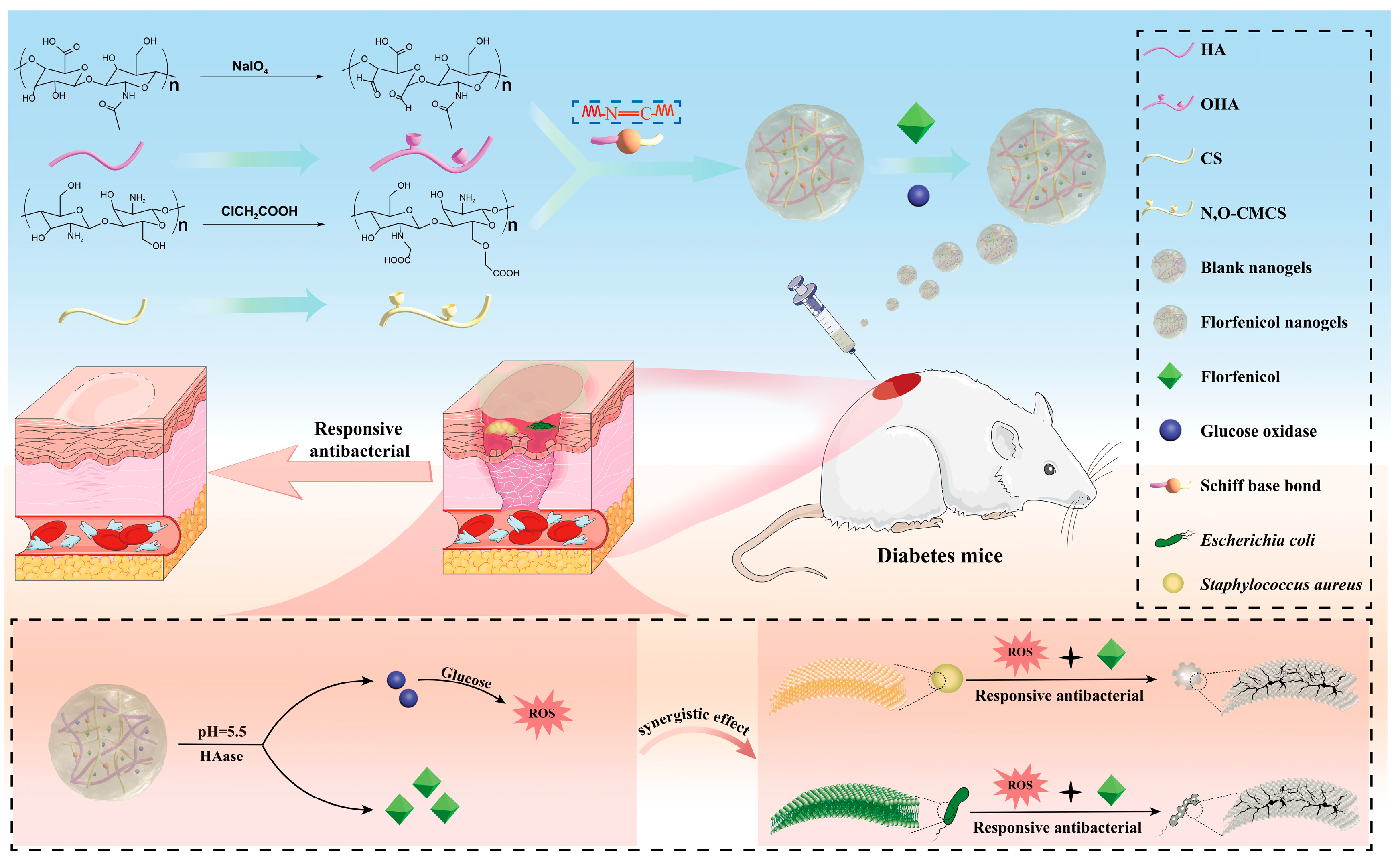
Nanogel Dressing with Targeted Glucose Reduction and pH/Hyaluronidase Dual-Responsive Release for Synergetic Therapy of Diabetic Bacterial Wounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of N, O-CMCS
2.2. Oxidation of HA
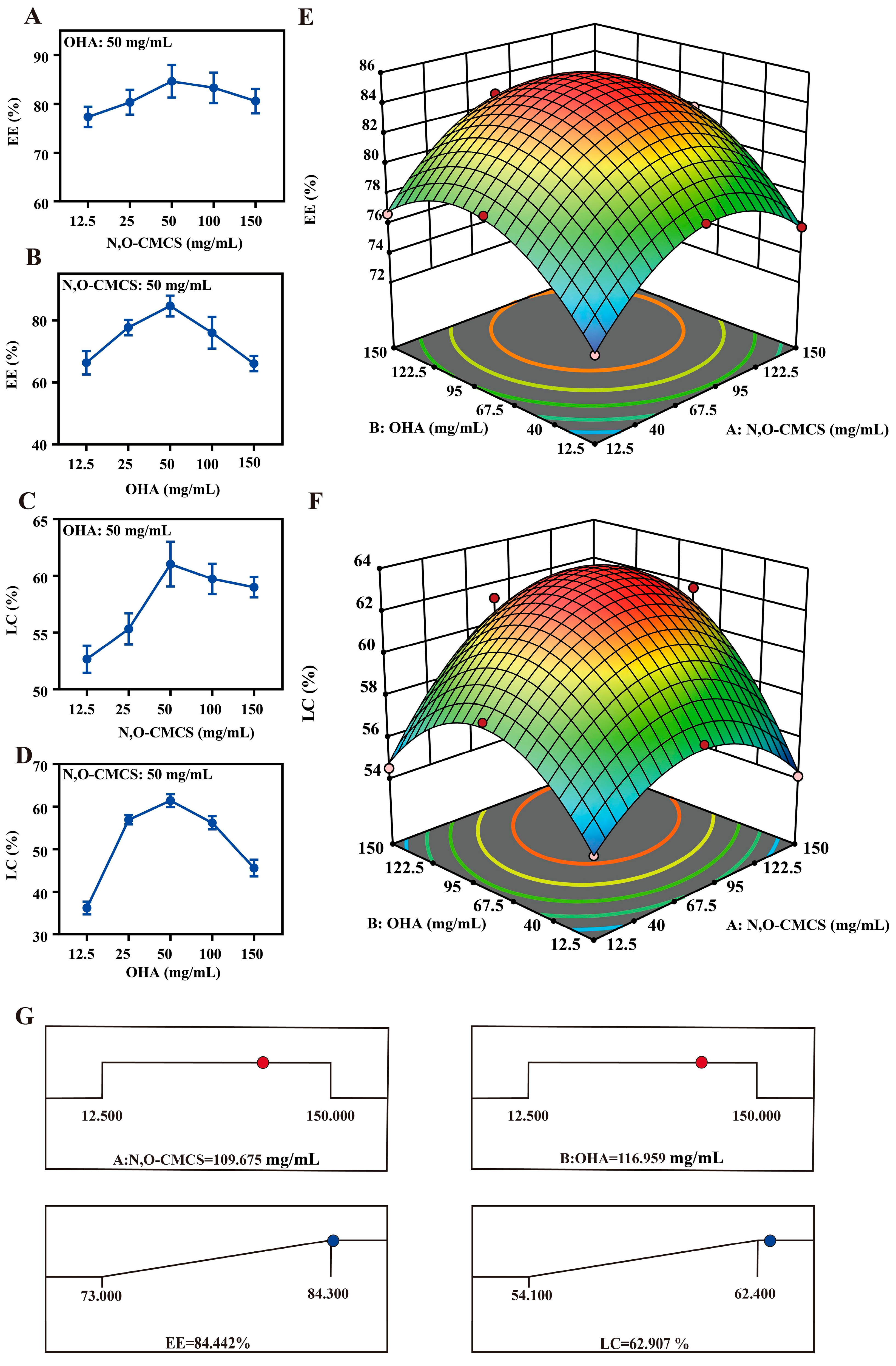
2.3. Formulation and Determination of Optimal Formulation
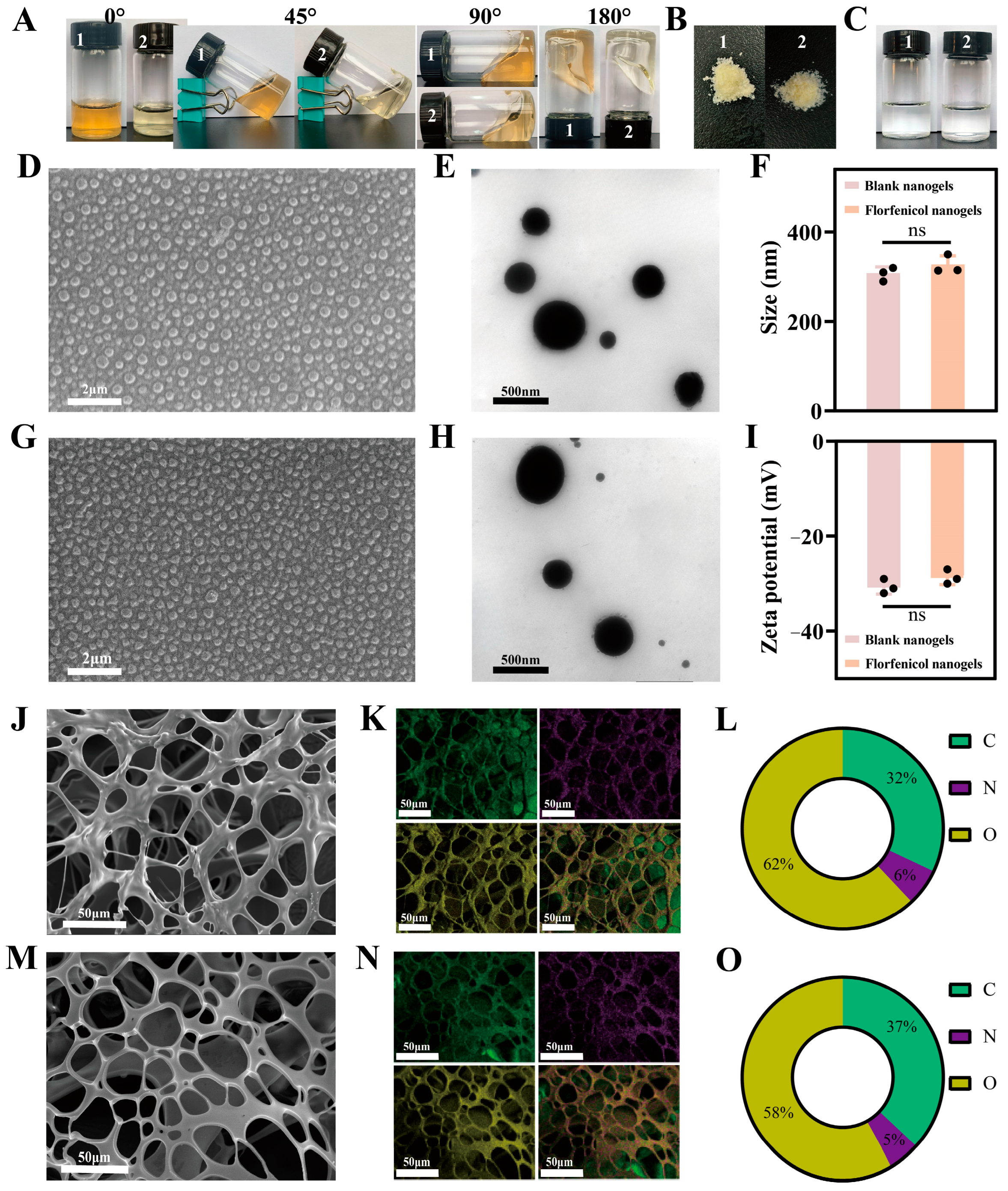
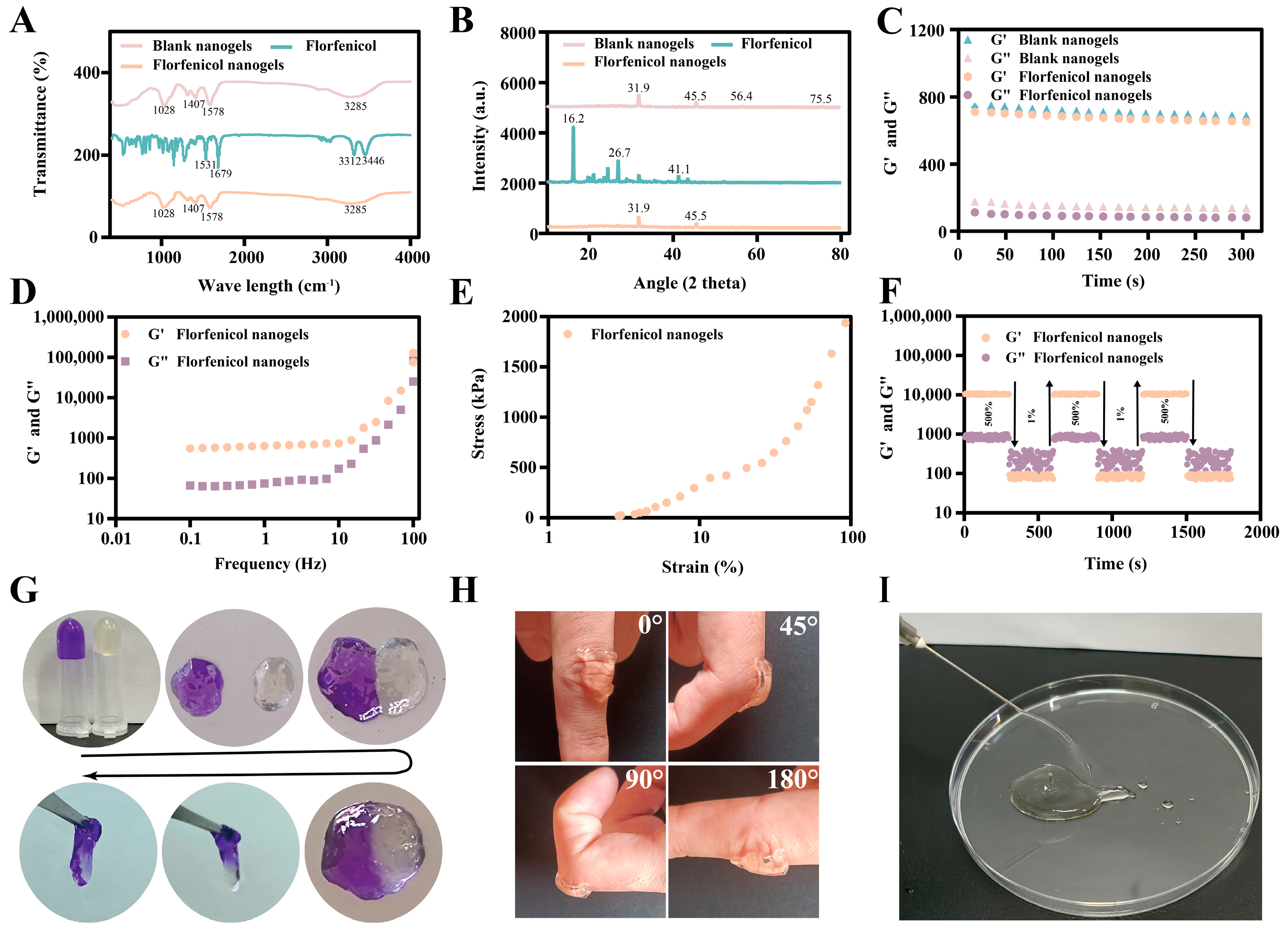
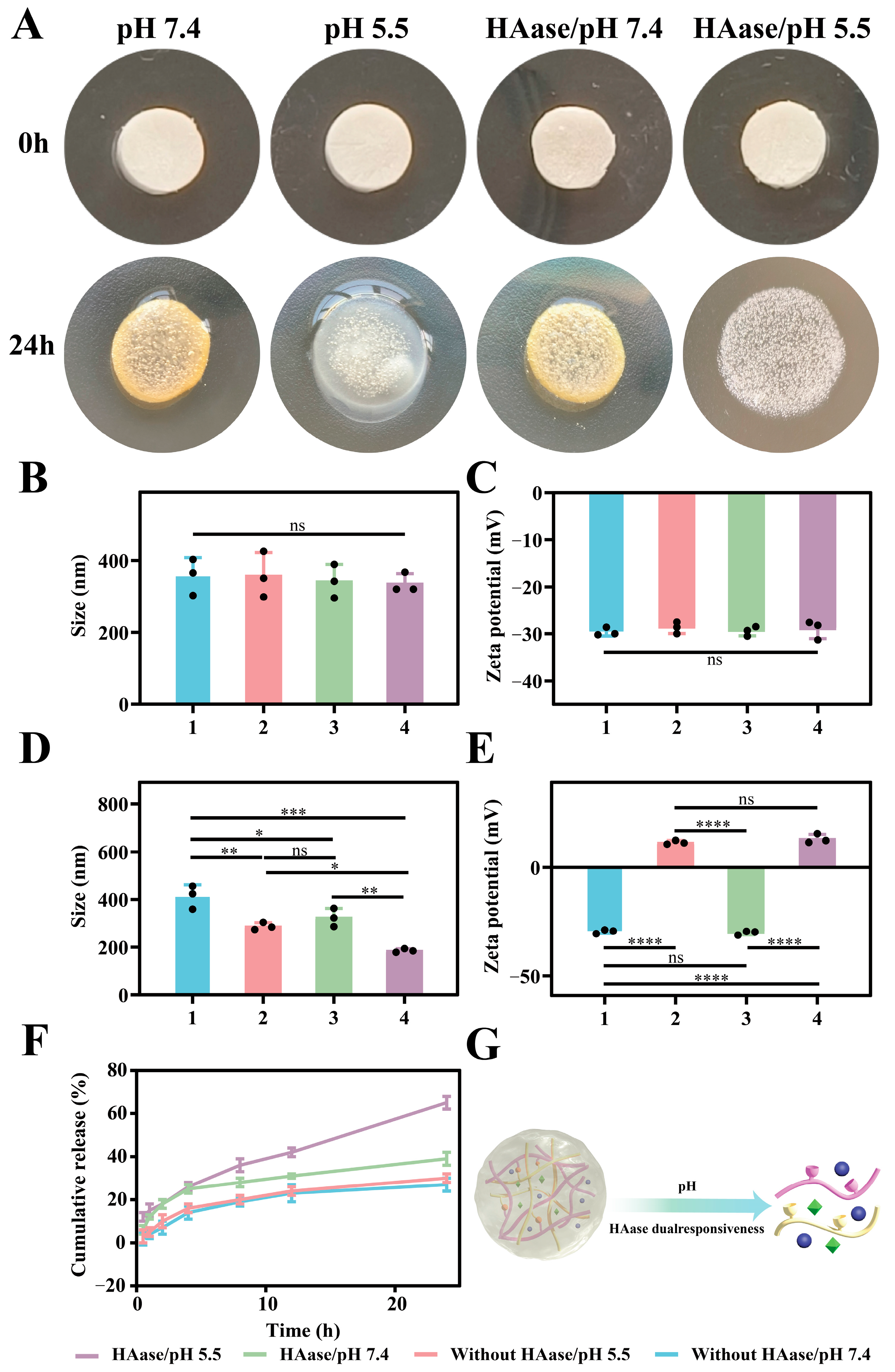
2.4. Characterization
2.5. pH/HAase Dual-Responsive Performances
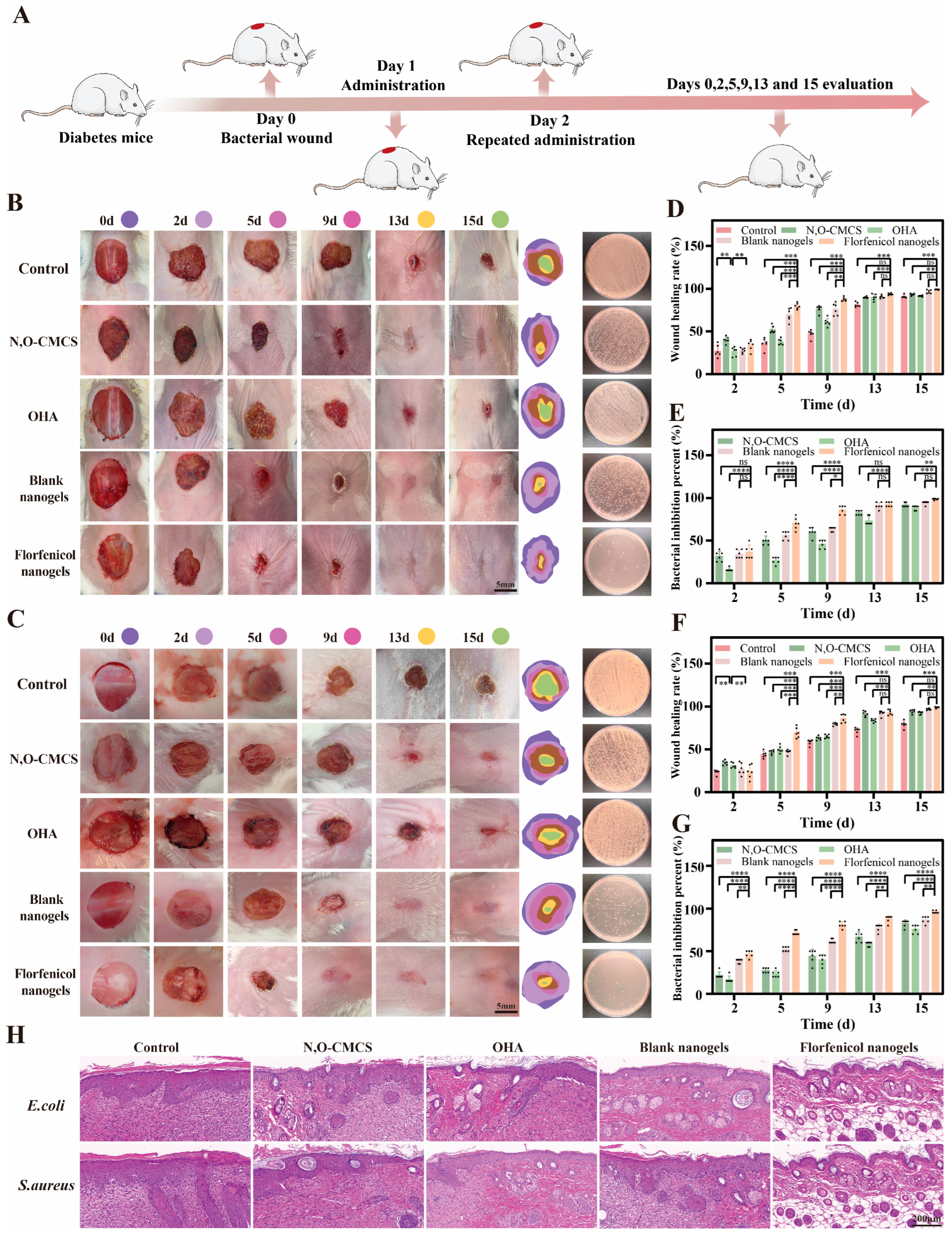
2.6. Antibacterial Activity
2.7. Evaluation of In Vivo Wound Healing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of N, O-CMCS
4.3. Oxidation of HA
4.4. Formulation of Florfenicol-Loaded N, O-CMCS@OHA Nanogels
4.5. Single-Factor Experiment
4.6. Box–Behnken Response Surface Analysis
4.7. Physicochemical Characterization
4.7.1. Surface Morphology
4.7.2. The Mean Size, ZP, and Polydispersity Index (PDI)
4.7.3. PXRD and FTIR
4.7.4. Rheological Analysis
4.7.5. Self-Healing Properties
4.7.6. Adhesiveness
4.7.7. Injectability
4.7.8. Stability
