Metal–Organic Frameworks (MOF)-Derived Gel Electrolyte via UV Cross-Linking for High-Performance Lithium Metal Batteries
Abstract
1. Introduction
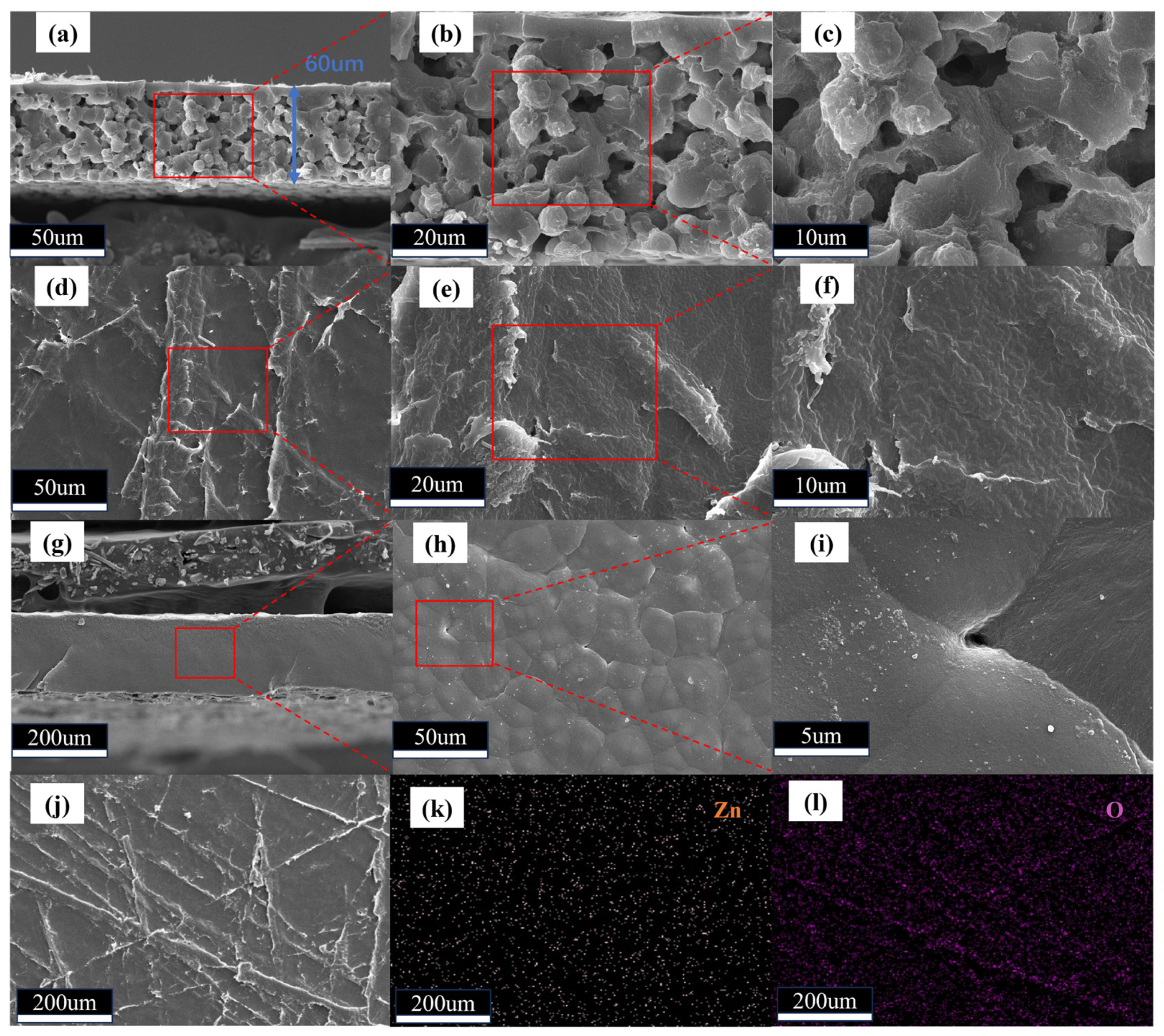
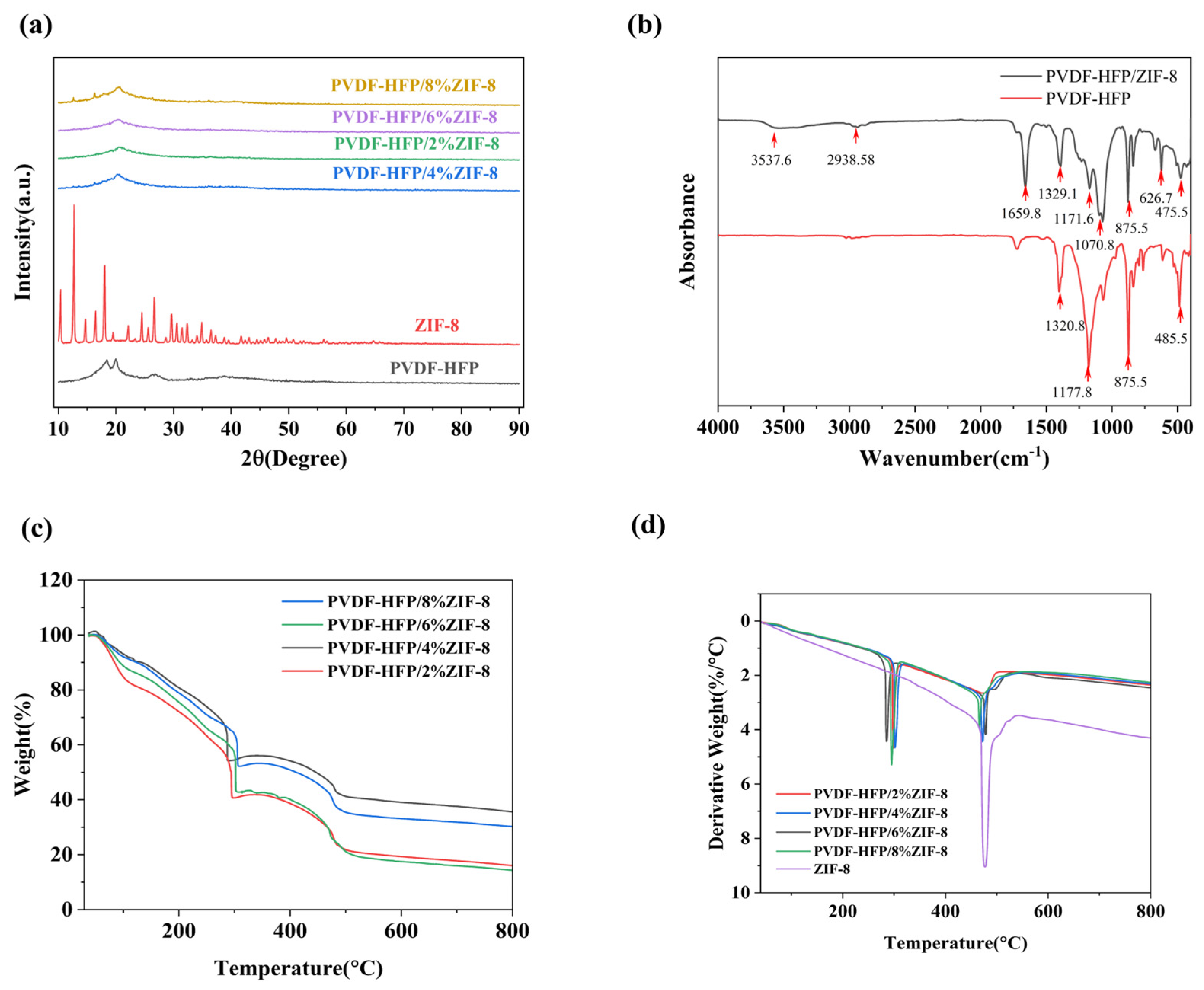
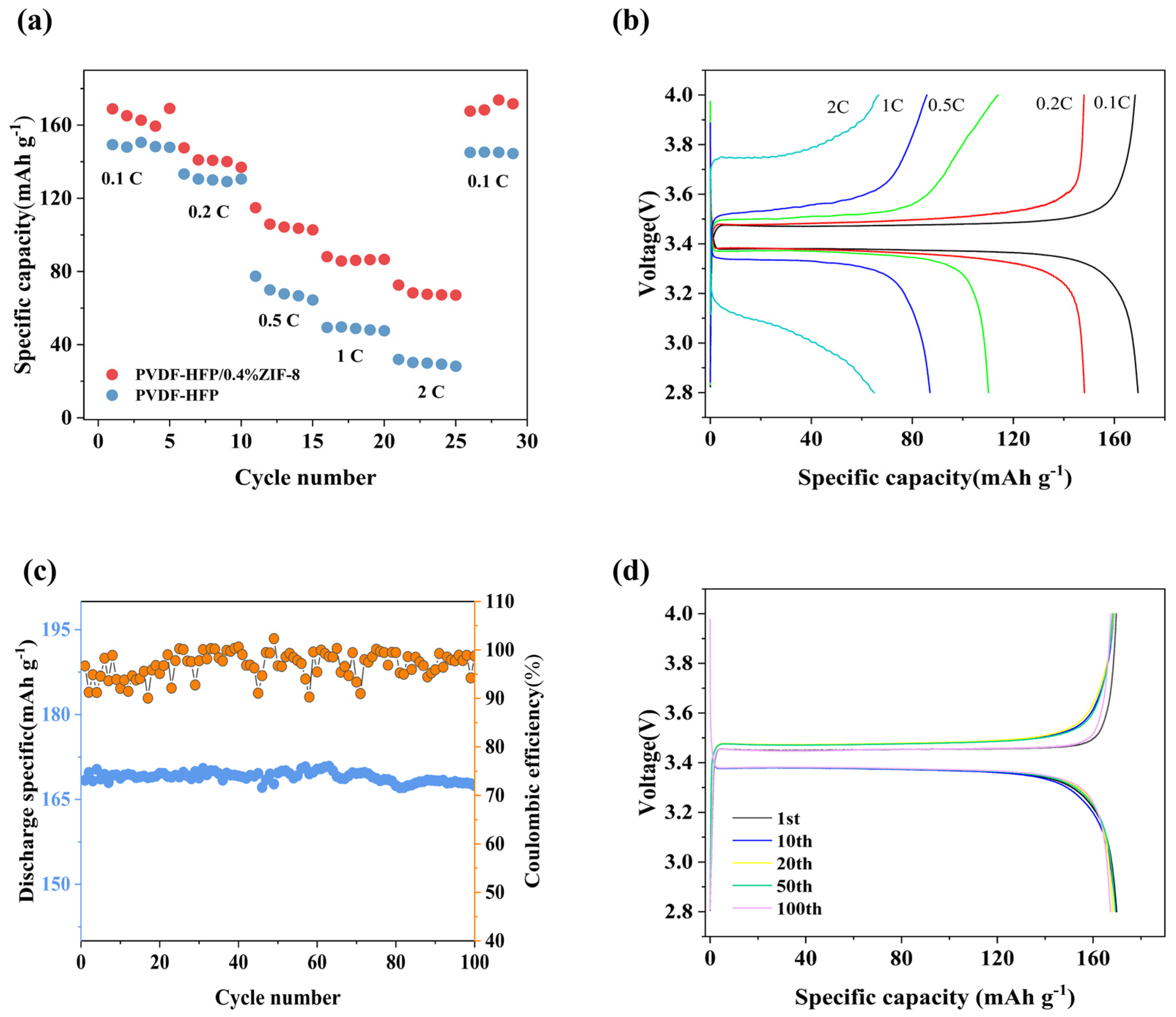
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
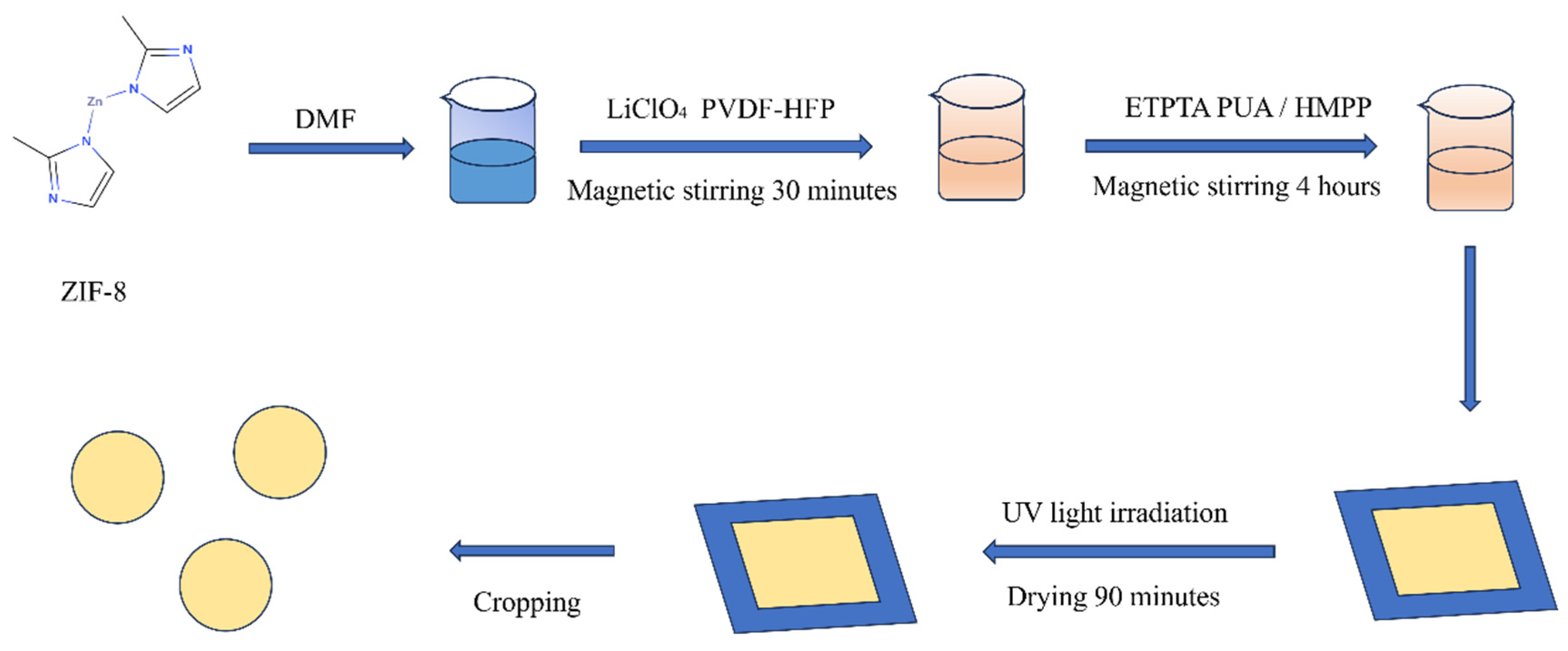
4.2. Preparation of Electrolyte Membrane
4.3. Battery Assembly
4.4. Material Characterization
4.5. Electrochemical Testing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamada, A. Hidden Negative Issues and Possible Solutions for Advancing the Development of High-Energy-Density in Lithium Batteries: A Review. Adv. Sci. 2024, 11, 2401739. [Google Scholar] [CrossRef] [PubMed]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mat. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Sun, N.; Liu, Q.; Cao, Y.; Lou, S.; Ge, M.; Xiao, X.; Lee, W.K.; Gao, Y.; Yin, G.; Wang, J.; et al. Anisotropically Electrochemical–Mechanical Evolution in Solid-State Batteries and Interfacial Tailored Strategy. Angew. Chem. Int. Ed. 2019, 58, 18647–18653. [Google Scholar] [CrossRef]
- Ma, J.; Chen, B.; Wang, L.; Cui, G. Progress and prospect on failure mechanisms of solid-state lithium batteries. J. Power Sources 2018, 392, 94–115. [Google Scholar] [CrossRef]
- Hao, F.; Han, F.; Liang, Y.; Wang, C.; Yao, Y. Architectural design and fabrication approaches for solid-state batteries. MRS Bull. 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Stegmaier, S.; Voss, J.; Reuter, K.; Luntz, A.C. Li+ Defects in a Solid-State Li Ion Battery: Theoretical Insights with a Li3OCl Electrolyte. Chem. Mat. 2017, 29, 4330–4340. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial Soft–Rigid Protective Layer for Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2018, 28, 1870049. [Google Scholar] [CrossRef]
