Development of Liposome-Based Hydrogel Patches Incorporating Essential Oils of African Plants and Deep Eutectic Solvents
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectral and Chromatographic Analysis of DES
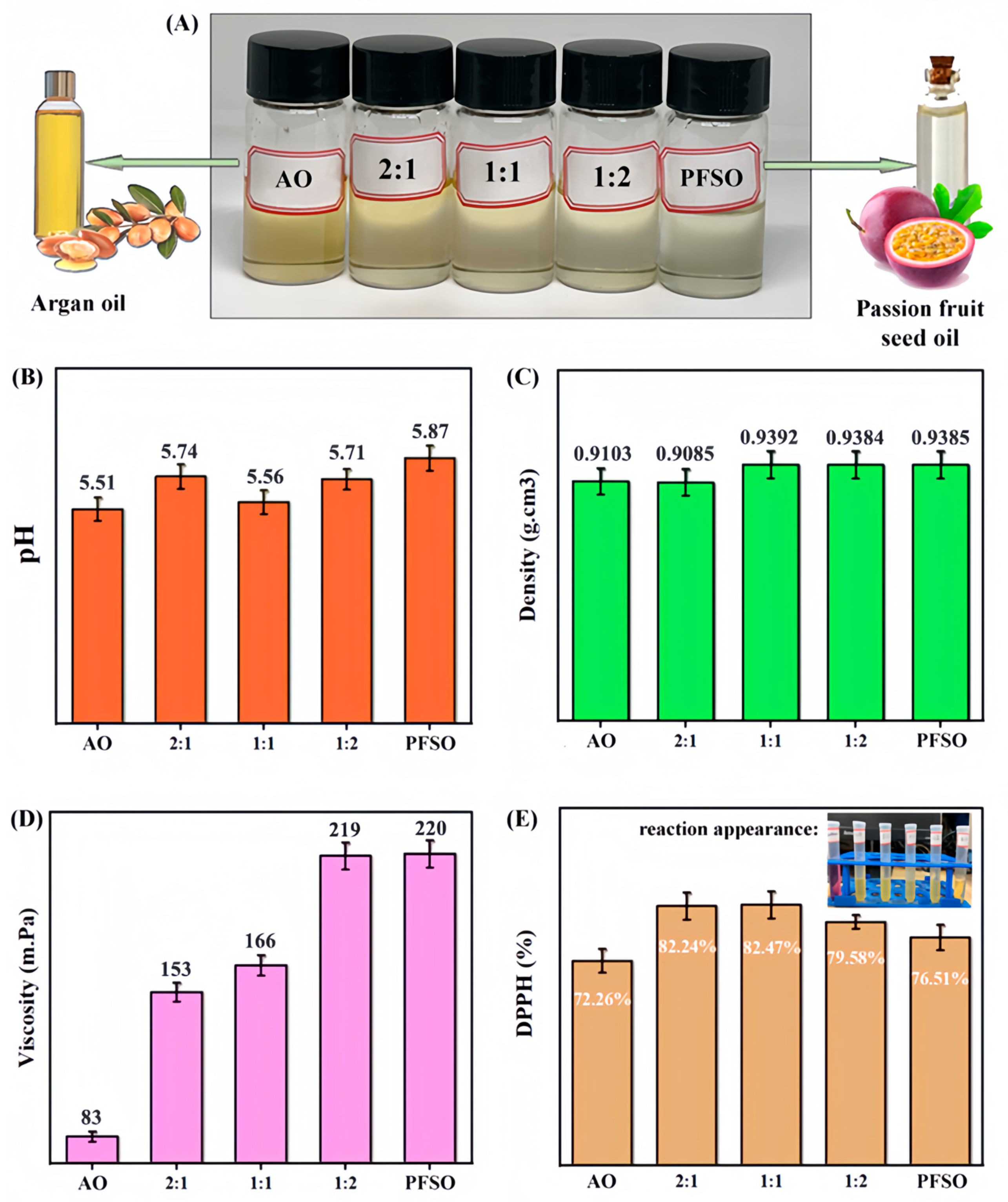
2.2. Basic Properties and Activities of EO Mixtures
2.2.1. Basic Properties
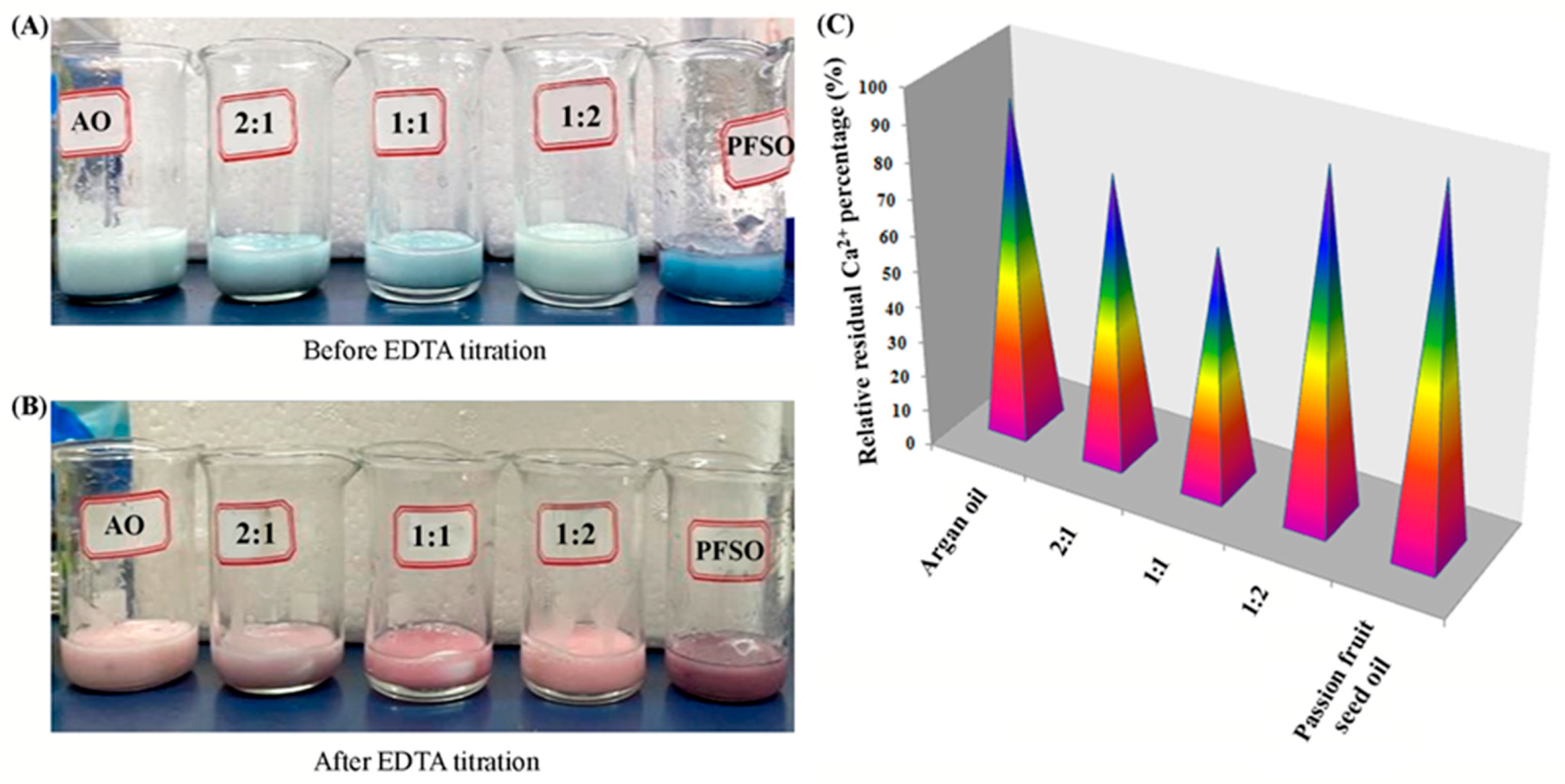
2.2.2. Typical Bioactivities
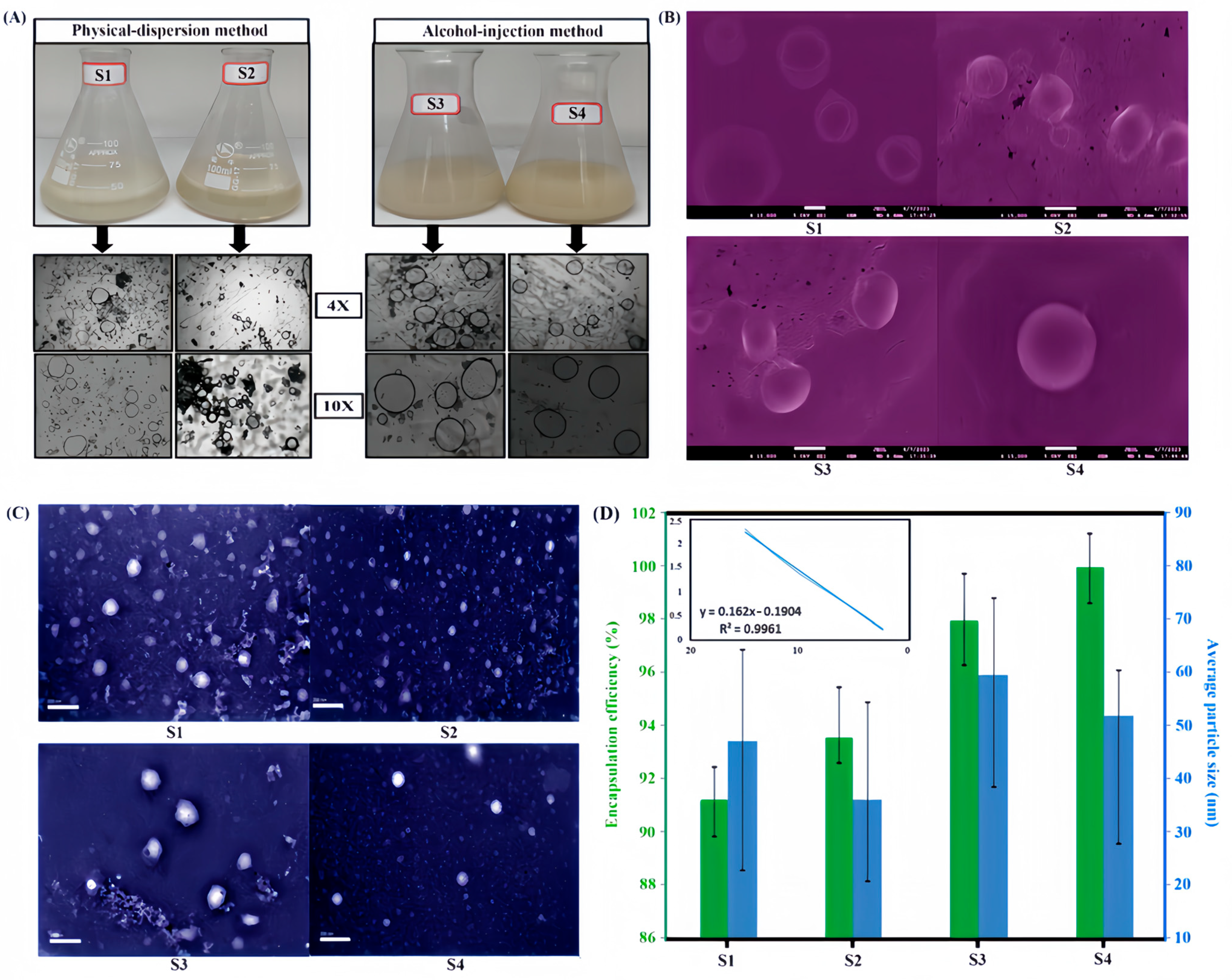
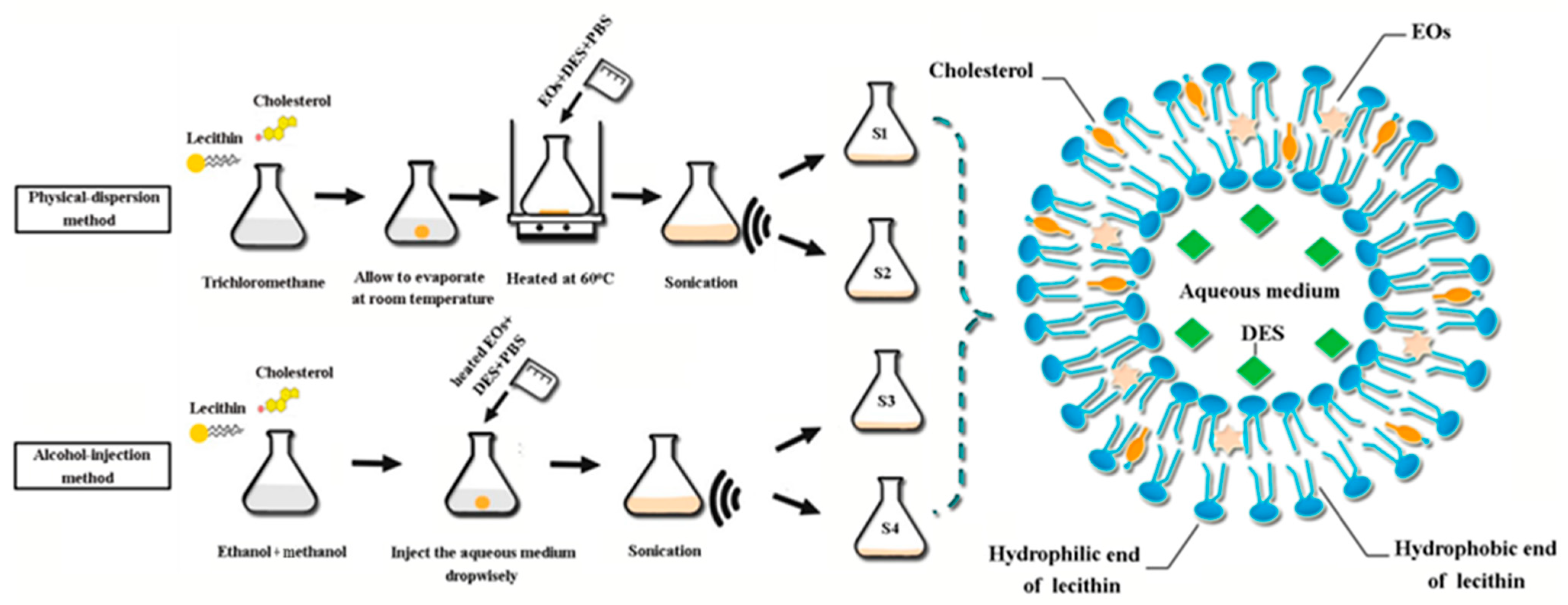
2.3. Characterization of Structure and Properties of Nanoliposomes
2.4. Characterizations and Measurements on Nanoliposome-Loaded Hydrogel Patches
2.4.1. Appearance and Microstructure
2.4.2. FT-IR and TGA
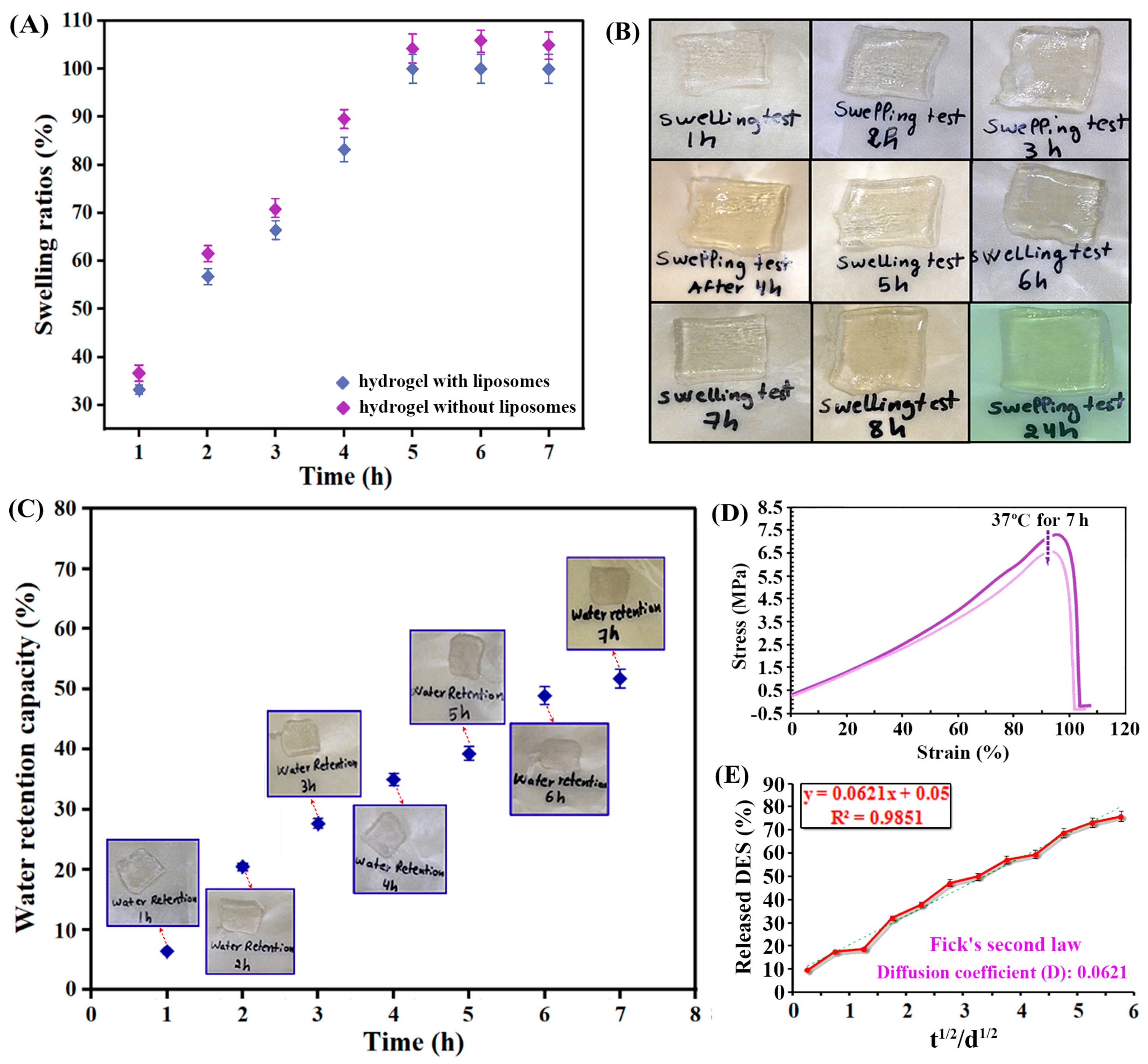
2.4.3. Swelling and Mechanical Properties
2.4.4. Skin Adaptability and DES Release
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Instruments
4.3. Preparation, Identification and Quantitation of DES
4.4. Preparation of Argan Oil-Passion Fruit Oil Mixtures and Their Basic Properties
4.4.1. Determination on pH of the Oil Mixtures
4.4.2. Determination on Density of the Oil Mixtures
4.4.3. Determination on Apparent Viscosity of the Oil Mixtures
4.5. Key Bioactivities of Argan Oil–Passion Fruit Seed Oil Mixtures
4.5.1. Diphenyl-1-Picrylhydrazyl Antioxidant Activity
4.5.2. Ca2+-Binding Activity (Antikeratotic Activity)
4.5.3. Sun Factor Protection Activity
4.6. Preparation and Characterization of Nanoliposomes Encapsulating Two EOs and DES
4.6.1. Preparation of Essential Oils and DES-Encapsulated Nanoliposomes
- (1)
- Physical-dispersion method
- (2)
- Alcohol-injection method
4.6.2. Characterization of Essential Oils and DES-Encapsulated Nanoliposomes
4.7. Preparation of Hydrogel Patches Containing the Formed Nanoliposomes
4.8. Characterization and Testing on the Prepared Hydrogel Patches
4.8.1. Thickness Measurement
4.8.2. SEM Observation
4.8.3. FT-IR Spectral and Thermogravimetric Analysis (TGA)
