Enhancing Wound Healing Through Secretome-Loaded 3D-Printed Biomaterials
Abstract
1. Introduction
2. Skin Structure and Physiology
3. Wound Healing Process
4. Treatments of Wound
4.1. Standard Treatments of Wound
4.2. Alternative Treatments of Wound
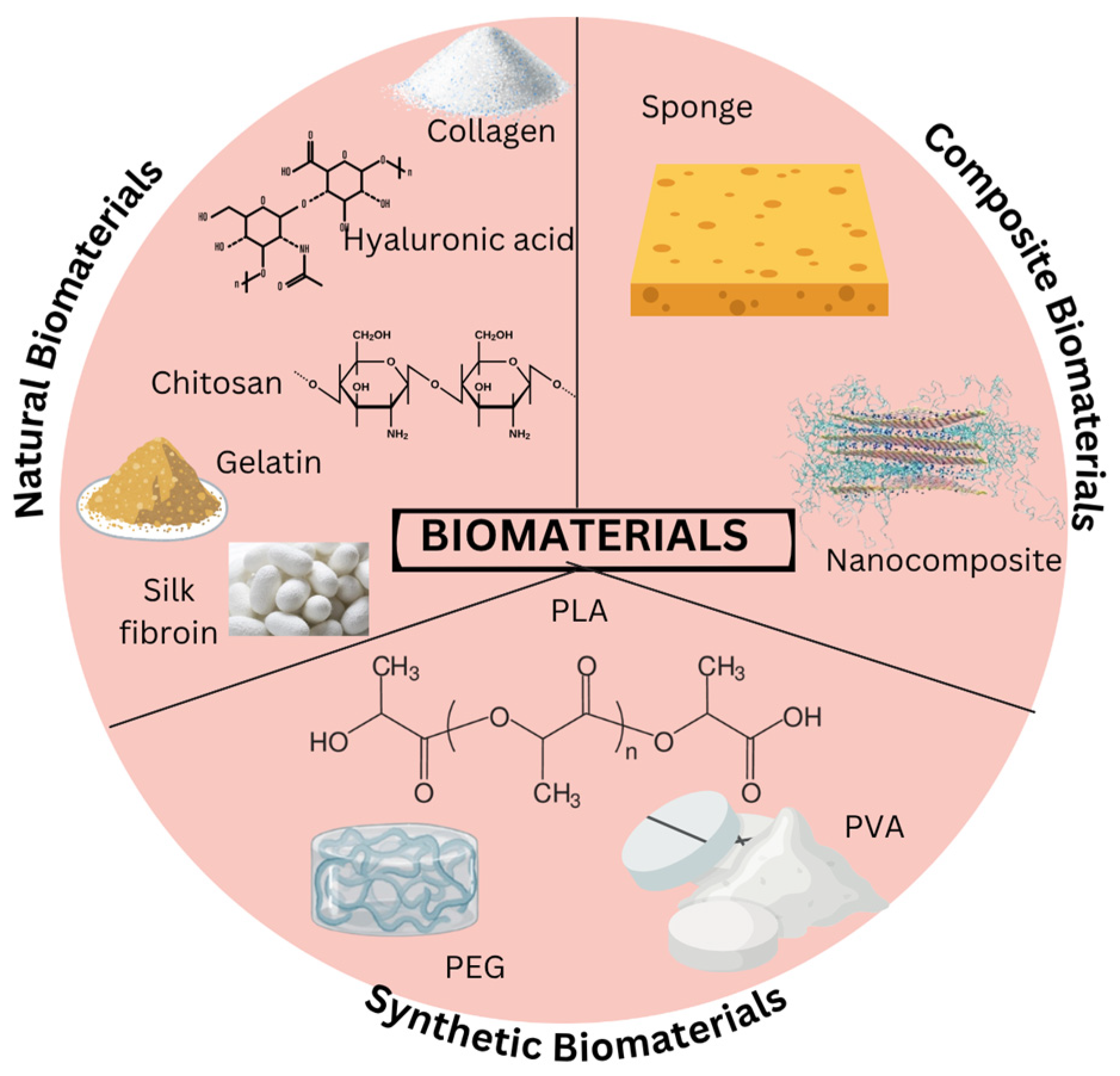
5. Biomaterials
5.1. Naturals Biomaterials
5.2. Synthetic Biomaterials
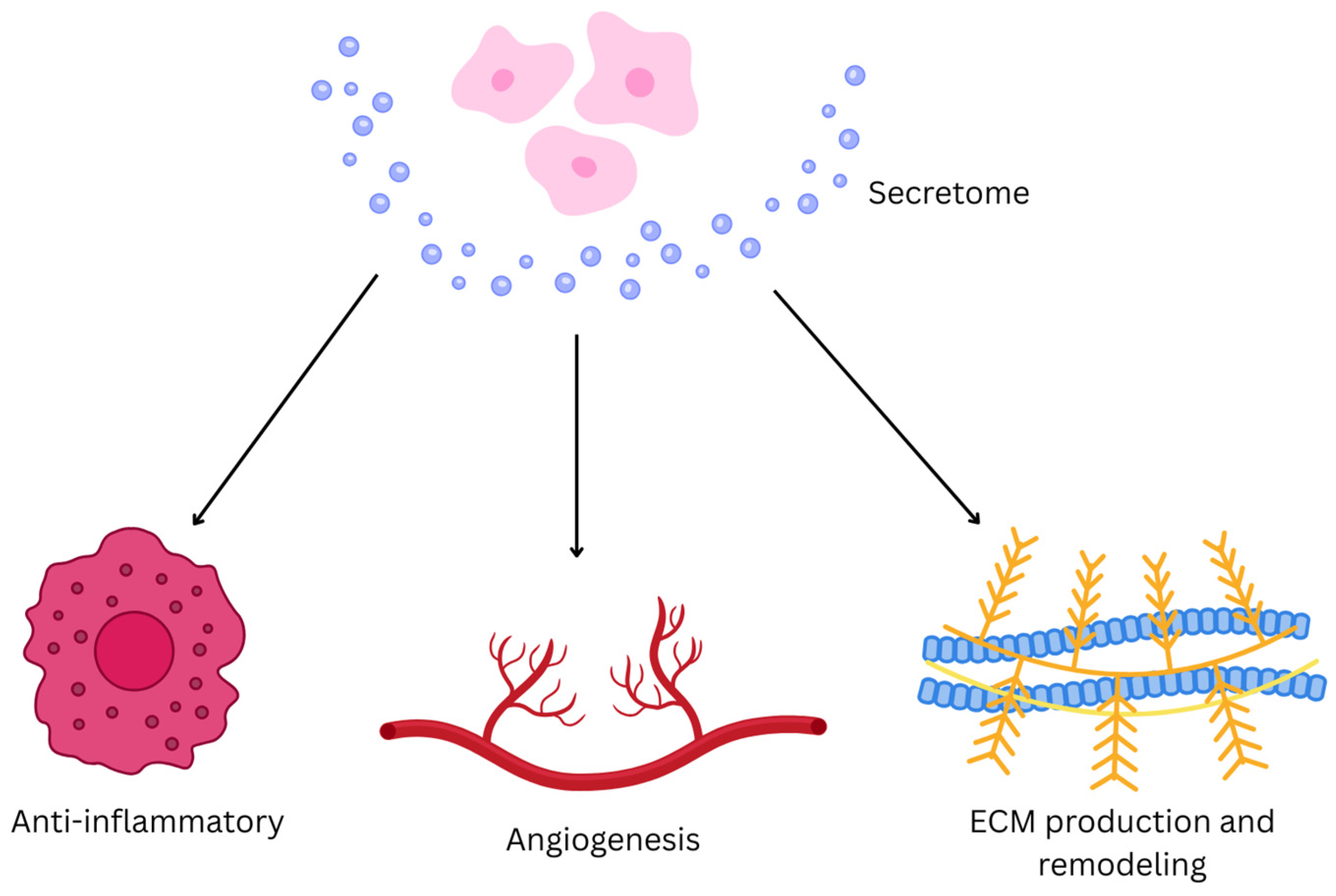
6. Secretome
6.1. Extracellular Vesicles (EVs)
6.2. Fundamental Mechanism of Secretome
6.3. Uses of Secretome
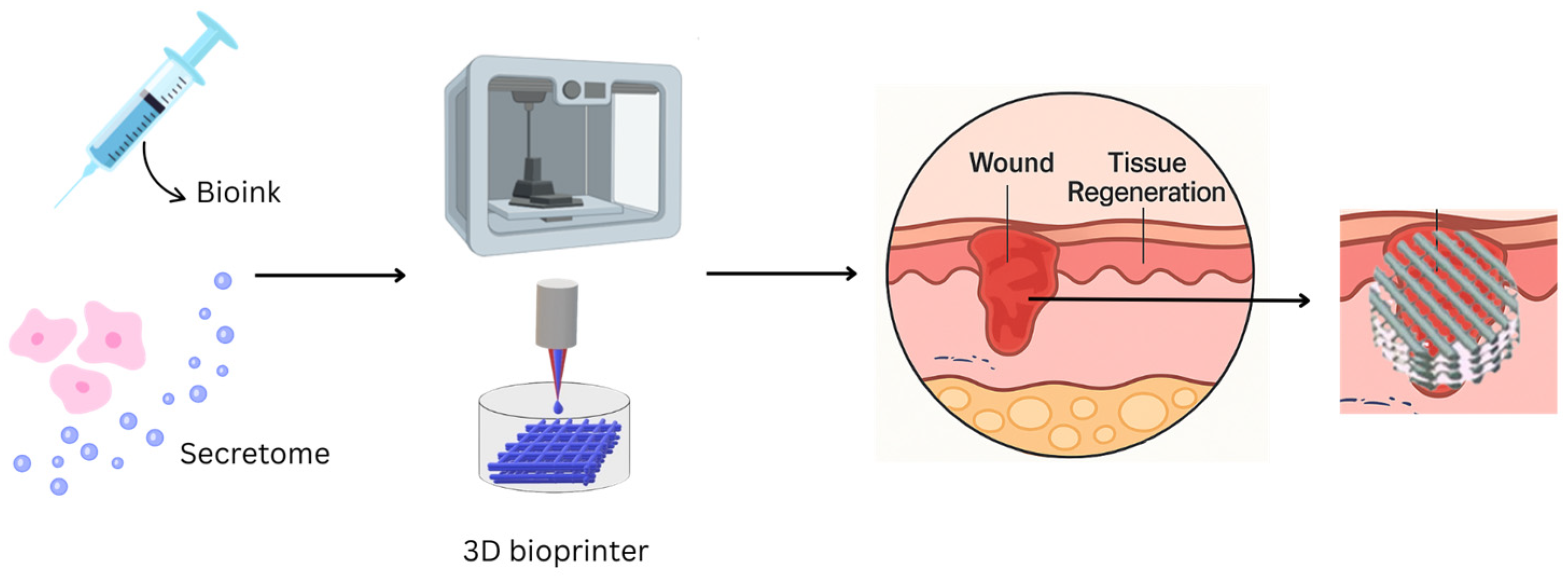
7. Secretome-Loaded Biomaterials
8. 3D Bioprinting Technology
8.1. Integration of Secretome with 3D-Printed Biomaterials
8.2. Preclinical and Clinal Applications of Secretome-Loaded 3D-Printed Biomaterials
8.3. Ideal 3D-Bioprinting Materials Requirements
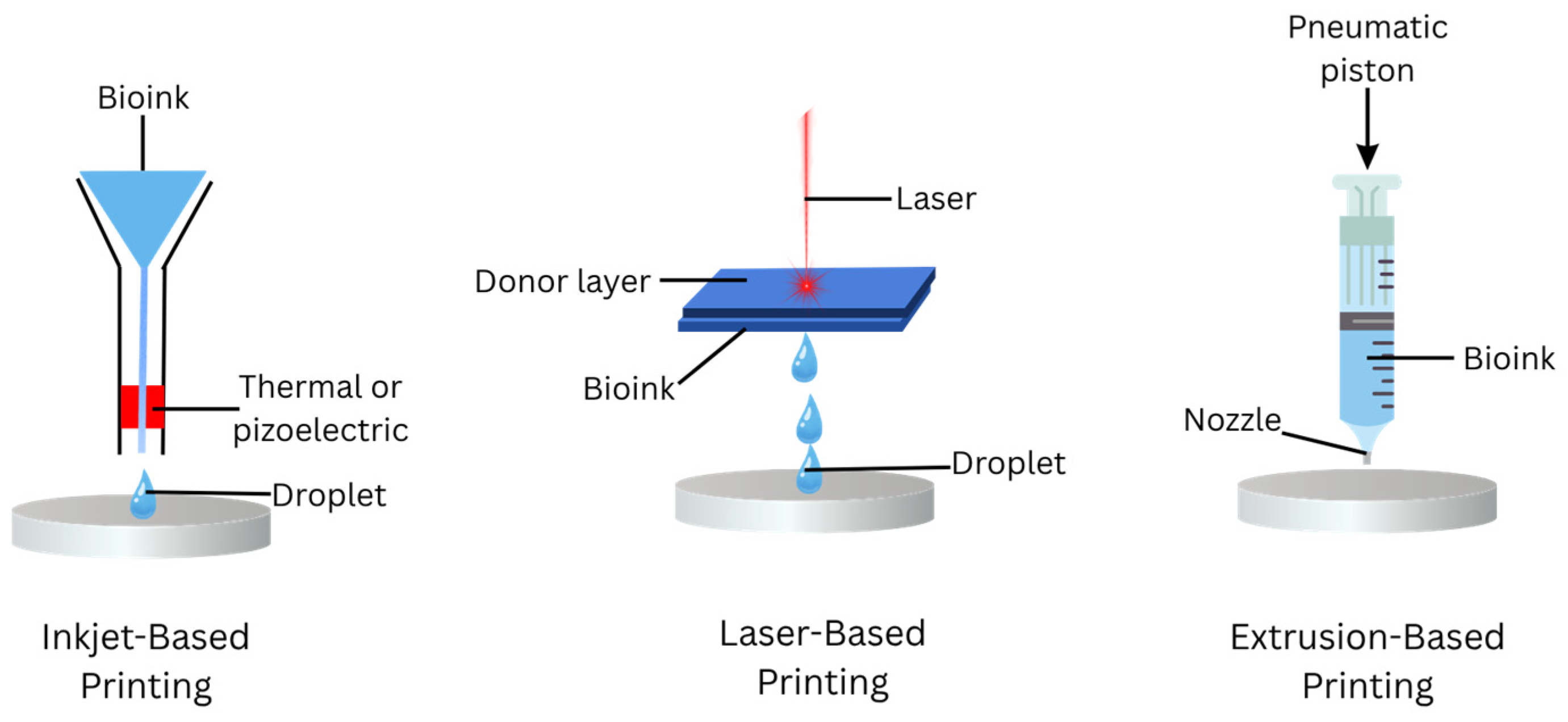
8.4. 3D-Bioprinting Techniques
9. Challenges and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| ECM | Extracellular matrix |
| 3D | Three-dimensional |
| PDGF | Platelets derived growth factor |
| TGF-α | Transforming growth alpha |
| TGF-β | Transforming growth beta |
| FGF | Fibroblast growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| ROS | Reactive oxygen species |
| MMPs | Matrix metalloproteinases |
| FTSG | Full-thickness skin graft |
| STSG | Split-thickness skin graft |
| MSCs | Mesenchymal stem cells |
| PVA | Polyvinyl alcohol |
| GAG | Glycosaminoglycan |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PEG | Poly(ethylene glycol) |
| CM | Conditioned medium |
| Th | T helper cells |
| IL | Interleukin |
| VEGF | Vascular endothelial growth factor |
| EGF | Epidermal growth factor |
| MVEs | Multivesicular endosomes |
| ILVs | Intraluminal vesicles |
| MVBs | Multivesicular bodies |
| RNA | Ribonucleic acid |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| CAD | Computer-aided design |
References
- Lotfollahi, Z. The anatomy, physiology and function of all skin layers and the impact of ageing on the skin. Wound Pract. Res. 2024, 32, 6–10. [Google Scholar] [CrossRef]
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Criolla-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Basilio Heredia, J. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Majerciak, V.; Zheng, Z.-M. HPV16 and HPV18 Genome Structure, Expression, and Post-Transcriptional Regulation. Int. J. Mol. Sci. 2022, 23, 4943, Erratum in Int. J. Mol. Sci. 2022, 23, 7903. [Google Scholar] [CrossRef]
- Sood, R.F.; Gibran, N.S. Mechanisms of burn injury. In Oxford Textbook of Plastic and Reconstructive Surgery; Rawlins, J., Ed.; Oxford University Press: Oxford, UK, 2021; pp. 129–138. [Google Scholar]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Healing, Inflammation, and Fibrosis: Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 144. [Google Scholar] [CrossRef]
- Dutra Alves, N.S.; Reigado, G.R.; Santos, M.; Caldeira, I.D.S.; Hernandes, H.d.S.; Freitas-Marchi, B.L.; Zhivov, E.; Chambergo, F.S.; Nunes, V.A. Advances in regenerative medicine-based approaches for skin regeneration and rejuvenation. Front. Bioeng. Biotechnol. 2025, 13, 1527854. [Google Scholar] [CrossRef]
- Arifka, M.; Wilar, G.; Elamin, K.M.; Wathoni, N. Polymeric Hydrogels as Mesenchymal Stem Cell Secretome Delivery System in Biomedical Applications. Polymers 2022, 14, 1218. [Google Scholar] [CrossRef]