- Ali, S.; Tan, C.; Waqas, M.; Lv, W.; Wei, Z.; Wu, S.; Boateng, B.; Liu, J.; Ahmed, J.; Xiong, J. Highly Efficient PVDF-HFP/Colloidal Alumina Composite Separator for High-Temperature Lithium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1701147. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Effect of polyaniline (PANI) on Poly(vinylidene fluoride-co-hexaflouro propylene) (PVDF-co-HFP) polymer electrolyte membrane prepared by breath figure method. Polym. Test. 2017, 60, 124–131. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2021, 64, 62–84. [Google Scholar] [CrossRef]
- Zhong, X.; Han, J.; Chen, L.; Liu, W.; Qin, W. Binding mechanisms of PVDF in lithium ion batteries. Appl. Surf. Sci. 2021, 553, 149564. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanba, M.; Matsuda, H.; Tsunemi, K.; Mizoguchi, K.; Tsuchida, E.; Shinohara, I. High lithium ionic conductivity of polymeric solid electrolytes. Die Makromol. Chem. Rapid Commun. 1981, 2, 741–744. [Google Scholar] [CrossRef]
- Zuofeng, C.; Yanxia, J.; Quanchao, Z.; Quanfeng, D.; Ye, W.; Shigang, S. Preparation and characterization of a novel composite microporous polymer electrolyte for Li-ion batteries. Sci. Bull. 2005, 50, 6. [Google Scholar]
- Gonçalves, R.; Miranda, D.; Almeida, A.M.; Silva, M.M.; Meseguer-Dueñas, J.M.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Costa, C.M. Solid polymer electrolytes based on lithium bis(trifluoromethanesulfonyl)imide/poly(vinylidene fluoride -co-hexafluoropropylene) for safer rechargeable lithium-ion batteries. Sustain. Mater. Technol. 2019, 21, e00104. [Google Scholar] [CrossRef]