4.7.9. Reduce Glucose Performance
4.8. pH/hyaluronidase Dual-Responsive Release
4.9. In Vitro Antibacterial Activity Studies
4.9.1. Broth Macrodilution Method
4.9.2. Inhibition Zones
4.9.3. Live/Dead Bacterial Staining Analysis
4.9.4. Morphological Analysis
4.9.5. Bacterial ROS Level Test
4.10. In Vivo Wound Healing
4.11. In Vivo Antibacterial Studies
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.; Liu, Y.Q.; Liu, P.; Cao, J.; Shen, A.G.; Chu, P.K. Blood-Glucose-Depleting Hydrogel Dressing as an Activatable Photothermal/Chemodynamic Antibacterial Agent for Healing Diabetic Wounds. ACS Appl. Mater. Interfaces 2023, 15, 24162–24174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wu, H.; Chen, X.; Chen, Z.; Pan, Y.; Qu, W.; Hao, H.; Chen, D.; Xie, S. Targeting and arginine-driven synergizing photodynamic therapy with nutritional immunotherapy nanosystems for combating MRSA biofilms. Sci. Adv. 2023, 9, eadg9116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zheng, C.; Hu, C.; Yang, L.; Kong, Q.; Zhang, H.; Wang, Y. A versatile glycopeptide hydrogel promotes chronic refractory wound healing through bacterial elimination, sustained oxygenation, immunoregulation, and neovascularization. Adv. Funct. Mater. 