Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments
Abstract
1. Introduction
1.1. Skin Disorders
1.2. Wound Care and Skin Cancer
1.3. Skin Infections and Inflammation
1.4. Targeted Drug Delivery in Dermatology
| Therapy | Side Effects | Skin Pathology |
|---|---|---|
| Topical Therapies | ||
| Corticosteroids (e.g., hydrocortisone and betamethasone) | Skin thinning, skin atrophy, telangiectasia, irritation, folliculitis, and striae | Psoriasis [19,21] and atopic dermatitis (AD) [13,14] |
| Phototherapy (e.g., narrow-band UVB, Psoralen + UV-A − PUVA) and photodynamic therapy (PDT) | Nausea, skin burning, and increased skin cancer risk | Atopic dermatitis (AD) [13], psoriasis [19,21], and skin cancer [8] |
| Antibiotics (e.g., clindamycin, tetracycline, macrolides, and isotretinoin) | Antibiotics resistance, gastrointestinal issues, and nausea | Rosacea [17], acne [44], skin infections [43], and skin wounds [9,25] |
| Chemotherapy (5-fluorouracil and imiquimod) | Irritation, redness, pigmentation changes, and skin ulceration | Squamous cells carcinoma, basal cell carcinoma [34] |
| Systemic Therapies | ||
| Immunomodulatory (e.g., cyclosporin, methotrexate, sulfasalazine, and cemiplimab) | Increased infection risk, renal impairment, hypertension, hypersensitivity reaction, and nausea | Psoriasis [19,21], atopic dermatitis (AD) [13], and skin cancer [8] |
| Chemotherapy (dacarbazine, paclitaxel, and cisplatin) | Nausea, skin rash, pruritus hyperpigmentation, hypersensitivity, and hair loss | Melanoma [38], squamous cells carcinoma, and basal cell carcinoma [34] |
| JAK inhibitors (e.g., abrocitinib, upadacitinib, baricitinib, deuruxolitinib, vemurafenib, and dabrafenib) | Nausea, headache, and increased risk of infections | Atopic dermatitis (AD) [13] and non-melanoma and melanoma skin cancer [8] |
| TNF-α inhibitors (e.g., adalimumab, infliximab, and remicade) | Injection site reactions and increased infection risk | Psoriasis [19,21] and acne [45] |
| IL-17 inhibitors (e.g., secukinumab, bimekizumab, and brodalumab) | Diarrhea, injection site reactions, and risk of infections | Psoriasis [19,21] |
2. Liposomal Formulations
2.1. Structural and Functional Properties of Liposomes
2.1.1. Classification of Liposomes Based on the Number of Lipid Bilayers
- Unilamellar Vesicles
- Multivesicular and Multivesicular Vesicles
2.1.2. Structural Variations on Liposome Functionality
2.1.3. Functional Modifications of Liposomes
2.1.4. Applications of Liposomes in Target Drug Delivery
| Type | Description | Preparations Methods |
|---|---|---|
| Conventional Liposomes | Basic liposomes used for drug delivery, typically composed of natural phospholipids | Thin-film hydration [77] and sonication |
| Fusogenic Liposomes | Designed to facilitate the fusion of the liposome with cellular membranes, enhancing drug delivery | Incorporation of fusogenic agents (e.g., DOPE) [98] |
| Prolonged-Circulating Liposomes | Modified to evade the immune system and prolong circulation time in the bloodstream | PEGylation during liposome preparation [99] |
| pH-sensitive Liposomes | Designed to release their contents in response to pH changes | Incorporation of pH-sensitive lipids during formation [100] |
| Thermosensitive Liposomes | Respond to elevated temperatures, releasing their encapsulated compounds upon hyperthermic stimuli | Positively charged liposomes that enhance cellular uptake and transfection efficiency [102] |
| Cationic Liposomes | Positively charged liposomes that enhance cellular uptake and transfection efficiency | Formed using cationic lipids (e.g., DOTAP and DODAC) [103] |
| Immunoliposomes | Engineered to target specific cells or tissues by attaching antibodies or ligands to their surface | Coupling antibodies to pre-formed liposomes [105] |
2.2. Therapeutic Applications of Liposomes in Dermatology
2.2.1. Skin Inflammation
2.2.2. Skin Cancer
2.2.3. Wound Care
2.2.4. Skin Infections
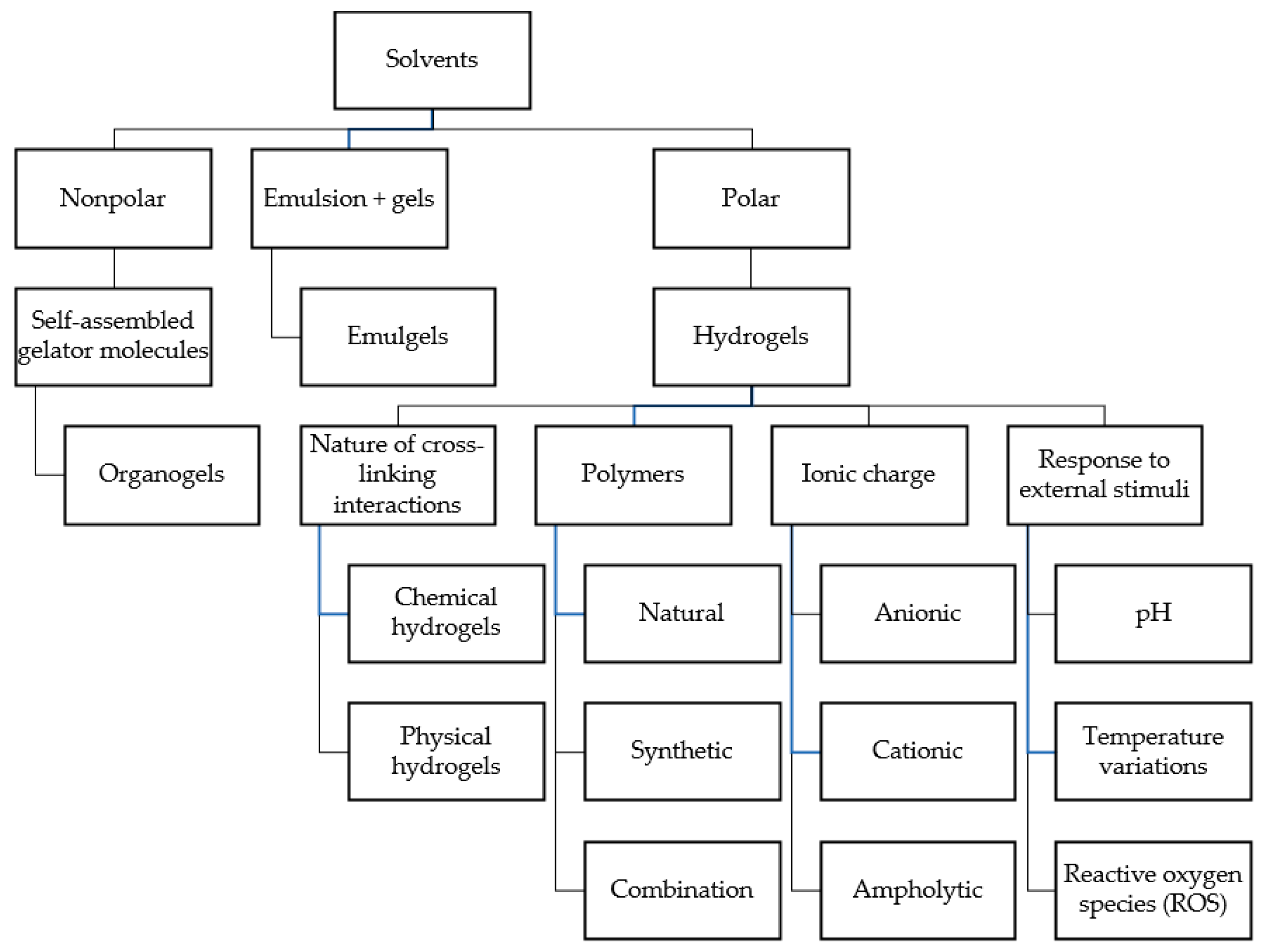
3. Gel-Based Formulations
3.1. Structural and Functional Properties of Gel-Based Formulations
3.2. Organogels
3.3. Hydrogels
3.3.1. Classification of Hydrogels Based on Cross-Linking and Ionic Charge
3.3.2. Responsive Hydrogels
3.3.3. Therapeutic Role of Hydrogels and Their Active Functionality
3.4. Emulgels
3.5. Therapeutic Applications of Gel-Based Formulations in Dermatology
| Type | Description | Gelling Agent Examples |
|---|---|---|
| Organogels | Gels formed with a nonpolar solvent immobilized within a three-dimensional network of gelator molecules | Stigmasterol [172] and lecithin [173] |
| “Natural“ Hydrogels | Water-insoluble natural polymer capable of absorbing significant amounts of water; highly biodegradable and biocompatible | Collagen [177], alginate [178], chitosan [179], gelatin [180], and silk fibroin [181] |
| Synthetic Hydrogels | Gel systems obtained by dispersing synthetic polymers in a liquid phase; more tunable than natural ones | Polycaprolactone [186], PVP [187], PLA [188], PEG [189], and PVA [190] |
| Charge-Holder Gels | Hydrogels categorized based on their ionic charge: anionic, cationic, and ampholytic; commonly used in drug delivery | Polyacrylic acid (anionic) [193], poly (2-vinyl pyridine) (cationic) [194], and SPE (ampholytic) [195] |
| Thermosensitive Gels | Complex systems that respond to temperature changes, often used in drug delivery systems | Poloxamers and poly N-isopropylacrylamide [200] |
