Elaboration of Conductive Hydrogels by 3D Printer for the Development of Strain Sensors
Abstract
1. Introduction
2. Results and Discussion
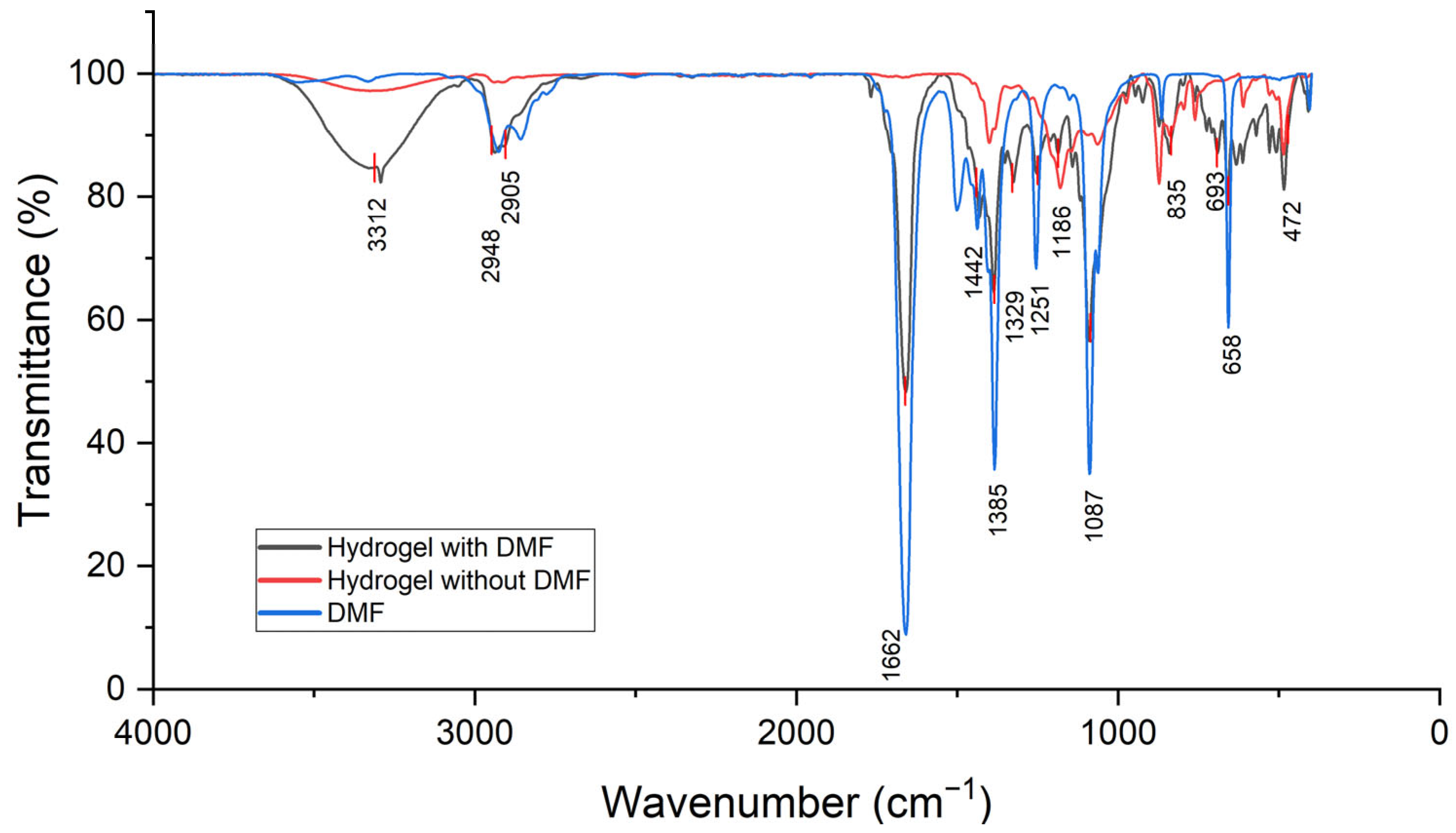
2.1. Efficiency of the DMF Removal Step
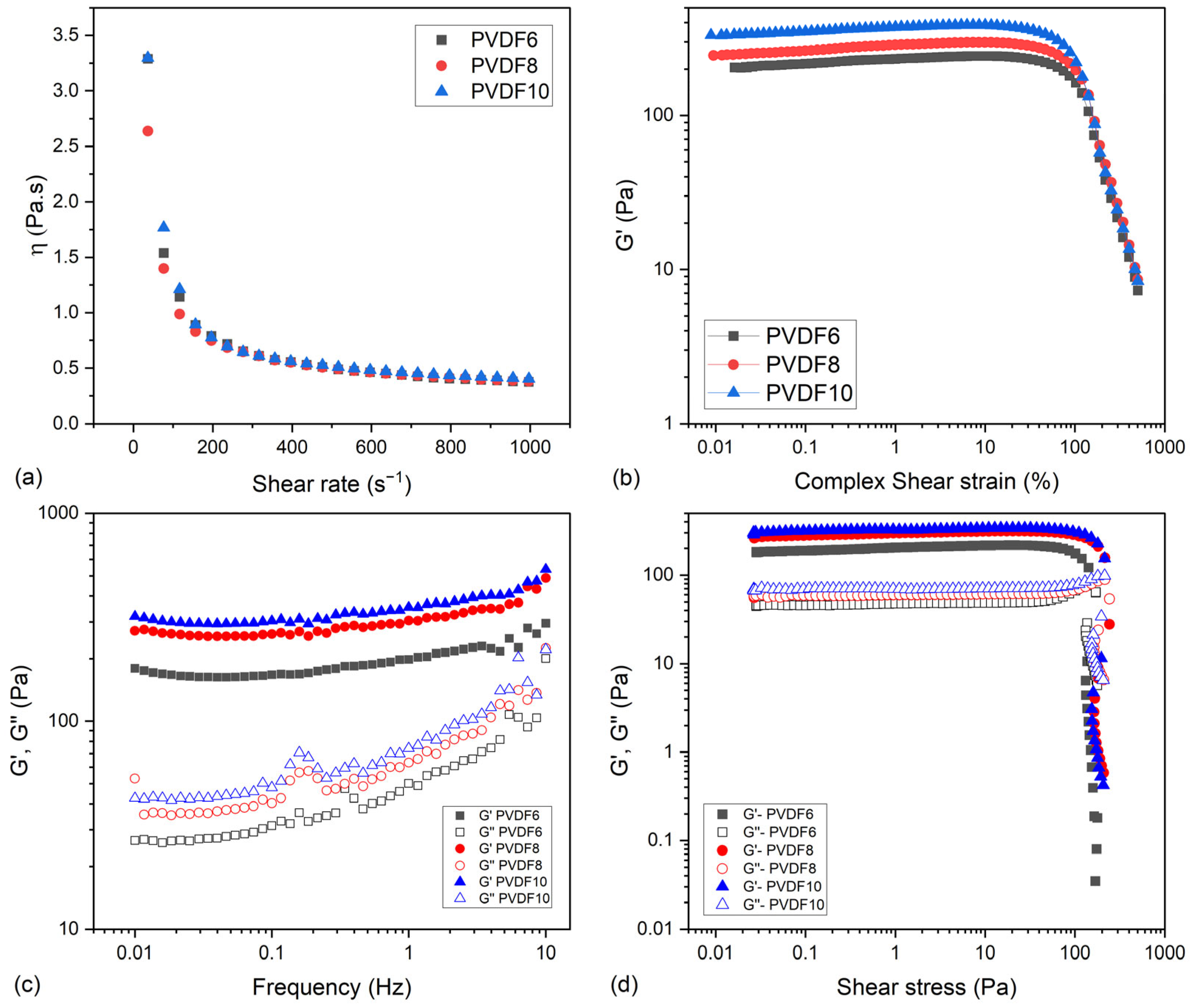
2.2. Rheological Assessment of Inks
2.2.1. Flow Test Curves
2.2.2. Amplitude and Frequency Sweep Measurements
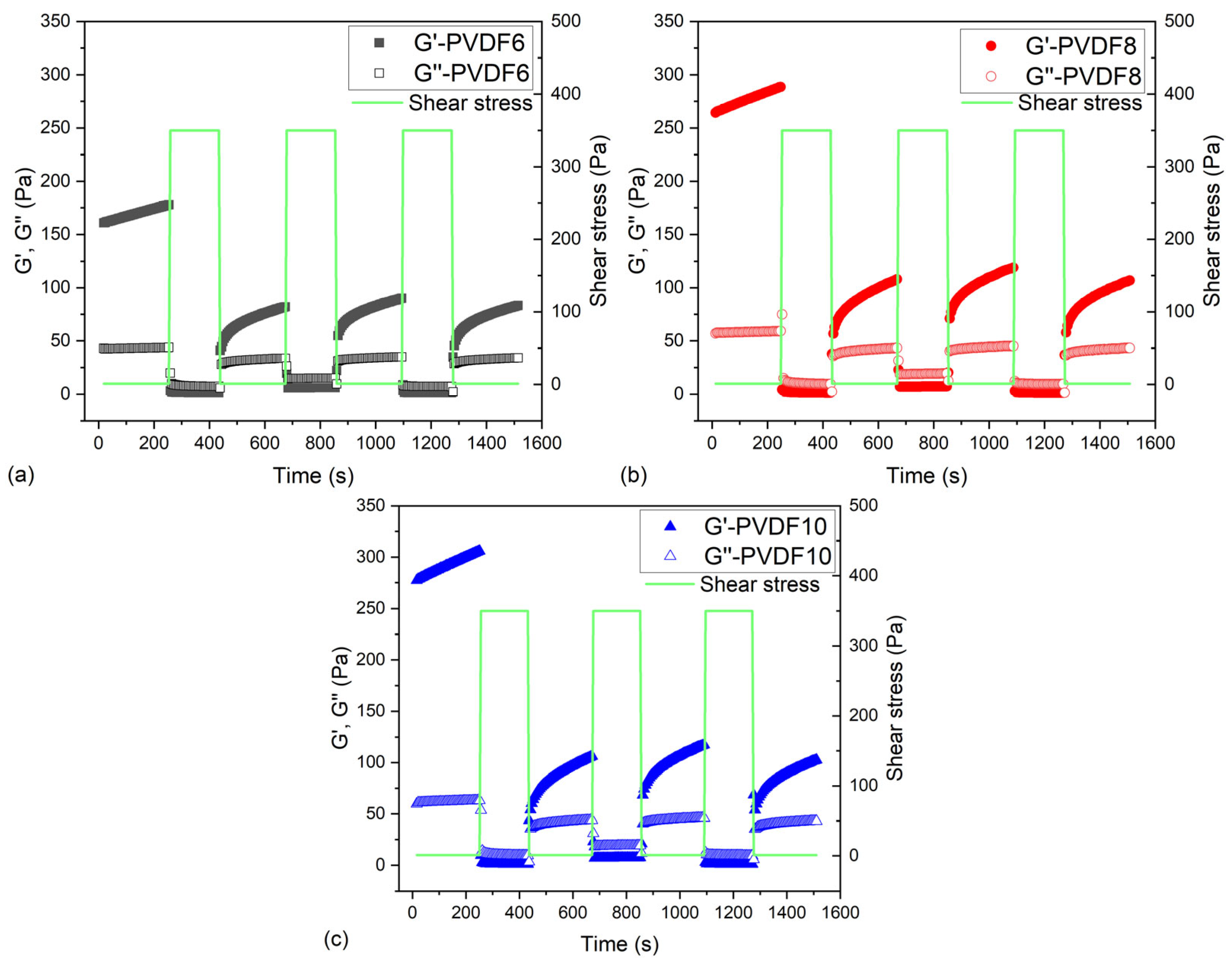
2.2.3. Shear Stress Sweep Measurements
2.3. Characterization of the Structured Hydrogels
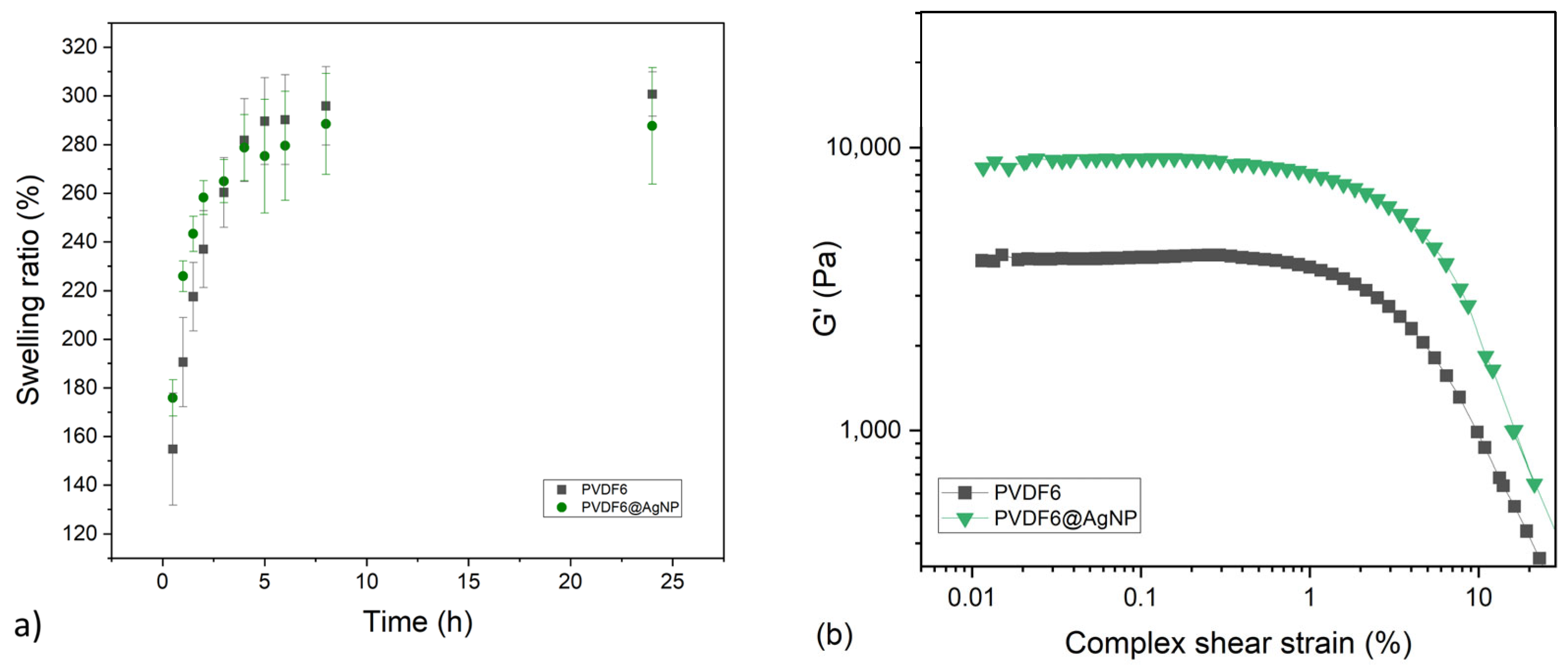
2.3.1. Amplitude Strain Sweep and Swelling Ratio Evaluation
2.3.2. Piezoelectricity and Electrical Resistance Evaluation
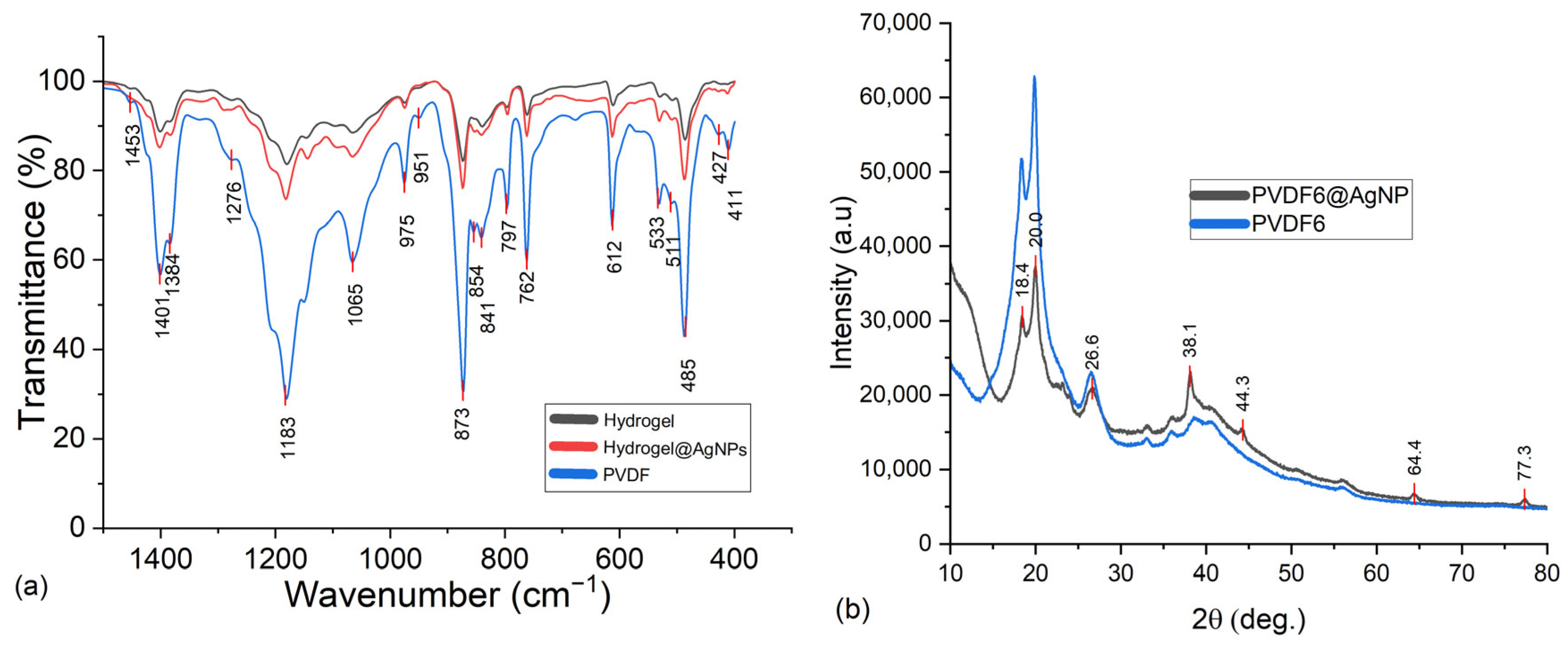
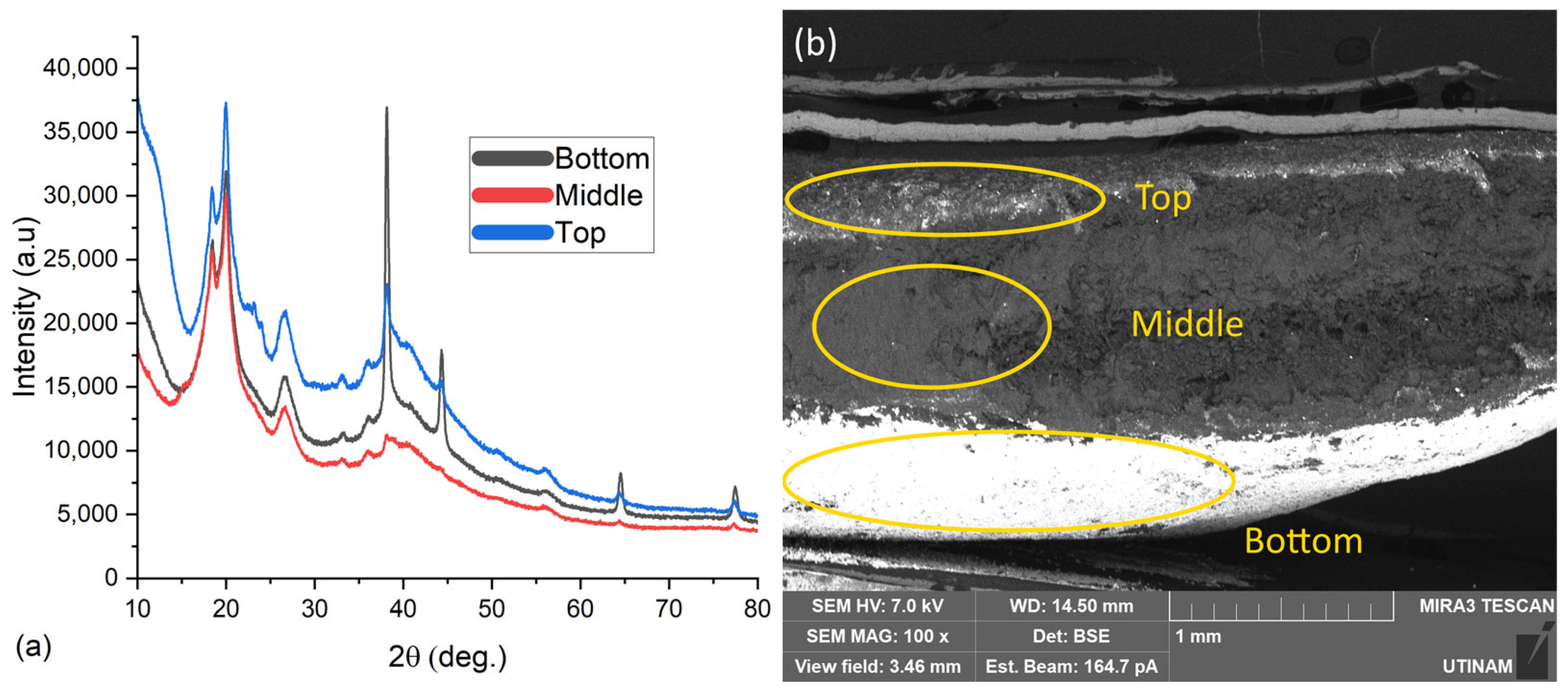
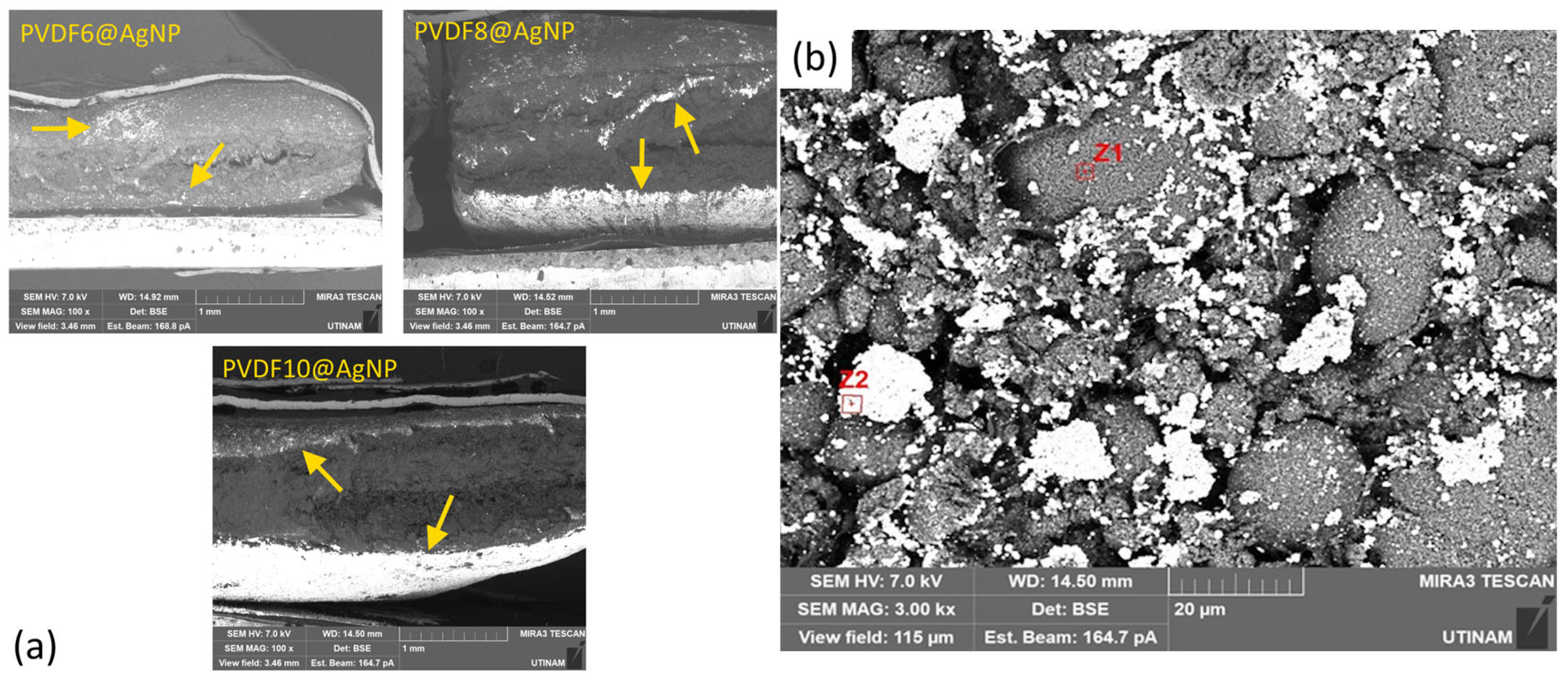
2.3.3. Evaluation of the Impact of AgNPs
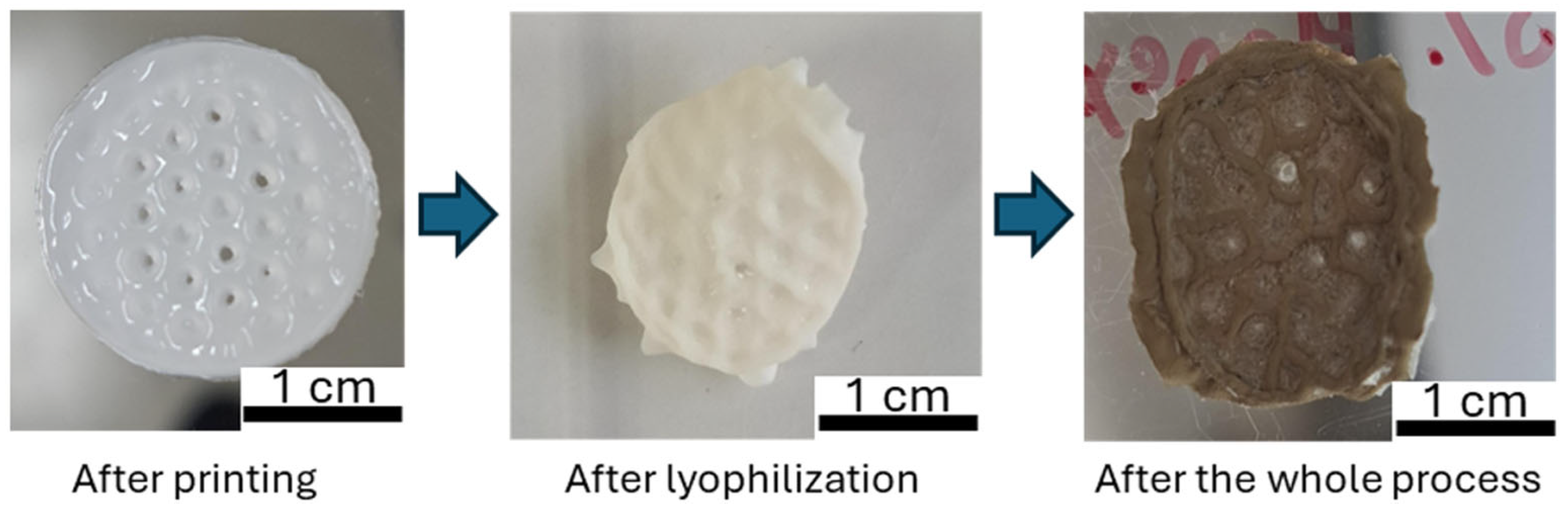
2.4. Three-Dimensional Printing Results
3. Conclusions
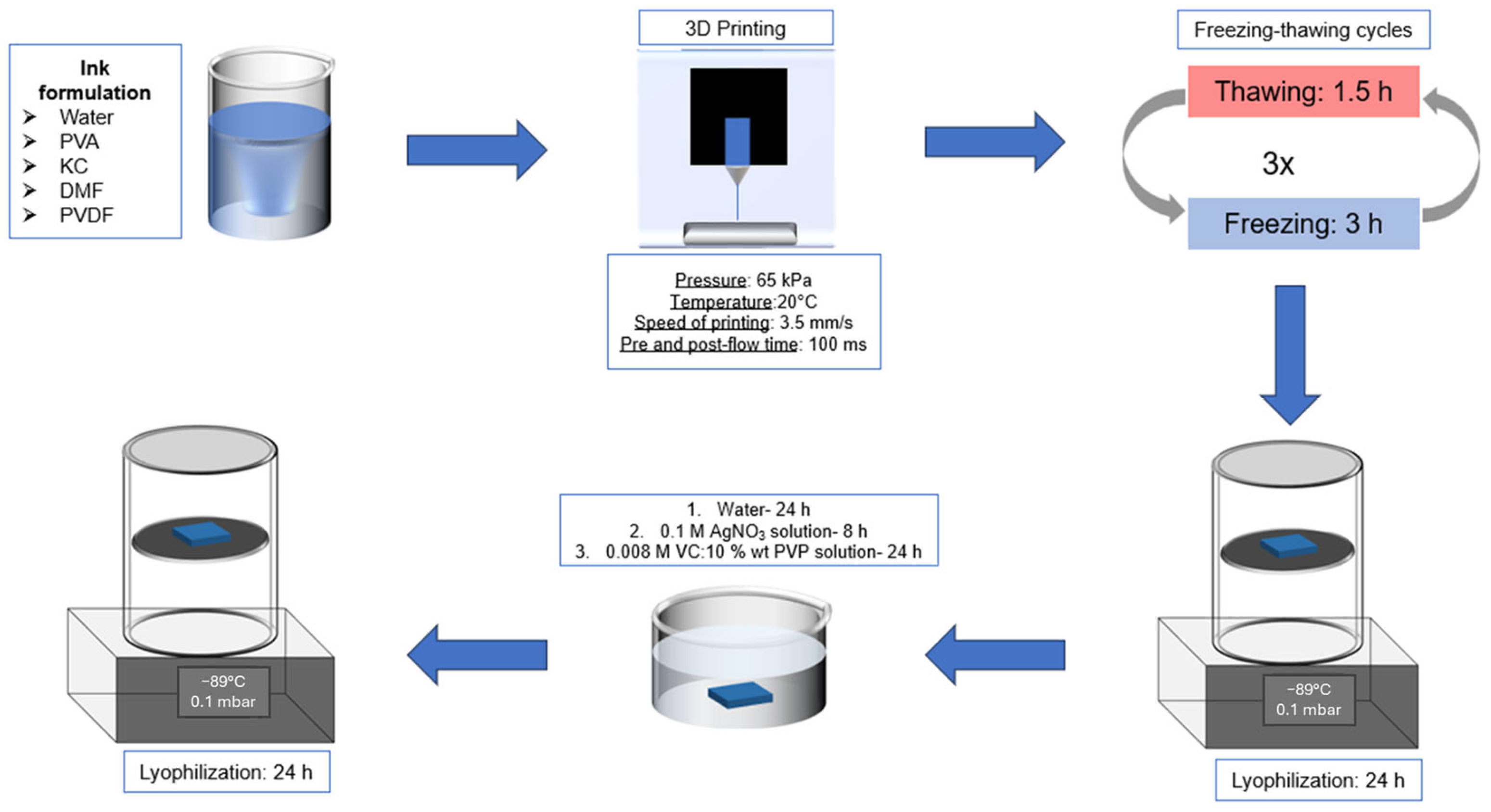
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Structured Hydrogels
4.3. Three-Dimensional Printing Protocol
4.4. Characterization of Inks and Hydrogels
4.4.1. Water Uptake of Hydrogels: Swelling Ratio Analysis
4.4.2. Rheology Measurements
- Flow test
- Shear stress sweep
- Amplitude sweep
- Frequency sweep
- Thixotropy loop
- Amplitude strain sweep measurements
- Geometry: Parallel plate with 20 mm diameter
- Temperature: 25 °C
- Frequency: 1 Hz
- Shear strain range: 0.01–1000%