- Liang, Y.F.; Deng, S.J.; Xia, Y.; Wang, X.L.; Xia, X.H.; Wu, J.B.; Gu, C.D.; Tu, J.P. A superior composite gel polymer electrolyte of Li7La3Zr2O12- poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for rechargeable solid-state lithium ion batteries. Mater. Res. Bull. 2018, 102, 412–417. [Google Scholar] [CrossRef]
- Sohn, J.Y.; Im, J.S.; Shin, J.; Nho, Y.C. PVDF-HFP/PMMA-coated PE separator for lithium ion battery. J. Solid State Electrochem. 2012, 16, 551–556. [Google Scholar] [CrossRef]
- Farina, M.; Duff, B.B.; Tealdi, C.; Pugliese, A.; Blanc, F.; Quartarone, E. Li+ Dynamics of Liquid Electrolytes Nanoconfined in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 53986–53995. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Wang, S.M.; Mu, X.T.; Liu, H.R.; Zheng, S.T.; Yang, Q.Y. Pore-Structure Control in Metal-Organic Frameworks (MOFs) for Capture of the Greenhouse Gas SF. Angew. Chem. 2022, 61, e202207066. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Review-Emerging Trends in the Design of Electrolytes for Lithium and Post-Lithium Batteries. J. Electrochem. Soc. 2020, 167, 50508. [Google Scholar] [CrossRef]
- Lv, X.L.; Yuan, S.; Xie, L.H.; Darke, H.F.; Chen, Y.; He, T.; Dong, C.; Wang, B.; Zhang, Y.Z.; Li, J.R. Ligand Rigidification for Enhancing the Stability of Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 10283–10293. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xue, J.; Xie, L.; Chen, H.; Deng, Z.; Yin, W. A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries. Gels 2024, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, P.; Fan, L.Z. Asymmetric Polymer Electrolyte Constructed by Metal-Organic Framework for Solid-State, Dendrite-Free Lithium Metal Battery. Adv. Funct. Mater. 2020, 31, 2007198. [Google Scholar] [CrossRef]