- Deguchi, K.; Zambaiti, E.; De Coppi, P. Regenerative medicine: Current research and perspective in pediatric surgery. Pediatr. Surg. Int. 2023, 39, 167. [Google Scholar] [CrossRef]
- Prado-Yupanqui, J.W.; Ramírez-Orrego, L.; Cortez, D.; Vera-Ponce, V.J.; Chenet, S.M.; Tejedo, J.R.; Tapia-Limonchi, R. The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine—A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1926. [Google Scholar] [CrossRef]
- Ma, H.; Lam, P.K.; Siu, W.S.; Tong, C.S.W.; Lo, K.K.Y.; Koon, C.M.; Wu, X.X.; Li, X.; Cheng, W.; Shum, W.T.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model—A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 2021, 14, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Iwasaki, K.; Peng, Y.; Honda, Y. Mesenchymal Stem Cell Extract Promotes Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 13745. [Google Scholar] [CrossRef] [PubMed]
- Cases-Perera, O.; Blanco-Elices, C.; Chato-Astrain, J.; Miranda-Fernández, C.; Campos, F.; Crespo, P.V.; Sánchez-Montesinos, I.; Alaminos, M.; Martín-Piedra, M.A.; Garzón, I. Development of secretome-based strategies to improve cell culture protocols in tissue engineering. Sci. Rep. 2022, 12, 10003. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.-H.; Kim, B.; Chun, H.-J.; Lim, D.; Han, D.-W. Advances and Innovations of 3D Bioprinting Skin. Biomolecules 2022, 13, 55. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef]
- Sachdeo, R.A.; Khanwelkar, C.; Shete, A. 3D Printing in Wound Healing: Innovations, Applications, and Future Directions. Cureus 2024, 16, e75331. [Google Scholar] [CrossRef]
- Lee, J.; van der Valk, W.H.; Serdy, S.A.; Deakin, C.C.; Kim, J.; Le, A.P.; Koehler, K.R. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat. Protoc. 2022, 17, 1266–1305. [Google Scholar] [CrossRef]
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, X.; Zhang, D.; Yu, Y.; Wang, L.; Zhao, M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics. Cell Prolif. 2024, 57, e13586. [Google Scholar] [CrossRef]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.-A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Davies, K.; Hewitt, C. Biological basis of child health 13: Structure and functions of the skin, and common children’s skin conditions. Nurs. Child. Young People 2022, 34, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Zawani, M.; Zulkiflee, I.; Salleh, A.; Fadilah, N.I.M.; Maarof, M.; Wen, A.P.Y.; Duman, F.; Tabata, Y.; Aziz, I.A.; et al. Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 476. [Google Scholar] [CrossRef]
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The insights of microbes’ roles in wound healing: A comprehensive review. Pharmaceutics 2021, 13, 981. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Fujita, T.; Yuki, T.; Honda, M. The construction of a microenvironment with the vascular network by co-culturing fibroblasts and endothelial cells. Regen. Ther. 2024, 25, 138–146. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Bakal, R.L.; Hatwar, P.R.; Shelke, P.G.; Bagmar, N.A. A review on “Topical gels: An emerging drug delivery system”. GSC Biol. Pharm. Sci. 2024, 28, 285–296. [Google Scholar] [CrossRef]