4.4.3. Infrared Spectroscopy
4.4.4. X-Ray Diffraction
4.4.5. Scanning Electron Microscopy Coupled with Energy-Dispersive X-Ray Analysis
4.4.6. Assessment of Piezoelectricity and Resistance Measurements
- Piezoelectrical measurements
- Resistance measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOAJ | Directory of open access journals |
| PVA | polyvinyl alcohol |
| PVDF | polyvinylidene fluoride |
| KC | κ-carrageenan |
| AgNPs | Silver nanoparticles |
| DIW | Direct ink writing |
| PVP | polyvinylpyrrolidone |
References
- Park, K.; Choi, H.; Kang, K.; Shin, M.; Son, D. Soft Stretchable Conductive Carboxymethylcellulose Hydrogels for Wearable Sensors. Gels 2022, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Duan, L.; D’Hooge, D.R.; Cardon, L. Recent progress on flexible and stretchable piezoresistive strain sensors: From design to application. Prog. Mater. Sci. 2020, 114, 100617. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, R.; Wu, X. Recent progress in the development of conductive hydrogels and the application in 3D printed wearable sensors. RSC Appl. Polym. 2023, 1, 132–157. [Google Scholar] [CrossRef]
- Jiang, P.; Yan, C.; Guo, Y.; Zhang, X.; Cai, M.; Jia, X.; Wang, X.; Zhou, F. Direct ink writing with high-strength and swelling-resistant biocompatible physically crosslinked hydrogels. Biomater. Sci. 2019, 7, 1805–1814. [Google Scholar] [CrossRef]
