A Novel Green In Situ Amine-Functionalized Aerogel UiO-66-NH2/TOCNF for the Removal of Azo Anionic Dyes
Abstract
1. Introduction
2. Results and Discussion
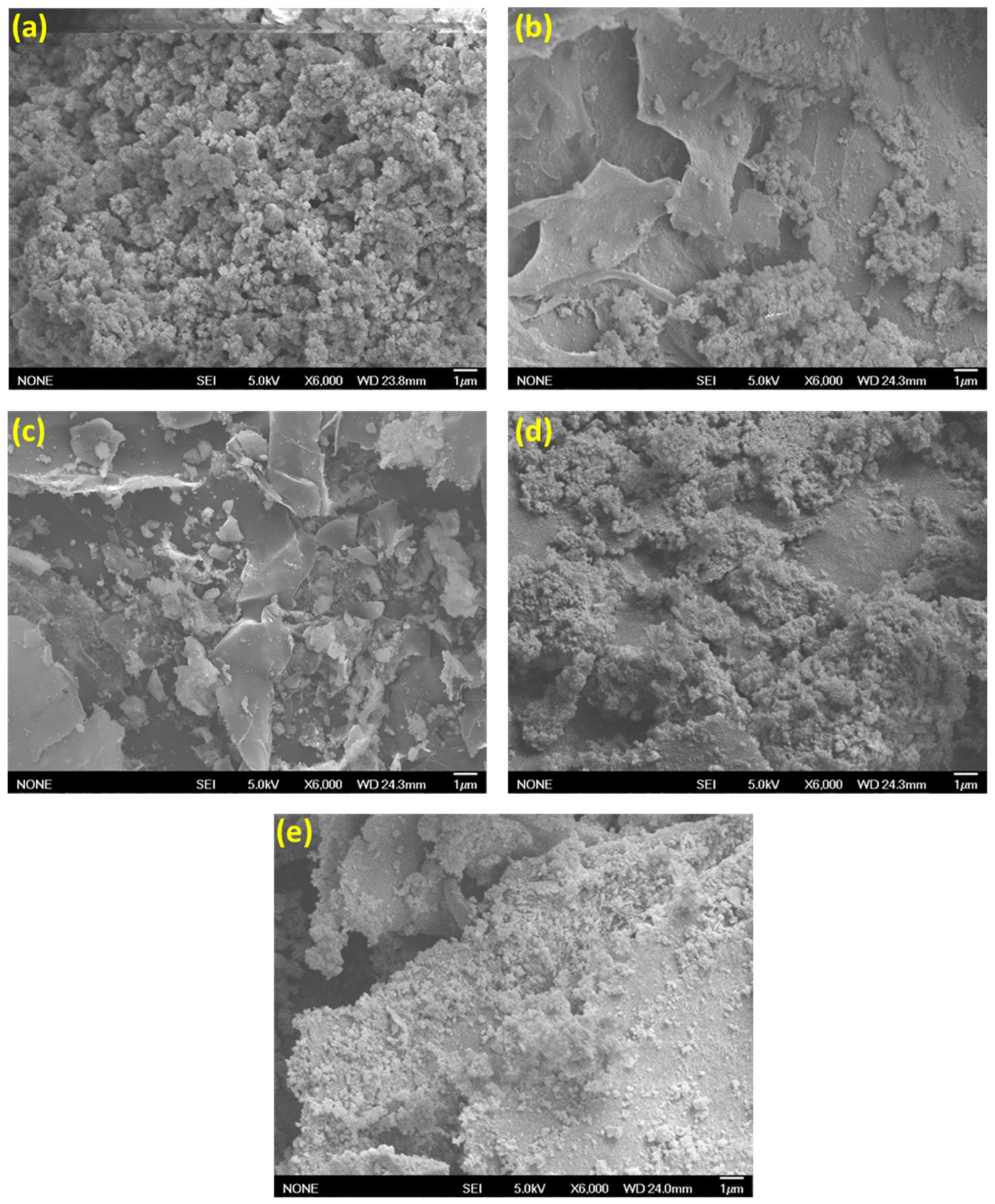
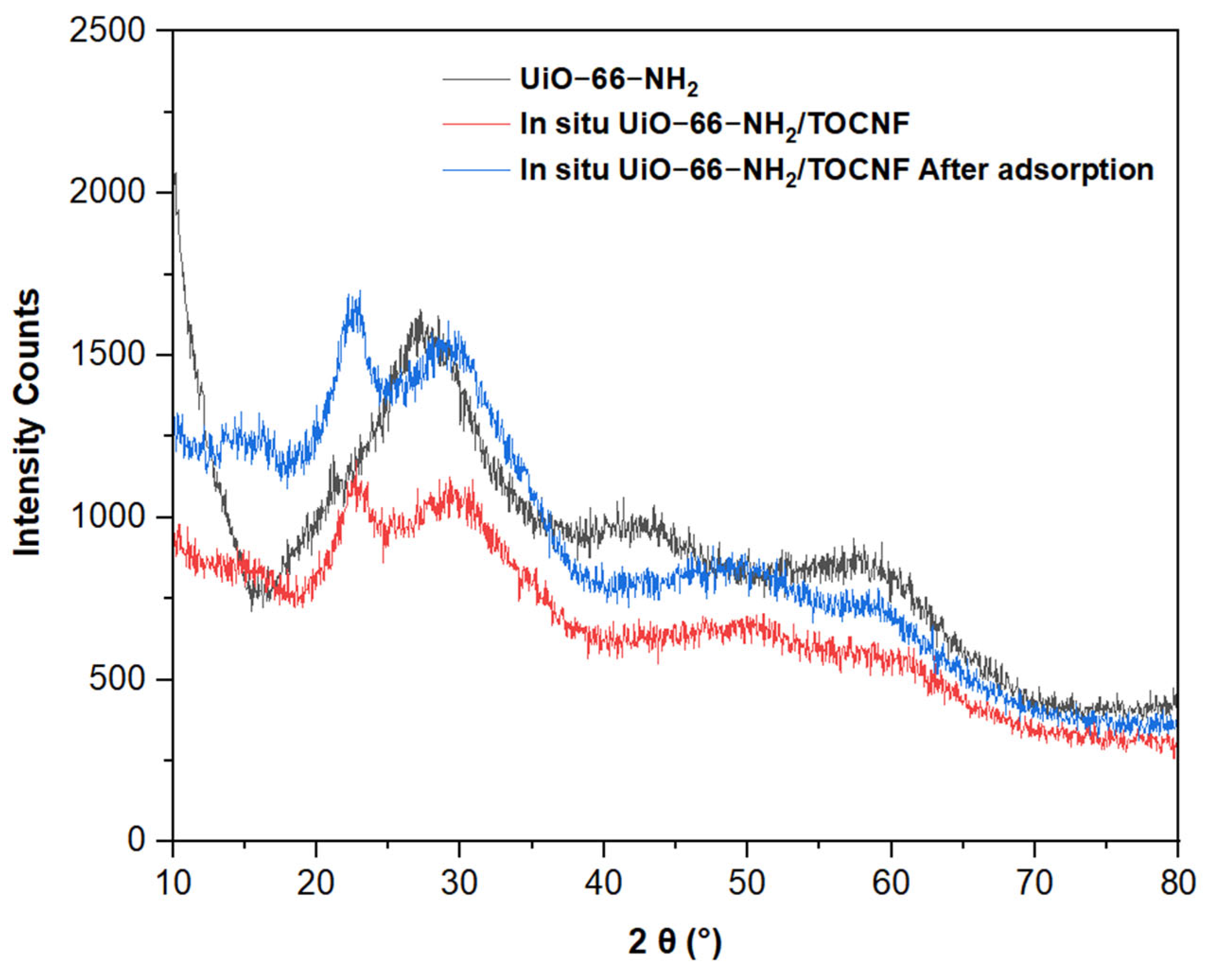
2.1. Characterization of UiO-66-NH2/TOCNF Adsorbents
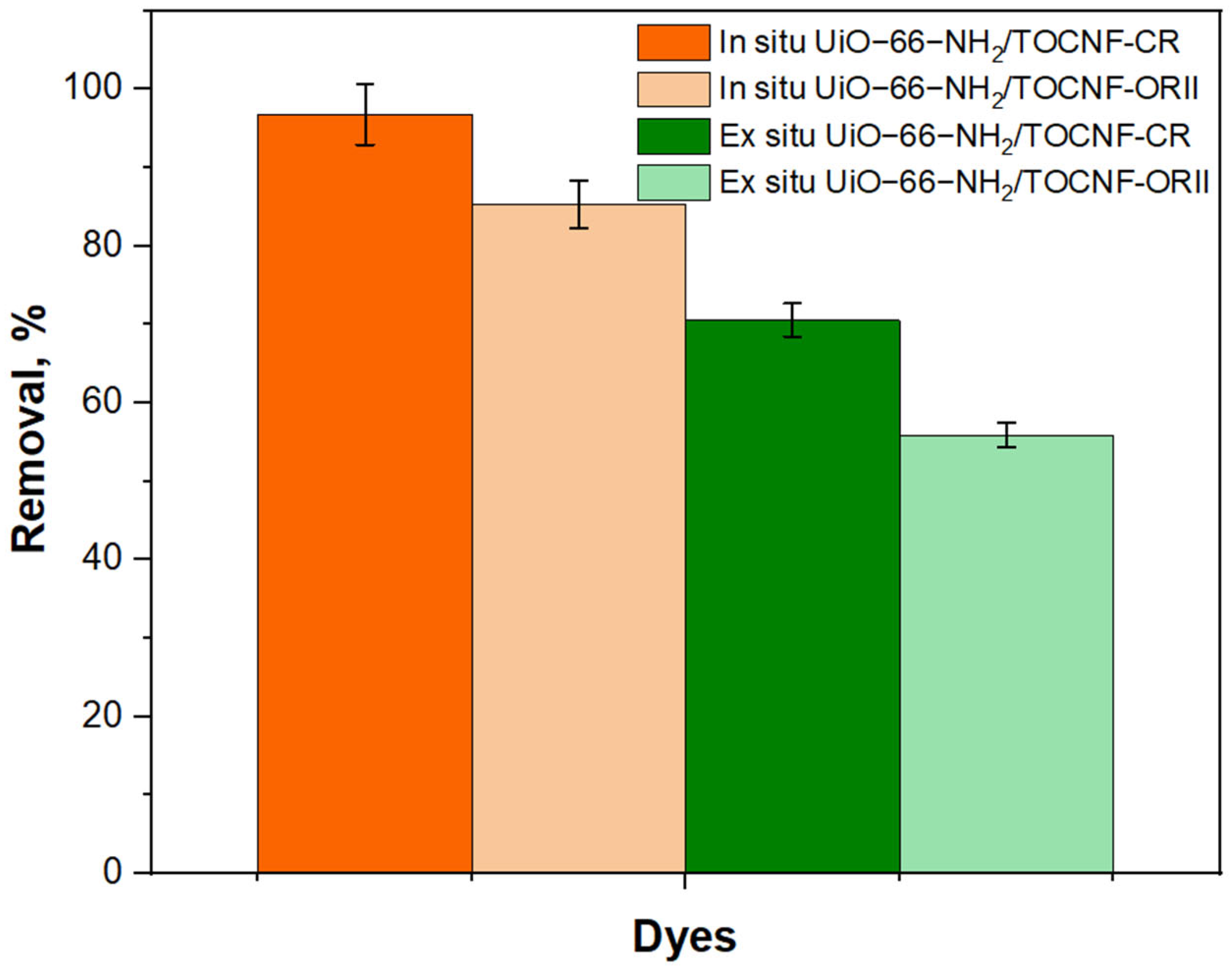
2.2. Adsorption Studies
2.2.1. Effect of pH
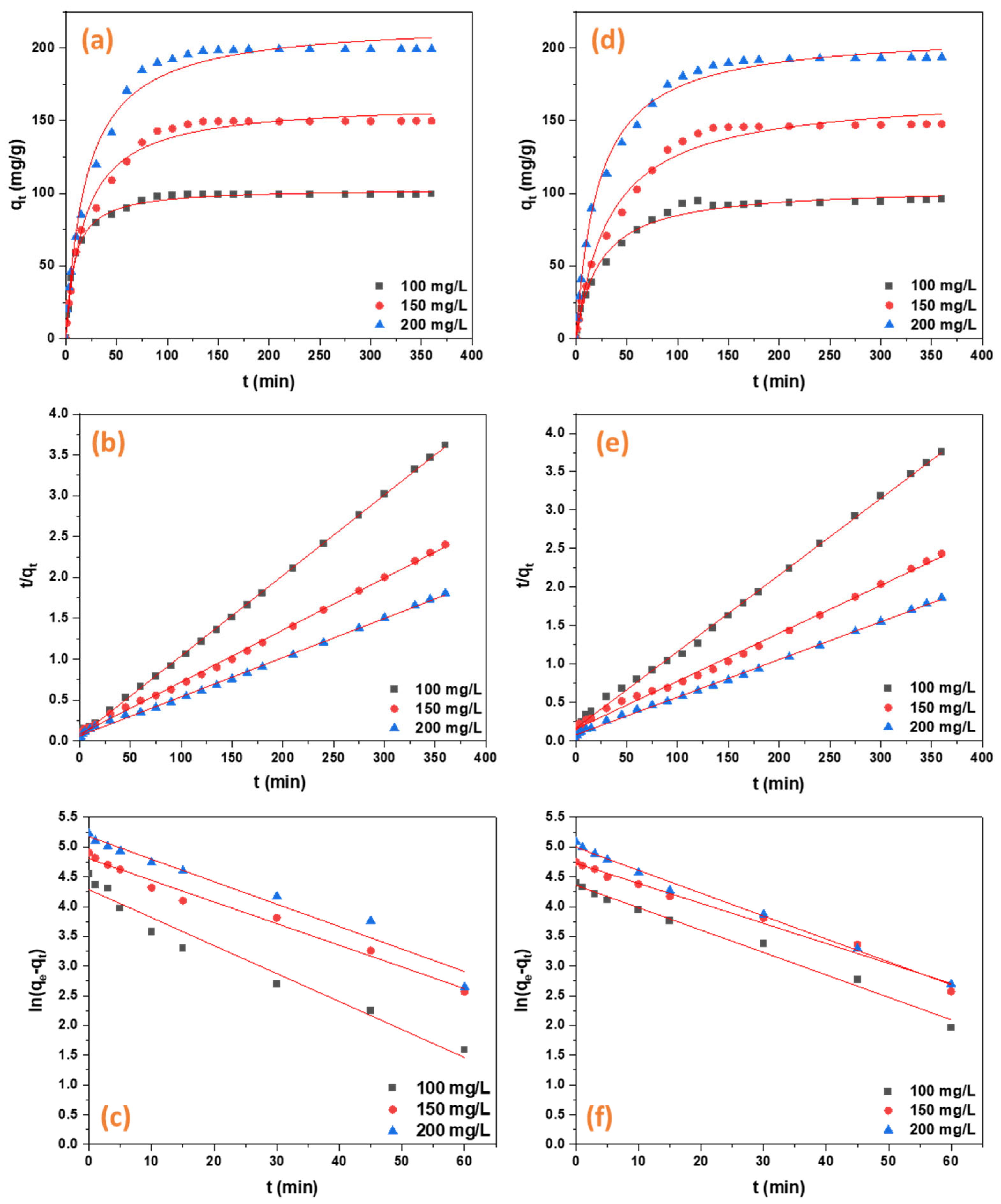
2.2.2. Adsorption Kinetics
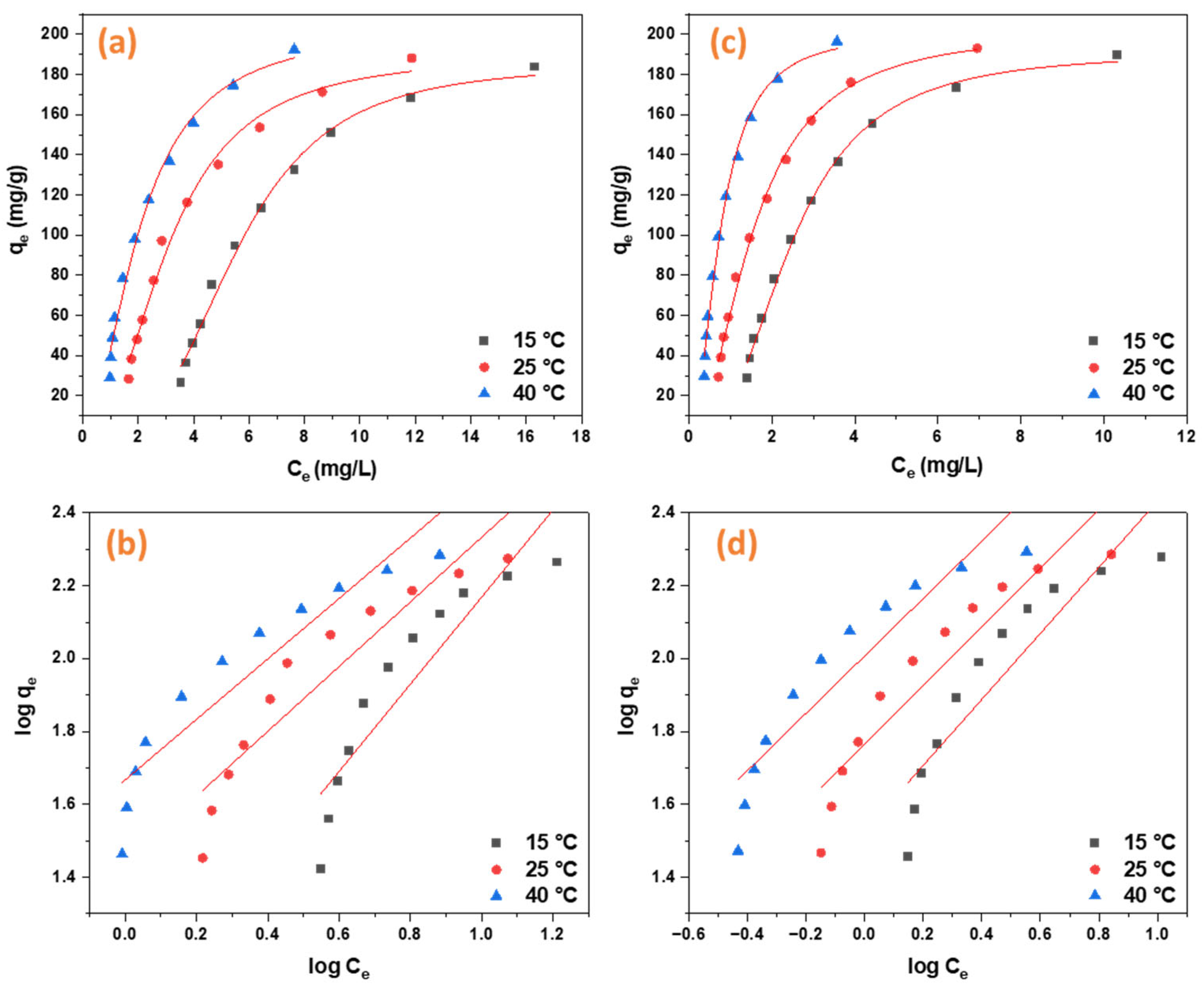
2.2.3. Adsorption Isotherms
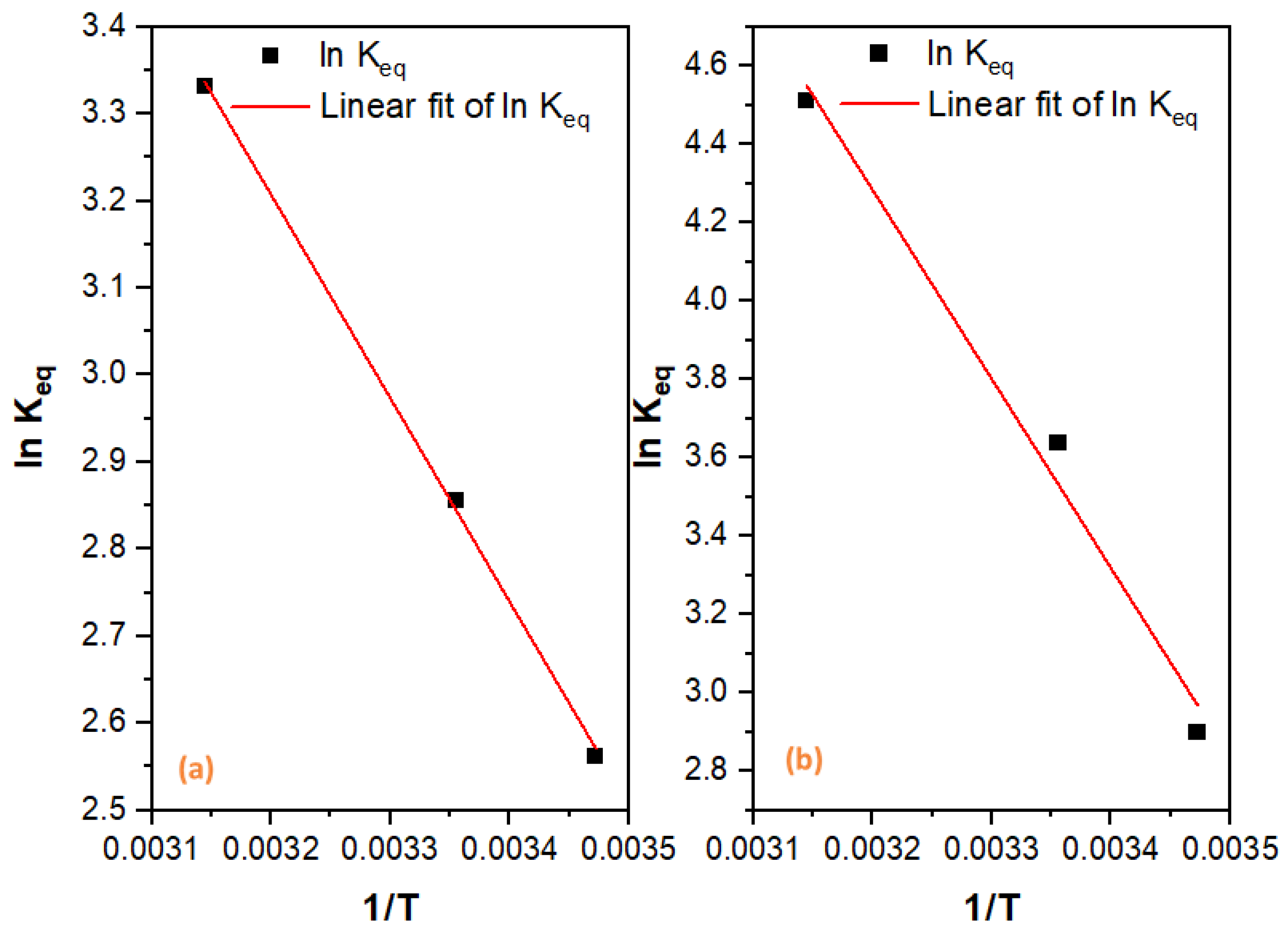
2.2.4. Adsorption Thermodynamics
2.2.5. Adsorption Mechanism
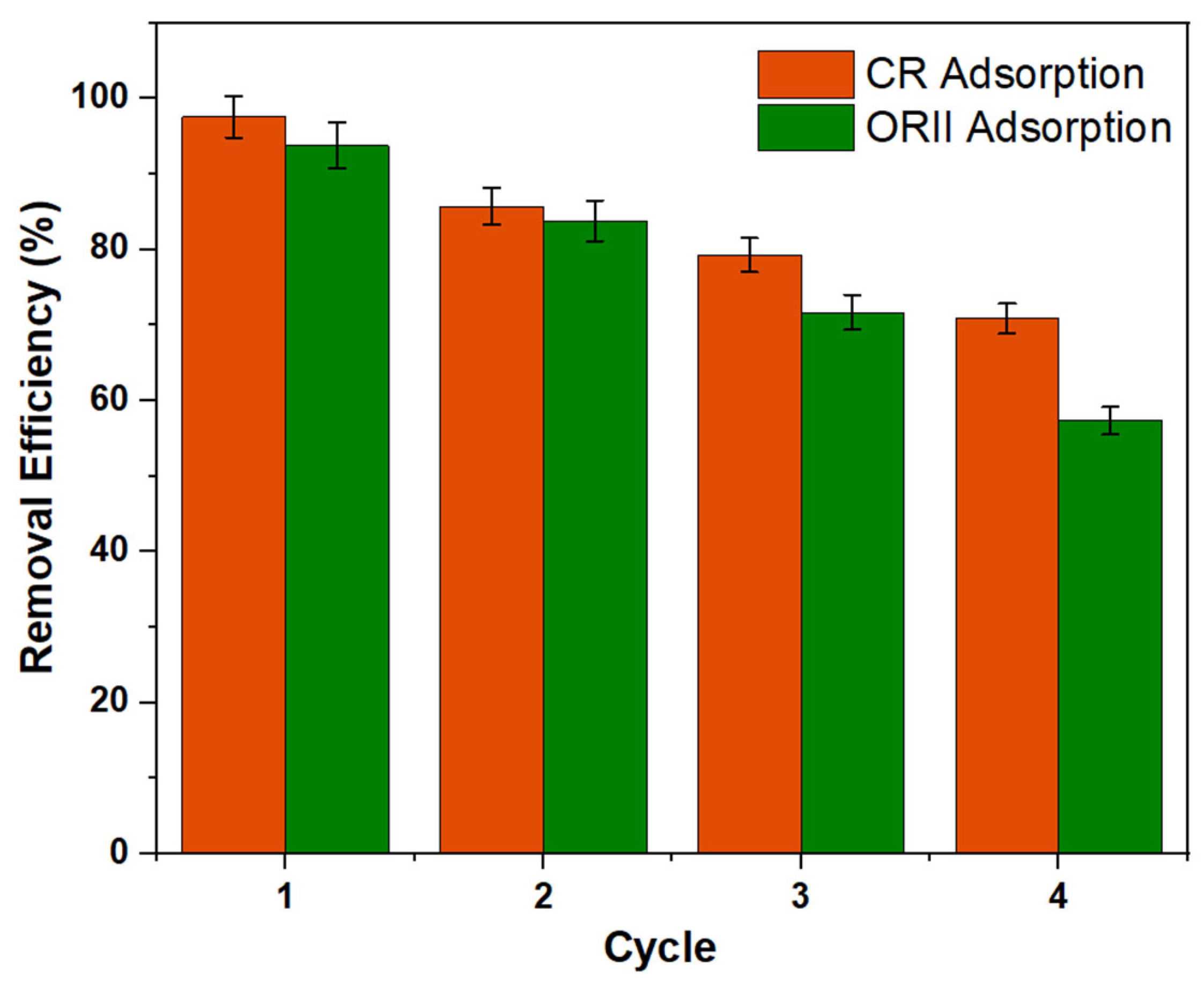
2.2.6. Regeneration Study
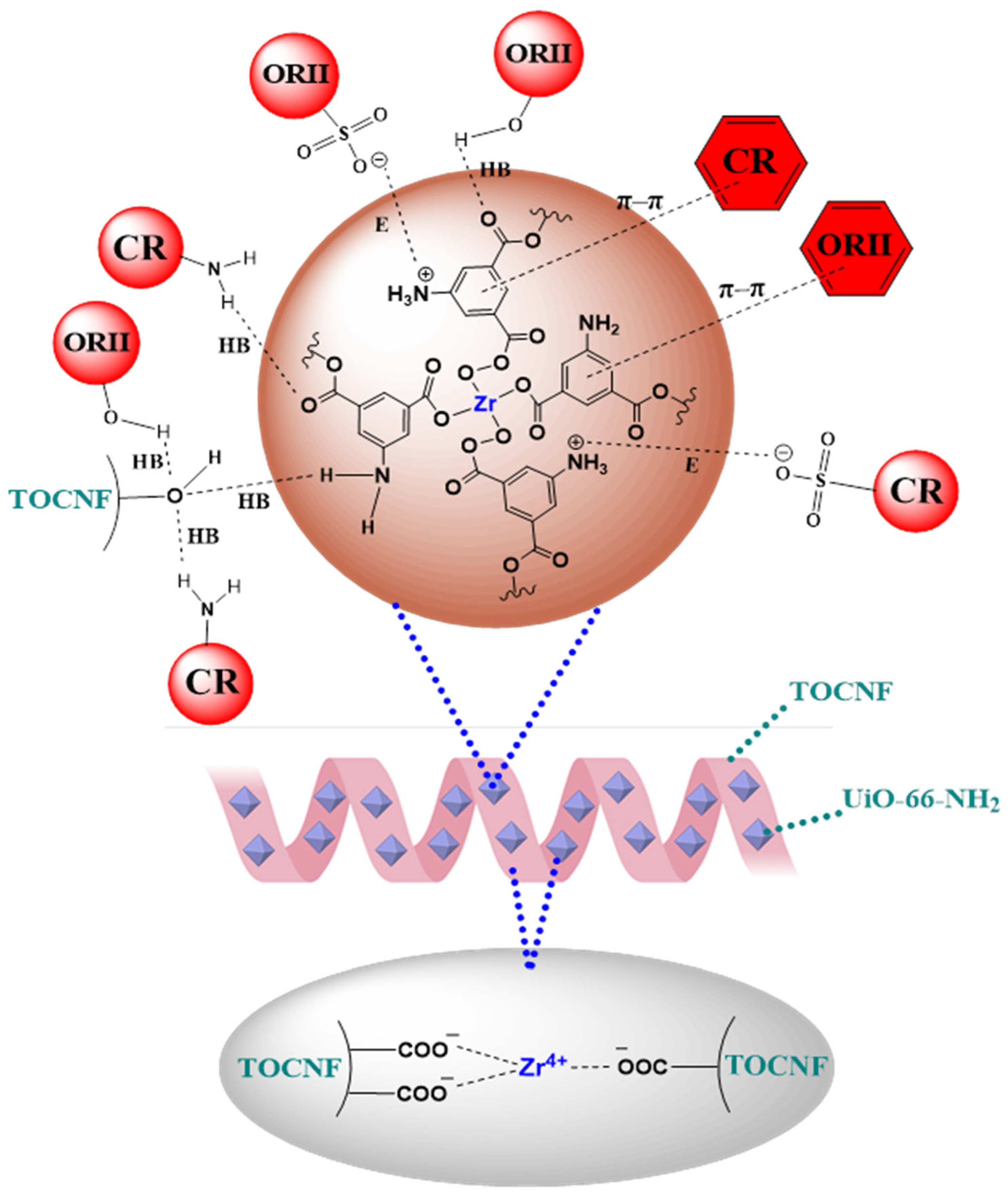
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of UiO-66-NH2 and In Situ UiO-66-NH2/TOCNF
4.3. Aerogel Characterization
4.4. Point of Zero Charge (PZC)
4.5. Adsorption Experiment
4.5.1. pH Influence
4.5.2. Adsorption Kinetics
4.5.3. Adsorption Isotherms
4.5.4. Adsorption Thermodynamics
4.6. Reusability Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Mosaffa, E.; Patel, D.; Ramsheh, N.A.; Patel, R.I.; Banerjee, A.; Ghafuri, H. Bacterial cellulose microfiber reinforced hollow chitosan beads decorated with cross-linked melamine plates for the removal of the Congo red. Int. J. Biol. Macromol. 2024, 254, 127794. [Google Scholar] [CrossRef]
- Wang, P.; Tan, L.; Yuan, G.; Feng, S.; Tang, H.; Wang, G.; Wang, C. ZIF-8 modified polyvinyl alcohol/chitosan composite aerogel for efficient removal of Congo red. J. Solid State Chem. 2022, 316, 123628. [Google Scholar] [CrossRef]