- Zhang, C.; Pandya, S.; Alessandri Bonetti, M.; Costantino, A.; Egro, F.M. Comparison of split thickness skin graft versus full thickness skin graft for radial forearm flap donor site closure: A systematic review and Meta-analysis. Am. J. Otolaryngol. 2024, 45, 104156. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Salloum, A.; Bazzi, N.; Squires, S.; Chu, T.; Benedetto, P.; Benedetto, A. Comparing the application of various engineered xenografts for skin defects: A systematic review. J. Cosmet. Dermatol. 2023, 22, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- P, A.; M, R.J.; Joy, J.M.; Visnuvinayagam, S.; Remya, S.; Mathew, S. Development of κ-carrageenan-based transparent and absorbent biodegradable films for wound dressing applications. Int. J. Biol. Macromol. 2024, 282, 137084. [Google Scholar] [CrossRef]
- Rashmi; Rimpy; Ahuja, M. Iodine Impregnated Poly(N-Vinylpyrrolidone) Grafted Antibacterial Cotton Gauze for Wound Dressing Applications. Fibers Polym. 2020, 21, 1411–1421. [Google Scholar] [CrossRef]
- Ibrahim, R.; Mndlovu, H.; Kumar, P.; Adeyemi, S.A.; Choonara, Y.E. Cell Secretome Strategies for Controlled Drug Delivery and Wound-Healing Applications. Polymers 2022, 14, 2929. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Wu, S.; Sun, S.; Fu, W.; Yang, Z.; Yao, H.; Zhang, Z. The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines 2024, 12, 743. [Google Scholar] [CrossRef]
- Palomeque Chávez, J.C.; McGrath, M.; Kearney, C.J.; Browne, S.; O’Brien, F.J. Biomaterial scaffold-based gene delivery for the repair of complex wounds: Challenges, progress, and future perspectives. Cell Biomaterials 2025, 1, 100073. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Guo, Z.; Li, Z. Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res. Ther. 2021, 12, 583. [Google Scholar] [CrossRef]
- Seet, W.T.; Maarof, M.; Khairul Anuar, K.; Chua, K.H.; Ahmad Irfan, A.W.; Ng, M.H.; Saim Aminuddin, B.; Idrus Ruszymah, B.H. Shelf-Life Evaluation of Bilayered Human Skin Equivalent, MyDermTM. PLoS ONE 2012, 7, e40978. [Google Scholar] [CrossRef]
- Masri, S.; Maarof, M.; Aziz, I.A.; Idrus, R.; Fauzi, M.B. Performance of hybrid gelatin-PVA bioinks integrated with genipin through extrusion-based 3D bioprinting: An in vitro evaluation using human dermal fibroblasts. Int. J. Bioprinting 2023, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Rossi, A.; Pescara, T.; Gambelli, A.M.; Gaggia, F.; Asthana, A.; Perrier, Q.; Basta, G.; Moretti, M.; Senin, N.; Rossi, F.; et al. Biomaterials for extrusion-based bioprinting and biomedical applications. Front. Bioeng. Biotechnol. 2024, 12, 1393641. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D Bioprinting and Nanotechnology in Tissue Engineering Scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef]
- Watkins, N.N.; Traxel, K.D.; Wilson-Heid, A.E.; Reeve, T.C.; Silva, C.M.; Jeffries, J.R.; Pascall, A.J. Process-structure-property relationships for droplet-on-demand liquid-metal-jetted parts. Addit. Manuf. 2023, 73, 103709. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Stepwise Proliferation and Chondrogenic Differentiation of Mesenchymal Stem Cells in Collagen Sponges under Different Microenvironments. Int. J. Mol. Sci. 2022, 23, 6406. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-printed bioinks for skin regeneration and wound healing: A systematic review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/bioinks and extrusion bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef]