- Baniasadi, H.; Madani, Z.; Ajdary, R.; Rojas, O.J.; Seppala, J. Ascorbic acid-loaded polyvinyl alcohol/cellulose nanofibril hydrogels as precursors for 3D printed materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112424. [Google Scholar] [CrossRef]
- Mehtani, D.; Seth, A.; Sharma, P.; Maheshwari, N.; Kapoor, D.; Shrivastava, S.K.; Tekade, R.K. Chapter 4—Biomaterials for Sustained and Controlled Delivery of Small Drug Molecules. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 89–152. [Google Scholar]
- Han, J.; Lei, T.; Wu, Q. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef]
- More, S.M.; Kulkarni, R.V.; Sa, B.; Kayane, N.V. Glutaraldehyde-crosslinked poly(vinyl alcohol) hydrogel discs for the controlled release of antidiabetic drug. J. Appl. Polym. Sci. 2010, 116, 1732–1738. [Google Scholar] [CrossRef]
- Babeli, I.; Ruano, G.; Casanovas, J.; Ginebra, M.-P.; García-Torres, J.; Alemán, C. Conductive, self-healable and reusable poly(3,4-ethylenedioxythiophene)-based hydrogels for highly sensitive pressure arrays. J. Mater. Chem. C 2020, 8, 8654–8667. [Google Scholar] [CrossRef]
- Kesavan, S.; Rajesh, D.; Shanmugam, J.; Aruna, S.; Gopal, M.; Vijayakumar, S. Biocompatible polysaccharide fabricated graphene oxide nanoparticles: A versatile nanodrug carrier to deliver κ-carrageenan against cancer cells. Int. J. Biol. Macromol. 2023, 244, 125322. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zeng, H.; Qiao, L.; Jiang, H.; Ye, Q.; Wang, Z.; Liu, B.; Fan, Z. 3D Printed Piezoelectric Wound Dressing with Dual Piezoelectric Response Models for Scar-Prevention Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 30507–30522. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lee, J.Y.; Kang, D.S.; Anil, S.; Kim, S.K.; Shim, M.S.; Kim, D.G. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol. 2017, 98, 515–525. [Google Scholar] [CrossRef]
- Lotfi, R.; Eslahi, N.; Koohkhezri, M.; Moghadasi, H. 9—3D printing of graphene polymer composites. In Innovations in Graphene-Based Polymer Composites; Rangappa, S.M., Parameswaranpillai, J., Ayyappan, V., Motappa, M.G., Siengchin, S., Soutis, C., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 247–281. [Google Scholar]
- Stuart, R. Lessons Learned from a Short-Term Exposure to DMF. ACS Chem. Health Saf. 2023, 30, 44–48. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Zhang, L.; Guo, R.; Lu, Y.; Liu, S.; Su, Z.; Ji, Z.; Wang, X. 3D printable conductive ionic hydrogels with self-adhesion performance for strain sensing. J. Mater. Chem. C 2022, 10, 14288–14295. [Google Scholar] [CrossRef]
- Muscolino, E.; Di Stefano, A.B.; Trapani, M.; Sabatino, M.A.; Giacomazza, D.; Alessi, S.; Cammarata, E.; Moschella, F.; Cordova, A.; Toia, F.; et al. κ-Carrageenan and PVA blends as bioinks to 3D print scaffolds for cartilage reconstruction. Int. J. Biol. Macromol. 2022, 222, 1861–1875. [Google Scholar] [CrossRef]