| ROS-sensitive | Hydrogels containing bonds sensitive to reactive oxygen species for therapeutic applications | Disulfide bonds and di-selenium bonds [201] |
| Hybrid Systems | Systems combining properties of emulsions and gels for encapsulation of active ingredients while maintaining stability | Natural polysaccharides and synthetic polymers [205] |
| Organogels | Gels formed with a nonpolar solvent immobilized within a three-dimensional network of gelator molecules | Stigmasterol [172] and lecithin [173] |
| Dermatological Disease | Advantages of Liposomes in the Treatment of Pathological Conditions | Advantages of Gels in the Treatment of Pathological Conditions |
|---|---|---|
| Inflammatory Skin Conditions Psoriasis, Atopic Dermatitis, and Eczema | Improved drug penetration and retention in the epidermis and dermis, boosting anti-inflammatory effects while minimizing side effects. | Advanced and enhanced skin penetration; the presence of moisturizing agent helps the overall anti-inflammatory activity of the active ingredients dispersed in the gel formulation. |
| Acne Treatment Acne Vulgaris | Liposomal formulations effectively deliver active ingredients to pilosebaceous units, enhancing treatment outcomes compared to traditional methods. | Possibility to incorporate multiple active ingredients (e.g., benzoyl peroxide and clindamycin), enhancing treatment effectiveness. Most of the formulations are oil-free, avoiding the risk of further clogging pores. |
| Skin Cancer Management Basal Cell Carcinoma and Squamous Cell Carcinoma | Chemotherapeutic agents, such as doxorubicin, are encapsulated to target tumors while minimizing systemic toxicity. | When inserted in a gel formulation, the antineoplastic agents can potentially be released in a more controlled manner, achieving reduced systemic exposure and toxicity. |
| Wound Healing Chronic Wounds and Burns | Vesicular systems deliver growth factors (e.g., EGF and TGF-β) and antimicrobial agents to promote tissue repair and reduce infection risks. | Gels can form a physical barrier, reducing wound infections while helping to maintain optimal moisture levels around the wound. These two aspects are critical for a faster healing process. |
| Hyperpigmentation Disorders Melasma and Post-Inflammatory Hyperpigmentation | Depigmenting agents (e.g., hydroquinone and kojic acid) in vesicular formulations offer improved stability and targeted action while reducing irritation. | Gel formulations are a suitable and alternative vehicle for the active ingredients used to treat this skin condition, allowing even application and absorption. |
| Aesthetic Dermatology Anti-Aging Treatments | Liposomes deliver antioxidants (e.g., vitamin C) and other cosmeceuticals that combat oxidative stress and enhance skin texture. | Injectable gels are being developed for deep injection techniques to lift and rejuvenate aging skin; this formulation is one of the most used in aesthetic medicine. |
3.5.1. Skin Inflammation
3.5.2. Skin Cancer
3.5.3. Wound Care
3.5.4. Skin Infections
4. Limitations and Challenges of Liposomal and Gel Formulations
4.1. Liposomal Formulations
4.2. Gel Formulation
5. Innovations and Emerging Trends
| Product Name | Type (Liposomal or Gel Based) | Indication | FDA Approval Status | Clinical Data Available | Comparative Analysis |
|---|---|---|---|---|---|
| Lidocaine ointment USP, 5% | Liposomal | Mucosal anesthesia, minor burns | ANDA approved (2019) | Bioequivalence study to reference drug; clinical use for intubation lubrication and sunburn relief | Liposomal delivery enhances penetration and prolongs anesthetic effect compared to non-liposomal alternatives [273] |
| Abreva® (Docosanol) | Liposomal cream | Herpes labialis | OTC FDA approved (2000) | Median healing time: 4.1 days vs. 4.8 days placebo (p = 0.0076); reduces pain, burning, and itching (p = 0.002) | Reduces healing time by 18 hrs vs. placebo; targets viral envelope fusion but less effective than Viroxyn [274] |
| Doxil® (Doxorubicin) | PEGylated liposomal | Cancer therapy | FDA approved (1995) | Progression-free survival of 8.6 months vs. 4.2 months for conventional doxorubicin; reduces cardiotoxicity by 60% [275] | Superior safety profile with reduced cardiotoxicity compared to conventional formulations |
| Twyneo® (Tretinoin and Benzoyl Protide) | Microencapsulated gel | Acne vulgaris | FDA approved (2021) | Phase 3 trials: 50% IGA success rate vs. 26% for vehicle; prevents benzoyl peroxide–tretinoin interaction | Combines efficacy with reduced irritation due to microencapsulation [276] |
| Emrosi (DFD-29) | Gel-based | Rosacea | FDA Approved (2024) | Late-stage trials showed superior efficacy in reducing inflammation and redness compared to doxycycline | Demonstrated better safety and efficacy compared to Oracea with no significant safety issues [277,278] |
| Dapsone Gel (Aczone) | Gel-based | Acne and photo-damage | FDA approved (2008) | A meta-analysis of randomized controlled trials was conducted to analyze the efficacy and adverse events of dapsone gel treatment compared with excipient and other drug therapies | Provides sustained release, reducing irritation and improving tolerability over traditional formulations [279,280] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tree, C. Skin and Its Appendages. Available online: https://clinicalpub.com/skin-and-its-appendages/ (accessed on 13 February 2025).