- Masri, S.; Maarof, M.; Mohd, N.F.; Hiraoka, Y.; Tabata, Y.; Fauzi, M.B. Injectable Crosslinked Genipin Hybrid Gelatin–PVA Hydrogels for Future Use as Bioinks in Expediting Cutaneous Healing Capacity: Physicochemical Characterisation and Cytotoxicity Evaluation. Biomedicines 2022, 10, 2651. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Rivas, F.; Erxleben, D.; Smith, I.; Rahbar, E.; DeAngelis, P.L.; Cowman, M.K.; Hall, A.R. Methods for isolating and analyzing physiological hyaluronan: A review. Am. J. Physiol. Physiol. 2022, 322, C674–C687. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Xie, Y.; Zeng, Z.; Chen, J. A new classification method of nanotechnology for design integration in biomaterials. Nanotechnol. Rev. 2020, 9, 820–832. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-based cellulose: Applications, and future perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Niu, M.; Wang, H.; Li, J.; Chen, H.; Li, L.; Yang, H.; Liu, X.; Chen, Z.; Liu, H.; Chen, J. Polyethylene glycol grafted with carboxylated graphene oxide as a novel interface modifier for polylactic acid/graphene nanocomposites. R. Soc. Open Sci. 2020, 7, 192154. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Mahmud, Z.; Gomes, V.G. Superhydrophilic 3D-printed scaffolds using conjugated bioresorbable nanocomposites for enhanced bone regeneration. Chem. Eng. J. 2022, 445, 136639. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Mat Ghani, S.M.; Rabat, N.E.; Ramli, R.A.; Majid, M.F.; Yahya, W.Z.N. Hydrophilic comonomer impact on poly(vinyl alcohol-co-methyl methacrylate) based hydrogel coating. Mater. Today Proc. 2020, 31, 54–59. [Google Scholar] [CrossRef]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D bioprinting of dual-crosslinked nanocellulose hydrogels for tissue engineering applications. J. Mater. Chem. B 2021, 9, 6163–6175. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Yetisgin, A.A.; Demir, E.; Sahin, S.B.; Cetinel, S. Polysaccharide-bioceramic composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2023, 250, 126237. [Google Scholar] [CrossRef]
- Venkata Prathyusha, E.; Gomte, S.S.; Ahmed, H.; Prabakaran, A.; Agrawal, M.; Chella, N.; Alexander, A. Nanostructured polymer composites for bone and tissue regeneration. Int. J. Biol. Macromol. 2025, 284, 137834. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Maarof, M.; Chowdhury, S.R.; Saim, A.; Bt Hj Idrus, R.; Lokanathan, Y. Concentration Dependent Effect of Human Dermal Fibroblast Conditioned Medium (DFCM) from Three Various Origins on Keratinocytes Wound Healing. Int. J. Mol. Sci. 2020, 21, 2929. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15, 1486. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, H.; Tirmizi, Z.; Arellano, J.A.; Egro, F.M.; Ejaz, A. Application of Adipose-Tissue Derived Products for Burn Wound Healing. Pharmaceuticals 2023, 16, 1302. [Google Scholar] [CrossRef] [PubMed]
- Ajit, A.; Ambika Gopalankutty, I. Adipose-derived stem cell secretome as a cell-free product for cutaneous wound healing. 3 Biotech 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Krizanova, O.; Penesova, A.; Sokol, J.; Hokynkova, A.; Samadian, A.; Babula, P. Signaling pathways in cutaneous wound healing. Front. Physiol. 2022, 13, 1030851. [Google Scholar] [CrossRef]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Hanachi, P.; Montaseri, A. Extracellular vesicles of immune cells; immunomodulatory impacts and therapeutic potentials. Clin. Immunol. 2023, 248, 109237. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Abaza, T.; Youssef, Y.A.; Rady, M.; Fahmy, S.A.; Kamel, R.; Hamdi, N.; Efthimiado, E.; Braoudaki, M.; Youness, R.A. Extracellular vesicles: From intracellular trafficking molecules to fully fortified delivery vehicles for cancer therapeutics. Nanoscale Adv. 2025, 7, 934–962. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Firmansyah, Y.; Sidharta, V.M.; Wijaya, L.; Tan, S.T. Unraveling the Significance of Growth Factors (TGF-β, PDGF, KGF, FGF, Pro Collagen, VEGF) in the Dynamic of Wound Healing. Asian J. Med. Health 2024, 22, 49–61. [Google Scholar] [CrossRef]