- Jurin, F.E.; Buron, C.C.; Frau, E.; del Rossi, S.; Schintke, S. The Electrical and Mechanical Characteristics of Conductive PVA/PEDOT:PSS Hydrogel Foams for Soft Strain Sensors. Sensors 2024, 24, 570. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Qi, F.; Zeng, Z.; Yao, J.; Cai, W.; Zhao, Z.; Peng, S.; Shuai, C. Constructing core-shell structured BaTiO3@carbon boosts piezoelectric activity and cell response of polymer scaffolds. Mater. Sci. Eng. C 2021, 126, 112129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, L.; Qi, F.; Qin, S.; Yang, X.; Xie, W.; Gai, K.; Han, Y.; Zhang, X.; Zhu, Z.; et al. Enhanced bone regeneration via ZIF-8 decorated hierarchical polyvinylidene fluoride piezoelectric foam nanogenerator: Coupling of bioelectricity, angiogenesis, and osteogenesis. Nano Energy 2023, 106, 108076. [Google Scholar] [CrossRef]
- Khazani, Y.; Rafiee, E.; Samadi, A.; Mahmoodi, M. Alginate-PVDF piezoelectric hydrogel containing calcium copper titanate- hydroxyapatite as a self-powered scaffold for bone tissue engineering and energy harvesting. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133537. [Google Scholar] [CrossRef]
- Netto, T.G.; Zimmerman, R.L. Effect of water on piezoelectricity in bone and collagen. Biophys. J. 1975, 15, 573–576. [Google Scholar] [CrossRef]
- Alakanandana, A.; Subrahmanyam, A.R.; Siva Kumar, J. Structural and Electrical Conductivity studies of pure PVA and PVA doped with Succinic acid polymer electrolyte system. Mater. Today Proc. 2016, 3 Pt B, 3680–3688. [Google Scholar] [CrossRef]