2023, 33, 2305992. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Hussain, M.; Wang, H.; Zhou, N.; Tao, J.; Jiang, H.; Zhu, J. Injectable and pH-Sensitive Hyaluronic Acid-Based Hydrogels with On-Demand Release of Antimicrobial Peptides for Infected Wound Healing. Biomacromolecules 2021, 22, 3049–3059. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Sun, X.; Jia, P.; Zhang, H.; Dong, M.; Wang, J.; Li, L.; Bu, T.; Wang, X.; Wang, L.; Lu, Q.; et al. Green regenerative hydrogel wound dressing functionalized by natural drug-food homologous small molecule self-assembled nanospheres. Adv. Funct. Mater. 2022, 32, 2106572. [Google Scholar] [CrossRef]
- Pranantyo, D.; Yeo, C.K.; Wu, Y.; Fan, C.; Xu, X.; Yip, Y.S.; Vos, M.I.G.; Mahadevegowda, S.H.; Lim, P.L.K.; Yang, L.; et al. Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing. Nat. Commun. 2024, 15, 954. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ding, Y.; Fan, X.; Ye, L.; Pan, Q.; Zhang, B.; Li, P.; Luo, K.; Hu, B.; et al. A Double Network Composite Hydrogel with Self-Regulating Cu2+/Luteolin Release and Mechanical Modulation for Enhanced Wound Healing. ACS Nano 2024, 18, 17251–17266. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Li, W.; Lin, H.; Ding, C.; Liu, Q.; Wang, L.; Li, Z.; Mei, L.; Chen, H.; et al. Biofilm microenvironment triggered self-enhancing photodynamic immunomodulatory microneedle for diabetic wound therapy. Nat. Commun. 2023, 14, 7658. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, X.; Ding, H.; Chen, Y.; He, W.; Wei, Y.; Chen, W.; Chan, Y.K.; Shi, Y.; Huang, D.; et al. Engineered Probiotic Bio-Heterojunction with Robust Antibiofilm Modality via “Eating” Extracellular Polymeric Substances for Wound Regeneration. Adv. Mater. 2024, 36, e2402530. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, J.; Yuan, Q.; Xin, P.; Cheng, H.; Gu, z.; Wu, J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2019, 392, 123775. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, P.; Yin, C.; Li, Y.; Liao, Z.; Yang, C.; Liu, H.; Wang, W.; Fan, C.; Sun, D.; et al. Antibiotic-derived carbon-nanodot-decorated hydrogel for reactive oxygen species-enhanced anti-infection through biofilm damage. Adv. Funct. Mater. 2023, 33, 2300341. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Jiang, Y.; Liu, J.; Ju, M.; Algharib, S.A.; Dawood, A.S. On-demand release of enrofloxacin-loaded chitosan oligosaccharide-oxidized hyaluronic acid composite nanogels for infected wound healing. Int. J. Biol. Macromol. 2023, 253, 127248. [Google Scholar] [CrossRef]
- Fan, Y.; Lüchow, M.; Zhang, Y.; Lin, J.; Fortuin, L.; Mohanty, S.; Brauner, A.; Malkoch, M. Nanogel encapsulated hydrogels as advanced wound dressings for the controlled delivery of antibiotics. Adv. Funct. Mater. 2021, 31, 2006453. [Google Scholar] [CrossRef]
- Xuan, Q.; Jiang, F.; Dong, H.; Zhang, W.; Zhang, F.; Ma, T.; Zhuang, J.; Yu, J.; Wang, Y.; Shen, H.; et al. Bioinspired intrinsic versatile hydrogel fabricated by amyloidal toxin simulant-based nanofibrous assemblies for accelerated diabetic wound healing. Adv. Funct. Mater. 2021, 31, 2106705. [Google Scholar] [CrossRef]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Chen, J.; Guo, J.; Yuan, M.; Wan, G.; Yan, C.; Li, W.; Machens, H.G.; Rinkevich, Y.; et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int. J. Biol. Macromol. 