Graphene Oxide-Modulated Nanocellulose/Polyacrylamide/Sodium Alginate Hierarchical Network Hydrogel for Flexible Sensing
Abstract
1. Introduction
2. Results and Discussion
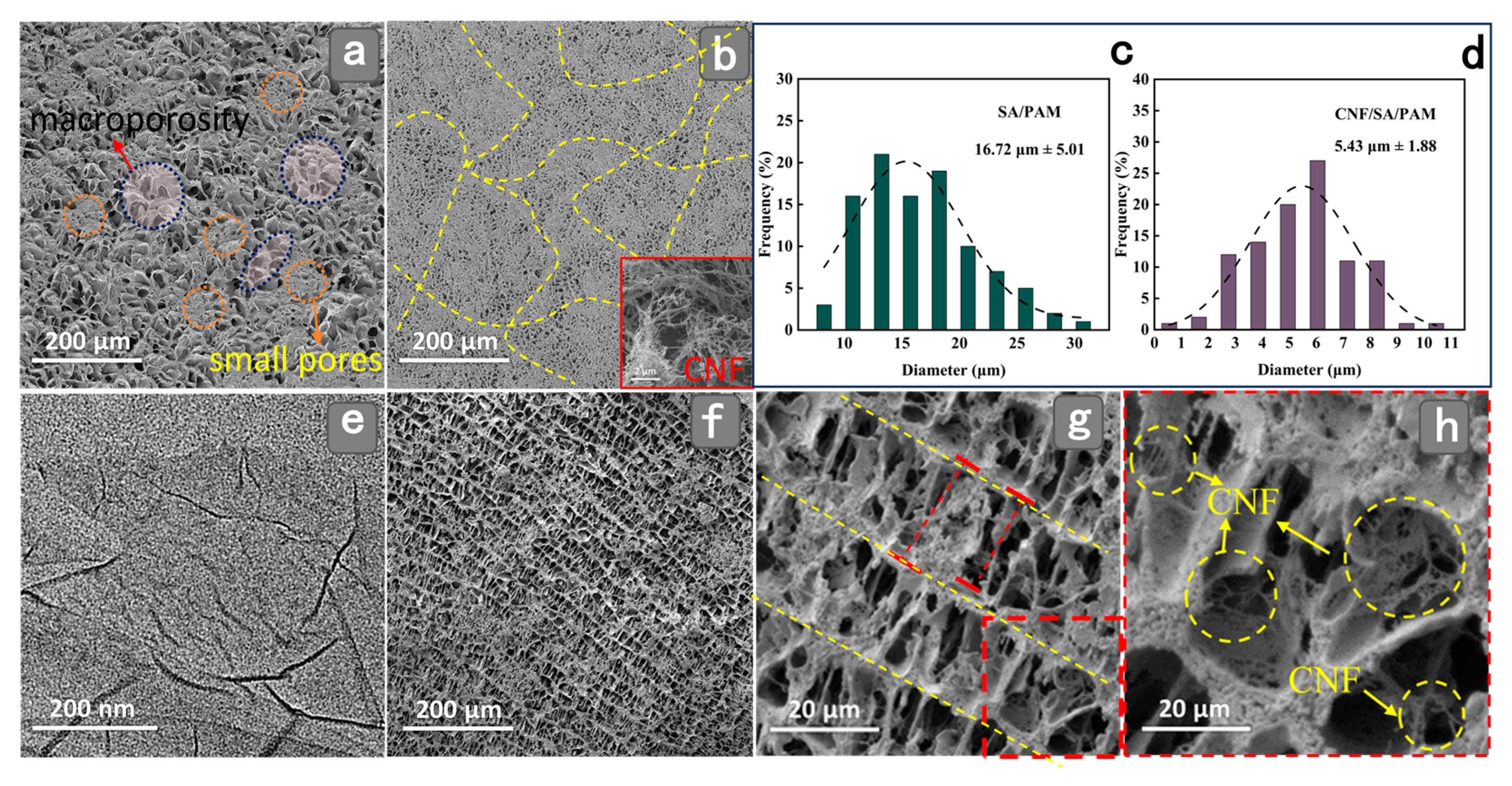
2.1. Micro-Morphological Analysis of Hydrogels
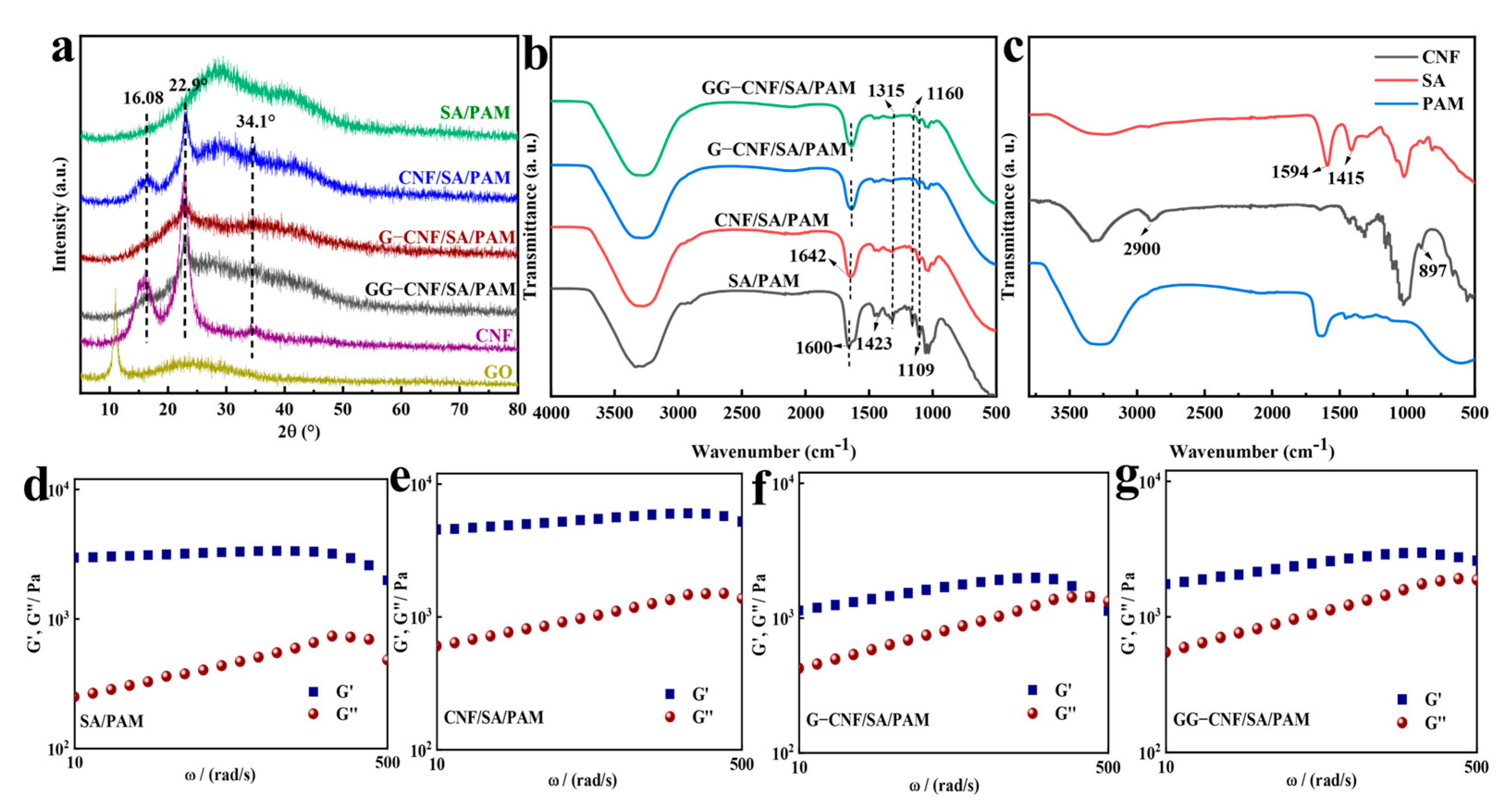
2.2. Chemical Structure Analysis of Hydrogels
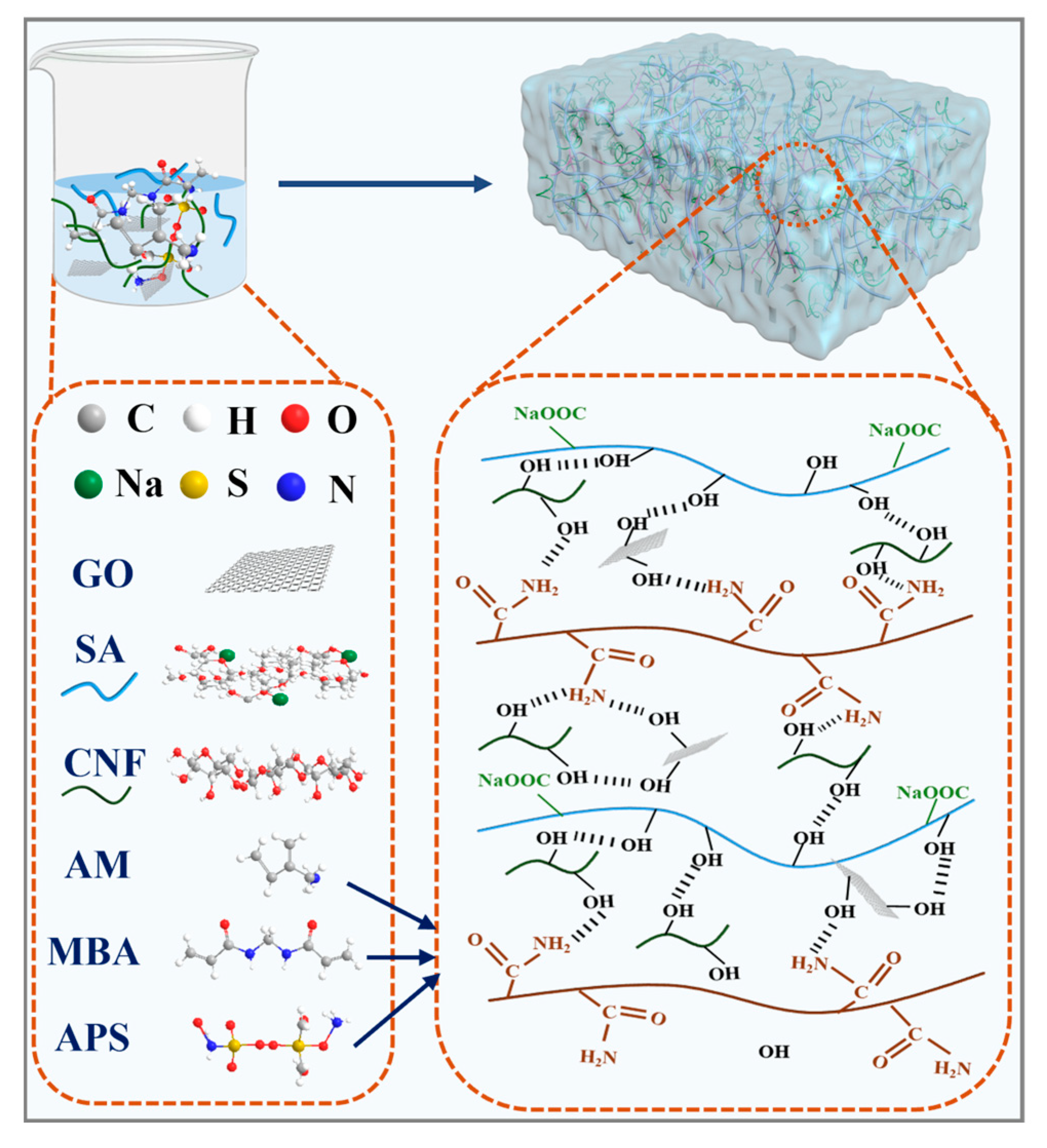
2.3. Reaction Mechanism Diagram of GG-CNF/SA/PAM
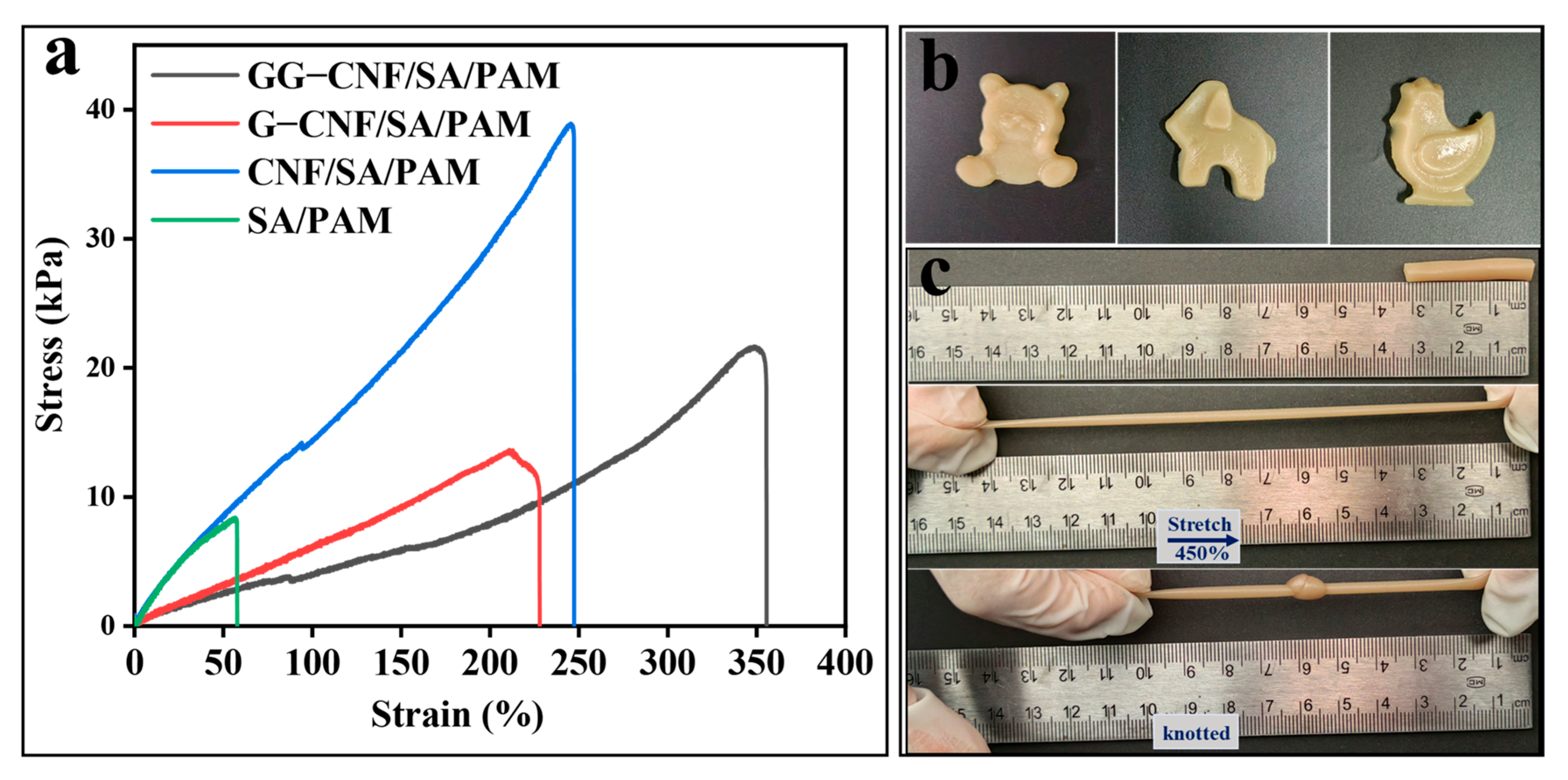
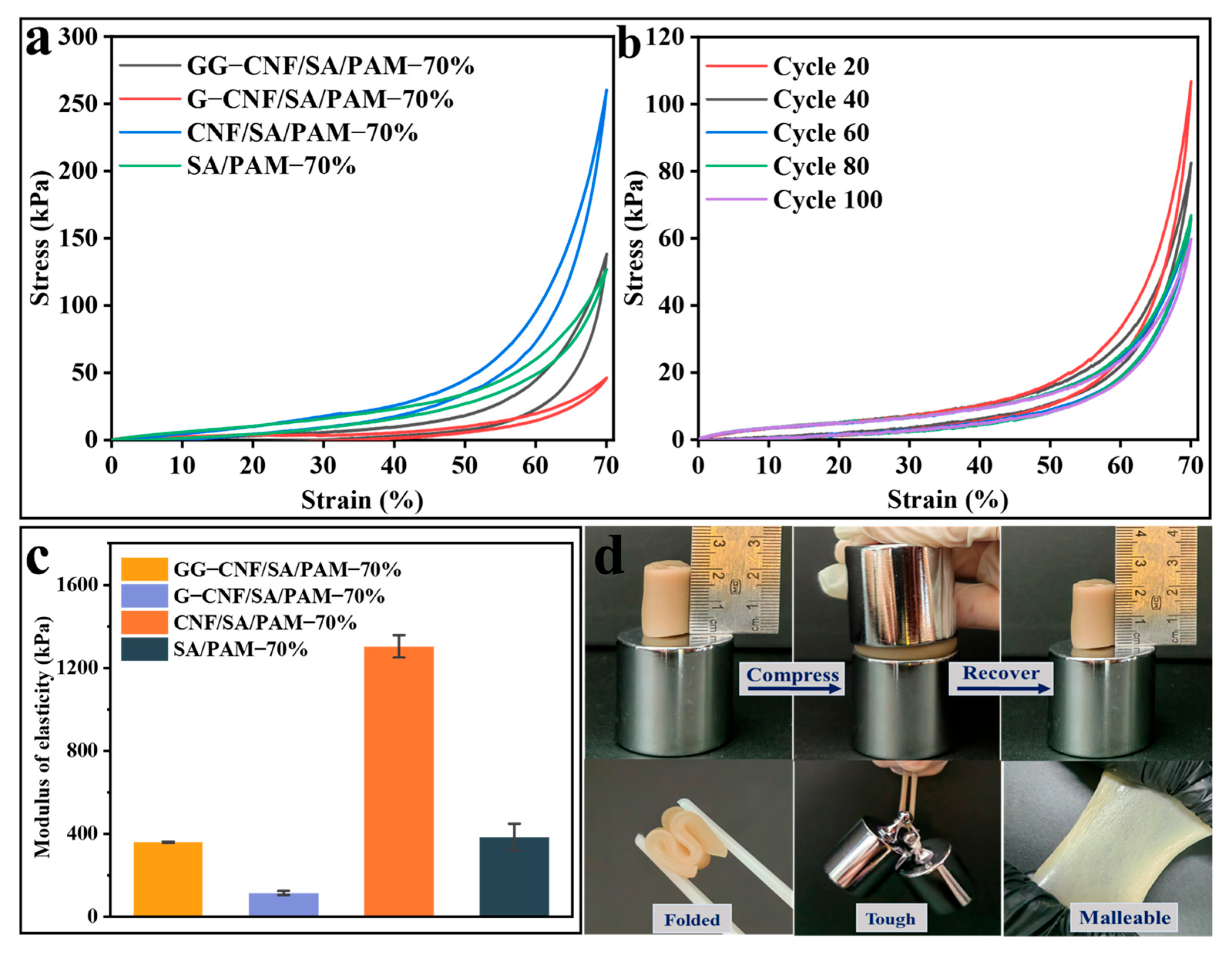
2.4. Analysis of Mechanical Properties of Hydrogels
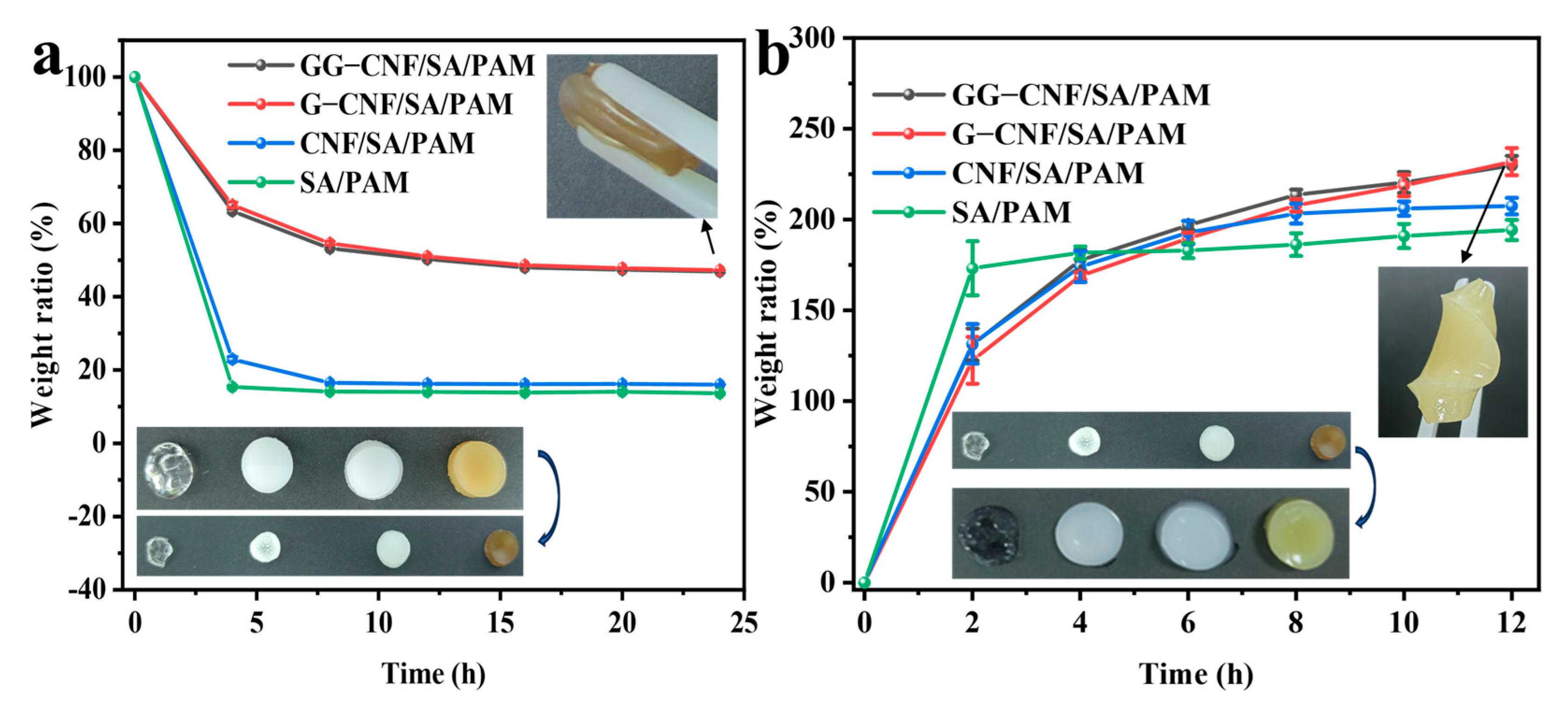
2.5. Analysis of the Anti-Drying Properties of Hydrogels
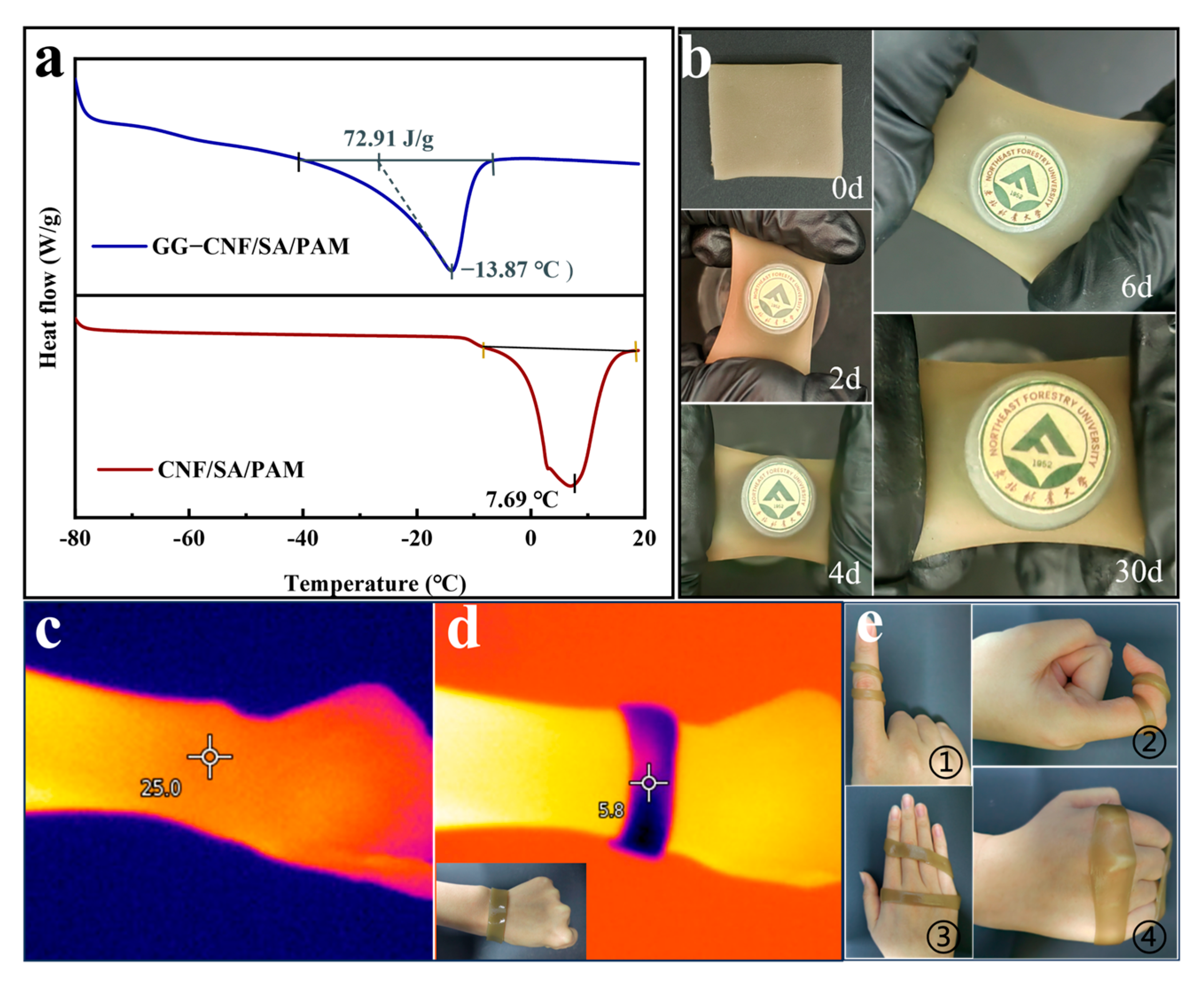
2.6. Analysis of Anti-Freezing Properties of Hydrogels
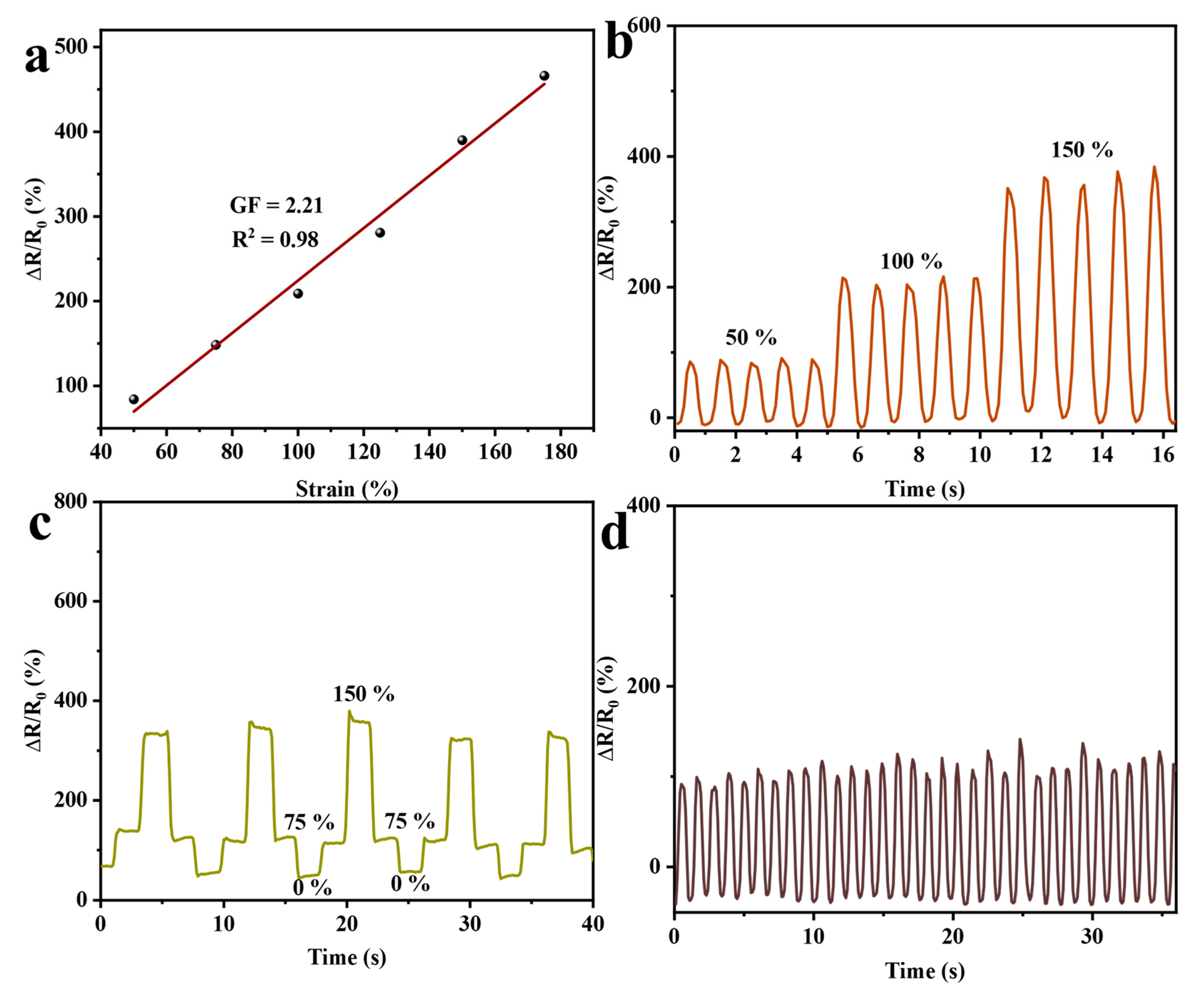
2.7. Analysis of the Sensing Properties of Hydrogels
2.8. Application Analysis of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Characterisation of Hydrogels
4.4. Anti-Drying Test
4.5. Swelling Test
4.6. Mechanical Test
4.7. Electrochemical Testing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Gao, G.; Xu, Z.; Tang, D.; Chen, T. Recent Progress in Bionic Skin Based on Conductive Polymer Gels. Macromol. Rapid Commun. 2021, 42, 2100480. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, Z.; Mahmoudpour, M.; Rahimpour, E.; Jouyban, A. Nanomaterial based PVA nanocomposite hydrogels for biomedical sensing: Advances toward designing the ideal flexible/wearable nanoprobes. Adv. Colloid Interface Sci. 2022, 305, 102705. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, F.; He, W.; Hang, T.; Li, Z.; Zheng, J.; Li, X.; Jiang, S.; Chen, Y. Anti-freezing, recoverable and transparent conductive hydrogels co-reinforced by ethylene glycol as flexible sensors for human motion monitoring. Int. J. Biol. Macromol. 2023, 230, 123117. [Google Scholar] [CrossRef]
