Advances in Carbon-Based Aerogels for CO2 Capture: Fundamental Design Strategies and Technological Progress
Abstract
1. Introduction
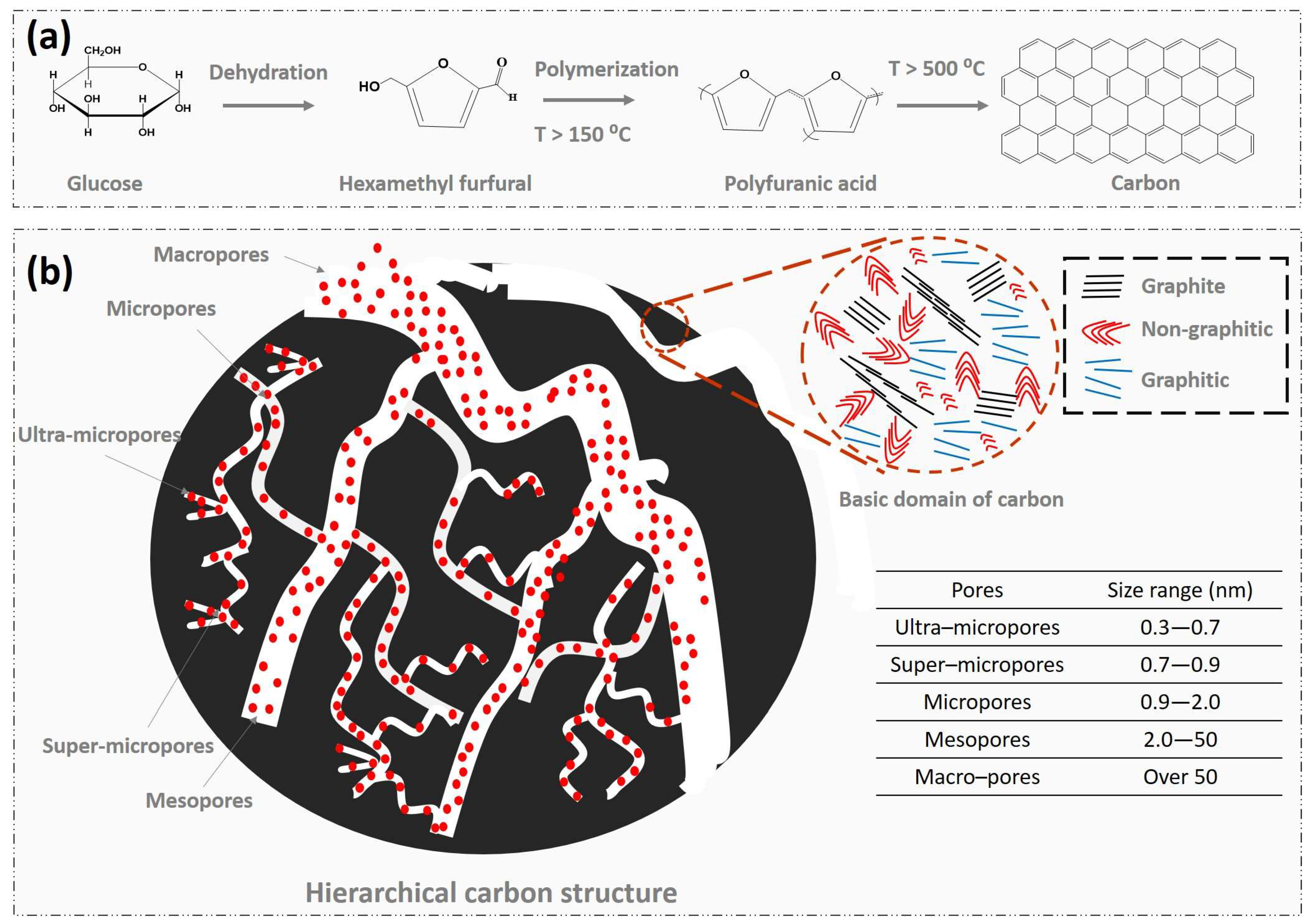
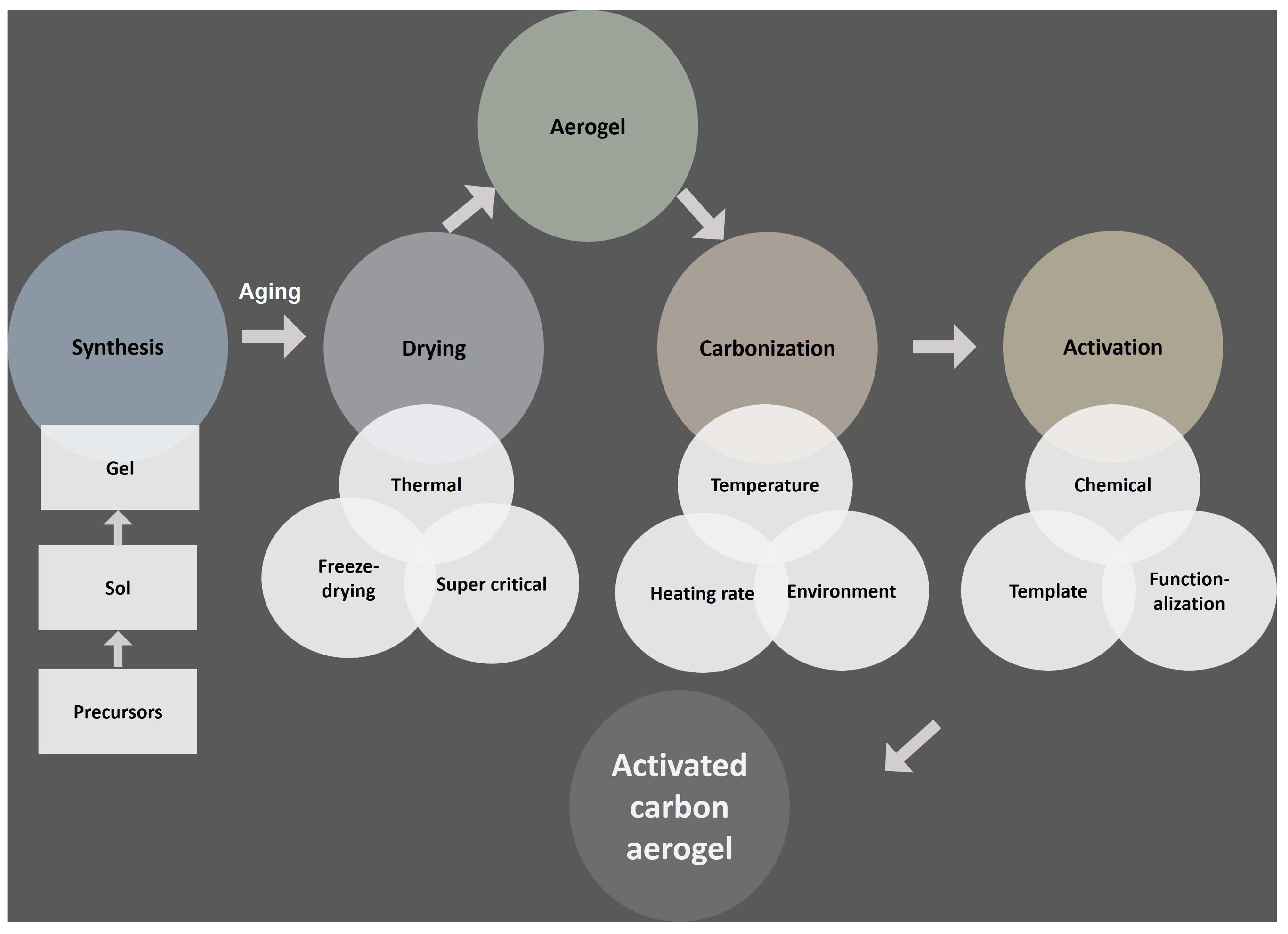
2. Fundamentals of Carbon Aerogels
3. Carbon Aerogels Design Strategies
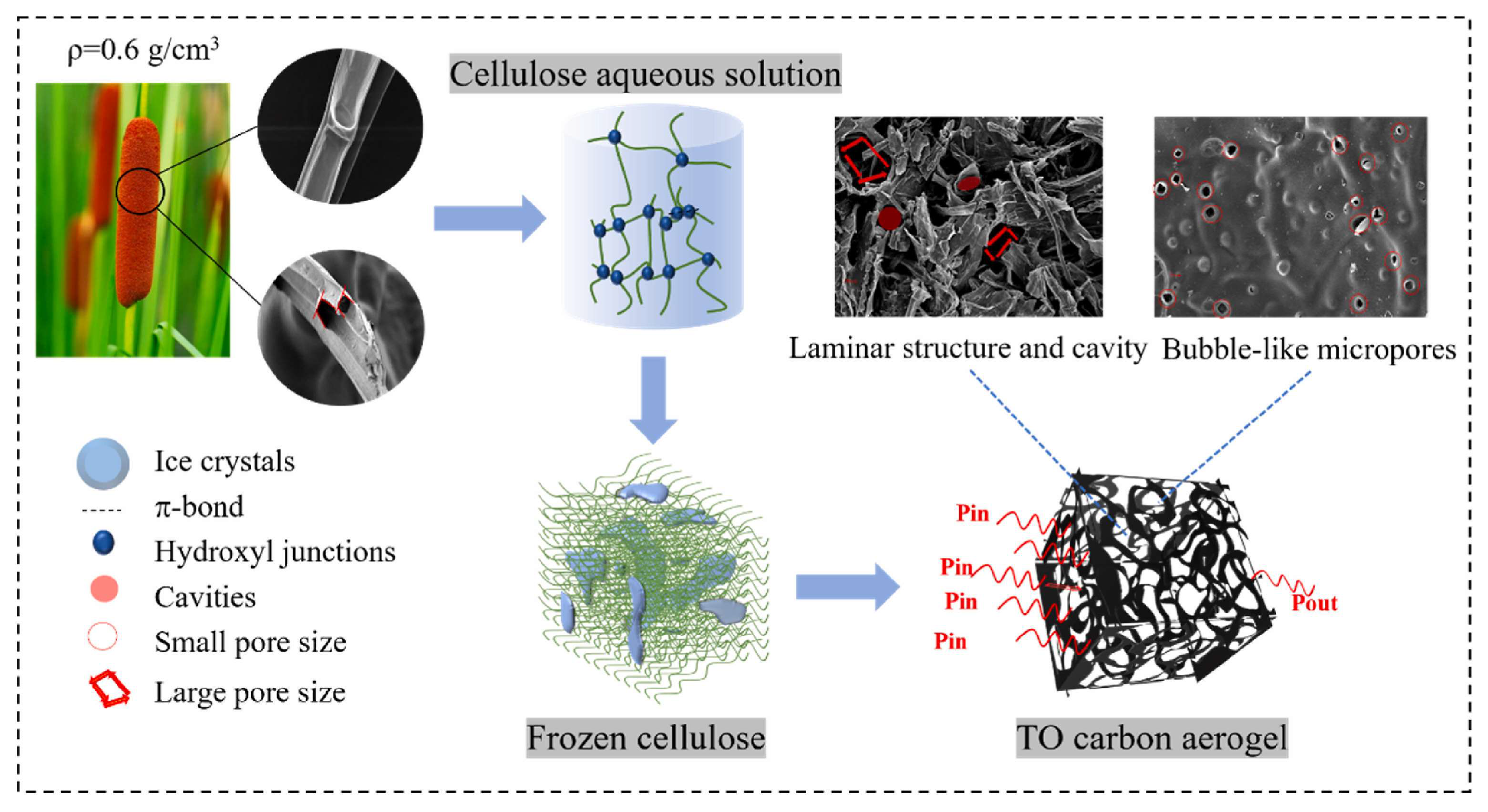
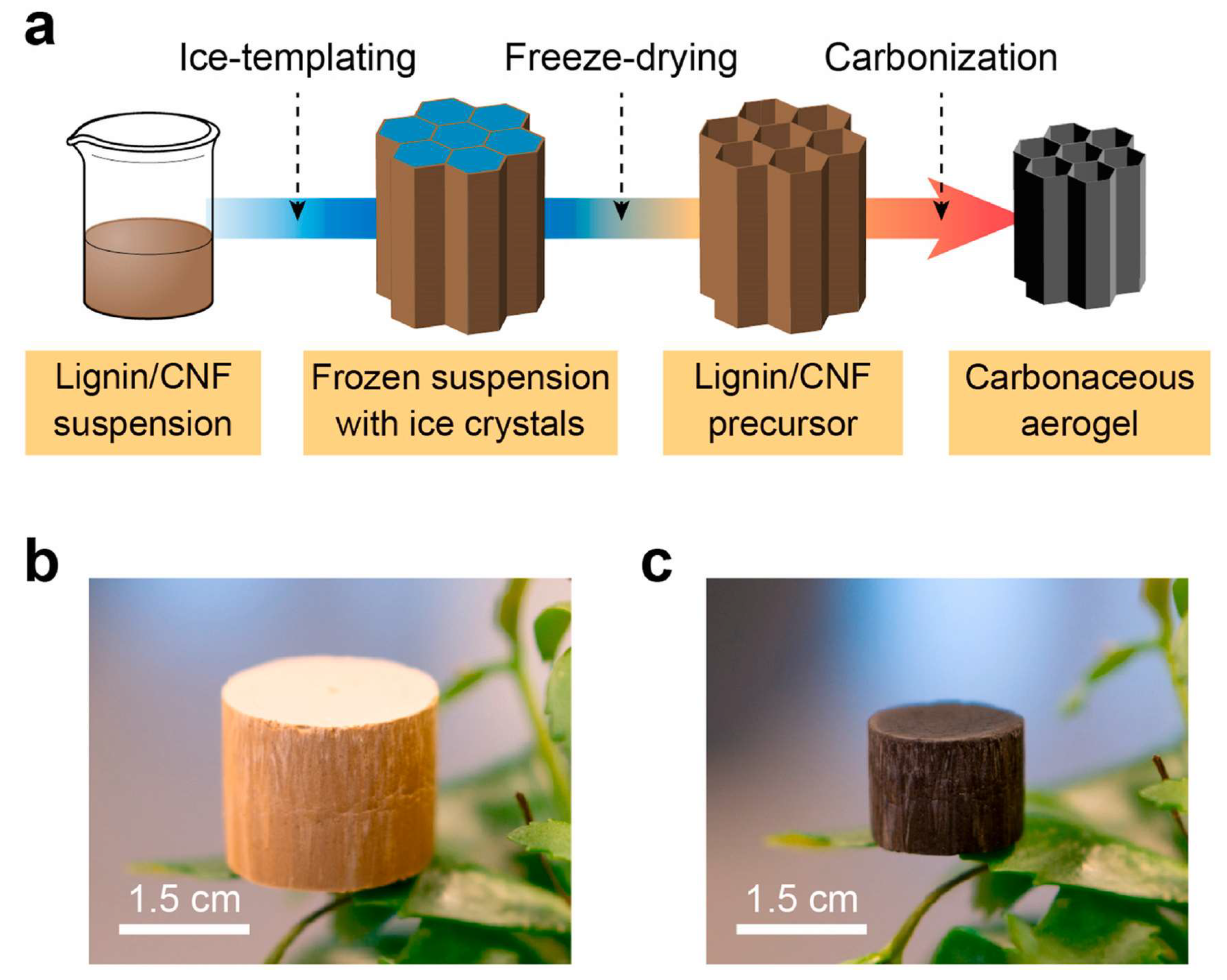