- Tan, K.; Chang, T.; Lin, X. Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Exp. Dermatol. 2022, 31, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Advani, D.; Farid, N.; Tariq, M.H.; Kohli, N. A systematic review of mesenchymal stem cell secretome: Functional annotations, gene clusters and proteomics analyses for bone formation. Bone 2025, 190, 117269. [Google Scholar] [CrossRef] [PubMed]
- Shoma Suresh, K.; Bhat, S.; Guru, B.R.; Muttigi, M.S.; Seetharam, R.N. A nanocomposite hydrogel delivery system for mesenchymal stromal cell secretome. Stem Cell Res. Ther. 2020, 11, 205. [Google Scholar] [CrossRef]
- Damayanti, R.H.; Rusdiana, T.; Wathoni, N. Mesenchymal stem cell secretome for dermatology application: A review. In Clinical, Cosmetic and Investigational Dermatology; Dove Medical Press Ltd.: London, UK, 2021; Volume 14, pp. 1401–1412. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Amico, G.; Gallo, A.; Iannolo, G.; Cuscino, N.; Vella, S.; Carcione, C.; Nascari, D.; Conaldi, P.G. Small Extracellular Vesicles from Human Fetal Dermal Cells and Their MicroRNA Cargo: KEGG Signaling Pathways Associated with Angiogenesis and Wound Healing. Stem Cells Int. 2020, 2020, 8889379. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Iannolo, G.; Cittadini, E.; Carreca, A.P.; Nascari, D.; Timoneri, F.; Di Bella, M.; Cuscino, N.; Amico, G.; Carcione, C.; et al. Extracellular vesicle-derived micrornas of human Wharton’s Jelly mesenchymal stromal cells may activate endogenous VEGF-A to promote angiogenesis. Int. J. Mol. Sci. 2021, 22, 2045. [Google Scholar] [CrossRef]
- Später, T.; Assunção, M.; Lit, K.K.; Gong, G.; Wang, X.; Chen, Y.-Y.; Rao, Y.; Li, Y.; Yiu, C.H.K.; Laschke, M.W.; et al. Engineering microparticles based on solidified stem cell secretome with an augmented pro-angiogenic factor portfolio for therapeutic angiogenesis. Bioact. Mater. 2022, 17, 526–541. [Google Scholar] [CrossRef]
- Kwon, J.W.; Savitri, C.; An, B.; Yang, S.W.; Park, K. Mesenchymal stem cell-derived secretomes-enriched alginate/extracellular matrix hydrogel patch accelerates skin wound healing. Biomater. Res. 2023, 27, 107. [Google Scholar] [CrossRef]
- Liu, J.; Guo, M.; Chen, C. Nano-bio interactions: A major principle in the dynamic biological processes of nano-assemblies. Adv. Drug Deliv. Rev. 2022, 186, 114318. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Carrillo, D.; Edwards, N.; Arancibia-Altamirano, D.; Otárola, F.; Villarroel, C.; Prieto, C.P.; Villamizar-Sarmiento, M.G.; Sauma, D.; Valenzuela, F.; Lattus, J.; et al. Efficacy of stem cell secretome loaded in hyaluronate sponge for topical treatment of psoriasis. Bioeng. Transl. Med. 2022, 8, e10443. [Google Scholar] [CrossRef] [PubMed]
- Sears, V.; Danaoui, Y.; Ghosh, G. Impact of mesenchymal stem cell-secretome-loaded hydrogel on proliferative and migratory activities of hyperglycemic fibroblasts. Mater. Today Commun. 2021, 27, 102285. [Google Scholar] [CrossRef]