- Amen, R.; Elsayed, I.; Barbary Hassan, E. Sustainable and economical dolomite-modified biochar for efficient removal of anionic dyes. Arab. J. Chem. 2023, 16, 105125. [Google Scholar] [CrossRef]
- Cheng, P.; Kim, M.; Lim, H.; Lin, J.; Torad, N.L.; Zhang, X.; Hossain, M.S.A.; Wu, C.-W.; Wang, C.; Na, J.; et al. A General Approach to Shaped MOF-Containing Aerogels toward Practical Water Treatment Application. Adv. Sustain. Syst. 2020, 4, 2000060. [Google Scholar] [CrossRef]
- Feng, C.; Lv, C.-P.; Li, Z.-Q.; Zhao, H.; Huang, H.-H. A porous 2D Ni-MOF material with a high supercapacitive performance. J. Solid State Chem. 2018, 265, 244–247. [Google Scholar] [CrossRef]
- Wu, W.-P.; Wu, J.; Liu, J.-Q.; Trivedi, M.; Kumar, A. Fabrication of a new metal–organic framework for sensitive sensing of nitroaromatics and efficient dye adsorption. RSC Adv. 2017, 7, 54522–54531. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Gong, F.-X.; Sun, X.-J.; Li, Y.; Zhang, T.; Zhao, X.-J.; Zhang, F.-M. Rapid and Low-Cost Electrochemical Synthesis of UiO-66-NH2 with Enhanced Fluorescence Detection Performance. Inorg. Chem. 2019, 58, 6742–6747. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, F.; Huelsenbeck, L.; Smith, N. Comparison between conventional solvothermal and aqueous solution-based production of UiO-66-NH2: Life cycle assessment, techno-economic assessment, and implications for CO2 capture and storage. J. Environ. Chem. Eng. 2021, 9, 105159. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, M.; Nie, J.; Yang, B.; Song, S.; Lu, P. Multifunctional cellulose-based air filters with high loadings of metal–organic frameworks prepared by in situ growth method for gas adsorption and antibacterial applications. Cellulose 2018, 25, 5999–6010. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted Applications: A review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Georgouvelas, D.; Edlund, U.; Mathew, A.P. CelloZIFPaper: Cellulose-ZIF hybrid paper for heavy metal removal and electrochemical sensing. Chem. Eng. J. 2022, 446, 136614. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Liu, S.; Low, Z.X.; Xie, Z.; Wang, H. TEMPO-oxidized cellulose nanofibers: A renewable nanomaterial for environmental and energy applications. Adv. Mater. Technol. 2021, 6, 2001180. [Google Scholar] [CrossRef]
- Shimizu, M.; Saito, T.; Isogai, A. Water-resistant and high oxygen-barrier nanocellulose films with interfibrillar cross-linkages formed through multivalent metal ions. J. Membr. Sci. 2016, 500, 1–7. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Berglund, L.A.; Isogai, A. Cellulose nanofibrils improve the properties of all-cellulose composites by the nano-reinforcement mechanism and nanofibril-induced crystallization. Nanoscale 2015, 7, 17957–17963. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Feng, Y.; Wang, Z.; Jia, M.; Yao, J. Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N2 separation. J. Membr. Sci. 2018, 568, 10–16. [Google Scholar] [CrossRef]
- Zhu, L.; Zong, L.; Wu, X.; Li, M.; Wang, H.; You, J.; Li, C. Shapeable fibrous aerogels of metal–organic-frameworks templated with nanocellulose for rapid and large-capacity adsorption. ACS Nano 2018, 12, 4462–4468. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, J.; Huang, X.; Zhang, R.; Liu, P. Mixed matrix membranes with highly dispersed UiO-66-NH2 filler for removal of dyes and molybdenum (VI) ions from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131959. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Hao, D.; Feng, Y.; Yao, J. Lightweight UiO-66/cellulose aerogels constructed through self-crosslinking strategy for adsorption applications. Chem. Eng. J. 2019, 371, 138–144. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Hegazy, S.; Ghannami, A.; dos Reis, G.S.; Hu, T.; Brahmi, R.; Tuomikoski, S.; Lassi, U.; Srivastava, V. Activation and Zr precursor influence on UiO-66-NH2 composites for efficient cationic and anionic dye removal. Chem. Eng. Sci. 2025, 302, 120785. [Google Scholar] [CrossRef]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-scale production of metal–organic frameworks. Adv. Funct. Mater. 2024, 34, 2309089. [Google Scholar] [CrossRef]
- Ibarra, I.A.; Bayliss, P.A.; Pérez, E.; Yang, S.; Blake, A.J.; Nowell, H.; Allan, D.R.; Poliakoff, M.; Schröder, M. Near-critical water, a cleaner solvent for the synthesis of a metal–organic framework. Green Chem. 2012, 14, 117–122. [Google Scholar] [CrossRef]
- Reinsch, H. “Green” synthesis of metal-organic frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4290–4299. [Google Scholar] [CrossRef]
- Lin, S.; Su, M.; Li, X.; Liang, S.-X. Solvent-free mechanochemical synthesis of a sodium disulfonate covalent organic framework for simultaneous highly efficient selective capture and sensitive fluorescence detection of fluoroquinolones. Sep. Purif. Technol. 2024, 336, 126167. [Google Scholar] [CrossRef]