- Zaidi, S.F.A.; Saeed, A.; Ho, V.-C.; Heo, J.H.; Cho, H.H.; Sarwar, N.; Lee, N.-E.; Mun, J.; Lee, J.H. Chitosan-reinforced gelatin composite hydrogel as a tough, anti-freezing, and flame-retardant gel polymer electrolyte for flexible supercapacitors. Int. J. Biol. Macromol. 2023, 234, 123725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, W.; Ma, Z.; Zhao, H.; Ren, L. Cartilage-bioinspired tenacious concrete-like hydrogel verified via in-situ testing. Nat. Commun. 2025, 16, 2309. [Google Scholar] [CrossRef]
- You, J.; Lu, M.; Dazhen, L.; Gao, M.; Zhang, R.; Li, W.; Lei, F.; Ren, W.; Li, G.; Yang, J. Anti-Motion Artifacts Iontronic Sensor for Long-Term Accurate Fingertip Pulse Monitoring. Adv. Sci. 2025, 12, e2414425. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, K.; Huang, J.; Sun, X.; Li, J.; Cheng, Y.; Sun, Y.; Shi, Y.; Pan, L. Mechanically Robust, Flexible, Fast Responding Temperature Sensor and High-Resolution Array with Ionically Conductive Double Cross-Linked Hydrogel. Adv. Funct. Mater. 2024, 34, 2314433. [Google Scholar] [CrossRef]
- Rao, V.K.; Shauloff, N.; Sui, X.; Wagner, H.D.; Jelinek, R. Polydiacetylene hydrogel self-healing capacitive strain sensor. J. Mater. Chem. C 2020, 8, 6034–6041. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W.; Yang, W.; Chen, J.; Peng, Q.; Wang, T.; Yang, D.; Wang, J.; Zhang, H.; Zeng, H. Stretchable, compressible, and conductive hydrogel for sensitive wearable soft sensors. J. Colloid Interface Sci. 2022, 618, 111–120. [Google Scholar] [CrossRef]
- Truskewycz, A.; Beker, S.A.; Ball, A.S.; Murdoch, B.; Cole, I. Incorporation of quantum carbon dots into a PVP/ZnO hydrogel for use as an effective hexavalent chromium sensing platform. Anal. Chim. Acta 2020, 1099, 126–135. [Google Scholar] [CrossRef]
- Liu, S.; Chen, B.; Wang, Y.; Wang, H.; Cheng, X.; Wang, H.; Qiu, J.; Du, Z. Ultrastrength High-Sensitivity Poly (acrylic acid)-Based Deep Eutectic Solvents Gel for Wearable Strain Sensing and Human Health Monitoring. ACS Appl. Polym. Mater. 2024, 6, 5385–5393. [Google Scholar] [CrossRef]