- Pang, Z.; Li, Y.; Zheng, H.; Qin, R. Microscopic Mechanism of Electrical Aging of PVDF Cable Insulation Material. Polymers 2023, 15, 1286. [Google Scholar] [CrossRef]
- Low, Y.K.A.; Meenubharathi, N.; Niphadkar, N.D.; Boey, F.Y.C.; Ng, K.W. α- and β-Poly(Vinylidene Fluoride) Evoke Different Cellular Behaviours. J. Biomater. Sci. Polym. Ed. 2011, 22, 1651–1667. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef]
- Janusas, G.; Ponelyte, S.; Brunius, A.; Guobiene, A.; Prosycevas, I.; Vilkauskas, A.; Palevicius, A. Periodical Microstructures Based on Novel Piezoelectric Material for Biomedical Applications. Sensors 2015, 15, 31699–31708. [Google Scholar] [CrossRef]
- Meng, H.; Chen, Z.; Lu, Z.; Wang, X. Piezoelectric effect enhanced plasmonic photocatalysis in the Pt/BaTiO3 heterojunctions. J. Mol. Liq. 2023, 369, 120846. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Liao, Y.; Wang, J.; Zhang, T.; Liu, X.; Zhang, Y. Unveiling the Dual-Enhancing Mechanisms of Kinetically Controlled Silver Nanoparticles on Piezoelectric PVDF Nanofibers for Optimized SERS Performance. ACS Sens. 2024, 9, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Iqbal, S.; Chen, X. Recent advances in piezoelectric wearable energy harvesting based on human motion: Materials, design, and applications. Energy Strategy Rev. 2024, 53, 101422. [Google Scholar] [CrossRef]