4.8.4. Tensile, Folding Strength, and Removability Analysis
4.8.5. Swelling Testing
4.8.6. Water Retention Testing
4.8.7. Releasing of Loaded Component from the Hydrogel Patch
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stacy, A.; Belkaid, Y. Microbial guardians of skin health. Science 2019, 363, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Balin, A.K.; Pratt, L.A. Physiological consequences of human-skin aging. Cutis 1989, 43, 431–436. [Google Scholar] [PubMed]
- Gilchrest, B.A. Skin aging and photoaging—An overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef]
- Meng, H.; Li, J.; Dong, Y.; He, Y. Poly traditional Chinese medicine formulation prepared with skin moisturizing properties. Dermatol. Ther. 2020, 33, e14105. [Google Scholar] [CrossRef]
- Khatibi, S.A.; Misaghi, A.; Akhondzadeh Basti, A. Effect of nanoliposomes containing Zataria multiflora Boiss. essential oil on gene expression of Shiga toxin 2 in Escherichia coli O157:H7. J. Appl. Microbiol. 2018, 124, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Goik, U.; Goik, T.; Zaleska, I. The properties and application of argan oil in cosmetology. Eur. J. Lipid Sci. Technol. 2019, 121, 1800313. [Google Scholar] [CrossRef]
- Vieira Lopes, R.d.V.; Zamian, J.R.; Resck, I.S.; Sales, M.J.A.; Santos, M.L.; Cunha, F.R. Physicochemical and rheological properties of passion fruit oil and its polyol. Eur. J. Lipid Sci. Technol. 2010, 112, 1253–1262. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.; Casabianca, H.; El Mousadik, A. Essential oils: From extraction to encapsulation. Int. J. Pharmaceut. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Aarabi, M.H. Preparation of nanoliposomes containing rosmarinus offi cinalis L essential oil; a comparative study. Biosci. Biotechnol. Res. Commun. 2017, 10, 105–110. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Jiang, M.; Ying, W. Research progress on delivery systems of plant essential oils and applications in fruit and vegetable preservation. Food Sci. 2024, 45, 293–305. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Technology, preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Devi, M.; Moral, R.; Thakuria, S.; Mitra, A.; Paul, S. Hydrophobic deep eutectic solvents as greener substitutes for conventional extraction media: Examples and techniques. ACS Omega 2023, 8, 9702–9728. [Google Scholar] [CrossRef]
- Zhuo, Y.; Cheng, H.L. Ionic liquids in pharmaceutical and biomedical applications: A review. Pharmaceutics 2024, 16, 151. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; Alsaadi, M.A.; Hashim, M.A. Functionalization of graphene using deep eutectic solvents. Nanoscale Res. Lett. 2015, 10, 324. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, Y.; Shi, X. Construction of electrospun organic/inorganic hybrid nanofibers for drug delivery and tissue engineering applications. Adv. Fiber Mater. 2019, 1, 32–45. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T. Effect of choline chloride/ethylene glycol or glycerol as deep eutectic solvents on the solubility and thermodynamic properties of acetaminophen. J. Mol. Liq. 2018, 249, 1222–1235. [Google Scholar] [CrossRef]
- Palmelund, H.; Andersson, M.P.; Asgreen, C.; Rantanen, J.; Lobmann, K. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents (DES). Int. J. Pharm. X 2019, 1, 100034. [Google Scholar] [CrossRef]
- Chen, C.; Yan, W.; Jiang, W.; Zhu, C.; Yao, S. Transdermal release behaviors of bioactive deep eutectic solvents as natural skin care and mechanism. J. Mol. Liq. 2022, 367, 120412. [Google Scholar] [CrossRef]
- Dave, G.; Modi, H. FT-IR method for estimation of phytic acid content during bread-making process. J. Food Meas. Charact. 2018, 12, 2202–2208. [Google Scholar] [CrossRef]
- Kitamura, Y.; Iwasaki, T. Standard infrared absorption spectrum of betaine and optimal conditions for its measurement. J. Food Hyg. Soc. Jpn. 2006, 47, 232–236. [Google Scholar] [CrossRef][Green Version]
- Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. The heterogeneity and complexity of skin surface lipids in human skin health and disease. Prog. Lipid Res. 2024, 93, 101264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chow, P.S. Developing eco-friendly skin care formulations with microemulsions of essential oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Maghazechi, A.; Mohammadi Nafchi, A.; Tan, T.C.; Easa, A.M. Rheological characterization and fouling deposition behavior of coconut cream emulsion at heat processing temperature range. Food Sci. Nutr. 2022, 10, 3801–3813. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Csakvari, A.C.; Lupitu, A. Fatty acids profile and antioxidant activity of almond oils obtained from six Romanian varieties. Farmacia 2019, 67, 882–887. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.H. Skin barrier and calcium. Ann. Dermatol. 2018, 30, 265. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1041. [Google Scholar]
- Mbanga, L.; Mulenga, M. Determination of sun protection factor (SPF) of some body creams and lotions marketed in Kinshasa by ultraviolet spectrophotometry. Int. J. Pharm. Res. 2014, 1, 7–13. [Google Scholar]
- Cefali, L.; Ataide, J.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Advan. Res. Chem. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.M.; Cautela, D. Determination of antioxidant activity and sun protection factor of commercial essential oils. Biol. Life. Sci. Forum 2021, 6, 96. [Google Scholar]
- Barbosa, A.I.; Lima, S.A.C. Evaluating the skin interactions and permeation of alginate/fucoidan hydrogels per se and associated with different essential oils. Pharmaceutics 2023, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Girotti, J.; Fan, Y.; Levy, E.S.; Zang, N.; Sethuraman, V.; Kou, P.; Zhang, K.; Gruenhagen, J.; Lin, J. Fast and versatile analysis of liposome encapsulation efficiency by nanoParticle exclusion chromatography. J. Chromatogr. A 2022, 1662, 462688. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Complexometric determination of calcium. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2009; pp. 61–67. [Google Scholar] [CrossRef]
- Emami, S.; Ahmadi, M.; Nasiraie, L.R.; Shahidi, S.A.; Jafarizadeh-Malmiri, H. Cinnamon extract and its essential oil nanoliposomes–preparation, characterization and bactericidal activity assessment. Biologia 2022, 77, 3015–3025. [Google Scholar] [CrossRef]
- Baek, Y.; Jeong, E.W.; Lee, H.G. Encapsulation of resveratrol within size-controlled nanoliposomes: Impact on solubility, stability, cellular permeability, and oral bioavailability. Colloid. Surf. B 2023, 224, 113205. [Google Scholar] [CrossRef]
- Chen, L.; Gan, L. Preparation and Drug Carrying Characteristics of Flurbiprofen Liposomes. Tongji Daxue Xuebao/J. Tongji Univ. 2011, 39, 1079–1083. [Google Scholar]
- Wang, Q.; Zhang, Y.; Wang, M.; Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Mater. Today Bio. 2023, 20, 100640. [Google Scholar] [CrossRef]
- El-Zawawy, W.K. Preparation of hydrogel from green polymer. Polym. Adv. Technol. 2005, 16, 48–54. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y. FT-IR spectrum analysis of the component content of acrylamide copolymers. Spec. Petrochem. 2012, 29, 79–82. [Google Scholar]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohyd. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Higginbotham, C.L. Fabrication and in vitro biological evaluation of photopolymerisable hydroxyapatite hydrogel composites for bone regeneration. J. Biomater. Appl. 2014, 28, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine–chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef]
- Hamdan, A.M.E. Design, Formulation and characterization of liposomal preparation of voriconazole (VRC). J. Pharmaceut. Biomed. Sci. 2015, 5. [Google Scholar]