- Sun, C.C.; Yusuf, A.; Li, S.W.; Qi, X.L.; Wang, D.Y. Metal Organic Frameworks Enabled Rational Design of Multifunctional PEO-Based Solid Polymer Electrolytes. Chem. Eng. J. 2021, 414, 128702. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, X.H.; Hiscox, W.; Liu, J.; Zamora, J.; Li, X.; Su, M.; Zhang, Q.; Guo, X.; Mccloy, J. Towards High-Performance Metal-Organic-Framework-Based Quasi-Solid-State Electrolytes: Tunable Structures and Electrochemical Properties. Adv. Mater. 2023, 35, e2211841. [Google Scholar] [CrossRef]
- Çakmakçı, E.; Uğur, M.H.; Güngör, A. UV-Cured polypropylene mesh-reinforced composite polymer electrolyte membranes. E-Polymers 2015, 15, 103–110. [Google Scholar] [CrossRef]
- Kim, K.; Chae, W.; Kim, J.; Kim, C.; Earmme, T. Gel Polymer Electrolytes for Lithium-Ion Batteries Enabled by Photo Crosslinked Polymer Network. Gels 2023, 9, 975. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Zhang, Z.; Liu, H.; Ling, S.D.; Liu, X.; Lian, C.; Xu, J. Anisotropic ZnS Nanoclusters/Ordered Macro-Microporous Carbon Superstructure for Fibrous Supercapacitor toward Commercial-Level Energy Density. Adv. Funct. Mater. 2023, 33, 2300329. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Wang, Z.; Sun, S.P.; Cui, Z.; Tavajohi, N. Poly(vinylidene fluoride-co-hexafluoro propylene) membranes prepared via thermally induced phase separation and application in direct contact membrane distillation. Front. Chem. Sci. Eng. 2022, 16, 720–730. [Google Scholar] [CrossRef]