- Li, X.; Yan, M.; Xiao, J.; Lian, H. Ultrafast fabrication of deep eutectic solvent flexible ionic gel with high-transmittance, freeze-resistant and conductivity by frontal polymerization. J. Colloid Interface Sci. 2023, 650, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Peng, H.; Chen, Z.; Zhang, J. Ultrasensitive Soft Sensor from Anisotropic Conductive Biphasic Liquid Metal-Polymer Gels. Adv. Mater. 2024, 36, 2305707. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanizamani, F.; Moulahoum, H.; Celik, E.G.; Timur, S. Ionic liquids enhancement of hydrogels and impact on biosensing applications. J. Mol. Liq. 2022, 357, 119075. [Google Scholar] [CrossRef]
- Lee, J.; Le, Q.T.; Lee, D.; Nam, S.; Nguyen, T.H.; Song, Y.; Sung, J.; Son, S.-W.; Kim, J. Micropyramidal flexible ion gel sensor for multianalyte discrimination and strain compensation. ACS Appl. Mater. Interfaces 2023, 15, 26138–26147. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Pakharenko, V.; Graziano, A.; Leão, A.L.; Tjong, J.; Jaffer, S.; Cui, T.; Filleter, T.; Sain, M. Molecular design and structural optimization of nanocellulose-based films fabricated via regioselective functionalization for flexible electronics. Chem. Eng. J. 2022, 440, 135950. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Gao, M.; Guo, Y.; Xu, T.; Jiang, H.; Zhang, Z.; Ji, X.; Si, C. Nanocellulose-based functional materials for physical, chemical, and biological sensing: A review of materials, properties, and perspectives. Ind. Crops Prod. 2024, 212, 118326. [Google Scholar] [CrossRef]
- Passornraprasit, N.; Siripongpreda, T.; Ninlapruk, S.; Rodthongkum, N.; Potiyaraj, P. γ-Irradiation crosslinking of graphene oxide/cellulose nanofiber/poly (acrylic acid) hydrogel as a urea sensing patch. Int. J. Biol. Macromol. 2022, 213, 1037–1046. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Xiao, C.; Xiao, S.; Wang, C.; Nie, Q.; Xu, P.; Chen, J.; You, R.; Zhang, G. Flexible SERS wearable sensor based on nanocomposite hydrogel for detection of metabolites and pH in sweat. Chem. Eng. J. 2023, 474, 145953. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, X.; Jin, W.; Mei, F.; Xu, Y.; He, L.; Tan, S.; Cai, G.; Cheng, D.; Wang, X.J.I.C.; et al. Conductive, self-healing and adhesive cellulose nanofibers-based hydrogels as wearable strain sensors and supercapacitors. Ind. Crops Prod. 2025, 225, 120547. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Sharma, T.S.K. Development of biocompatible cellulose microfiber stabilized carbon nanofiber hydrogel for the efficient electrochemical determination of nicotinamide adenine dinucleotide in physiological fluids. J. Electrochem. Soc. 2019, 166, B581. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Nasiri, N.; Duffin, R.N.; Raghuwanshi, V.S.; Mata, J.; Simon, G.P.; Hooper, J.F.; Garnier, G. Multifunctional graft-IPN hydrogels of cellulose nanofibers and poly (N-isopropyl acrylamide) via silver-promoted decarboxylative radical polymerization. Mater. Today Chem. 2024, 37, 102014. [Google Scholar] [CrossRef]
- Träger, A.; Carlmark, A.; Wågberg, L. Interpenetrated networks of nanocellulose and polyacrylamide with excellent mechanical and absorptive properties. Macromol. Mater. Eng. 2018, 303, 1700594. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Sun, X.; Yue, Y.; Tubana, B.; Yang, R.; Cheng, H.N. Novel alginate-cellulose nanofiber-poly (vinyl alcohol) hydrogels for carrying and delivering nitrogen, phosphorus and potassium chemicals. Int. J. Biol. Macromol. 2021, 172, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, S.; Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023, 300, 120243. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, H.; Lai, J.; Jin, X.; Liu, H.; Wu, P.; Chen, W.; Ma, A. Self-recoverable, stretchable, and sensitive wearable sensors based on ternary semi-interpenetrating ionic hydrogels. ACS Appl. Polym. Mater. 2021, 3, 2732–2741. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, Y. Ion-based double network hydrogel with compressive, conductive, and sensing properties for sports monitoring. AIP Adv. 2023, 13, 0168550. [Google Scholar] [CrossRef]