- Stander, S.; Schmelz, M. Skin Innervation. J. Investig. Dermatol. 2024, 144, 1716–1723. [Google Scholar] [CrossRef]
- Nagashima, K.; Tokizawa, K.; Marui, S. Thermal comfort. Handb. Clin. Neurol. 2018, 156, 249–260. [Google Scholar] [PubMed]
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2008, 18, 11–21. [Google Scholar] [PubMed]
- Ito, S.; Wakamatsu, K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Valeyrie-Allanore, L.; Sassolas, B.; Roujeau, J.C. Drug-induced skin, nail and hair disorders. Drug Saf. 2007, 30, 1011–1030. [Google Scholar] [CrossRef]
- Griffiths, C.E.; van de Kerkhof, P.; Czarnecka-Operacz, M. Psoriasis and Atopic Dermatitis. Dermatol. Ther. Heidelb. 2017, 7 (Suppl. 1), 31–41. [Google Scholar] [CrossRef]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of skin cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Tirupathi, R.; Areti, S.; Salim, S.A.; Palabindala, V.; Jonnalagadda, N. Acute bacterial skin and soft tissue infections: New drugs in ID armamentarium. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 310–313. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef]
- Elahi, N.; Astaneh, M.E.; Ai, J.; Rizwan, M. Atopic dermatitis treatment: A comprehensive review of conventional and novel bioengineered approaches. Int. J. Biol. Macromol. 2024, 282, 137083. [Google Scholar]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15, quiz 16–18. [Google Scholar] [CrossRef]
- Loden, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [PubMed]
- Holmes, A.D.; Steinhoff, M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp. Dermatol. 2017, 26, 659–667. [Google Scholar] [PubMed]
- Hoover, R.M.; Erramouspe, J. Role of Topical Oxymetazoline for Management of Erythematotelangiectatic Rosacea. Ann. Pharmacother. 2018, 52, 263–267. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Jee, S.H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schon, M.P.; Stander, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. 3), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The impact of psychological stress on wound healing: Methods and mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81–93. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging treatment strategies in wound care. Int. Wound J. 2022, 19, 1934–1954. [Google Scholar] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar]
- Kim, R.H.; Armstrong, A.W. Nonmelanoma skin cancer. Dermatol. Clin. 2012, 30, 125–139, ix. [Google Scholar]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 29–56. [Google Scholar]
- Anna, B.; Blazej, Z.; Jacqueline, G.; Andrew, C.J.; Jeffrey, R.; Andrzej, S. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev. Dermatol. 2007, 2, 451–469. [Google Scholar] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar]
- Naseri, H.; Safaei, A.A. Diagnosis and prognosis of melanoma from dermoscopy images using machine learning and deep learning: A systematic literature review. BMC Cancer 2025, 25, 75. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecinska-Pirog, J.; Walecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Aly, R. Microbial Infections of Skin and Nails. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Sandoval, A.G.W.; Vaughn, L.T.; Huang, J.T.; Barbieri, J.S. Role of Tumor Necrosis Factor-alpha Inhibitors in the Treatment and Occurrence of Acne: A Systematic Review. JAMA Dermatol. 2023, 159, 504–509. [Google Scholar]
- Ianiri, G.; LeibundGut-Landmann, S.; Dawson, T.L., Jr. Malassezia: A Commensal, Pathogen, and Mutualist of Human and Animal Skin. Annu. Rev. Microbiol. 2022, 76, 757–782. [Google Scholar]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar]
- Zhou, S.; Yao, Z. Roles of Infection in Psoriasis. Int. J. Mol. Sci. 2022, 23, 6955. [Google Scholar] [CrossRef] [PubMed]
- Spada, F.; Barnes, T.M.; Greive, K.A. Emollient formulations containing antiseptics reduce effectively the level of Staphylococcus aureus on skin. Clin. Cosmet. Investig. Dermatol. 2019, 12, 639–645. [Google Scholar]
- Lei, V.; Petty, A.J.; Atwater, A.R.; Wolfe, S.A.; MacLeod, A.S. Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Front. Immunol. 2020, 11, 593901. [Google Scholar]
- Whitley, R.J. Herpes simplex virus infection. Semin. Pediatr. Infect. Dis. 2002, 13, 6–11. [Google Scholar] [CrossRef]
- Rana, H.; Truong, N.R.; Sirimanne, D.R.; Cunningham, A.L. Breaching the Barrier: Investigating Initial Herpes Simplex Viral Infection and Spread in Human Skin and Mucosa. Viruses 2024, 16, 1790. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Kist, L.F.; Pereira, S.B.; Quessada, M.A.; Petek, H.; Pille, A.; Maccari, J.G.; Mutlaq, M.P.; Nasi, L.A. Human papillomavirus infection: Epidemiology, biology, host interactions, cancer development, prevention, and therapeutics. Rev. Med. Virol. 2024, 34, e2537. [Google Scholar]
- Trizna, Z. Viral diseases of the skin: Diagnosis and antiviral treatment. Paediatr. Drugs 2002, 4, 9–19. [Google Scholar] [PubMed]
- Nazarko, L. Fungal skin infections and HCAs: Identify, treat and act. Br. J. Healthc. Assist. 2010, 4, 551–553. [Google Scholar] [CrossRef]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Med. 2015, 25, e44–e58. [Google Scholar]
- Howell, S.A. Dermatopathology and the Diagnosis of Fungal Infections. Br. J. Biomed. Sci. 2023, 80, 11314. [Google Scholar]
- Tuor, M.; LeibundGut-Landmann, S. The skin mycobiome and intermicrobial interactions in the cutaneous niche. Curr. Opin. Microbiol. 2023, 76, 102381. [Google Scholar]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell Infect. Microbiol. 2022, 12, 873253. [Google Scholar]
- Frasca, D.; Strbo, N. Effects of Obesity on Infections with Emphasis on Skin Infections and Wound Healing. J. Dermatol. Skin Sci. 2022, 4, 5–10. [Google Scholar]
- Kyle, A.A.; Dahl, M.V. Topical therapy for fungal infections. Am. J. Clin. Dermatol. 2004, 5, 443–451. [Google Scholar] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [PubMed]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar]
- de Freitas, C.F.; Calori, I.R.; Tessaro, A.L.; Caetano, W.; Hioka, N. Rapid formation of Small Unilamellar Vesicles (SUV) through low-frequency sonication: An innovative approach. Colloids Surf. B Biointerfaces 2019, 181, 837–844. [Google Scholar]
- Ong, S.G.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, H. Preparation of Small Unilamellar Vesicle Liposomes Using Detergent Dialysis Method. Methods Mol. Biol. 2023, 2622, 49–56. [Google Scholar]
- Lapinski, M.M.; Castro-Forero, A.; Greiner, A.J.; Ofoli, R.Y.; Blanchard, G.J. Comparison of liposomes formed by sonication and extrusion: Rotational and translational diffusion of an embedded chromophore. Langmuir 2007, 23, 11677–11683. [Google Scholar] [PubMed]
- Seltzer, S.E.; Gregoriadis, G.; Dick, R. Evaluation of the dehydration-rehydration method for production of contrast-carrying liposomes. Investig. Radiol. 1988, 23, 131–138. [Google Scholar]
- Xiang, B.; Cao, D.Y. Preparation of Drug Liposomes by Thin-Film Hydration and Homogenization; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Pidgeon, C.; McNeely, S.; Schmidt, T.; Johnson, J.E. Multilayered vesicles prepared by reverse-phase evaporation: Liposome structure and optimum solute entrapment. Biochemistry 1987, 26, 17–29. [Google Scholar]
- Giuliano, C.B.; Cvjetan, N.; Ayache, J.; Walde, P. Multivesicular Vesicles: Preparation and Applications. ChemSystemsChem 2021, 3, e2000049. [Google Scholar]
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar]
- Machin, J.M.; Kalli, A.C.; Ranson, N.A.; Radford, S.E. Protein-lipid charge interactions control the folding of outer membrane proteins into asymmetric membranes. Nat. Chem. 2023, 15, 1754–1764. [Google Scholar]
- Li, Y.; Wang, J.; Gao, Y.; Zhu, J.; Wientjes, M.G.; Au, J.L. Relationships between liposome properties, cell membrane binding, intracellular processing, and intracellular bioavailability. AAPS J. 2011, 13, 585–597. [Google Scholar]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar]