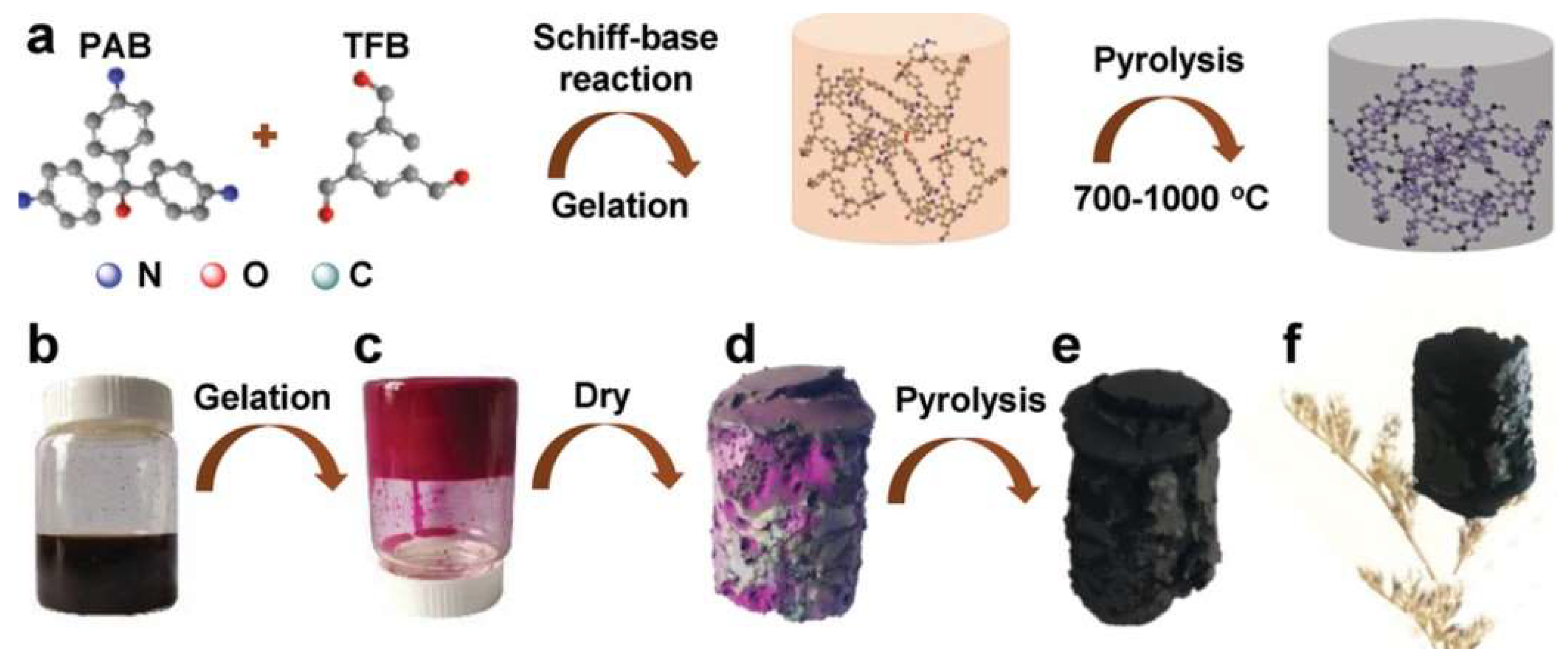
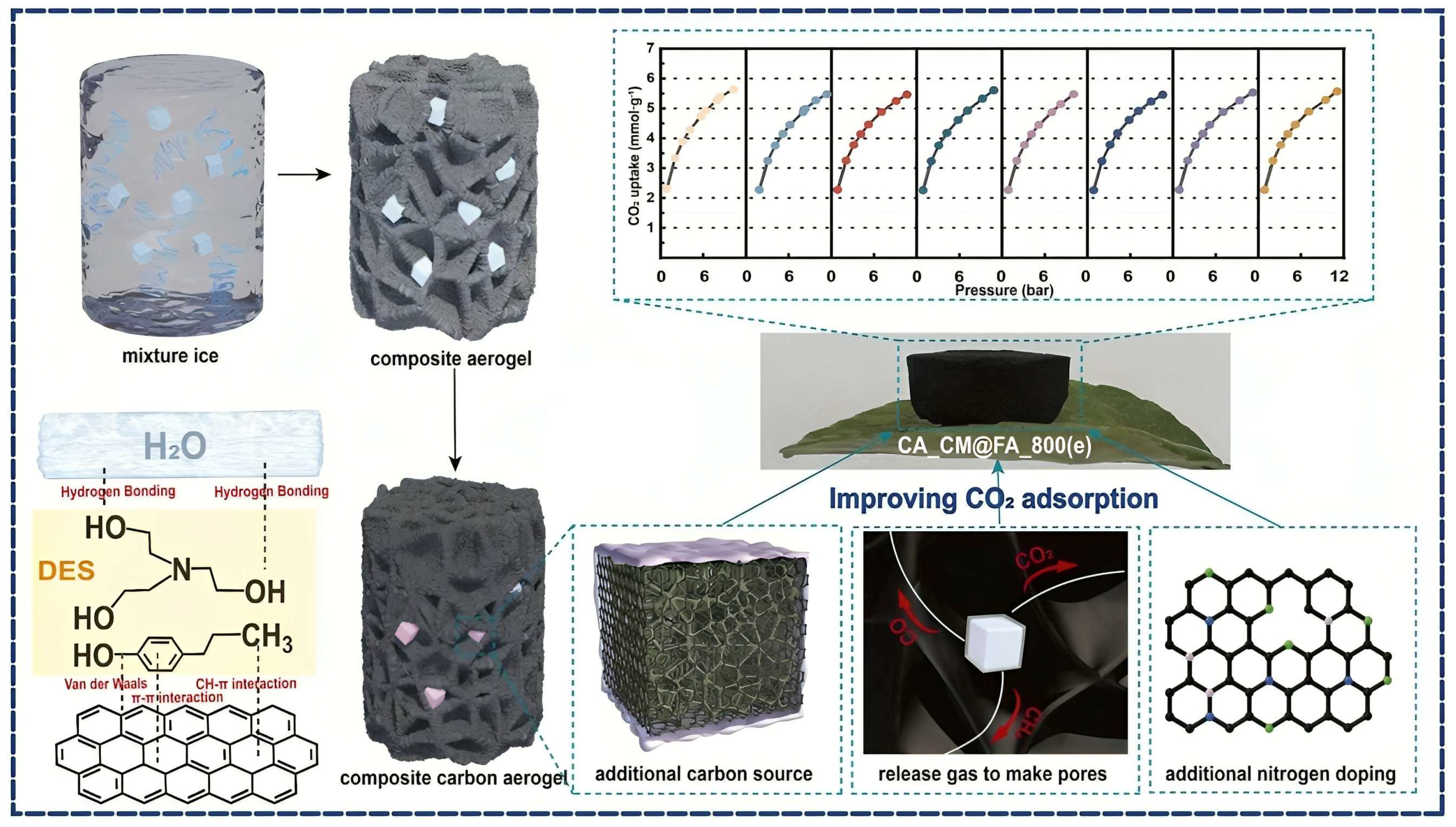
3.1. Carbon Aerogels Form Biomass
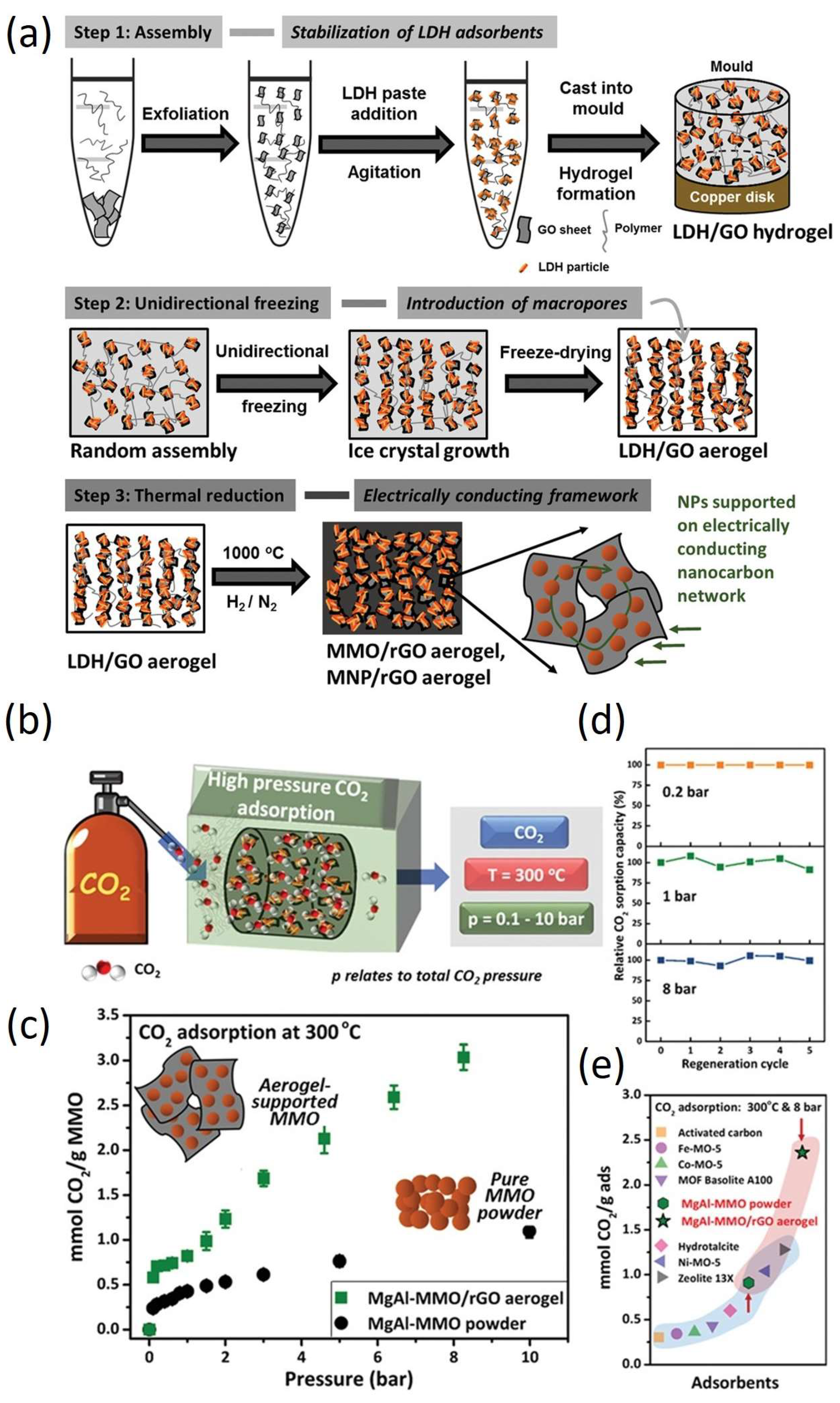
3.2. High-Pressure CO2 Adsorption for Pre-Combustion Capture Using Aerogels
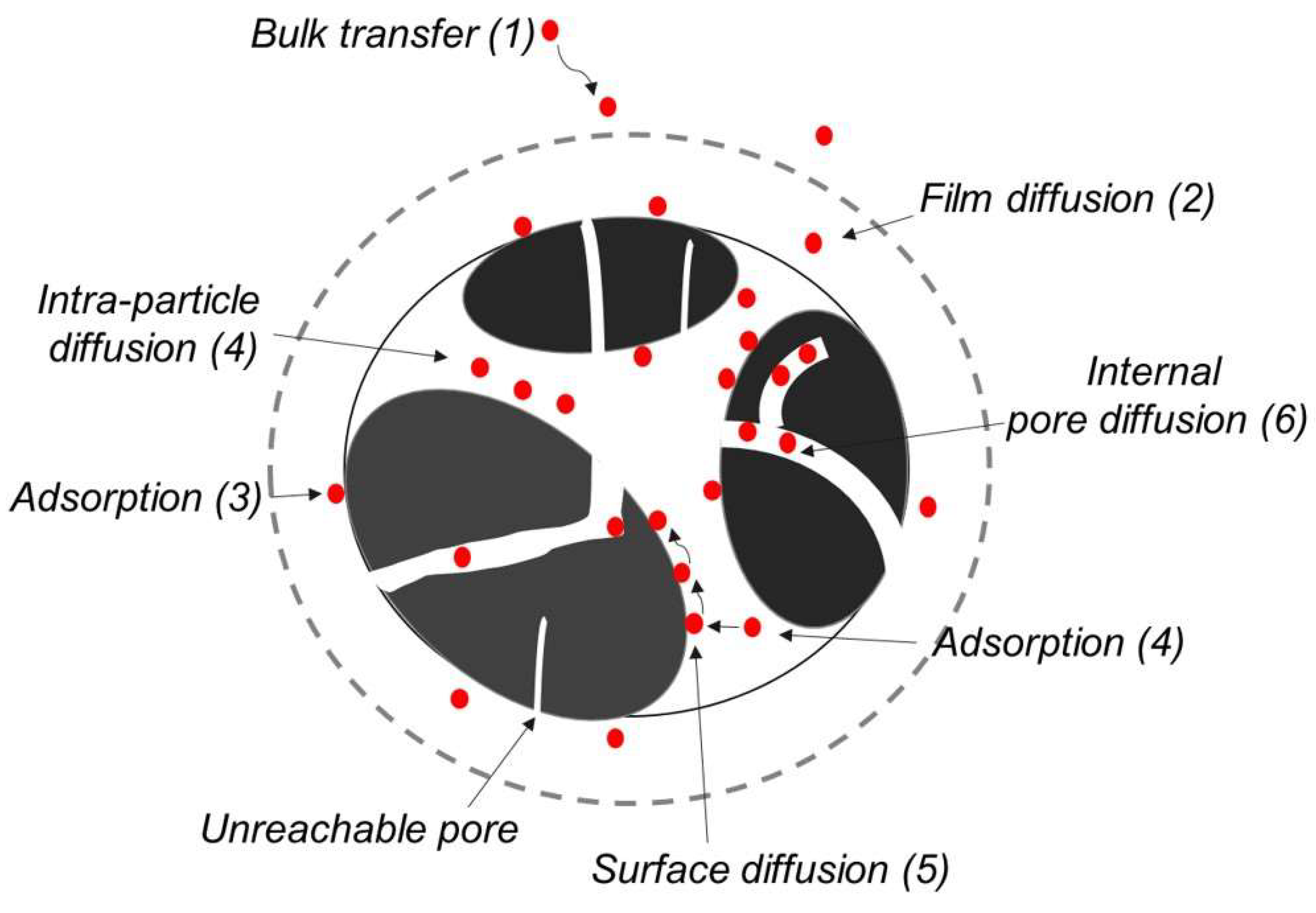
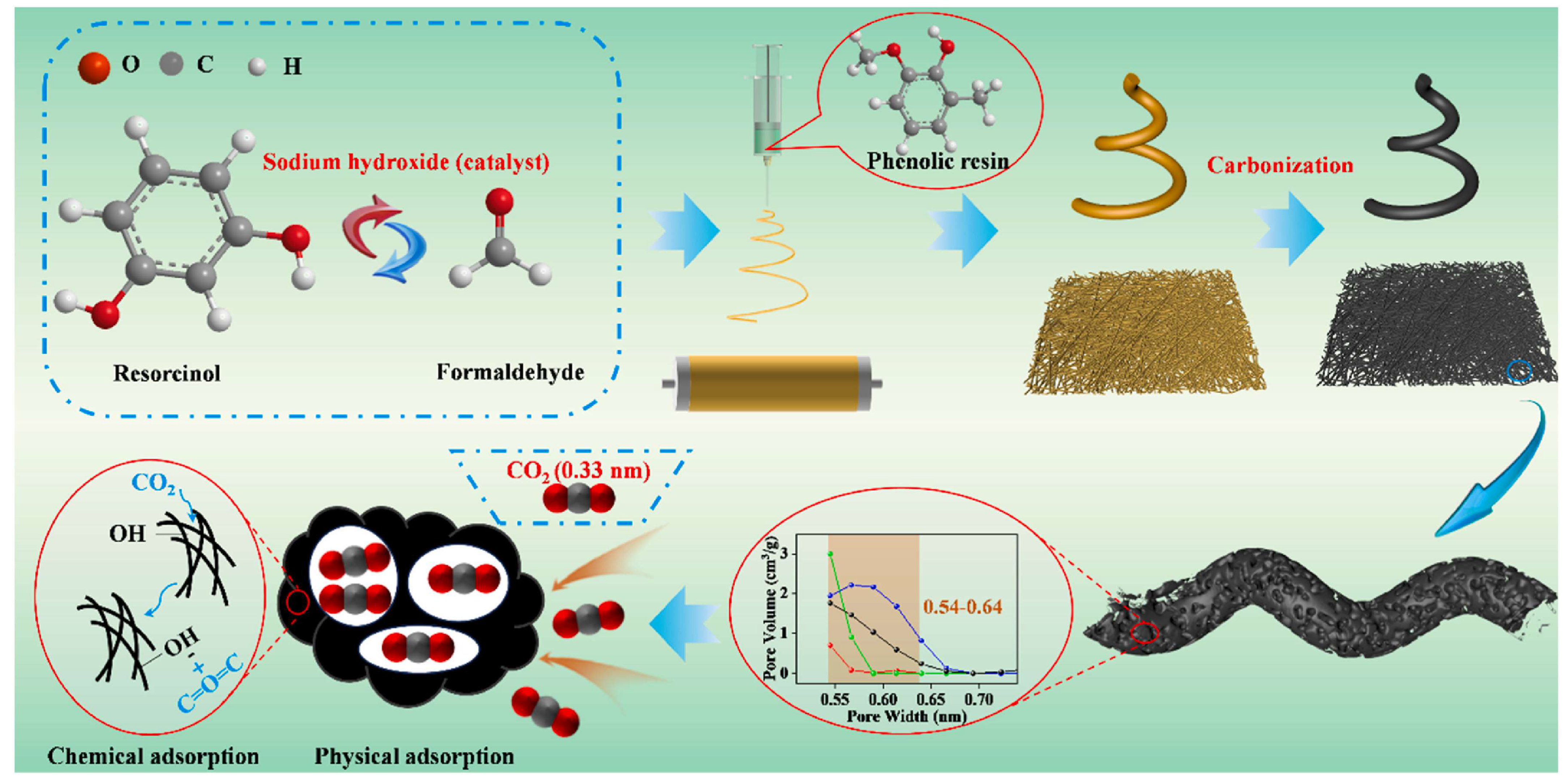
3.3. Effects of Pores and Surface Properties