2022, 202, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N, O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.H.; Nisar, S.; Imtiyaz, K.; Nadeem, M.; Mazumdar, N.; Rizvi, M.M.A.; Ahmad, S. Injectable, Self-Healing, and Biocompatible N, O-Carboxymethyl Chitosan/Multialdehyde Guar Gum Hydrogels for Sustained Anticancer Drug Delivery. Biomacromolecules 2021, 22, 3731–3745. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.; Li, Z.; Gu, Z.; Yang, J.; Luo, K. Chitosan-based hydrogel dressings for diabetic wound healing via promoting M2 macrophage-polarization. Carbohydr. Polym. 2024, 331, 121873. [Google Scholar] [CrossRef]
- Chen, R.F.; Wang, C.T.; Chen, Y.H.; Chien, C.M.; Lin, S.D.; Lai, C.S.; Wang, C.J.; Kuo, Y.R. Hyaluronic Acid-Povidone-Iodine Compound Facilitates Diabetic Wound Healing in a Streptozotocin-Induced Diabetes Rodent Model. Plast. Reconstr. Surg. 2019, 143, 1371–1382. [Google Scholar] [CrossRef]
- Bai, Q.; Gao, Q.; Hu, F.; Zheng, C.; Chen, W.; Sun, N.; Liu, J.; Zhang, Y.; Wu, X.; Lu, T. Chitosan and hyaluronic-based hydrogels could promote the infected wound healing. Int. J. Biol. Macromol. 