- Abd El-Alim, S.H.; Kassem, A.A.; Basha, M.; Salama, A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 563, 293–303. [Google Scholar] [PubMed]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar]
- Kuksis, A. Yolk lipids. Biochim. Biophys. Acta 1992, 1124, 205–222. [Google Scholar] [PubMed]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [PubMed]
- Luo, D.; Li, N.; Carter, K.A.; Lin, C.; Geng, J.; Shao, S.; Huang, W.C.; Qin, Y.; Atilla-Gokcumen, G.E.; Lovell, J.F. Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin-Phospholipids. Small 2016, 12, 3039–3047. [Google Scholar]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar]
- Marquez, M.G.; Dotson, R.; Pias, S.; Frolova, L.V.; Tartis, M.S. Phospholipid prodrug conjugates of insoluble chemotherapeutic agents for ultrasound targeted drug delivery. Nanotheranostics 2020, 4, 40–56. [Google Scholar]
- Purusottam, R.N.; Senicourt, L.; Lacapere, J.J.; Tekely, P. Probing the gel to liquid-crystalline phase transition and relevant conformation changes in liposomes by (13)C magic-angle spinning NMR spectroscopy. Biochim. Biophys. Acta 2015, 1848, 3134–3139. [Google Scholar]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Wlodarczyk, M.; Witkowski, S.; Polewski, K. Phase Transitions and Structural Changes in DPPC Liposomes Induced by a 1-Carba-Alpha-Tocopherol Analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What We Need to Know about Liposomes as Drug Nanocarriers: An Updated Review. Adv. Pharm. Bull. 2023, 13, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Nogueira, E.; Hartl, I.; Gomes, A.C.; Preto, A.; Steinhuber, E.; Muhlgrabner, V.; Repic, M.; Kuttke, M.; Zwirzitz, A.; et al. Fab antibody fragment-functionalized liposomes for specific targeting of antigen-positive cells. Nanomedicine 2018, 14, 123–130. [Google Scholar] [CrossRef]
- Wiedenhoeft, T.; Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Kiss, T.; Csiszar, A.; Csiszar, A.; et al. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 2019, 41, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-X.; Zhang, C.-t.; Yu, X.-N.; Guo, J.-b.; Ma, H.; Liu, K.; Luo, P.; Ren, J. Preparation and pharmacokinetic study of diosmetin long-circulating liposomes modified with lactoferrin. J. Funct. Foods 2022, 91, 105027. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, C. Preparation and Characterization of pH Sensitive Drug Liposomes; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1450. [Google Scholar] [CrossRef]
- Haemmerich, D.; Ramajayam, K.K.; Newton, D.A. Review of the Delivery Kinetics of Thermosensitive Liposomes. Cancers 2023, 15, 398. [Google Scholar] [CrossRef]
- Ho, E.A.; Ramsay, E.; Ginj, M.; Anantha, M.; Bregman, I.; Sy, J.; Woo, J.; Osooly-Talesh, M.; Yapp, D.T.; Bally, M.B. Characterization of cationic liposome formulations designed to exhibit extended plasma residence times and tumor vasculature targeting properties. J. Pharm. Sci. 2010, 99, 2839–2853. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar]
- Petrilli, R.; Eloy, J.O.; Lee, R.J.; Lopez, R.F.V. Preparation of Immunoliposomes by Direct Coupling of Antibodies Based on a Thioether Bond. Methods Mol. Biol. 2018, 1674, 229–237. [Google Scholar] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control Release 2019, 303, 130–150. [Google Scholar] [PubMed]
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Bawab, A.A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicas, L.G.; Marian, E.; Ganea, M.; Frent, O.D.; Maghiar, P.B.; Bodea, F.I.; Dejeu, G.E. Innovative Approaches to Enhancing the Biomedical Properties of Liposomes. Pharmaceutics 2024, 16, 1525. [Google Scholar] [CrossRef]
- de la Maza, A.; Coderch, L.; Lopez, O.; Baucells, J.; Parra, J.L. Permeability changes caused by surfactants in liposomes that model the stratum corneum lipid composition. J. Am. Oil Chem. Soc. 1997, 74, 1–8. [Google Scholar]
- van Alem, C.M.A.; Metselaar, J.M.; van Kooten, C.; Rotmans, J.I. Recent Advances in Liposomal-Based Anti-Inflammatory Therapy. Pharmaceutics 2021, 13, 1004. [Google Scholar] [CrossRef]
- Tran, M.A.; Watts, R.J.; Robertson, G.P. Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigment. Cell Melanoma Res. 2009, 22, 388–399. [Google Scholar] [CrossRef]
- Khasawneh, D.M.; Oweis, R.J.; Alsmadi, M. A Comprehensive Analysis of Liposomal-Based Nanocarriers for Treating Skin and Soft Tissue Infection. Curr. Drug Deliv. 2024, 22, 552–573. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar]
- Faye, O.; Flohr, C.; Kabashima, K.; Ma, L.; Paller, A.S.; Rapelanoro, F.R.; Steinhoff, M.; Su, J.C.; Takaoka, R.; Wollenberg, A.; et al. Atopic dermatitis: A global health perspective. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 801–811. [Google Scholar]
- Sibbald, C. Alopecia Areata: An Updated Review for 2023. J. Cutan Med. Surg. 2023, 27, 241–259. [Google Scholar] [PubMed]
- Di Agosta, E.; Salvati, L.; Corazza, M.; Baiardini, I.; Ambrogio, F.; Angileri, L.; Antonelli, E.; Belluzzo, F.; Bonamonte, D.; Bonzano, L.; et al. Quality of life in patients with allergic and immunologic skin diseases: In the eye of the beholder. Clin. Mol. Allergy 2021, 19, 26. [Google Scholar]
- Ferrucci, S.M.; Tavecchio, S.; Marzano, A.V.; Buffon, S. Emerging Systemic Treatments for Atopic Dermatitis. Dermatol. Ther. Heidelb. 2023, 13, 1071–1081. [Google Scholar]
- Lima, X.T.; Seidler, E.M.; Lima, H.C.; Kimball, A.B. Long-term safety of biologics in dermatology. Dermatol. Ther. 2009, 22, 2–21. [Google Scholar] [PubMed]
- Laniado-Laborin, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Samir, B.; El-Kamel, A.; Zahran, N.; Heikal, L. Resveratrol-loaded invasome gel: A promising nanoformulation for treatment of skin cancer. Drug Deliv. Transl. Res. 2024, 14, 3354–3370. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.D.; Fahr, A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin A. J. Control. Release 2004, 97, 55–66. [Google Scholar] [CrossRef]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta 1973, 298, 1015–1019. [Google Scholar] [PubMed]
- Brotzu, G.; Fadda, A.M.; Manca, M.L.; Manca, T.; Marongiu, F.; Campisi, M.; Consolaro, F. A liposome-based formulation containing equol, dihomo-gamma-linolenic acid and propionyl-l-carnitine to prevent and treat hair loss: A prospective investigation. Dermatol. Ther. 2019, 32, e12778. [Google Scholar] [PubMed]
- Thum, J.; Caspary, L.; Creutzig, A.; Alexander, K. Intra-arterial and intravenous administration of prostaglandin E1 cause different changes to skin microcirculation in patients with peripheral arterial occlusive disease. Vasa 1998, 27, 100–105. [Google Scholar]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal delivery from liposomal formulations—Evolution of the technology over the last three decades. J. Control Release 2016, 242, 126–140. [Google Scholar]
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, M.S.; Mahmud, M.Z.; Amin, M.N.; Mahmud, M.A. Overview of skin cancer types and prevalence rates across continents. Cancer Pathog. Ther. 2024, 2, E01–E36. [Google Scholar]
- Ali, Z.; Yousaf, N.; Larkin, J. Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013, 11, 81–91. [Google Scholar] [PubMed]
- Elder, D. Tumor progression, early diagnosis and prognosis of melanoma. Acta Oncol. 1999, 38, 535–547. [Google Scholar]
- Garbe, C.; Eigentler, T.K. Diagnosis and treatment of cutaneous melanoma: State of the art 2006. Melanoma Res. 2007, 17, 117–127. [Google Scholar]
- Hsan, K.M.; Chen, C.C.; Shyur, L.F. Current research and development of chemotherapeutic agents for melanoma. Cancers 2010, 2, 397–419. [Google Scholar] [CrossRef]
- Matos, C.M. Special Issue “Application Progress of Liposomes in Drug Development”. Int. J. Mol. Sci. 2024, 25, 3454. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar]