- Chen, J.; Shen, K.; Li, Y. Greening the processes of metal–organic framework synthesis and their use in sustainable catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Alvaro, M.; Garcia, H. Metal organic frameworks as catalysts in solvent-free or ionic liquid assisted conditions. Green Chem. 2018, 20, 86–107. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A promising green solvent for the deconstruction of lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Yamada, S.; Shinomiya, N.; Ohba, K.; Sekikawa, M.; Oda, Y. Enzymatic hydrolysis and ethanol fermentation of by-products from potato processing plants. Food Sci. Technol. Res. 2009, 15, 653–658. [Google Scholar] [CrossRef]
- Bui, L.T.; Novi, G.; Lombardi, L.; Iannuzzi, C.; Rossi, J.; Santaniello, A.; Mensuali, A.; Corbineau, F.; Giuntoli, B.; Perata, P. Conservation of ethanol fermentation and its regulation in land plants. J. Exp. Bot. 2019, 70, 1815–1827. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Microwave assisted functionalization of carboxylated-multiwalled carbon nanotubes with 5-aminoisophthalic acid and its application for the preparation of chiral poly (ester-imide)/CNT nanocomposites. Polym. Compos. 2016, 37, 835–843. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Liu, J.; Zhou, Y.; Zhao, D.; Li, C. An acid-base resistant Zn-based metal-organic framework as a luminescent sensor for mercury (II). J. Solid State Chem. 2020, 283, 121153. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Tian, X.; Guo, F.; Wang, C.; Zhang, J.; Jiang, M. Facile in-situ growth of metal–organic framework layer on carboxylated nanocellulose/chitosan aerogel spheres and their high-efficient adsorption and catalytic performance. Appl. Surf. Sci. 2022, 599, 153974. [Google Scholar] [CrossRef]
