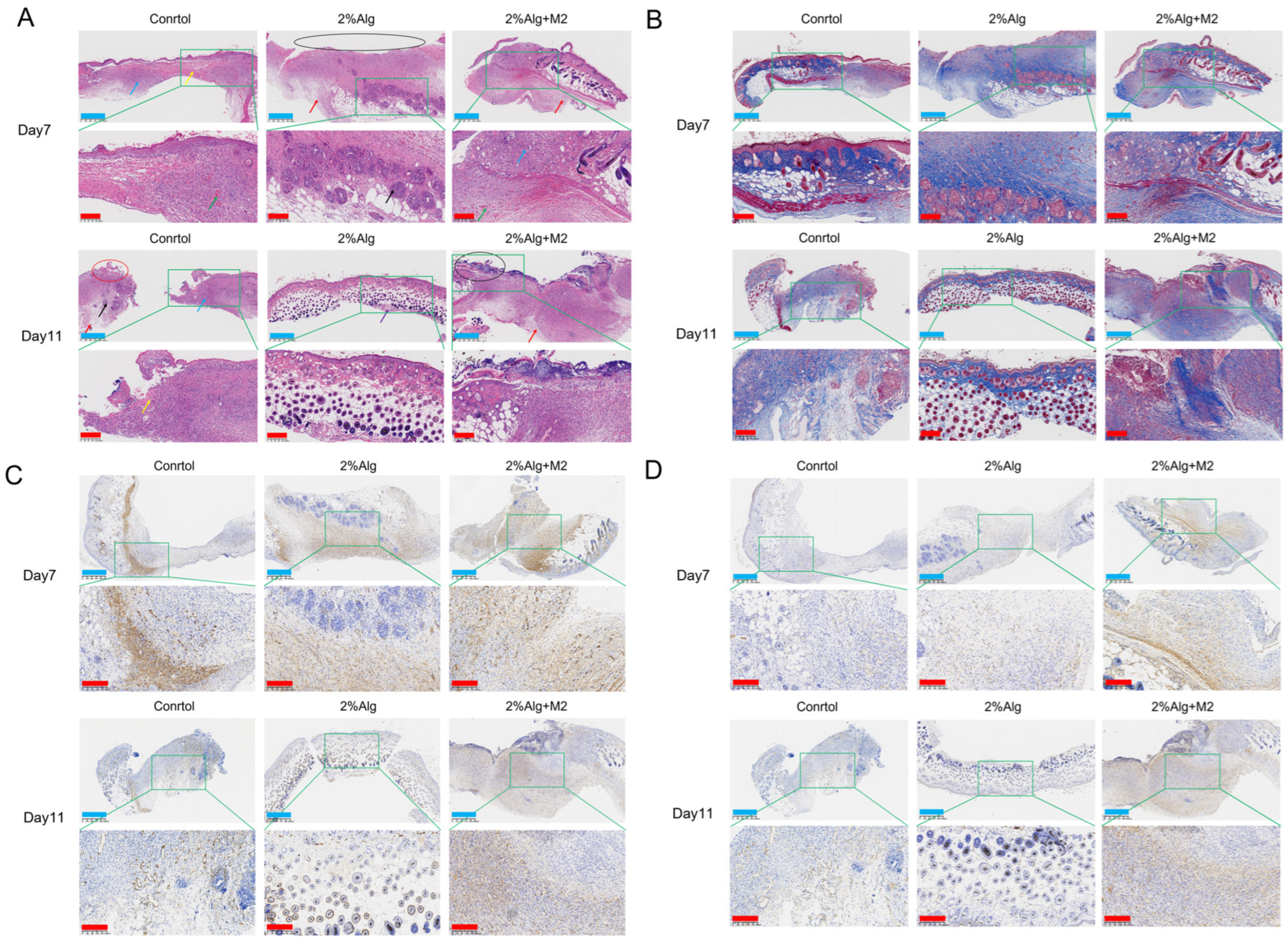
2.3. In Vivo Wound Healing Evaluation of the Diabetic Wound Model
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Preparation and Characterization of Hydrogel Sponges
4.2.1. Preparation of Hydrogel Sponges
4.2.2. Internal Morphology Observations
4.2.3. Rheological Properties
4.2.4. Porosity
4.2.5. Swelling Property
4.2.6. Water Holding Capacity
4.2.7. Sterility Examination
4.2.8. Blood Compatibility
4.2.9. In Vitro Cytocompatibility
Preparation of Fibroblasts
CCK-8 Assay for Detection of In Vitro Cytocompatibility of Hydrogel Sponges
Live/Dead Assay to Detect In Vitro Cytocompatibility of Hydrogel Sponges
4.3. In Vitro Study of M2 Macrophage-Hydrogel Sponges
4.3.1. Polarization of Macrophage M2 Type
4.3.2. Cell Encapsulation Rate
4.3.3. Cell Adhesion Rate
4.3.4. Cell Proliferation Rate
4.3.5. Cell Growth
4.3.6. Cell Distribution
4.3.7. Expression of Cell Marker Genes
4.3.8. Expression of Cytokine VEGF
4.3.9. Cryopreservation and Recovery of M2 Macrophage-Hydrogel Sponges
4.4. Application of M2 Macrophage-Hydrogel Sponge in Diabetic Wound Healing
4.4.1. Model Establishment of Chronic Wound
4.4.2. Histological Analysis
4.5. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, K.; Das, A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy 2019, 21, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Chen, J.; Wang, C.; Yuan, M.; Kang, Y.; Wu, Z.; Li, W.; Zhang, G.; Machens, H.-G.; Rinkevich, Y.; et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022, 29, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qiao, W.; Wang, Y.; Li, T.; Li, X.; Gong, T.; Zhang, Z.-R.; Fu, Y. An Extracellular Matrix-Mimicking Hydrogel for Full Thickness Wound Healing in Diabetic Mice. Macromol. Biosci. 2018, 18, e1800047. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Jeong, M.-J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Joshi, A.; Carson, W.F.; Schaller, M.; Allen, R.; Mukerjee, S.; Kittan, N.; Feldman, E.L.; Henke, P.K.; Hogaboam, C.; et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015, 64, 1420–1430. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Zhang, H.; Ding, Y.; Tan, Q. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int. Immunopharmacol. 2022, 106, 108605. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.V.; Ramkumar, K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020, 69, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.J.; Su, Q.; Zhang, C.N.; Huang, P.S.; Song, H.J.; Dong, A.J.; Kong, D.L.; Wang, W.W. Bioinspired Nanofibrous Glycopeptide Hydrogel Dressing for Accelerating Wound Healing: A Cytokine-Free, M2-Type Macrophage Polarization Approach. Adv. Funct. Mater. 2020, 30, 2006454. [Google Scholar] [CrossRef]
- Liu, W.; Gao, R.; Yang, C.; Feng, Z.; Ou-Yang, W.; Pan, X.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus-infected chronic skin wound healing. Sci. Adv. 2022, 8, eabn7006. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Dura, G.; Crespo-Cuadrado, M.; Waller, H.; Peters, D.T.; Ferreira-Duarte, A.; Lakey, J.H.; Fulton, D.A. Exploiting Meltable Protein Hydrogels to Encapsulate and Culture Cells in 3D. Macromol. Biosci. 2022, 22, e2200134. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter. 2019, 15, 7852–7862. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, H.J.; An, H.; Lee, K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993; Biological Evaluation of Medical Devices. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Li, W.; Zhang, L.; Gan, K.; Zhang, Y.; Wang, S. Sodium Alginate Hydrogel Sponges Embedded with M2 Macrophages: An Adoptive Cell Therapy Strategy for Accelerated Diabetic Wound Healing. Gels 2025, 11, 502. https://doi.org/10.3390/gels11070502
Tian Q, Li W, Zhang L, Gan K, Zhang Y, Wang S. Sodium Alginate Hydrogel Sponges Embedded with M2 Macrophages: An Adoptive Cell Therapy Strategy for Accelerated Diabetic Wound Healing. Gels. 2025; 11(7):502. https://doi.org/10.3390/gels11070502
Chicago/Turabian StyleTian, Qingchang, Wenqi Li, Lijiaqi Zhang, Kefen Gan, Yiting Zhang, and Shuling Wang. 2025. "Sodium Alginate Hydrogel Sponges Embedded with M2 Macrophages: An Adoptive Cell Therapy Strategy for Accelerated Diabetic Wound Healing" Gels 11, no. 7: 502. https://doi.org/10.3390/gels11070502
APA StyleTian, Q., Li, W., Zhang, L., Gan, K., Zhang, Y., & Wang, S. (2025). Sodium Alginate Hydrogel Sponges Embedded with M2 Macrophages: An Adoptive Cell Therapy Strategy for Accelerated Diabetic Wound Healing. Gels, 11(7), 502. https://doi.org/10.3390/gels11070502