- Shiraga, E.; Barichello, J.M.; Ishida, T.; Kiwada, H. A metronomic schedule of cyclophosphamide combined with PEGylated liposomal doxorubicin has a highly antitumor effect in an experimental pulmonary metastatic mouse model. Int. J. Pharm. 2008, 353, 65–73. [Google Scholar] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar]
- Li, X.Y.; Tan, L.C.; Dong, L.W.; Zhang, W.Q.; Shen, X.X.; Lu, X.; Zheng, H.; Lu, Y.G. Susceptibility and Resistance Mechanisms During Photodynamic Therapy of Melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar]
- Rensen, P.C.; Love, W.G.; Taylor, P.W. In vitro interaction of zinc(II)-phthalocyanine-containing liposomes and plasma lipoproteins. J. Photochem. Photobiol. B 1994, 26, 29–35. [Google Scholar]
- Goncalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar]
- Zhang, J.; Chen, B.; Gan, C.; Sun, H.; Zhang, J.; Feng, L. A Comprehensive Review of Small Interfering RNAs (siRNAs): Mechanism, Therapeutic Targets, and Delivery Strategies for Cancer Therapy. Int. J. Nanomed. 2023, 18, 7605–7635. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y. Virosomes: Evolution of the liposome as a targeted drug delivery system. Adv. Drug Deliv. Rev. 2000, 43, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Liu, X.; Guo, Y.; Wu, Y.; Zhang, L.; Yang, Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014, 35, 3840–3850. [Google Scholar] [CrossRef]
- Golestani, P. Lipid-based nanoparticles as a promising treatment for the skin cancer. Heliyon 2024, 10, e29898. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Ternullo, S.; Basnet, P.; Holsaeter, A.M.; Flaten, G.E.; de Weerd, L.; Skalko-Basnet, N. Deformable liposomes for skin therapy with human epidermal growth factor: The effect of liposomal surface charge. Eur. J. Pharm. Sci. 2018, 125, 163–171. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Wang, H.; Du, S. Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: Preparation optimization, in vitro dermal permeation, and in vivo bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar]
- Bian, D.; Liu, M.; Li, Y.; Xia, Y.; Gong, Z.; Dai, Y. Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J. Biochem. Mol. Toxicol. 2012, 26, 399–406. [Google Scholar] [PubMed]
- Günal, M.Y.; Ayla, Ş.; Bedri, N.; Beker, M.Ç.; Çağlayan, A.B.; Aslan, İ.; Özdemir, E.M.; Yeşilada, E.; Kılıç, Ü. The effects of topical liposomal resveratrol on incisional and excisional wound healing process. Turkderm 2019, 53, 128–134. [Google Scholar]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar]
- Rizkita, L.D.; Putri, R.G.P.; Farid, M.; Rizkawati, M.; Wikaningtyas, P. Liposome drug delivery in combating the widespread topical antibiotic resistance: A narrative review. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 90. [Google Scholar]
- Panthi, V.K.; Fairfull-Smith, K.E.; Islam, N. Liposomal drug delivery strategies to eradicate bacterial biofilms: Challenges, recent advances, and future perspectives. Int. J. Pharm. 2024, 655, 124046. [Google Scholar]
- Del Giudice, P. Skin Infections Caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, adv00110. [Google Scholar]
- Spernovasilis, N.; Psichogiou, M.; Poulakou, G. Skin manifestations of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2021, 34, 72–79. [Google Scholar] [PubMed]
- Hajiahmadi, F.; Alikhani, M.Y.; Shariatifar, H.; Arabestani, M.R.; Ahmadvand, D. The bactericidal effect of liposomal vancomycin as a topical combating system against Methicillin-resistant Staphylococcus aureus skin wound infection in mice. Med. J. Islam. Repub. Iran 2019, 33, 153. [Google Scholar]
- Rajan, S. Skin and soft-tissue infections: Classifying and treating a spectrum. Cleve Clin. J. Med. 2012, 79, 57–66. [Google Scholar] [CrossRef]
- Ayatollahi Mousavi, S.A.; Mokhtari, A.; Barani, M.; Izadi, A.; Amirbeigi, A.; Ajalli, N.; Amanizadeh, A.; Hadizadeh, S. Advances of liposomal mediated nanocarriers for the treatment of dermatophyte infections. Heliyon 2023, 9, e18960. [Google Scholar] [PubMed]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Erratum: Candida auris: The recent emergence of a multidrug-resistant fungal Pathogen. Med. Mycol. 2019, 57, e7. [Google Scholar]
- Jaromin, A.; Zarnowski, R.; Markowski, A.; Zagorska, A.; Johnson, C.J.; Etezadi, H.; Kihara, S.; Mota-Santiago, P.; Nett, J.E.; Boyd, B.J.; et al. Liposomal formulation of a new antifungal hybrid compound provides protection against Candida auris in the ex vivo skin colonization model. Antimicrob. Agents Chemother. 2024, 68, e0095523. [Google Scholar]
- Tofanica, B.M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Rushikesh Khemnar, S.S.; Nikhil, S.; Pallavi, K.; Avdhut, K.; Vitthal, D. Review Article On Organogels Methodology And Types Of Organogelators. Int. J. Pharm. Sci 2024, 2, 435–444. [Google Scholar]
- Tang, C.; Wan, Z.; Chen, Y.; Tang, Y.; Fan, W.; Cao, Y.; Song, M.; Qin, J.; Xiao, H.; Guo, S.; et al. Structure and Properties of Organogels Prepared from Rapeseed Oil with Stigmasterol. Foods 2022, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Barai, A.C. Self-assembly of naturally occurring stigmasterol in liquids yielding a fibrillar network and gel. RSC Adv. 2020, 10, 4755–4762. [Google Scholar]
- Raut, S.; Bhadoriya, S.S.; Uplanchiwar, V.; Mishra, V.; Gahane, A.; Jain, S.K. Lecithin organogel: A unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharm. Sin. B 2012, 2, 8–15. [Google Scholar]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Rando, G.; Scalone, E.; Sfameni, S.; Plutino, M.R. Functional Bio-Based Polymeric Hydrogels for Wastewater Treatment: From Remediation to Sensing Applications. Gels 2024, 10, 498. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Hu, X.; Jin, M.; Sun, K.; Zhang, Z.; Wu, Z.; Shi, J.; Liu, P.; Yao, H.; Wang, D.A. Type II collagen scaffolds repair critical-sized osteochondral defects under induced conditions of osteoarthritis in rat knee joints via inhibiting TGF-beta-Smad1/5/8 signaling pathway. Bioact. Mater. 2024, 35, 416–428. [Google Scholar]
- Hernandez-Gonzalez, A.C.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [PubMed]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Oprita, E.I.; Iosageanu, A.; Craciunescu, O. Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing. Gels 2023, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Ahearne, M. Silk fibroin based interpenetrating network hydrogel for corneal stromal regeneration. Int. J. Biol. Macromol. 2022, 223, 583–594. [Google Scholar]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D.; Cai, B. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar]
- Wang, S.; Tavakoli, S.; Parvathaneni, R.P.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Dynamic covalent crosslinked hyaluronic acid hydrogels and nanomaterials for biomedical applications. Biomater. Sci. 2022, 10, 6399–6412. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint 2019, 5, 229. [Google Scholar] [CrossRef]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Munim, S.A.; Raza, Z.A. Poly(lactic acid) based hydrogels: Formation, characteristics and biomedical applications. J. Porous Mater. 2018, 26, 881–901. [Google Scholar] [CrossRef]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; García-Carvajal, Z.Y.; Jiménez-Palomar, I.; Jiménez-Avalos, J.A.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2018, 136, 47149. [Google Scholar] [CrossRef]
- Berkovitch, Y.; Seliktar, D. Semi-synthetic hydrogel composition and stiffness regulate neuronal morphogenesis. Int. J. Pharm. 2017, 523, 545–555. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magana, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Suhail, M.; Fang, C.W.; Chiu, I.H.; Hung, M.C.; Vu, Q.L.; Lin, I.L.; Wu, P.C. Designing and In Vitro Characterization of pH-Sensitive Aspartic Acid-Graft-Poly(Acrylic Acid) Hydrogels as Controlled Drug Carriers. Gels 2022, 8, 521. [Google Scholar] [CrossRef]