- Shan, C.; Bauman, L.; Che, M.; Kim, A.-R.; Su, R.; Zhao, B. Organohydrogels with cellulose nanofibers enhanced supramolecular interactions toward high performance self-adhesive sensing pads. Carbohydr. Polym. 2023, 320, 121211. [Google Scholar] [CrossRef]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan–carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef]
- Puspitasari, F.H.; Nurdiansyah; Salamah, U.; Sari, N.R.; Maddu, A.; Solikhin, A.J.F. Potential of chitosan hydrogel based activated carbon nanoparticles and non-activated carbon nanoparticles for water purification. Polymers 2020, 21, 701–708. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Jiao, R.; Yu, H. A Flexible Microstructured Pressure Sensor with High Performance Based on Vertically Aligned Carbon Nanotubes and Ion Gel. IEEE Sens. Lett. 2023, 7, 2502104. [Google Scholar] [CrossRef]
- Tong, H.; Li, S.; Dong, X.; Xing, Z.; Li, H.; Liu, X. Enhanced carbon dispersion of polyacrylamide/sodium alginate hydrogels via irregular copolymerization imidazolyl ionic liquid for flexible sensor. J. Polym. Res. 2025, 32, 119. [Google Scholar] [CrossRef]
- Mianehrow, H.; Lo Re, G.; Carosio, F.; Fina, A.; Larsson, P.T.; Chen, P.; Berglund, L.A. Strong reinforcement effects in 2D cellulose nanofibril–graphene oxide (CNF–GO) nanocomposites due to GO-induced CNF ordering. J. Mater. Chem. A 2020, 8, 17608–17620. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Ali, G.A.; Algarni, H.; Sarkar, S.M.; Chong, K.F. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019, 12, 973–990. [Google Scholar] [CrossRef]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z.J.P. Anisotropic cellulose nanofibers/polyvinyl alcohol/graphene aerogels fabricated by directional freeze-drying as effective oil adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef]