- Qiao, J.; Song, Q.; Zhang, X.; Zhao, S.; Liu, J.; Nyström, G.; Zeng, Z. Enhancing Interface Connectivity for Multifunctional Magnetic Carbon Aerogels: An In Situ Growth Strategy of Metal-Organic Frameworks on Cellulose Nanofibrils. Adv. Sci. 2024, 11, 2400403. [Google Scholar] [CrossRef]
- Tian, S.; Yi, Z.; Chen, J.; Fu, S. In situ growth of UiO-66-NH(2) in wood-derived cellulose for iodine adsorption. J. Hazard. Mater. 2023, 443, 130236. [Google Scholar] [CrossRef]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef]
- Belaye, M.; Taddesse, A.M.; Teju, E.; Sanchez-Sanchez, M.; Yassin, J.M. Preparation and adsorption behavior of Ce (III)-MOF for phosphate and fluoride ion removal from aqueous solutions. ACS Omega 2023, 8, 23860–23869. [Google Scholar] [CrossRef]
- Amanda, P.; Kurniawan, Y.D.; Prasetiyo, K.W.; Hasan, F.; Amelia, A. Adsorption of Congo Red from Aqueous Solutions by Alginate-Nanocellulose-Polyethyleneimine Hydrogel Beads. Trends Sci. 2024, 21, 7262. [Google Scholar] [CrossRef]
- Lee, J.; Ka, D.; Jung, H.; Cho, K.; Jin, Y.; Kim, M. UiO-66-NH2 and Zeolite-Templated Carbon Composites for the Degradation and Adsorption of Nerve Agents. Molecules 2021, 26, 3837. [Google Scholar] [CrossRef]
- Ning, R.; Wu, C.-N.; Takeuchi, M.; Saito, T.; Isogai, A. Preparation and characterization of zinc oxide/TEMPO-oxidized cellulose nanofibril composite films. Cellulose 2017, 24, 4861–4870. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Shao, L.; Li, X.; Zhu, M.; Liu, Y.; Feng, X.; Zhu, X. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281. [Google Scholar] [CrossRef]