- Gohy, J.-F.; Varshney, S.K.; Antoun, S.; Jérôme, R. Water-Soluble Complexes Formed by Sodium Poly(4-styrenesulfonate) and a Poly(2-vinylpyridinium)-block-poly(ethyleneoxide) Copolymer. Macromolecules 2000, 33, 9298–9305. [Google Scholar] [CrossRef]
- Baker, J.; Stephens, D.; Blanch, H.; Prausnitz, J. Swelling Equilibria for Acrylamide-Based Polyampholyte Hydrogels. Macromolecules 1991, 25, 1955–1958. [Google Scholar]
- Casolaro, M.; Bottari, S.; Ito, Y. Vinyl polymers based on L-histidine residues. Part 2. Swelling and electric behavior of smart poly(ampholyte) hydrogels for biomedical applications. Biomacromolecules 2006, 7, 1439–1448. [Google Scholar] [CrossRef]
- Alaghawani, N.A.; Alkhatib, H.; Elmancy, L.; Daou, A. Harmonizing Innovations: An In-Depth Comparative Review on the Formulation, Applications, and Future Perspectives of Aerogels and Hydrogels in Pharmaceutical Sciences. Gels 2024, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [PubMed]
- Zhang, J.T.; Huang, S.W.; Zhuo, R.X. Temperature-sensitive polyamidoamine dendrimer/poly(N-isopropylacrylamide) hydrogels with improved responsive properties. Macromol. Biosci. 2004, 4, 575–578. [Google Scholar]
- Lu, L.; Wang, T.; Fang, C.; Song, L.; Qian, C.; Lv, Z.; Fang, Y.; Liu, X.; Yu, X.; Xu, X.; et al. Oncolytic Impediment/Promotion Balance Disruption by Sonosensitizer-Free Nanoplatforms Unfreezes Autophagy-Induced Resistance to Sonocatalytic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 36462–36472. [Google Scholar]
- Kumbhar, P.R.; Desai, H.; Desai, V.M.; Priya, S.; Rana, V.; Singhvi, G. Versatility of emulgel in topical drug delivery transforming its expedition from bench to bedside. Expert Opin. Drug Deliv. 2025, 22, 55–68. [Google Scholar]
- Charyulu, N.R.; Joshi, P.; Dubey, A.; Shetty, A. Emulgel: A Boon for Enhanced Topical Drug Delivery. J. Young Pharm. 2021, 13, 76–79. [Google Scholar]
- Milutinov, J.; Krstonosic, V.; Cirin, D.; Pavlovic, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 2023, 15, 2302. [Google Scholar] [CrossRef] [PubMed]
- Zoller, K.; To, D.; Bernkop-Schnurch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar]
- Olteanu, G.; Neacsu, S.M.; Joita, F.A.; Musuc, A.M.; Lupu, E.C.; Ionita-Mindrican, C.B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174. [Google Scholar] [CrossRef]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef]
- Robert, B.; Warfield, I.; Louis, F.; Stumpf Westfield, N.J. Acrylic Acid Polymer Laxative Compositions. Patent Application 8-12-1955; serial No. 551736, 1955. [Google Scholar]
- Gabriel, D.; Mugnier, T.; Courthion, H.; Kranidioti, K.; Karagianni, N.; Denis, M.C.; Lapteva, M.; Kalia, Y.; Moller, M.; Gurny, R. Improved topical delivery of tacrolimus: A novel composite hydrogel formulation for the treatment of psoriasis. J. Control. Release 2016, 242, 16–24. [Google Scholar]
- Stein Gold, L.; Weiss, J.; Rueda, M.J.; Liu, H.; Tanghetti, E. Moderate and Severe Inflammatory Acne Vulgaris Effectively Treated with Single-Agent Therapy by a New Fixed-Dose Combination Adapalene 0.3%/Benzoyl Peroxide 2.5% Gel: A Randomized, Double-Blind, Parallel-Group, Controlled Study. Am. J. Clin. Dermatol. 2016, 17, 293–303. [Google Scholar]
- Palakkal, S.; Cortial, A.; Frusic-Zlotkin, M.; Soroka, Y.; Tzur, T.; Nassar, T.; Benita, S. Effect of cyclosporine A—Tempol topical gel for the treatment of alopecia and anti-inflammatory disorders. Int. J. Pharm. 2023, 642, 123121. [Google Scholar] [PubMed]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar]
- Hasan, N.; Nadaf, A.; Imran, M.; Jiba, U.; Sheikh, A.; Almalki, W.H.; Almujri, S.S.; Mohammed, Y.H.; Kesharwani, P.; Ahmad, F.J. Skin cancer: Understanding the journey of transformation from conventional to advanced treatment approaches. Mol. Cancer 2023, 22, 168. [Google Scholar] [PubMed]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; DiMaio, D.; et al. Basal Cell Skin Cancer, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1181–1203. [Google Scholar] [PubMed]
- Garcia-Mouronte, E.; Berna-Rico, E.; de Nicolas-Ruanes, B.; Azcarraga-Llobet, C.; Alonso-Martinez de Salinas, L.; Bea-Ardebol, S. Imiquimod as Local Immunotherapy in the Management of Premalignant Cutaneous Conditions and Skin Cancer. Int. J. Mol. Sci. 2023, 24, 835. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Wang, L. Rare Cutaneous Side Effects of Imiquimod: A Review on Its Mechanisms, Diagnosis, and Management. Dermatol. Ther. Heidelb. 2023, 13, 1909–1934. [Google Scholar]
- Werbel, T.; Cohen, P.R. Topical Application of 5-Fluorouracil Associated with Distant Seborrheic Dermatitis-like Eruption: Case Report and Review of Seborrheic Dermatitis Cutaneous Reactions after Systemic or Topical Treatment with 5-Fluorouracil. Dermatol. Ther. Heidelb. 2018, 8, 495–501. [Google Scholar]
- Saraiva, M.I.R.; Portocarrero, L.K.L.; Vieira, M.; Swiczar, B.C.C.; Westin, A.T. Ingenol mebutate in the treatment of actinic keratoses: Clearance rate and adverse effects. An. Bras. Dermatol. 2018, 93, 529–534. [Google Scholar]
- Pansuriya, R.; Doutch, J.; Parmar, B.; Kailasa, S.K.; Mahmoudi, N.; Hoskins, C.; Malek, N.I. A bio-ionic liquid based self-healable and adhesive ionic hydrogel for the on-demand transdermal delivery of a chemotherapeutic drug. J. Mater. Chem. B 2024, 12, 5479–5495. [Google Scholar]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef]
- Blohm-Mangone, K.; Burkett, N.B.; Tahsin, S.; Myrdal, P.B.; Aodah, A.; Ho, B.; Janda, J.; McComas, M.; Saboda, K.; Roe, D.J.; et al. Pharmacological TLR4 Antagonism Using Topical Resatorvid Blocks Solar UV-Induced Skin Tumorigenesis in SKH-1 Mice. Cancer Prev. Res. 2018, 11, 265–278. [Google Scholar]
- Ruiz, V.H.; Encinas-Basurto, D.; Sun, B.; Eedara, B.B.; Dickinson, S.E.; Wondrak, G.T.; Chow, H.S.; Curiel-Lewandrowski, C.; Mansour, H.M. Design, Physicochemical Characterization, and In Vitro Permeation of Innovative Resatorvid Topical Formulations for Targeted Skin Drug Delivery. Pharmaceutics 2022, 14, 700. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, S.A.; Dawaba, A.M.; Mansour, A.; Zalat, Z.A.; Ammar, A.A. Ectoine gel transdermal formulation as a novel therapeutic approach in melanoma using 3D printed microneedles. Pharm. Dev. Technol. 2022, 27, 1110–1124. [Google Scholar] [PubMed]
- Mishra, M.; Barkat, M.A.; Misra, C.; Alanezi, A.A.; Ali, A.; Chaurawal, N.; Ali, A.; Preet, S.; Barkat, H.; Raza, K. Lipid-based microemulsion gel for the topical delivery of methotrexate: An optimized, rheologically acceptable formulation with conducive dermatokinetics. Arch. Dermatol. Res. 2024, 316, 316. [Google Scholar]
- Zhang, W.; Qian, S.; Chen, J.; Jian, T.; Wang, X.; Zhu, X.; Dong, Y.; Fan, G. Photo-Crosslinked Pro-Angiogenic Hydrogel Dressing for Wound Healing. Int. J. Mol. Sci. 2024, 25, 9948. [Google Scholar] [CrossRef] [PubMed]
- Gocmen, G.; Gonul, O.; Oktay, N.S.; Yarat, A.; Goker, K. The antioxidant and anti-inflammatory efficiency of hyaluronic acid after third molar extraction. J. Craniomaxillofac. Surg. 2015, 43, 1033–1037. [Google Scholar]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar]
- Liu, S.; Wei, L.; Huang, J.; Luo, J.; Weng, Y.; Chen, J. Chitosan/Alginate-Based Hydrogel Loaded With VE-Cadherin/FGF as Scaffolds for Wound Repair in Different Degrees of Skin Burns. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35533. [Google Scholar]
- Esposito, S.; De Simone, G.; Pan, A.; Brambilla, P.; Gattuso, G.; Mastroianni, C.; Kertusha, B.; Contini, C.; Massoli, L.; Francisci, D.; et al. Epidemiology and Microbiology of Skin and Soft Tissue Infections: Preliminary Results of a National Registry. J. Chemother. 2019, 31, 9–14. [Google Scholar] [PubMed]
- Grossi, A.P.; Ruggieri, A.; Vecchio, A.D.; Comandini, A.; Corio, L.; Calisti, F.; Loreto, G.D.; Almirante, B. Skin infections in Europe: A retrospective study of incidence, patient characteristics and practice patterns. Int. J. Antimicrob. Agents 2022, 60, 106637. [Google Scholar] [PubMed]