3.4. Synthesis Approach
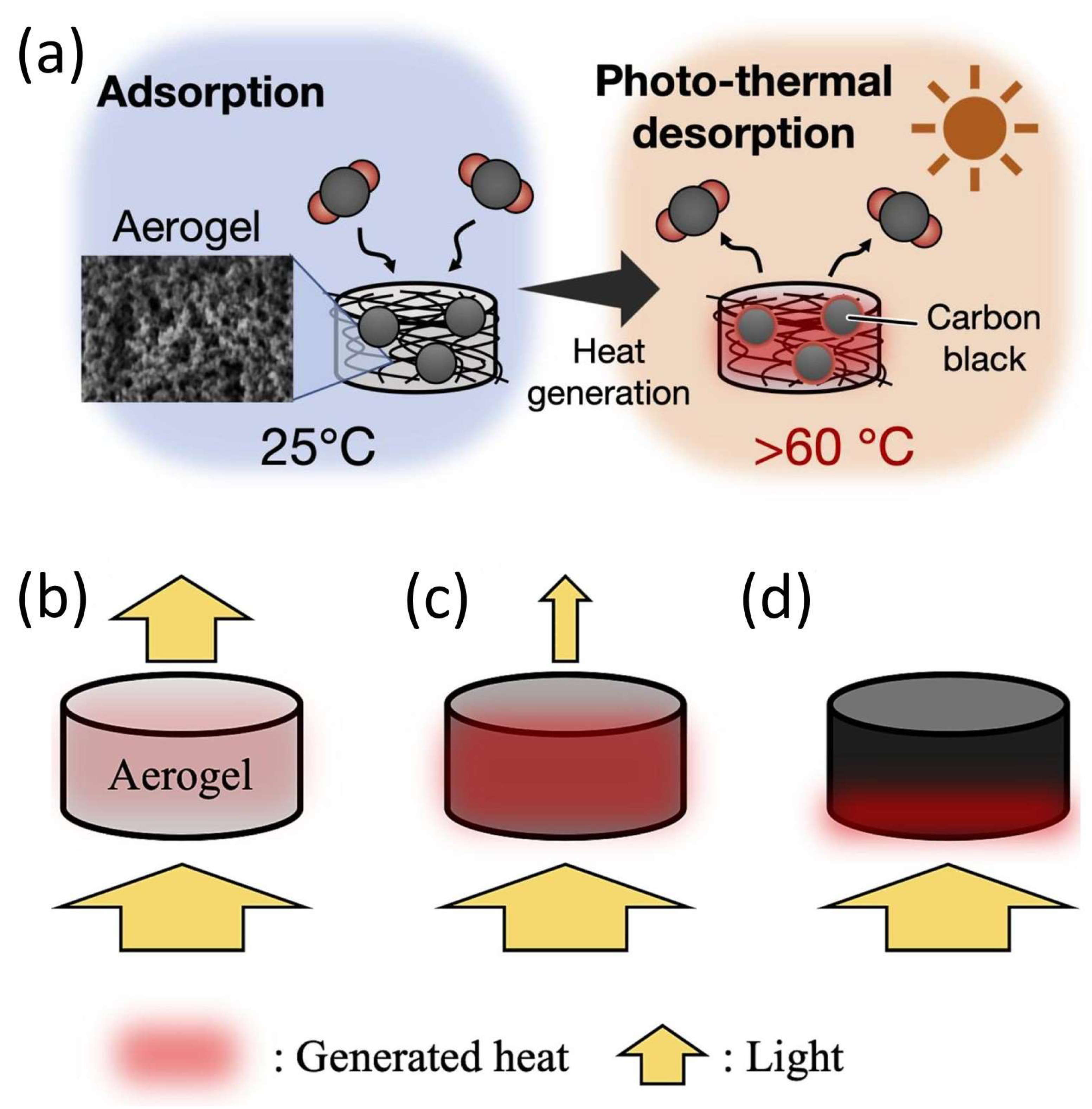
3.5. Aerogel-Assisted Photothermal CO2 Desorption for Regenerative Capture Systems
3.6. Multifunctional Aerogels for Integrated CO2 Capture and Conversion
3.7. Artificial Intelligence for Aerogels Design
4. Summary and Future Research Strategies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jasmine, K. The Concentration of CO2 in the Atmosphere Could Reach 550 Parts per Million by 2050. A 32 % Increase Compared to 2021. The World Counts • Impact through Awareness. Zugegriffen: 28. Oktober 2024. [Online]. Available online: https://www.theworldcounts.com/challenges/global-warming/CO2-concentration (accessed on 28 January 2025).
- Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Hwang, H.J.; An, B.-H.; Seo, H.-W.; Ko, C.H.; Hong, W.H.; Jun, Y.-S. Dual-templating-derived porous carbons for low-pressure CO2 capture. Carbon. Lett. 2023, 33, 811–822. [Google Scholar] [CrossRef]
- Brethomé, F.M.; Williams, N.J.; Seipp, C.A.; Kidder, M.K.; Custelcean, R. Direct air capture of CO2 via aqueous-phase absorption and crystalline-phase release using concentrated solar power. Nat. Energy 2018, 3, 553–559. [Google Scholar] [CrossRef]
- Clifford, C. Carbon Capture Challenges Are Not Deterring Investor at Bill Gates’ Firm. CNBC, USA, 2022. [Online]. Available online: https://www.cnbc.com/2022/05/07/what-is-carbon-capture-eric-toone-investor-at-gates-firm-explains.html?_hsenc=p2ANqtz-_lvXFc1Ucw6m2b_evUgXwAL1FyrCI2aZw4fZftQ19CbbEwLpZyCN1jgpc-xgGJUq4z78fu (accessed on 28 January 2025).
- Buis, A. Examining the Viability of Planting Trees to Help Mitigate Climate Change. NASA’s Jet. Propuls. Lab. 2019. Available online: https://science.nasa.gov/earth/climate-change/examining-the-viability-of-planting-trees-to-help-mitigate-climate-change/ (accessed on 20 January 2025).
- Islam, M.; Han, D.; Bari, G.A.K.M.R.; Nam, K. Electrochemical Storage Behavior of a High-Capacity Mg-Doped P2-Type Na2/3Fe1−yMnyO2 Cathode Material Synthesized by a Sol–Gel Method. Gels 2024, 10, 24. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Bari, G.A.K.M.R.; Jeong, J. Fundamental Understanding of Marine Applications of Molten Salt Reactors: Progress, Case Studies, and Safety. J. Mar. Sci. Eng. Sci. Eng. 2024, 12, 1835. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive Insight and Advancements in Material Design for Electrocatalytic Ammonia Production Technologies: An Alternative Clean Energy. Int. J. Energy Res. 2024, 5685619. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels 2024, 10, 63. [Google Scholar] [CrossRef]
- Khan, T.T.; Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-doped TiO2 for efficient photocatalytic degradation of atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces 2023, 6, 316–340. [Google Scholar] [CrossRef]
- Liu, C. Multi-stage solvent circulation absorption enhancement: System optimization for energy-saving CO2 capture. Sep. Purif. Technol. 2024, 332, 125644. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Li, N.; Qi, T.; Wang, R.; Wang, L.; An, S. Energy-efficient biphasic solvents for industrial CO2 capture: Absorption mechanism and stability characteristics. Energy 2024, 293, 130710. [Google Scholar] [CrossRef]
- Buvik, V.; Høisæter, K.K.; Vevelstad, S.J.; Knuutila, H.K. A review of degradation and emissions in post-combustion CO2 capture pilot plants. Int. J. Greenh. Gas Control. 2021, 106, 103246. [Google Scholar] [CrossRef]
- Fytianos, G.; Ucar, S.; Grimstvedt, A.; Hyldbakk, A.; Svendsen, H.F.; Knuutila, H.K. Corrosion and degradation in MEA based post-combustion CO2 capture. Int. J. Greenh. Gas Control 2016, 46, 48–56. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. Stability of aqueous amine solutions to thermal and oxidative degradation in the absence and the presence of CO2. Int. J. Greenh. Gas Control 2014, 29, 16–21. [Google Scholar] [CrossRef]
- Grimstvedt, A.; Zahlsen, K.; Vevelstad, S.J.; Vernstad, K.; Holten, T.; Brunsvik, A. Exploration of Degradation Chemistry by Advanced Analytical Methodology. Energy Procedia 2017, 114, 1785–1793. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Liu, Y.; Fang, Y.; Zhou, X.; Bao, J. Rice husk-derived hard carbons as high-performance anode materials for sodium-ion batteries. Carbon 2018, 127, 658–666. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated Amorphous Carbon With High-Porosity Derived From Camellia Pollen Grains as Anode Materials for Lithium/Sodium Ion Batteries. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Rehman, A.; Heo, Y.J.; Nazir, G.; Park, S.J. Solvent-free, one-pot synthesis of nitrogen-tailored alkali-activated microporous carbons with an efficient CO2 adsorption. Carbon 2021, 172, 71–82. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; Angelo, D.S.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A comparative energy and costs assessment and optimization for direct air capture technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Honda, R.; Hamasaki, A.; Miura, Y.; Hoshino, Y. Thermoresponsive CO2 absorbent for various CO2 concentrations: Tuning the pK a of ammonium ions for effective carbon capture. Polym. J. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Hou, C.; Liu, J. Quaternary functionalized mesoporous adsorbents for ultra-high kinetics of CO2 capture from air. Sci. Rep. 2020, 10, 21429. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol–Formaldehyde Organic and Carbon Gels. Adv. Funct. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Naeimirad, M.; Kotek, R.; Ramakrishna, S. A Review on Aerogel: 3D Nanoporous Structured Fillers in Polymer-Based Nanocomposites. Polym. Compos. 2018, 39, 3383–3408. [Google Scholar] [CrossRef]
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in Tailoring Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Kang, H.J.; Huh, Y.S.; Im, W.B.; Jun, Y.S. Molecular cooperative assembly-mediated synthesis of ultra-high-performance hard carbon anodes for dual-carbon sodium hybrid capacitors. ACS Nano 2019, 13, 11935–11946. [Google Scholar] [CrossRef]
- Vilian, A.; Song, J.Y.; Lee, Y.S.; Hwang, S.-K.; Kim, H.J.; Jun, Y.-S.; Huh, Y.S.; Han, Y.-K. Salt-templated three-dimensional porous carbon for electrochemical determination of gallic acid. Biosens. Bioelectron. 2018, 117, 597–604. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zou, R.; Huang, Y.; Lai, H.; Chen, Z. Cellulose nanofiber-derived carbon aerogel for advanced room-temperature sodium–sulfur batteries. Carbon Energy 2023, 5, e203. [Google Scholar] [CrossRef]
- Ye, J.; Li, X.; Gao, W.; Zhu, Y. In Situ Nitrogen-Doping Carbon Aerogel as an Effective Sulfur Host to Immobilize Polysulfides for High Performance Lithium-Sulfur Battery. ChemistrySelect 2020, 5, 14729–14734. [Google Scholar] [CrossRef]