- Miastkowska, M.; Konieczna, M.; Lason, E. The release of perillyl alcohol from the different kind of vehicles. Curr. Pharm. Biotechnol. 2018, 19, 573–580. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, J.; Tang, S. Densities and surface tensions of ionic liquids/sulfuric acid binary mixtures. Chin. J. Chem. Eng. 2018, 26, 1513–1521. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-carotene bleaching methods. Monatsh. Chem. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Era, B.; Floris, S. Anti-aging potential of extracts from Washingtonia filifera seeds. Plants 2021, 10, 151. [Google Scholar] [CrossRef]
- Schreml, S.; Kemper, M. Skin pH in the elderly and appropriate skin care. EMJ Dermatol. 2014, 2, 86–94. [Google Scholar] [CrossRef]
- Taribak, C.; Casas, L.; Elfadli, Z.; Metni, R.E. Quality of cosmetic argan oil extracted by supercritical fluid extraction from Argania spinosa L. J. Chem. 2013, 2013, 408194. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T. Resveratrol loaded liposomes produced by different techniques. Food Sci. Emerg. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Shivhare, U.; Ambulkar, D.; Altamimi, M.A.; Hussain, A. Formulation and evaluation of pentoxifylline liposome formulation. Dig. J. Nanomater. Bios. 2009, 4, 857–862. [Google Scholar]
- Fattahian, A.; Fazlara, A.; Maktabi, S.; Pourmahdi, M.; Bavarsad, N. The effects of chitosan containing nano-capsulated Cuminum cyminum essential oil on the shelf-life of veal in modified atmosphere packaging. J. Food Meas. Charact. 2022, 16, 920–933. [Google Scholar] [CrossRef]
- Biswas, G.; Chakraborty, S.; Ghosh, N.; Majee, S. Fabrication of bucco-matrix tablets of amoxicillin trihydrate on the basis of release and permeation kinetics. J. Appl. Pharm. Sci. 2015, 5, 48–52. [Google Scholar] [CrossRef]