- Furnica, D.T.; Dittmer, S.; Scharmann, U.; Meis, J.F.; Steinmann, J.; Rath, P.M.; Kirchhoff, L. In Vitro and In Vivo Effect of the Imidazole Luliconazole against Lomentospora prolificans and Scedosporium spp. Microbiol. Spectr. 2023, 11, e0513022. [Google Scholar]
- Kaur, M.; Singh, G.; Shivgotra, R.; Singh, M.; Thakur, S.; Jain, S.K. Prolonged Skin Retention of Luliconazole from SLNs Based Topical Gel Formulation Contributing to Ameliorated Antifungal Activity. AAPS PharmSciTech 2024, 25, 229. [Google Scholar] [CrossRef]
- Cross, E.R.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Unravelling the antimicrobial activity of peptide hydrogel systems: Current and future perspectives. Soft Matter 2021, 17, 8001–8021. [Google Scholar]
- Albadr, A.A.; Coulter, S.M.; Porter, S.L.; Thakur, R.R.S.; Laverty, G. Ultrashort Self-Assembling Peptide Hydrogel for the Treatment of Fungal Infections. Gels 2018, 4, 48. [Google Scholar] [CrossRef]
- Chiloeches, A.; Zagora, J.; Placha, D.; Torres, M.D.T.; de la Fuente-Nunez, C.; Lopez-Fabal, F.; Gil-Romero, Y.; Fernandez-Garcia, R.; Fernandez-Garcia, M.; Echeverria, C.; et al. Synergistic Combination of Antimicrobial Peptides and Cationic Polyitaconates in Multifunctional PLA Fibers. ACS Appl. Bio Mater. 2023, 6, 4805–4813. [Google Scholar]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Alshaer, W.; Nsairat, H.; Lafi, Z.; Hourani, O.M.; Al-Kadash, A.; Esawi, E.; Alkilany, A.M. Quality by Design Approach in Liposomal Formulations: Robust Product Development. Molecules 2022, 28, 10. [Google Scholar] [CrossRef]
- Kant Shashi, K.S. A complete review on: Liposomes. Int. Res. J. Pharm. 2012, 3, 10–16. [Google Scholar]
- Sharma, A. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Bozo, T.; Meszaros, T.; Mihaly, J.; Bota, A.; Kellermayer, M.S.Z.; Szebeni, J.; Kalman, B. Aggregation of PEGylated liposomes driven by hydrophobic forces. Colloids Surf. B Biointerfaces 2016, 147, 467–474. [Google Scholar] [PubMed]
- Eugster, R.; Orsi, M.; Buttitta, G.; Serafini, N.; Tiboni, M.; Casettari, L.; Reymond, J.L.; Aleandri, S.; Luciani, P. Leveraging machine learning to streamline the development of liposomal drug delivery systems. J. Control. Release 2024, 376, 1025–1038. [Google Scholar]
- Wang, Y.; Grainger, D.W. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Front. Drug Deliv. 2022, 2, 901281. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Silva-Lima, B.; Magro, F.; Alcobia, A.; da Costa, F.L.; Feio, J. Non-biological Complex Drugs (NBCDs): Complex Pharmaceuticals in Need of Individual Robust Clinical Assessment Before Any Therapeutic Equivalence Decision. Front. Med. 2020, 7, 590527. [Google Scholar]
- Klein, K.; Stolk, P.; De Bruin, M.L.; Leufkens, H.G.M.; Crommelin, D.J.A.; De Vlieger, J.S.B. The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: Observations and recommendations. Eur. J. Pharm. Sci. 2019, 133, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT(1). Int. J. Toxicol. 2002, 21 (Suppl. 2), 19–94. [Google Scholar]
- Atre, P.; Rizvi, S.A.A. A brief overview of quality by design approach for developing pharmaceutical liposomes as nano-sized parenteral drug delivery systems. RSC Pharm. 2024, 1, 675–688. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Baliga, V.; Londhe, V.Y. Liposomal Formulations: A Recent Update. Pharmaceutics 2024, 17, 36. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzman, M.L.; Perez, A.P.; Apezteguia, G.A.; Formica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes can both enhance or reduce drugs penetration through the skin. Sci. Rep. 2018, 8, 13253. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research 2018, 7, 1746. [Google Scholar]
- Gopinathan, U.; Stapleton, F.; Sharma, S.; Willcox, M.D.; Sweeney, D.F.; Rao, G.N.; Holden, B.A. Microbial contamination of hydrogel contact lenses. J. Appl. Microbiol. 1997, 82, 653–658. [Google Scholar] [PubMed]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar]
- Dantas, M.G.; Reis, S.A.; Damasceno, C.M.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar]
- Das, S.; Wong, A.B.H. Stabilization of ferulic acid in topical gel formulation via nanoencapsulation and pH optimization. Sci. Rep. 2020, 10, 12288. [Google Scholar]
- Khadivi, Y.; Shakeri, S.; Arjmandmazidi, S.; Shokri, J.; Monajjemzadeh, F. The effect of emulgel preparation on the stability of Kojic acid in the topical anti-hyperpigmentation products. J. Cosmet. Dermatol. 2024, 23, 2145–2155. [Google Scholar]
- European Medicines Agency. ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products. 2003. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-and-products-step-5_en.pdf (accessed on 20 March 2025).
- U.S. Food and Drug Administration (Center for Drug Evaluation and Research.). In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs; Department of Health and Human Services; Food and Drug Administration: Silver Spring, MD, USA, 24 October 2022. [Google Scholar]
- Suman, D.; Office of Generic Drugs; Division of Bioequivalence. In Vitro Bioequivalence Data for a Topical Product: Bioequivalence Review Perspective-Presentation Food and drug Administration on fda.gov Ocrober 20th; Office of Generic Drugs: Silver Spring, MD, USA, 2017.
- Han, J.Y.; La Fiandra, J.N.; DeVoe, D.L. Microfluidic vortex focusing for high throughput synthesis of size-tunable liposomes. Nat. Commun. 2022, 13, 6997. [Google Scholar] [PubMed]
- van Swaay, D.; deMello, A. Microfluidic methods for forming liposomes. Lab Chip 2013, 13, 752–767. [Google Scholar]
- Wang, T.; Yu, T.; Li, W.; Liu, Q.; Sung, T.C.; Higuchi, A. Design and lyophilization of mRNA-encapsulating lipid nanoparticles. Int. J. Pharm. 2024, 662, 124514. [Google Scholar]
- Massing, U.; Cicko, S.; Ziroli, V. Dual asymmetric centrifugation (DAC)—A new technique for liposome preparation. J. Control. Release 2008, 125, 16–24. [Google Scholar] [PubMed]
- Ingebrigtsen, S.G.; Skalko-Basnet, N.; de Albuquerque Cavalcanti Jacobsen, C.; Holsaeter, A.M. Successful co-encapsulation of benzoyl peroxide and chloramphenicol in liposomes by a novel manufacturing method—Dual asymmetric centrifugation. Eur. J. Pharm. Sci. 2017, 97, 192–199. [Google Scholar] [PubMed]
- Attri, N.; Das, S.; Banerjee, J.; Shamsuddin, S.H.; Dash, S.K.; Pramanik, A. Liposomes to Cubosomes: The Evolution of Lipidic Nanocarriers and Their Cutting-Edge Biomedical Applications. ACS Appl. Bio Mater. 2024, 7, 2677–2694. [Google Scholar] [PubMed]
- Palma, A.S.; Casadei, B.R.; Lotierzo, M.C.; de Castro, R.D.; Barbosa, L.R.S. A short review on the applicability and use of cubosomes as nanocarriers. Biophys. Rev. 2023, 15, 553–567. [Google Scholar]
- Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in Drug Delivery-A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines 2023, 11, 1114. [Google Scholar] [CrossRef]
- Globe Newswire-2019.-SunGen Pharma Receives Eighth ANDA Approval from US FDA. Available online: https://www.globenewswire.com/news-release/2019/10/31/1939048/0/en/SunGen-Pharma-Receives-Eighth-ANDA-Approval-from-US-FDA.html (accessed on 22 March 2025).
- Sacks, S.L.; Thisted, R.A.; Jones, T.M.; Barbarash, R.A.; Mikolich, D.J.; Ruoff, G.E.; Jorizzo, J.L.; Gunnill, L.B.; Katz, D.H.; Khalil, M.H.; et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2001, 45, 222–230. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 4. [Google Scholar] [CrossRef]
- Kontzias, C.; Zaino, M.; Feldman, S.R. Tretinoin 0.1% and Benzoyl Peroxide 3% Cream for the Treatment of Facial Acne Vulgaris. Ann. Pharmacother. 2023, 57, 1088–1093. [Google Scholar]
- Bosslett, M. FDA Approves Journey Medical’s DFD-29 for Rosacea. Available online: https://www.dermatologytimes.com/view/fda-approves-journey-medical-s-dfd-29-for-rosacea (accessed on 17 March 2025).