- Cai, T.; Kuang, L.; Wang, C.; Jin, C.; Wang, Z.; Sun, Q. Cellulose as an Adhesive for the Synthesis of Carbon Aerogel with a 3D Hierarchical Network Structure for Capacitive Energy Storage. ChemElectroChem 2019, 6, 2586–2594. [Google Scholar] [CrossRef]
- Kamran, U.; Rhee, K.Y.; Lee, S.Y.; Park, S.J. Solvent-free conversion of cucumber peels to N-doped microporous carbons for efficient CO2 capture performance. J. Clean. Prod. 2022, 369, 133367. [Google Scholar] [CrossRef]
- Yang, I.; Jung, M.; Kim, M.S.; Choi, D.; Jung, J.C. Physical and chemical activation mechanisms of carbon materials based on the microdomain model. J. Mater. Chem. A Mater. 2021, 9, 9815–9825. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Bari, G.A.K.M.R.; Vattikuti, S.V.P.; Kim, H. Synthesis of carbon-doped SnO2 nanostructures for visible-light-driven photocatalytic hydrogen production from water splitting. Int. J. Hydrogen Energy 2020, 45, 32789–32796. [Google Scholar] [CrossRef]
- Mandari, K.K.; Son, N.; Pandey, S.; Kim, Y.S.; Bari, G.A.K.M.R.; Kang, M. Nb2O5–SnS2–CdS heteronanostructures as efficient visible-light-harvesting materials for production of H2 under solar light irradiation. J. Alloys Compd 2020, 835, 155399. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Islam, M.; Jeong, J.-H. Materials Design and Development of Photocatalytic NOx Removal Technology. Metals 2024, 14, 4. [Google Scholar] [CrossRef]
- Liu, X.; Giordano, C.; Antonietti, M. A facile molten-salt route to graphene synthesis. Small 2014, 10, 193–200. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. “Salt templating”: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Sonu, S.S.; Rai, N.; Chauhan, I. Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Solgel Sci. Technol. 2023, 105, 324–336. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Zhang, W.; Hu, X.; Zhang, X.; Gou, Z.; Xu, W.; Zheng, H.; Ding, X. A review: Recent advances in conductive aerogels: Assembly strategies, conductive mechanisms, influencing factors and applications. J. Mater. Sci. 2024, 59, 4431–4460. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.; Barai, H.R. Conductive Gels for Energy Storage, Conversion, and Generation: Materials Design Strategies, Properties, and Applications. Materials 2024, 17, 2268. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Lee, T.-G.; Bari, G.A.K.M.R.; Seo, H.-W.; Park, J.-W.; Hwang, H.J.; An, B.-H.; Suzuki, N.; Fujishima, A.; Kim, J.-H.; et al. Sulfuric acid treated G-CN as a precursor to generate high-efficient G-CN for hydrogen evolution from water under visible light irradiation. Catalysts 2021, 11, 37. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Thomas, B.; Geng, S.; Sain, M.; Oksman, K. Hetero-porous, high-surface area green carbon aerogels for the next-generation energy storage applications. Nanomaterials 2021, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cheng, X.; Wang, Z.; Hussain, M.B.; Wang, M. Multifunctional carbon aerogels from typha orientalis for applications in adsorption: Hydrogen storage, CO2 capture and VOCs removal. Energy 2023, 263, 125984. [Google Scholar] [CrossRef]
- Geng, S.; Maennlein, A.; Yu, L.; Hedlund, J.; Oksman, K. Monolithic carbon aerogels from bioresources and their application for CO2 adsorption. Microporous Mesoporous Mater. 2021, 323, 111236. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Wahby, A.; Ramos-Fernández, J.M.; Martínez-Escandell, M.; Sepúveda-Escribano, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-surface-area carbon molecular sieves for selective CO2 adsorption. ChemSusChem 2010, 3, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose aerogels from aqueous alkali hydroxide-urea solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Thomas, A. Ultra-High Surface Area Nitrogen-Doped Carbon Aerogels Derived From a Schiff-Base Porous Organic Polymer Aerogel for CO2 Storage and Supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, T.; Li, H.; Sun, H.; Wang, Y.; Niu, L.; Wen, X.; Bai, G. Biotemplate-Assisted Synthesis of Layered Double Oxides Confining Ultrafine Ni Nanoparticles as a Stable Catalyst for Phenol Hydrogenation. Ind. Eng. Chem. Res. 2019, 58, 14688–14694. [Google Scholar] [CrossRef]
- Xia, D.; Li, H.; Mannering, J.; Huang, P.; Zheng, X.; Kulak, A.; Baker, D.; Iruretagoyena, D.; Menzel, R. Electrically Heatable Graphene Aerogels as Nanoparticle Supports in Adsorptive Desulfurization and High-Pressure CO2 Capture. Adv. Funct. Mater. 2020, 30, 1–15. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications. Gels 2024, 10, 389. [Google Scholar] [CrossRef]
- Liu, Q.; Han, Y.; Qian, X.; He, P.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. CO2 Adsorption over Carbon Aerogels: The Effect of Pore and Surface Properties. ChemistrySelect 2019, 4, 3161–3168. [Google Scholar] [CrossRef]
- Begag, P.N.R.; Krutka, H.; Dong, W.; Mihalcik, D.; Rhine, W.; Gould, G.; Baldic, J. Superhydrophobic amine functionalized aerogels as sorbents for CO2 capture. Greenh. Gases Sci. Technol. 2013, 3, 30–39. [Google Scholar] [CrossRef]
- Yang, G.; Luo, H.; Ohba, T.; Kanoh, H. CO2 Capture by Carbon Aerogel-Potassium Carbonate Nanocomposites. Int. J. Chem. Eng. 2016, 2016, 4012967. [Google Scholar] [CrossRef]
- Singh, G.; Ruban, A.M.; Geng, X.; Vinu, A. Recognizing the potential of K-salts, apart from KOH, for generating porous carbons using chemical activation. Chem. Eng. J. 2023, 451, 139045. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S.A. Nitrogen-Enriched Carbon Aerogels Derived from Polybenzoxazine Cross-Linked Graphene Oxide-Chitosan Hybrid Matrix with Superior CO2 Capture Performance. ACS Appl. Eng. Mater. 2024, 2, 1672–1686. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Miao, Y.; Pudukudy, M.; Zhi, Y.; Miao, Y.; Shan, S.; Jia, Q.; Ni, Y. A facile method for in situ fabrication of silica/cellulose aerogels and their application in CO2 capture. Carbohydr. Polym. 2020, 236, 116079. [Google Scholar] [CrossRef]