| Sample | Λ (nm) | Abs 1 | Abs 2 | Abs 3 | Aver Abs | SD Abs | CF × EE(λ) × I(λ) × Ab (λ) | Total SPF | p |
|---|---|---|---|---|---|---|---|---|---|
| AO | 290 | 0.240 | 0.248 | 0.248 | 0.245 | 0.005 | 0.037 | 1.503 | <0.01 |
| 295 | 0.242 | 0.240 | 0.243 | 0.242 | 0.002 | 0.197 | |||
| 300 | 0.181 | 0.179 | 0.194 | 0.185 | 0.008 | 0.531 | |||
| 305 | 0.141 | 0.151 | 0.152 | 0.148 | 0.006 | 0.485 | |||
| 310 | 0.096 | 0.105 | 0.094 | 0.098 | 0.006 | 0.183 | |||
| 315 | 0.065 | 0.075 | 0.068 | 0.069 | 0.005 | 0.058 | |||
| 320 | 0.057 | 0.072 | 0.069 | 0.066 | 0.008 | 0.012 | |||
| AO:PFSO = 2:1 | 290 | 0.272 | 0.269 | 0.264 | 0.268 | 0.004 | 0.040 | 1.622 | <0.01 |
| 295 | 0.232 | 0.235 | 0.236 | 0.234 | 0.002 | 0.191 | |||
| 300 | 0.180 | 0.190 | 0.191 | 0.187 | 0.006 | 0.537 | |||
| 305 | 0.167 | 0.157 | 0.166 | 0.163 | 0.006 | 0.535 | |||
| 310 | 0.119 | 0.109 | 0.113 | 0.114 | 0.005 | 0.212 | |||
| 315 | 0.113 | 0.101 | 0.109 | 0.108 | 0.006 | 0.090 | |||
| 320 | 0.081 | 0.089 | 0.085 | 0.085 | 0.004 | 0.015 | |||
| AO:PFSO = 1:1 | 290 | 0.268 | 0.271 | 0.278 | 0.272 | 0.005 | 0.041 | 1.511 | <0.01 |
| 295 | 0.233 | 0.231 | 0.234 | 0.233 | 0.002 | 0.190 | |||
| 300 | 0.166 | 0.175 | 0.163 | 0.168 | 0.006 | 0.483 | |||
| 305 | 0.135 | 0.158 | 0.153 | 0.149 | 0.121 | 0.487 | |||
| 310 | 0.110 | 0.113 | 0.107 | 0.110 | 0.003 | 0.205 | |||
| 315 | 0.098 | 0.109 | 0.111 | 0.106 | 0.007 | 0.089 | |||
| 320 | 0.084 | 0.087 | 0.088 | 0.086 | 0.002 | 0.016 | |||
| AO:PFSO = 1:2 | 290 | 0.296 | 0.305 | 0.299 | 0.300 | 0.005 | 0.045 | 1.430 | <0.01 |
| 295 | 0.237 | 0.249 | 0.237 | 0.241 | 0.007 | 0.197 | |||
| 300 | 0.153 | 0.148 | 0.155 | 0.152 | 0.004 | 0.437 | |||
| 305 | 0.132 | 0.134 | 0.134 | 0.133 | 0.001 | 0.437 | |||
| 310 | 0.102 | 0.114 | 0.111 | 0.109 | 0.006 | 0.203 | |||
| 315 | 0.116 | 0.106 | 0.117 | 0.113 | 0.006 | 0.095 | |||
| 320 | 0.096 | 0.093 | 0.089 | 0.093 | 0.004 | 0.017 | |||
| PFSO | 290 | 0.313 | 0.308 | 0.314 | 0.312 | 0.003 | 0.047 | 1.679 | <0.01 |