- Wang, T.; Jung, J.; Zhao, Y. Isolation, characterization, and applications of holocellulose nanofibers from apple and rhubarb pomace using eco-friendly approach. Food Bioprod. Process. 2022, 136, 166–175. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Functional gels based on chemically modified graphenes. Adv. Mater. 2014, 26, 3992–4012. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly (vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Chutturi, M.; Gillela, S.; Yadav, S.M.; Wibowo, E.S.; Sihag, K.; Rangppa, S.M.; Bhuyar, P.; Siengchin, S.; Antov, P.; Kristak, L. A comprehensive review of the synthesis strategies, properties, and applications of transparent wood as a renewable and sustainable resource. Sci. Total. Environ. 2023, 864, 161067. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, L.; Zhang, P.; Li, D.; Guo, Z.; Hu, L. Graphene oxide-based composite organohydrogels with high strength and low temperature resistance for strain sensors. Soft Matter 2022, 18, 1201–1208. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf. B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef]
- Yu, W.; Yi, Y.; Wang, H.; Yang, Y.; Xing, C.; Zeng, L.; Tang, J.; Tan, Z. Effects of residual pectin composition and content on the properties of cellulose nanofibrils from ramie fibers. Carbohydr. Polym. 2022, 298, 120112. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, C.; Zhao, Y.; Li, Y.; Yu, S.; Huang, L. Cellulose nanofiber-based hybrid hydrogel electrode with superhydrophilicity enabling flexible high energy density supercapacitor and multifunctional sensors. Int. J. Biol. Macromol. 2024, 276, 134003. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly (vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-pot assembly of microfibrillated cellulose reinforced PVA–borax hydrogels with self-healing and pH-responsive properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Wang, A.; Huang, A.; Chen, J.; Xu, J.; Wong, C.-P. Anti-freezing flexible aqueous Zn–MnO2 batteries working at −35 °C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J. Mater. Chem. A 2020, 8, 6828–6841. [Google Scholar] [CrossRef]