- Li, T.; An, X.; Chen, J.; Fan, L.; Fu, D. One-Step Synthesis of Cellulosed-Based Nitrogen-Doped Carbon Aerogel and Its CO2 Adsorption Performance. Energy Fuels 2024, 38, 555–564. [Google Scholar] [CrossRef]
- Ma, H.; Cui, B.; Li, J.; Ju, X.; Wang, D.; Yang, Z. Enhancing CO2 adsorption in hierarchical carbon aerogels via deep eutectic solvent-encapsulated carbon source pyrolysis. J. Environ. Chem. Eng. 2025, 13, 115789. [Google Scholar] [CrossRef]
- Chai, S.; Dai, X.; Wu, T.; Liu, B.; Yao, H.; Yuan, Y.; Wu, Q. Synthesis of Si/O/C/N Quaternary Composite Aerogels with Micro/Mesoporous Structures and Their Selective Adsorption Property for Volatile Carbonyl Compounds in Cigarette Smoke. Microporous Mesoporous Mater. 2020, 301, 110164. [Google Scholar] [CrossRef]
- Ekabutr, P.; Ariyathanakul, T.; Chaiyo, S.; Niamlang, P.; Rattanaveeranon, S.; Chailapakul, O.; Supaphol, P. Carbonized Electrospun Polyvinylpyrrolidone/Metal Hybrid Nanofiber Composites for Electrochemical Applications. J. Appl. Polym. Sci. 2018, 135, 3–9. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, A.P.; Hudson, M.S.L.; Soni, P.K.; Verma, S.K.; Shukla, V.; Sekkar, V.; Tripathi, M.; Srivastava, O.N. Economical Synthesis of Highly Efficient and Tunable Carbon Aerogels for Enhanced Storage of CO2 Emitted from Energy Sources. Int. J. Energy Res. 2021, 45, 6285–6292. [Google Scholar] [CrossRef]
- Robertson, C.; Mokaya, R. Microporous Activated Carbon Aerogels via a Simple Subcritical Drying Route for CO2 Capture and Hydrogen Storage. Microporous Mesoporous Mater. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Dixit, V.; Shukla, V.; Shaz, M.A.; Sinha, A.S.K.; Srivastava, O.N.; Sekkar, V. Synthesis, Characterization and Hydrogen Storage Characteristics of Ambient Pressure Dried Carbon Aerogel. Int. J. Hydrogen Energy 2016, 41, 3561–3570. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Use of Monolithic Silicon Carbide Aerogel as a Reusable Support for Development of Regenerable CO2 Adsorbent. RSC Adv. 2014, 4, 64193–64199. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Gao, S.; Jiang, L.; Yi, Y. Study of CO2 adsorption on carbon aerogel fibers prepared by electrospinning. J. Environ. Manag. 2024, 349, 119432. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wei, J.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional Carbon Aerogels with Hierarchical Anisotropic Structure Derived from Lignin and Cellulose Nanofibers for CO2 Capture and Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Gu, Y.; Mu, X.; Wang, P.; Wang, X.; Liu, J.; Shi, J.; Wei, A.; Tian, Y.; Zhu, G.; Xu, H.; et al. Integrated photothermal aerogels with ultrahigh-performance solar steam generation. Nano Energy 2020, 74, 104857. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Truong, R.; Lee, R.; Goetz, S.A.; Esser-Kahn, A.P. Photothermal release of CO2 from capture solutions using nanoparticles. Energy Environ. Sci. 2014, 7, 2603–2607. [Google Scholar] [CrossRef]
- Campbell, Z.S.; Han, S.; Marre, S.; Abolhasani, M. Continuous Flow Solar Desorption of CO2 from Aqueous Amines. ACS Sustain. Chem. Eng. 2021, 9, 2570–2579. [Google Scholar] [CrossRef]
- Kataoka, T.; Orita, Y.; Shimoyama, Y. Photo-thermal CO2 desorption from amine-modified silica / carbon aerogel for direct air capture. Chem. Eng. J. 2024, 482, 148710. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Li, J.; Wang, B.; Jin, M.; Liang, Q.; Zhang, D.; Han, Z.; Deng, L.; Qu, X.; et al. Insights into Hierarchical Structure–Property–Application Relationships of Advanced Bacterial Cellulose Materials. Adv. Funct. Mater. 2023, 33, 1–32. [Google Scholar] [CrossRef]
- Bahadur, R.; Singh, G.; Li, M.; Chu, D.; Yi, J.; Karakoti, A.; Vinu, A. BCN Nanostructures Conjugated Nanoporous Carbon with Oxygenated Surface and High Specific Surface Area for Enhanced CO2 Capture and Supercapacitance. Chem. Eng. J. 2023, 460, 141793. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.H.; Müller, J.O.; Antonietti, M. A Direct Synthesis of Mesoporous Carbons with Bicontinuous Pore Morphology from Crude Plant Material by Hydrothermal Carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Ummah, M.S. 3Dfibrousaerogels from 1Dpolymernanofibersfor Energy and Environmental Applications. J. Mater. Chem. A 2023, 11, 512–547. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the Well-Crystallized Ordered Mesoporous TiO2 with the Confined Space Effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Sharma, N.K.; Verma, C.S.; Chariar, V.M.; Prasad, R. Eco-Friendly Flame-Retardant Treatments for Cellulosic Green Building Materials. Indoor Built Environ. 2015, 24, 422–432. [Google Scholar] [CrossRef]
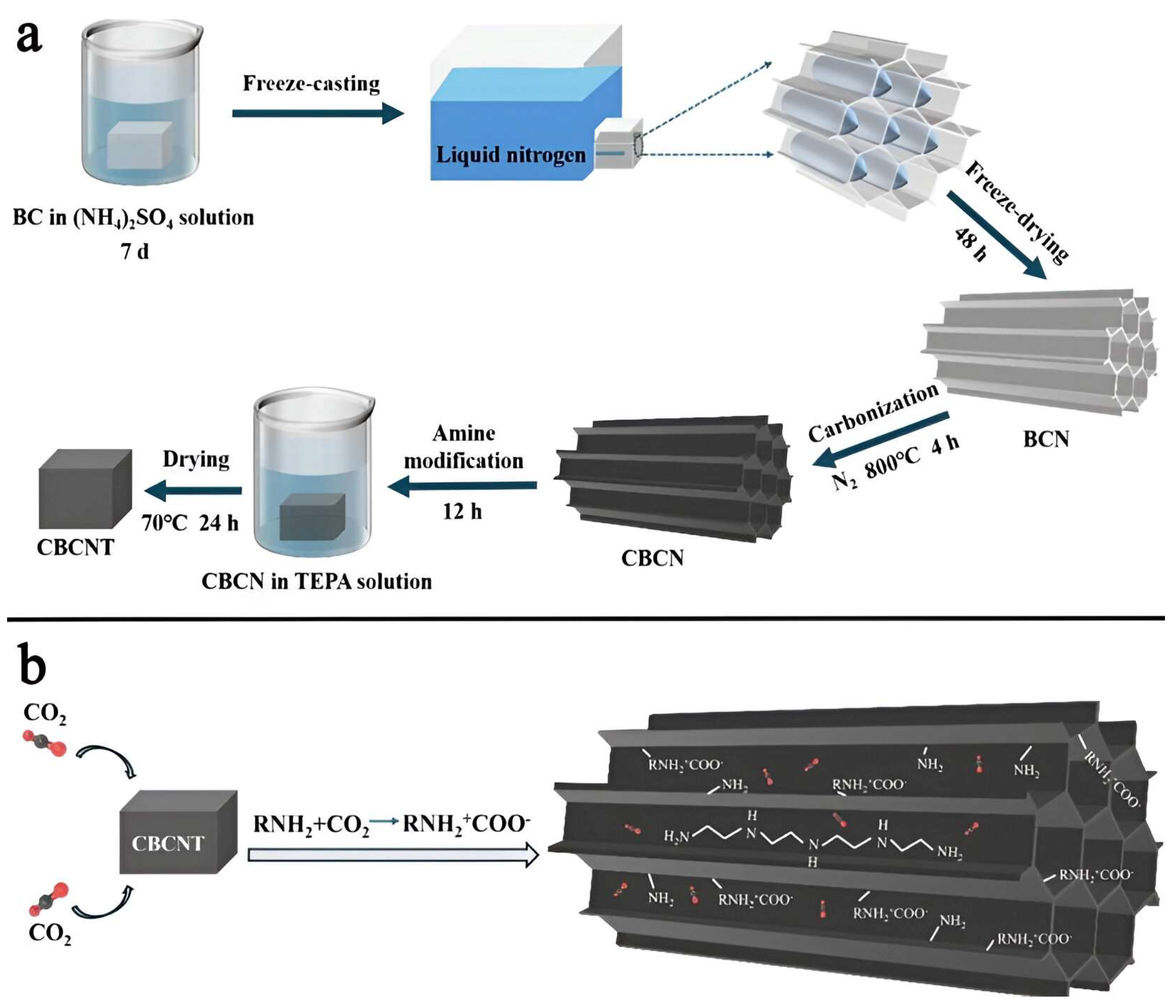
- Bao, S.; Zheng, X.; Xu, Z.; Ji, B.; Yang, Z.; Sun, W.; Mei, J.; Rong, J.; Li, Z. Amine-impregnated elastic carbon nanofiber aerogel templated by bacterial cellulose for CO2 adsorption and separation. Fuel 2025, 381, 133320. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A Critical Review on Deployment Planning and Risk Analysis of Carbon Capture, Utilization, and Storage (CCUS) toward Carbon Neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.; Ahn, Y.H.; Park, Y. Abnormal Thermodynamic Promotion and Tuning Behavior of Epoxycyclopentane for Its Implications in CO2 Storage. Chem. Eng. J. 2021, 425, 130647. [Google Scholar] [CrossRef]
- Chakrabortty, S.; Kumar, R.; Nayak, J.; Jeon, B.H.; Dargar, S.K.; Tripathy, S.K.; Pal, P.; Ha, G.S.; Kim, K.H.; Jasiński, M. Green Synthesis of MeOH Derivatives through in Situ Catalytic Transformations of Captured CO2 in a Membrane Integrated Photo-Microreactor System: A State-of-Art Review for Carbon Capture and Utilization. Renew. Sustain. Energy Rev. 2023, 182, 113417. [Google Scholar] [CrossRef]
- Das, S.; Wan Daud, W.M.A. A Review on Advances in Photocatalysts towards CO2 Conversion. RSC Adv. 2014, 4, 20856–20893. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Cao, S.; Chen, G.; Li, C.; Izquierdo, R.; Sun, S. Advanced Semiconductor Catalyst Designs for the Photocatalytic Reduction of CO2. Mater. Rep. Energy 2023, 3, 100193. [Google Scholar] [CrossRef]