- Zeng, Q.; Shi, L.; Wang, J.; Zha, X.; Yang, W.; Yang, Y. High-performance MOF-derived polymer electrolytes with modified ionic transport for solid-state lithium metal batteries. J. Energy Storage 2025, 110, 115314. [Google Scholar] [CrossRef]
- Huang, C.; Li, H.; Teng, Z.; Luo, Y.; Chen, W. MOF-modified dendrite-free gel polymer electrolyte for zinc-ion batteries. RSC Adv. 2024, 14, 15337–15346. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chang, X.; Chen, X.; Liu, X.; Jia, M.; Bi, Z.; Guo, X. Hierarchical-structural design of ultrathin composite electrolytes for high-stability solid-state lithium batteries: From “polymer-in-salt” to “polymer-in-ceramic”. Nano Energy 2025, 135, 110644. [Google Scholar] [CrossRef]
- Xu, B.; Mei, Y.; Xiao, Z.; Kang, Z.; Wang, R.; Sun, D. Monitoring thermally induced structural deformation and framework decomposition of ZIF-8 through in situ temperature dependent measurements. Phys. Chem. Chem. Phys. 2017, 19, 27178–27183. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S. Study on the Structure Activity Relationship of ZIF-8 Synthesis and Thermal Stability. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1317–1322. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Q.; Du, G.; Xu, B.; Wang, S.; Chen, Z.; Wang, L.; Huang, W.; Pang, H. Stabilizing Solid-state Lithium Metal Batteries through In Situ Generated Janus-heterarchical LiF-rich SEI in Ionic Liquid Confined 3D MOF/Polymer Membranes. Angew. Chem. Int. Ed. 2023, 62, e202304947. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Mo, Y. Lithium crystallization at solid interfaces. Nat. Commun. 2023, 14, 2986. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Nolan, A.M.; Mo, Y. Interfacial Atomistic Mechanisms of Lithium Metal Stripping and Plating in Solid-State Batteries. Adv. Mater. 