- Riddle, M.; MacDermid, J.; Robinson, S.; Szekeres, M.; Ferreira, L.; Lalone, E. Evaluation of individual finger forces during activities of daily living in healthy individuals and those with hand arthritis. J. Hand Ther. 2020, 33, 188–197. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Q.; Liang, Y.; Ren, L.; Ren, L. Hypersensitized Strain Sensors Based on Conductive Hydrogels with Excellent Conductivity and Good Mechanical Properties. ACS Sustain. Chem. Eng. 2022, 10, 4425–4437. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. 2—VISCOELASTICITY. In Rheology, 4th ed.; Malkin, A.Y., Isayev, A.I., Eds.; ChemTec Publishing: New York, NY, USA, 2022; pp. 45–131. [Google Scholar]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Huang, Z.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Flexible electrically resistive-type strain sensors based on reduced graphene oxide-decorated electrospun polymer fibrous mats for human motion monitoring. Carbon 2018, 126, 360–371. [Google Scholar] [CrossRef]



| Sample | Average Resistance (GΩ) | Average Resistance (kΩ) |
|---|---|---|
| Dry State | Wet State | |
| PVDF6 | 58 ± 18 | 227 ± 44.2 |
| PVDF8 | 127 ± 19 | 120 ± 25.1 |
| PVDF10 | 29 ± 5 | 344 ± 56.4 |
| PVDF6@AgNP | 1.7 ± 0.8 | 1.29 ± 0.65 |
| PVDF8@AgNP | 1.2 ± 1.1 | 1.34 ± 0.54 |
| PVDF10@AgNP | 1.8 ± 1.2 | 1.38 ± 0.37 |
| Zone | Atomic Composition (%) | |||
|---|---|---|---|---|
| C | O | F | Ag | |
| Z1 | 69.8370 | 03.1950 | 26.3426 | 00.6253 |