- Nguena, K.L.T.; Fotsop, C.G.; Bopda, A.; Tchuifon Tchuifon, D.R.; Kamgang Djioko, F.H.; Soukoua Ngueabouo, A.M.; Ada Madu, C.; Ezema, F.I.; Oguzie, E.E. Unraveling the sorption mechanism of industrial dyes onto Zr-based MOFs: Computational and experimental modelling for highly efficient removal. Mater. Adv. 2025, 6, 579–597. [Google Scholar] [CrossRef]
- Bessaha, F.; Mahrez, N.; Marouf-Khelifa, K.; Çoruh, A.; Khelifa, A. Removal of Congo red by thermally and chemically modified halloysite: Equilibrium, FTIR spectroscopy, and mechanism studies. Int. J. Environ. Sci. Technol. 2019, 16, 4253–4260. [Google Scholar] [CrossRef]
- Molavi, H.; Eskandari, A.; Shojaei, A.; Mousavi, S.A. Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66 (Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, K.; Qin, Y.; Xie, L.; Chai, X.; Zhang, L.; Du, G.; Ge, S.; Rezakazemi, M.; Aminabhavi, T.M. Amine-functionalized UiO-66 incorporated electrospun cellulose/chitosan porous nanofibrous membranes for removing copper ions. Chem. Eng. J. 2024, 480, 148077. [Google Scholar] [CrossRef]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.; Meireles, C.S.; Cerqueira, D.A.; Rodrigues Filho, G.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Han, F.; Yan, P.; Liu, B.; Zhou, Q.; Min, F.; He, F.; Wu, Z. Investigation on the adsorption of phosphorus in all fractions from sediment by modified maifanite. Sci. Rep. 2018, 8, 15619. [Google Scholar] [CrossRef]