| 295 | 0.273 | 0.274 | 0.279 | 0.275 | 0.003 | 0.225 | |||
| 300 | 0.172 | 0.172 | 0.188 | 0.177 | 0.009 | 0.510 | |||
| 305 | 0.153 | 0.158 | 0.159 | 0.157 | 0.003 | 0.514 | |||
| 310 | 0.128 | 0.137 | 0.134 | 0.133 | 0.005 | 0.248 | |||
| 315 | 0.126 | 0.139 | 0.136 | 0.134 | 0.007 | 0.112 | |||
| 320 | 0.128 | 0.132 | 0.134 | 0.131 | 0.003 | 0.024 |
| Ingredients | Physical-Dispersion Method | Alcohol-Injection Method | ||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Cholesterol (mg) | 100 | 100 | 100 | 100 |
| Lecithin (mg) | 100 | 200 | 100 | 200 |
| EOs (g) | 1 | 1 | 1 | 1 |
| DES (g) | 1 | 1 | 1 | 1 |
| Ethanol (mL) | - | - | 7 | 7 |
| Trichloromethane (mL) | 5 | 5 | - | - |
| Methanol (mL) | - | - | 3 | 3 |
| PBS (mL) | 50 | 50 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Toufouki, S.; Mahmood, S.; Ahmad, A.; Yohannes, A.; Xiang, Y.; Yao, S. Development of Liposome-Based Hydrogel Patches Incorporating Essential Oils of African Plants and Deep Eutectic Solvents. Gels 2025, 11, 364. https://doi.org/10.3390/gels11050364
Jiang W, Toufouki S, Mahmood S, Ahmad A, Yohannes A, Xiang Y, Yao S. Development of Liposome-Based Hydrogel Patches Incorporating Essential Oils of African Plants and Deep Eutectic Solvents. Gels. 2025; 11(5):364. https://doi.org/10.3390/gels11050364
Chicago/Turabian StyleJiang, Wanhang, Sara Toufouki, Subhan Mahmood, Ali Ahmad, Alula Yohannes, Yang Xiang, and Shun Yao. 2025. "Development of Liposome-Based Hydrogel Patches Incorporating Essential Oils of African Plants and Deep Eutectic Solvents" Gels 11, no. 5: 364. https://doi.org/10.3390/gels11050364
APA StyleJiang, W., Toufouki, S., Mahmood, S., Ahmad, A., Yohannes, A., Xiang, Y., & Yao, S. (2025). Development of Liposome-Based Hydrogel Patches Incorporating Essential Oils of African Plants and Deep Eutectic Solvents. Gels, 11(5), 364. https://doi.org/10.3390/gels11050364