2023, 232, 123271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, R.; Song, X.; Gong, J.; Wang, J.; Lin, M.; Yang, F.; Cheng, X.; Gao, X.; Peng, L.; et al. Optimization of Maduramicin Ammonium-Loaded Nanostructured Lipid Carriers Using Box-Behnken Design for Enhanced Anticoccidial Effect against Eimeria tenella in Broiler Chickens. Pharmaceutics 2022, 14, 1330. [Google Scholar] [CrossRef]
- Asfour, M.H.; Kassem, A.A.; Salama, A. Topical nanostructured lipid carriers/inorganic sunscreen combination for alleviation of all-trans retinoic acid-induced photosensitivity: Box-Behnken design optimization, in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 134, 219–232. [Google Scholar] [CrossRef]
- Li, C.; Yuan, L.; Zhang, X.; Zhang, A.; Pan, Y.; Wang, Y.; Qu, W.; Hao, H.; Algharib, S.A.; Chen, D.; et al. Core-shell nanosystems designed for effective oral delivery of polypeptide drugs. J. Control. Release 2022, 352, 540–555. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, M.; Jiang, Y.; Ma, G.; Liu, J.; Dawood, A.S.; Xie, S.; Algharib, S.A. Manipulated Slow Release of Florfenicol Hydrogels for Effective Treatment of Anti-Intestinal Bacterial Infections. Int. J. Nanomed. 2025, 20, 541–555. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Jiang, Y.; Liu, J.; Algharib, S.A.; Dawood, A.S.; Xie, S. Nanogel Dressing with Targeted Glucose Reduction and pH/Hyaluronidase Dual-Responsive Release for Synergetic Therapy of Diabetic Bacterial Wounds. Gels 2025, 11, 380. https://doi.org/10.3390/gels11060380
Luo W, Jiang Y, Liu J, Algharib SA, Dawood AS, Xie S. Nanogel Dressing with Targeted Glucose Reduction and pH/Hyaluronidase Dual-Responsive Release for Synergetic Therapy of Diabetic Bacterial Wounds. Gels. 2025; 11(6):380. https://doi.org/10.3390/gels11060380
Chicago/Turabian StyleLuo, Wanhe, Yongtao Jiang, Jinhuan Liu, Samah Attia Algharib, Ali Sobhy Dawood, and Shuyu Xie. 2025. "Nanogel Dressing with Targeted Glucose Reduction and pH/Hyaluronidase Dual-Responsive Release for Synergetic Therapy of Diabetic Bacterial Wounds" Gels 11, no. 6: 380. https://doi.org/10.3390/gels11060380
APA StyleLuo, W., Jiang, Y., Liu, J., Algharib, S. A., Dawood, A. S., & Xie, S. (2025). Nanogel Dressing with Targeted Glucose Reduction and pH/Hyaluronidase Dual-Responsive Release for Synergetic Therapy of Diabetic Bacterial Wounds. Gels, 11(6), 380. https://doi.org/10.3390/gels11060380