- Aoki, D.; Lossada, F.; Hoenders, D.; Ajiro, H.; Walther, A. Efficient softening and toughening strategies of cellulose nanofibril nanocomposites using comb polyurethane. Biomacromolecules 2022, 23, 1693–1702. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, H.; Zhao, R.; Ye, Z.; Deng, H.; Xiao, B.; Zhu, J. Amine-caged ZrO2@GO multilayer core-shell hybrids in epoxy matrix for enhancing interfacial adhesion of carbon fiber composites. Compos. Part B Eng. 2022, 245, 110207. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Wang, L.; Ge, X.; Fu, M.; Wang, P.; Wang, Q. High-strength nanocomposite hydrogels with swelling-resistant and anti-dehydration properties. Polymers 2018, 10, 1025. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2017, 28, 1704195. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Belyi, V.A.; Kononov, L.O.; Martinson, E.A.; Litvinets, S.G. Pectin–glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT: Sulfonated lignin as conductive materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Kościelniak, P.; Więckowska, A.; Karbarz, M.; Kaniewska, K. Nanocomposite hydrogel for skin motion sensing–An antifreezing, nanoreinforced hydrogel with decorated AuNP as a multicrosslinker. J. Colloid Interface Sci. 2024, 674, 392–404. [Google Scholar] [CrossRef]
- Khan, M.; Rahman, T.U.; Shah, L.A.; Akil, H.M.; Fu, J.; Yoo, H.-M. Multi-role conductive hydrogels for flexible transducers regulated by MOFs for monitoring human activities and electronic skin functions. J. Mater. Chem. B 2024, 12, 6190–6202. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhong, H.; Guo, M.; Li, J. Efficient Adsorption and Utilisation of Methylene Blue by NaOH-Modified Nanocellulose–Polyacrylamide Interpenetrating Network Gels. Gels 2025, 11, 252. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhong, H.; Guo, M.; Chen, X.; Lu, Y. Construction of a non-toxic interpenetrating network hydrogel drug carrier supported by carbon microspheres and nanocellulose. Carbohydr. Polym. 2025, 350, 123035. [Google Scholar] [CrossRef]