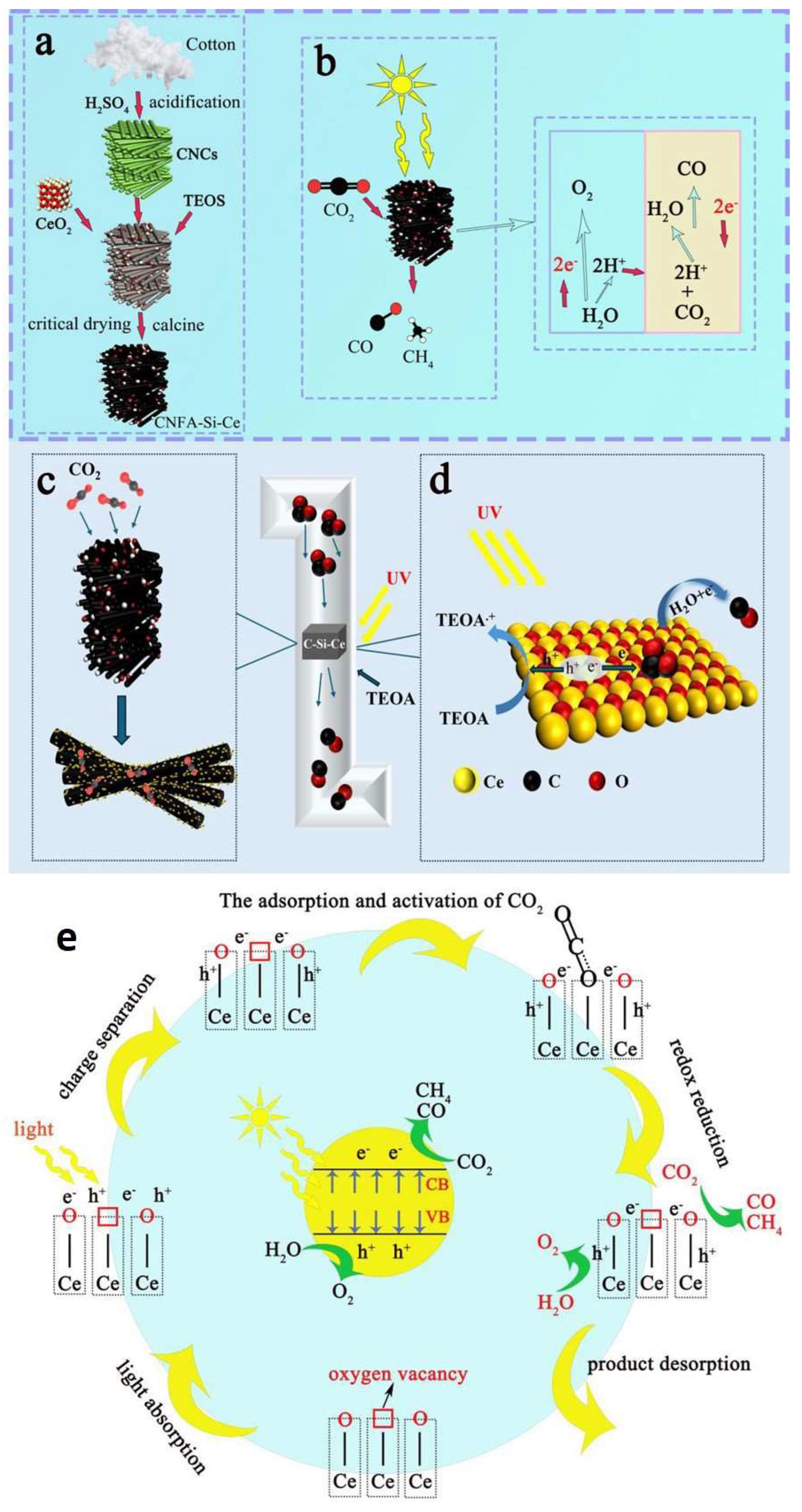
- Xu, Z.; Zheng, X.; Ji, B.; Bao, S.; Mei, J.; Yang, Z.; Rong, J.; Li, Z. Development of cerium-doped porous composite aerogel using cellulose nanocrystals for enhanced CO2 capture and conversion. J. Colloid Interface Sci. 2025, 683, 322–334. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, P.; Khan, A.A.P.; Van Le, Q.; Nguyen, V.H.; Thakur, S.; Raizada, P. CO2 Photoreduction into Solar Fuels via Vacancy Engineered Bismuth-Based Photocatalysts: Selectivity and Mechanistic Insights. Chem. Eng. J. 2022, 439, 135563. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, T.; Gu, L.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M. Surface-Structure Sensitivity of CeO2 Nanocrystals in Photocatalysis and Enhancing the Reactivity with Nanogold. ACS Catal. 2015, 5, 4385–4393. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium Oxide Nanoparticle: A Remarkably Versatile Rare Earth Nanomaterial for Biological Applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. High Surface Area Metal Salt Templated Carbon Aerogels via a Simple Subcritical Drying Route: Preparation and CO2 Uptake Properties. RSC Adv. 2013, 3, 17677–17681. [Google Scholar] [CrossRef]
- Jeon, D.H.; Min, B.G.; Oh, J.G.; Nah, C.; Park, S.J. Influence of Nitrogen Moieties on CO2 Capture of Carbon Aerogel. Carbon Lett. 2015, 16, 57–61. [Google Scholar] [CrossRef]
- He, P.; Qian, X.; Fei, Z.; Liu, Q.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X. Structure Manipulation of Carbon Aerogels by Managing Solution Concentration of Precursor and Its Application for CO2 Capture. Processes 2018, 6, 35. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, X.; Zhuo, H.; Zhong, L.; Peng, X.; Wang, S.; Sun, R. 3D Hierarchical Porous N-Doped Carbon Aerogel from Renewable Cellulose: An Attractive Carbon for High-Performance Supercapacitor Electrodes and CO2 Adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, W.; Jiang, G.; Zhang, T.; He, K.; Mu, L.; Zhu, J.; Huang, D.; Qian, H.; Lu, X. Machine Learning Prediction of the Yield and BET Area of Activated Carbon Quantitatively Relating to Biomass Compositions and Operating Conditions. Ind. Eng. Chem. Res. 2023, 62, 11016–11031. [Google Scholar] [CrossRef]
- Chang, J.; Lee, J.-Y. Machine Learning-Based Prediction of the Adsorption Characteristics of Biochar from Waste Wood by Chemical Activation. Materials 2024, 17, 21. [Google Scholar] [CrossRef]
- Vishnyakov, A. Machine Learning in Computational Design and Optimization of Disordered Nanoporous Materials. Materials 2025, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, O.A.; Saadatnia, Z.; Ghaffari-Mosanenzadeh, S.; Okhovatian, S.; Park, C.B. Machine learning-based model for predicting the material properties of nanostructured aerogels. SPE Polym. 2022, 4, 24–37. [Google Scholar] [CrossRef]
- Jiang, W.; Xing, X.; Li, S.; Zhang, X.; Wang, W. Synthesis, characterization and machine learning based performance prediction of straw activated carbon. J. Clean. Prod. 2019, 212, 1210–1223. [Google Scholar] [CrossRef]
| Materials | Performances |
|---|---|
| N-doped C aerogels [54] | 6.1 mmol g−1 (0 °C, 1 bar), 33.1 mmol g−1 (50 °C, 30 bar), selectivity 47.8 (CO2/N2) at ambient pressure |
| MgAl-MMO/rGO aerogel [56] | 2.4 mmol g−1 (300 °C, 8 bar) |
| Microemulsion templated C aerogel [58] | 63 mmol g−1 (0 °C, 1 bar), selectivity (15%CO2/N2) |
| C aerogels-K2CO3 [60] | Total CO2 capture 2.7 mmol g−1, CO2 capture capacity by K2CO3 14.5 mmol g−1 |
| Hierarchical C aerogels [66] | 5.7 mmol g−1 (0 °C, 1 bar), selectivity (15%CO2/N2) |
| C aerogel based on formaldehyde /resorcinol/triethyl amine [69] | 6.7 mmol g−1 at 25 °C, 40 bar |
| C aerogel based on formaldehyde/melamine [101] | 2.2 mmol g−1 at 25 °C, 1 bar |
| Microporous activated C aerogel [102] | 3 mmol g−1 at 25 °C, 1 bar |
| C aerogel based on chitosan/polybenzoxazine [62] | 7.3 mmol g−1 at 25 °C, 1 bar |
| Cellulose based N-doped C aerogel [65] | 3.6 mmol g−1 (20 °C, 1 bar), selectivity (15%CO2/85N2) |
| Lignin/TOCNF C aerogel [74] | 5.2 mmol g−1 (0 °C, 1 bar) |
| C aerogel based on chitosan/polybenzoxazine [63] | 5.7 mmol g−1 at 25 °C, 1 bar |
| N-doped C aerogel [103] | 118 mg g−1 (2.68 mmol g−1) at 25 °C, 1 bar |
| C aerogel based on formaldehyde/resorcinol [104] | 83.7 cm3 g−1 (3.73 mmol g−1) at (0 °C), 56.5 (25 °C), 18.5 (50 °C) at 1 bar |
| N-doped C aerogel [105] | 4.8 mmol g−1 at 1 bar |
| C aerogel fiber [69] | 4.2 mmol g−1 at 0 °C, 1 bar |
| Cellulose based C aerogel [47] | 15 mmol g−1 at 25 °C, 30 bar |
| Monolithic C aerogel [48] | 4.5 mmol g−1 at 25 °C, 1 bar |
| Cellulose based hierarchical C aerogels [51] | 3.4 mmol g−1 at 25 °C, 1 bar |
| C aerogel [70] | 3 mmol g−1 at 25 °C, 1 bar |
| Bacterial cellulose C naofiber aerogel/TEPA [86] | 4.88 mmol g−1 at 0 °C, 1 bar, selectivity (15CO2/85N2) |
| Ce-doped porous C aerogel [92] | 3.18 mmol g−1 at 0 °C, 1 bar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Bari, G.A.K.M.R. Advances in Carbon-Based Aerogels for CO2 Capture: Fundamental Design Strategies and Technological Progress. Gels 2025, 11, 361. https://doi.org/10.3390/gels11050361
Asrafali SP, Periyasamy T, Bari GAKMR. Advances in Carbon-Based Aerogels for CO2 Capture: Fundamental Design Strategies and Technological Progress. Gels. 2025; 11(5):361. https://doi.org/10.3390/gels11050361
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, and Gazi A. K. M. Rafiqul Bari. 2025. "Advances in Carbon-Based Aerogels for CO2 Capture: Fundamental Design Strategies and Technological Progress" Gels 11, no. 5: 361. https://doi.org/10.3390/gels11050361
APA StyleAsrafali, S. P., Periyasamy, T., & Bari, G. A. K. M. R. (2025). Advances in Carbon-Based Aerogels for CO2 Capture: Fundamental Design Strategies and Technological Progress. Gels, 11(5), 361. https://doi.org/10.3390/gels11050361