2021, 33, e2008081. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Chen, P.; Li, Z.; Chen, A.; Guan, J.; Wang, A.; Zhang, L. Modified MOF-Based Composite All-Solid-State Polymer Electrolyte with Improved Comprehensive Performance for Dendrite-Free Li-Ion Batteries. Macromol. Chem. Phys. 2021, 223, 2100325. [Google Scholar] [CrossRef]
- Zugmann, S.; Fleischmann, M.; Amereller, M.; Gschwind, R.M.; Wiemhoefer, H.D.; Gores, H.J. Measurement of transference numbers for lithium ion electrolytes via four different methods, a comparative study. Electrochim. Acta 2011, 56, 3926–3933. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, N.; Lan, L.; Hun, Q.; Wei, J.; Liang, X.; Guo, Y. Metal–Organic Frameworks (MOF)-Derived Gel Electrolyte via UV Cross-Linking for High-Performance Lithium Metal Batteries. Gels 2025, 11, 409. https://doi.org/10.3390/gels11060409
Mao N, Lan L, Hun Q, Wei J, Liang X, Guo Y. Metal–Organic Frameworks (MOF)-Derived Gel Electrolyte via UV Cross-Linking for High-Performance Lithium Metal Batteries. Gels. 2025; 11(6):409. https://doi.org/10.3390/gels11060409
Chicago/Turabian StyleMao, Naiyao, Lingxiao Lan, Qiankun Hun, Jianghua Wei, Xinghua Liang, and Yifeng Guo. 2025. "Metal–Organic Frameworks (MOF)-Derived Gel Electrolyte via UV Cross-Linking for High-Performance Lithium Metal Batteries" Gels 11, no. 6: 409. https://doi.org/10.3390/gels11060409
APA StyleMao, N., Lan, L., Hun, Q., Wei, J., Liang, X., & Guo, Y. (2025). Metal–Organic Frameworks (MOF)-Derived Gel Electrolyte via UV Cross-Linking for High-Performance Lithium Metal Batteries. Gels, 11(6), 409. https://doi.org/10.3390/gels11060409