- Wang, J.; Qu, J.; Liu, Y.; Wang, S.; Liu, X.; Chen, Y.; Qi, P.; Miao, G.; Liu, X. “Crocodile skin” ultra-tough, rapidly self-recoverable, anti-dry, anti-freezing, MoS2-based ionic organohydrogel as pressure sensors. Eng. Asp. 2021, 625, 126458. [Google Scholar] [CrossRef]
- Li, H.; Fang, S.; Wang, P.; Feng, S.; Yu, Y.; Zhang, H.; Liu, Y.; Guo, J. Preparation of hydrogen-bonded supramolecular dual-network hydrogels with tunable mechanical properties. Polym. Eng. Sci. 2024, 64, 184–195. [Google Scholar] [CrossRef]
- Du, X.-Y.; Zhang, X.-M.; Du, C.; Song, Y.-J.; Liu, J.-D.; Zhao, Z.-B.; Gao, J. Facile preparation of self-healing, adhesive and patterned gels via frontal polymerization for wearable sensing and actuating applications. Chem. Eng. J. 2024, 498, 155185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lu, Y.; Wang, J.; Huang, C.; Guo, M.; Gao, X. Graphene Oxide-Modulated Nanocellulose/Polyacrylamide/Sodium Alginate Hierarchical Network Hydrogel for Flexible Sensing. Gels 2025, 11, 379. https://doi.org/10.3390/gels11060379
Wang Y, Lu Y, Wang J, Huang C, Guo M, Gao X. Graphene Oxide-Modulated Nanocellulose/Polyacrylamide/Sodium Alginate Hierarchical Network Hydrogel for Flexible Sensing. Gels. 2025; 11(6):379. https://doi.org/10.3390/gels11060379
Chicago/Turabian StyleWang, Yanan, Yanan Lu, Jiaming Wang, Chensen Huang, Minghui Guo, and Xing Gao. 2025. "Graphene Oxide-Modulated Nanocellulose/Polyacrylamide/Sodium Alginate Hierarchical Network Hydrogel for Flexible Sensing" Gels 11, no. 6: 379. https://doi.org/10.3390/gels11060379
APA StyleWang, Y., Lu, Y., Wang, J., Huang, C., Guo, M., & Gao, X. (2025). Graphene Oxide-Modulated Nanocellulose/Polyacrylamide/Sodium Alginate Hierarchical Network Hydrogel for Flexible Sensing. Gels, 11(6), 379. https://doi.org/10.3390/gels11060379