- Sousa, R.; Lakha, D.R.; Brette, S.; Hitier, S. A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of Ambroxol Hard-Boiled Lozenges in Patients with Acute Pharyngitis. Pulm Ther. 2019, 5, 201–211. [Google Scholar]
- Wang, X.; Wang, Z.; Sun, L.; Liu, H.; Zhang, F. Efficacy and safety of dapsone gel for acne: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 611–620. [Google Scholar] [CrossRef]
- Stotland, M.; Shalita, A.R.; Kissling, R.F. Dapsone 5% gel: A review of its efficacy and safety in the treatment of acne vulgaris. Am. J. Clin. Dermatol. 2009, 10, 221–227. [Google Scholar]
- Mahmoudi, C.; Tahraoui Douma, N.; Mahmoudi, H.; Iurciuc Tincu, C.E.; Popa, M. Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 7839. [Google Scholar] [CrossRef]
- Liu, M.; Jin, J.; Zhong, X.; Liu, L.; Tang, C.; Cai, L. Polysaccharide hydrogels for skin wound healing. Heliyon 2024, 10, e35014. [Google Scholar] [PubMed]
- Qin, D.; Cui, Y.; Zheng, M.; Yang, Z.; Wang, X. Preparation of Ethosome Gel with Total Flavonoids from Vernonia anthelmintica (L.) Willd. for the Treatment of Vitiligo. Gels 2025, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Perez, E.; Rubio-Retama, J.; Cusso, L.; Igartua, M.; Hernandez, R.M.; Santos-Vizcaino, E. 3D-printed Laponite/Alginate hydrogel-based suppositories for versatile drug loading and release. Drug Deliv. Transl. Res. 2024, 14, 3385–3403. [Google Scholar] [PubMed]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Landolina, J.A. In-Situ Cross-Linkable Polymeric Compositions and Methods Thereof; Cresilon Inc.: Brooklyn, NY, USA, 2020. [Google Scholar]
- Landolina, J.A.; Ahmad, O.M. Highly Efficacious Hemostatic Adhesive Polymer Scaffold; WO 2016/209198 A1; Cresilon Inc.: Brooklyn, NY, USA, 29 December 2016. [Google Scholar]
- Shin, B.; Hillyer, T.; Shin, W.S. Rational Design and Testing of Antibacterial Aloe Vera Hemostatic Hydrogel. Gels 2024, 10, 409. [Google Scholar] [CrossRef]
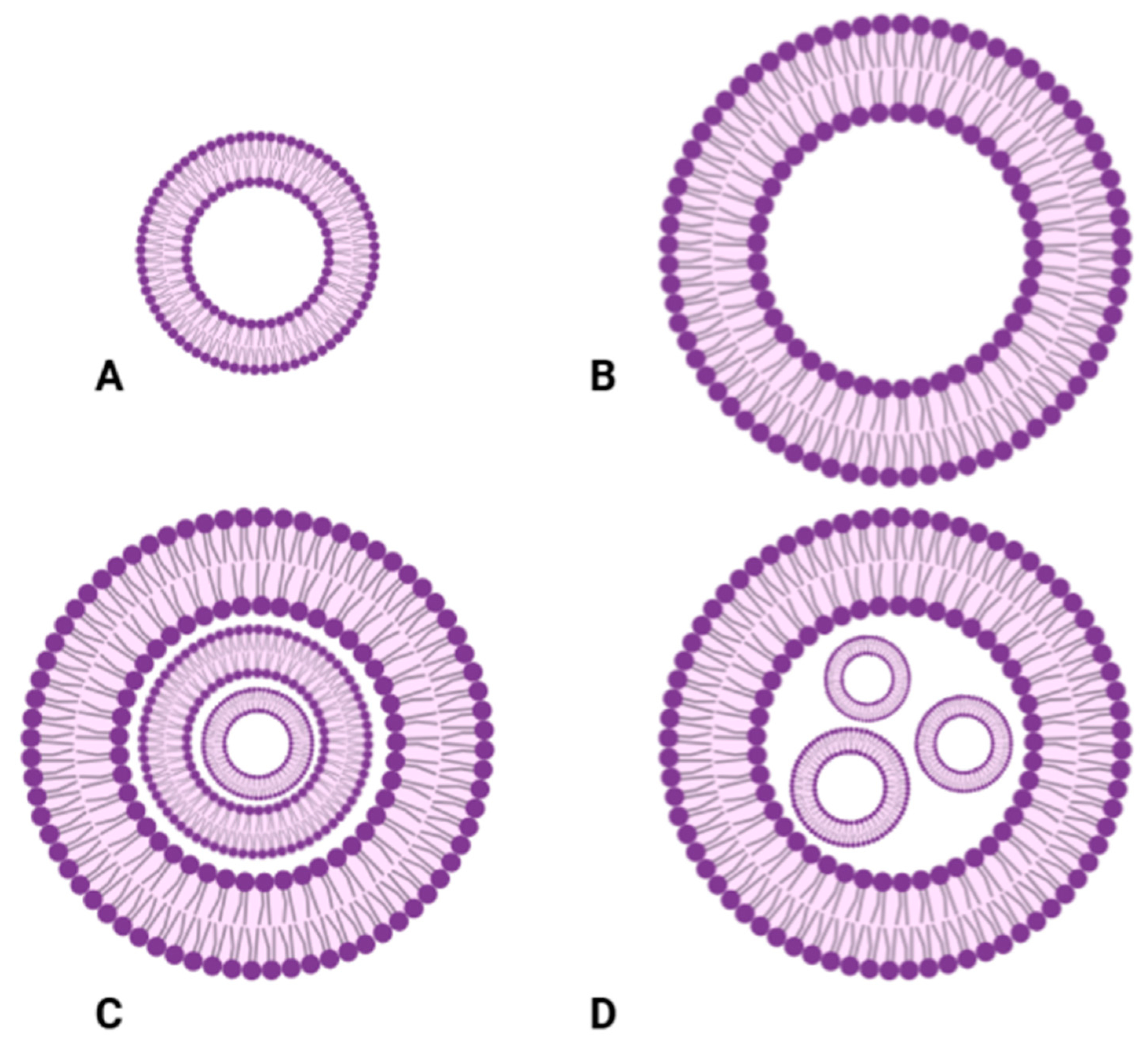

| Type | Description | Preparations Methods |
|---|---|---|
| Small Unilamellar Vesicles (SUVs) | Composed of a single lipid bilayer surrounding an aqueous compartment, typically sized 20–100 nm | Sonication [72], extrusion [73], and detergent dialysis method [74] |
| Large Unilamellar Vesicles (LUVs) | Enclosed by a single lipid bilayer with a size range of 100–250 nm | Extrusion [75] and dehydration–rehydration [76] |
| Multilamellar Vesicles (MLVs) | Composed of multiple concentric phospholipid bilayers, ranging from 1 to 5 µm in size | Thin-film hydration [77] and reverse-phase evaporation [78] |
| Multivesicular Vesicles (MVVs) | Contain multiple unilamellar vesicles within a larger liposome structure | Cochleate cylinder, interdigitated bilayer, bulk w/wo double emulsion, and microfluidic w/wo emulsion [79] |
| Feature | Liposomal Formulations | Gel-Based Formulations |
|---|---|---|
| Mechanism | Bilayer fusion, destabilization, and enzymatic action | Swelling, diffusion, and matrix erosion |
| Release Profile | Initial burst followed by sustained release | Gradual and prolonged release |
| Penetration | Deep penetration via cell membrane interaction | Localized retention in upper skin layers |
| Targeting Ability | Can be engineered for site-specific delivery | Limited targeting, with mainly localized effect |
| Stability | Susceptible to environmental factors | More stable but prone to microbial contamination |
| Applications | Systemic and localized delivery | Mostly topical, wound healing |
| Advantages | Enhanced skin penetration; enhanced drug delivery; reduced side effects; versatility in formulations; controlled release; and improved stability | Enhanced drug delivery; non-greasy texture; cooling and soothing effect; stability/long shelf life; moisturizing properties; bio adhesive properties; simple composition; and versatility |
| Disadvantages | Complexity in formulations; variability in drug penetration due to environmental factors; physical instability/short shelf life; limited knowledge of long-term effects; and production cost | Partial release of active ingredients; potential irritants (preservatives and few gelling agents); microbial contamination risk; physical stability issues; limited applicability; and limited penetration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strazzabosco, G.; Liboni, A.; Pezzi, G.; Alogna, A.; Bortolotti, D. Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments. Gels 2025, 11, 245. https://doi.org/10.3390/gels11040245
Strazzabosco G, Liboni A, Pezzi G, Alogna A, Bortolotti D. Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments. Gels. 2025; 11(4):245. https://doi.org/10.3390/gels11040245
Chicago/Turabian StyleStrazzabosco, Giovanni, Alessia Liboni, Giulia Pezzi, Andrea Alogna, and Daria Bortolotti. 2025. "Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments" Gels 11, no. 4: 245. https://doi.org/10.3390/gels11040245
APA StyleStrazzabosco, G., Liboni, A., Pezzi, G., Alogna, A., & Bortolotti, D. (2025). Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments. Gels, 11(4), 245. https://doi.org/10.3390/gels11040245