- Doshi, R.B.; Vakil, D.; Molley, T.G.; Islam, M.S.; Kilian, K.A.; Cunningham, C.; Sidhu, K.S. Mesenchymal stem cell-secretome laden photopolymerizable hydrogels for wound healing. J. Biomed. Mater. Res. Part A 2024, 112, 1484–1493. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, H.; Feng, X.; Liang, X.; Wei, D.; Xia, T.; Yang, B.; Yan, L.; Zhao, X.; Liu, H. Mesenchymal stem cell secretome-loaded fibrin glue improves the healing of intestinal anastomosis. Front. Bioeng. Biotechnol. 2023, 11, 1103709. [Google Scholar] [CrossRef]
- Masri, S.; Fauzi, M.B. Current Insight of Printability Quality Improvement Strategies in Natural-Based Bioinks for Skin Regeneration and Wound Healing. Polymers 2021, 13, 1011. [Google Scholar] [CrossRef]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, 9, 2000734. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; You, T.; Zhao, B.; Dong, L.; Huang, C.; Zhou, X.; Xing, M.; Qian, W.; Luo, G. 3D Printing-Based Hydrogel Dressings for Wound Healing. Adv. Sci. 2024, 11, e2404580. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D bioprinting of HAP/collagen scaffold in gelation bath for bone tissue engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef]
- Niu, C.; Wang, L.; Ji, D.; Ren, M.; Ke, D.; Fu, Q.; Zhang, K.; Yang, X. Fabrication of SA/Gel/C scaffold with 3D bioprinting to generate micro-nano porosity structure for skin wound healing: A detailed animal in vivo study. Cell Regen. 2022, 11, 10. [Google Scholar] [CrossRef]
- Noor Azlan, N.A.B.; Vitus, V.; Nor Rashid, N.; Nordin, F.; Tye, G.J.; Wan Kamarul Zaman, W.S. Human mesenchymal stem cell secretomes: Factors affecting profiling and challenges in clinical application. Cell Tissue Res. 2024, 395, 227–250. [Google Scholar] [CrossRef]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Aldana, A.A.; Valente, F.; Dilley, R.; Doyle, B. Development of 3D bioprinted GelMA-alginate hydrogels with tunable mechanical properties. Bioprinting 2021, 21, e00105. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wei, P.; Hao, L.; Zhong, L.; Zhong, K.; Liu, C.; Liu, P.; Feng, Q.; Wang, S.; et al. 3D-printed collagen/silk fibroin/secretome derived from bFGF-pretreated HUCMSCs scaffolds enhanced therapeutic ability in canines traumatic brain injury model. Front. Bioeng. Biotechnol. 2022, 10, 995099. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; D’Amora, U.; Gardin, C.; Leo, S.; Dalla Paola, L.; Tremoli, E.; Giuliani, A.; Calzà, L.; Ronca, A.; Ambrosio, L.; et al. Stem cell-derived small extracellular vesicles embedded into methacrylated hyaluronic acid wound dressings accelerate wound repair in a pressure model of diabetic ulcer. J. Nanobiotechnol. 2023, 21, 469. [Google Scholar] [CrossRef]
- Vrbjar, N.; Vlkovicova, J.; Snurikova, D.; Kalocayova, B.; Zorad, S.; Culafic, T.; Tepavcevic, S.; Tothova, L.; Radosinska, D.; Kollarova, M.; et al. Alterations in Oxidative Stress Markers and Na,K-ATPase Enzyme Properties in Kidney after Fructose Intake and Quercetin Intervention in Rats. Life 2023, 13, 931. [Google Scholar] [CrossRef]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. Mater. Int. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Alaaeddine, N. Study Details|Skin Rejuvenation Using Stem Cell Secretome|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06217627?intr=secretome&rank=3 (accessed on 6 June 2025).