| Z2 | 12.5521 | 00.0001 | 00.3055 | 87.1423 |
| Final Sample Composition | Sample’s Code |
|---|---|
| PVA, KC, PVDF (6% wt) | PVDF6 |
| PVA, KC, PVDF (8% wt) | PVDF8 |
| PVA, KC, PVDF (10% wt) | PVDF10 |
| PVA, KC, PVDF (6% wt), AgNPs | PVDF6@AgNP |
| PVA, KC, PVDF (8% wt), AgNPs | PVDF8@AgNP |
| PVA, KC, PVDF (10% wt), AgNPs | PVDF10@AgNP |
| Sample | Measurement Gap (mm) |
|---|---|
| PVDF6 | 1.84 |
| PVDF8 | 1.26 |
| PVDF10 | 1.39 |
| PVDF6@AgNP | 1.06 |
| PVDF8@AgNP | 1.48 |
| PVDF10@AgNP | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carravero Costa, L.; Pochard, I.; Buron, C.C.; Jurin, F.E. Elaboration of Conductive Hydrogels by 3D Printer for the Development of Strain Sensors. Gels 2025, 11, 474. https://doi.org/10.3390/gels11070474
Carravero Costa L, Pochard I, Buron CC, Jurin FE. Elaboration of Conductive Hydrogels by 3D Printer for the Development of Strain Sensors. Gels. 2025; 11(7):474. https://doi.org/10.3390/gels11070474
Chicago/Turabian StyleCarravero Costa, Lucas, Isabelle Pochard, Cédric C. Buron, and Florian E. Jurin. 2025. "Elaboration of Conductive Hydrogels by 3D Printer for the Development of Strain Sensors" Gels 11, no. 7: 474. https://doi.org/10.3390/gels11070474
APA StyleCarravero Costa, L., Pochard, I., Buron, C. C., & Jurin, F. E. (2025). Elaboration of Conductive Hydrogels by 3D Printer for the Development of Strain Sensors. Gels, 11(7), 474. https://doi.org/10.3390/gels11070474