- Nurudhin, A. Study Details|the Effects of Mesenchymal Stem Cell Secretome in Lupus Patients|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05921058?intr=secretome&page=1&rank=5 (accessed on 6 June 2025).
- Cinzia, C.; Giulio, C.P.; Calogero, F.; Giovanna, P.; Salvatore, P.F. WO 2025/061827 A1—Kit for the Reconstitution of a Cell-Free Biomedical Device for Use in Regenerative Medicine, Biomedical Device Thus Reconstituted and Related Synthesis Process|Full-Text. Available online: https://www.lens.org/lens/patent/155-790-469-861-562/fulltext (accessed on 6 June 2025).
- Jain, S.; Fuoco, T.; Yassin, M.A.; Mustafa, K.; Finne-Wistrand, A. Printability and Critical Insight into Polymer Properties during Direct-Extrusion Based 3D Printing of Medical Grade Polylactide and Copolyesters. Biomacromolecules 2020, 21, 388–396. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Iberite, F.; Badiola-Mateos, M.; Loggini, S.; Paci, C.; Ruspi, J.; Iachetta, D.; Mannini, A.; Gruppioni, E.; Ricotti, L. 3D bioprinting of thermosensitive inks based on gelatin, hyaluronic acid, and fibrinogen: Reproducibility and role of printing parameters. Bioprinting 2024, 39, e00338. [Google Scholar] [CrossRef]
- Murali, R.; Balasubramanian, R.V.; V.S., H.; Kasoju, N.; N.S., R.; Kartha, R.S.; A., P.; A., S.; V., A.K.; Nair, R.P.; et al. Unravelling the wound healing efficiency of 3D bioprinted alginate-gelatin-diethylaminoethyl cellulose-fibrinogen based skin construct in a rat’s full thickness wound model. Int. J. Biol. Macromol. 2025, 305, 140816. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Guazzelli, L.; Soldani, G.; Losi, P. Fibrinogen-Based Bioink for Application in Skin Equivalent 3D Bioprinting. J. Funct. Biomater. 2023, 14, 459. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements, 5th ed. International Organization for Standardization: Geneva, Switzerland, 2015.

| Function | Structure | Description | References |
|---|---|---|---|
| Protection | Epidermis | Serves as a physical barrier, protecting against environmental threats such as pathogens, chemicals, and physical injuries | [3,23] |
| Sensation | Dermis | Enables the perception of touch, pressure, temperature, and pain, facilitating interaction with the environment | [24,25] |
| Thermoregulation | Dermis and hypodermis | Contributes to body temperature regulation through mechanisms such as sweating and modulation of blood flow | [24,25,26,27,28] |
| Excretion | Dermis | Aids in the excretion of metabolic waste products through sweat glands | [26] |
| Immune function | Epidermis and dermis | Serves as an immunological barrier detecting and responding to pathogens | [3,29] |
| Fluid balance | Epidermis | Prevents excessive water loss, thus maintaining proper hydration and fluid balance within the body | [24] |
| Phases | Key Events | References |
|---|---|---|
| Hemostasis phase | Platelets are activated, and the collagen fibers then draw the platelets to form blood clots which are made up of fibronectin, fibrin, vitronectin, and thrombospondin | [4,8] |
| Inflammation phase |
| [4,31] |
| Proliferative phase |
| [4,32] |
| Remodeling phase |
| [33] |
| Treatments | Types | Description | Examples | References |
|---|---|---|---|---|
| Full-thickness Skin Graft (FTSG) | Autograft | Involves transplanting both the epidermis and entire dermis layers of the skin | Abdomen | [36] |
| Split-thickness Skin Graft (STSG) | Autograft | Skin taken from the patient’s own body and can be used for large wounds, burns, and ulcers | Thigh skin grafts | [37] |
| Allografts | Skin obtained from a human donor | Cryopreserved cadaveric skin | [38] | |
| Xenografts | Skin derived from an animal source used as a temporary biological dressing | Porcine skin grafts | [38] | |
| Wound Dressings | Hydrocolloid | Forms a gel upon contact with wound exudate, maintaining moisture. Best for wounds with minimal exudate | Duoderm | [39] |
| Hydrogel | Provides moisture to dry wounds. Best for wounds with minimal exudate | Intrasite Gel, Aquaform | [40] | |
| Transparent Film | Thin, adhesive, and waterproof dressing that allows wound visualization while preventing contamination | Tegaderm | [41] | |
| Antimicrobial Dressing | Contains agents like iodine to reduce bacterial load and prevent infection | Iodoflex | [42] | |
| Foam Dressing | Absorbs moderate to heavy exudate, maintains a moist environment, and provides cushioning | Mepilex | [37] |
| Biomaterials | Types | Source | Key Properties | Limitations | Applications | References |
|---|---|---|---|---|---|---|
| Natural biomaterials | Collagen | Human and animal ECM (bovine, pig, mouse, marine) | Biocompatible and can be used in 3D-printed scaffolds for bone or tendon repair | Lack mechanical strength and requires modifications | Widely used in tissue engineering. | [51,52,53] |
| Gelatin | Derived from partial hydrolysis of collagen | Biodegradable, biocompatible, and low immunogenicit | Poor viscosity and mechanical strength at high temperatures | Skin repair, tissue engineering, GelMA for cell encapsulation | [53,54,55] | |
| Silk | Extracted from silkworm cocoons | Biocompatible, promotes wound healing phases, antibacterial with nanodiamond | Less effective against Gram-positive bacteria | Wound dressings and tissue engineering | [50,56,57] | |
| Hyaluronic acid | Found in ECM of connective and epithelial tissues | Enhances cell adhesion, proliferation, differentiation, and water soluble | Immunoevasive in pathogens | Wound healing, 3D bioprinting, and viscosity enhancer | [54,58,59,60] | |
| Alginate | Extracted from brown algae | Biocompatible, biodegradable, and supports cell growth | Poor cell adhesion | Wound healing and tissue regeneration | [61] | |
| Chitosan | Crustacean shells | Antibacterial, modifiable, bioadhesive, and enhances drug delivery | Limited mechanical strength | Hydrogels, nanofibers, and drug delivery | [56,62,63] | |
| Synthetic biomaterials | PLA | Plant-based from lactic acid monomers | Biodegradable thermoplastic and supports bone regeneration | Weak mechanical properties | Bone scaffolds and 3D printing with additives | [62,64,65] |
| PVA | Synthetic polymer | Biocompatible, non-toxic and water soluble | Poor haemostasis, antibacterial activity, and hydrophilicity | Hemostatic dressings and wound healing with modifications | [66,67] | |
| Polyglycolic acid (PGA) | Synthetic polymer | Fast degradation and high mechanical strength | Produced acidic degradation products | Tissue engineering | [68] | |
| PEG | Synthetic polymer | Tunable, cell-encapsulating, and non-toxic | Requires modification to optimize its performance | Scaffolds and diabetic wound healing | [54,56,69] | |
| Composite biomaterials | Polysaccharide-bioceramic composites | Natural polysaccharides with ceramic phases | Enhanced bioactivity, osteoconductivity, and mechanical reinforcement | Brittleness and complex fabrication | Bone tissue engineering and scaffold reinforcement | [70] |
| Nanostructured polymer composites | Polymers with nanoparticles or nanofillers | Improved mechanical, thermal, and biological properties | Cost and scale-up challenges | Advanced wound healing, scaffold fabrication, and drug delivery | [71] |
| Growth Factors | Role in Wound Repair | References |
|---|---|---|
| IL-1, IL-6, IL-8 | Promotes angiogenesis of wounds and regeneration of epithelium | [79,80] |
| PDGF | Increased fibroblast and endothelial cell proliferation, migration, and invasion ability | [79] |
| TGF | Promoted ECM remodeling, ultimately promotes wound healing and reduces scar formation | [80] |
| bFGF | Migration and proliferation of fibroblasts | [81] |
| VEGF | Proliferation and migration of endothelial cells, acceleration of wound angiogenesis, promotes migration of fibroblasts | [79] |
| EGF | Promotes proliferation of fibroblasts | [79] |
| Study | Biomaterials | Model | Findings | Limitations | References |
|---|---|---|---|---|---|
| Hyaluronic Acid Sponge with MSC Secretome | Hyaluronic acid sponge | In vivo (psoriasis skin model) | Porous sponge enables sustained release of MSC secretome, promoted 50% increase in keratinocyte proliferation, angiogenesis, and inflammation needed for dermal wound repair | Clinical efficacy not yet validated, limited to psoriasis model | [97] |
| GelMA-PEGDA Hydrogels with MSC Secretome | GelMA and poly(ethylene glycol) diacrylate (PEGDA) hybrid hydrogels | In vitro (hyperglycemic human dermal fibroblasts) | Restored proliferation and migration of hyperglycemic fibroblasts to more than 85% wound closure, potential for diabetic wound healing | In vivo efficacy and long-term effects not assessed | [98] |
| Alginate/ECM Hydrogel Patch with hMSC Secretome | Alginate combined with decellularized ECM | In vivo (rat skin wound model) | Accelerated wound closure rate of 92% by day 14, improved angiogenesis, and increased in collagen deposition | Limited to skin wound model | [94] |
| Photopolymerizable GelMA Hydrogels with hADSC Secretome | GelMA hydrogels | In vitro (scratch assay, tube formation) | Enhanced fibroblast migration by 65% and angiogenesis, tunable release of secretome components | Requires in vivo validation, potential variability in hydrogel formulations | [99] |
| Fibrin Glue with MSC secretome | Fibrin-based hydrogels | In vivo (rat intestinal anastomosis model) | Improved anastomotic healing, increased granulation tissue and collagen deposition, and promoted 1.8 fold angiogenesis | Focused on intestinal model, broader applications need exploration | [100] |
| Bioprinting Techniques | Description | Advantages | Disadvantages | Examples of Biomaterials | References |
|---|---|---|---|---|---|
| Extrusion-Based Printing | Utilizes a fluid-dispensing mechanism and robotic system to extrude bioink as continuous cylindrical filaments |
| Increased mechanical stress reduces cell viability | GelMA-alginate and gelatin-fibrin | [108,117,118] |
| Inkjet-Based Printing | Deposits bioink onto a substrate either as a continuous flow or discrete droplets using electronically controlled ink cartridges |
| Limited to low viscosity bioinks that can be ejected through a nozzle | Collagen, fibrinogen-alginate, hyaluronic acid | [53,119,120] |
| Laser-Based Bioprinting | Uses laser-induced forward transfer to deposit bioink without physical contact, minimizing cellular stress |
| Expensive and complex control systems limit accessibility | Collagen-gelatin, alginat-MSC secretome | [53,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanachot, T.; Lokanathan, Y.; Fauzi, M.B.; Maarof, M. Enhancing Wound Healing Through Secretome-Loaded 3D-Printed Biomaterials. Gels 2025, 11, 476. https://doi.org/10.3390/gels11070476
Rattanachot T, Lokanathan Y, Fauzi MB, Maarof M. Enhancing Wound Healing Through Secretome-Loaded 3D-Printed Biomaterials. Gels. 2025; 11(7):476. https://doi.org/10.3390/gels11070476
Chicago/Turabian StyleRattanachot, Tithteeya, Yogeswaran Lokanathan, Mh Busra Fauzi, and Manira Maarof. 2025. "Enhancing Wound Healing Through Secretome-Loaded 3D-Printed Biomaterials" Gels 11, no. 7: 476. https://doi.org/10.3390/gels11070476
APA StyleRattanachot, T., Lokanathan, Y., Fauzi, M. B., & Maarof, M. (2025). Enhancing Wound Healing Through Secretome-Loaded 3D-Printed Biomaterials. Gels, 11(7), 476. https://doi.org/10.3390/gels11070476