- Yang, Q.; Song, H.; Li, Y.; Pan, Z.; Dong, M.; Chen, F.; Chen, Z. Flower-like core-shell Fe3O4@MnO2 microspheres: Synthesis and selective removal of Congo red dye from aqueous solution. J. Mol. Liq. 2017, 234, 18–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zhu, J.; Li, N.; Yin, Y. Fabrication of wood-inspired nanocellulose-based aerogels for efficient adsorption and filtration removal of Congo red. Ind. Crops Prod. 2023, 205, 117482. [Google Scholar] [CrossRef]
- Elsayed, I.; Madduri, S.; El-Giar, E.M. Effective removal of anionic dyes from aqueous solutions by novel polyethylenimine-ozone oxidized hydrochar (PEI-OzHC) adsorbent. Arab. J. Chem. 2022, 15, 103757. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Tang, S.; Ruiz-Hitzky, E.; Luo, J.; Wang, X. Pod-inspired MXene/porous carbon microspheres with ultrahigh adsorption capacity towards crystal violet. Chem. Eng. J. 2021, 426, 130776. [Google Scholar] [CrossRef]
- Chen, Y.; Hanshe, M.; Sun, Z.; Zhou, Y.; Mei, C.; Duan, G.; Zheng, J.; Shiju, E.; Jiang, S. Lightweight and anisotropic cellulose nanofibril/rectorite composite sponges for efficient dye adsorption and selective separation. Int. J. Biol. Macromol. 2022, 207, 130–139. [Google Scholar] [CrossRef]
- Bellaj, M.; Aziz, K.; El Achaby, M.; El Haddad, M.; Gebrati, L.; Kurniawan, T.A.; Chen, Z.; Yap, P.-S.; Aziz, F. Cationic and anionic dyes adsorption from wastewater by clay-chitosan composite: An integrated experimental and modeling study. Chem. Eng. Sci. 2024, 285, 119615. [Google Scholar] [CrossRef]
- Alanazi, K.D.; Alshammari, B.H.; Ahmad, O.A.S.; Aljohani, M.M.; Alsharief, H.H.; Al-Bagawi, A.H.; Alsehli, A.H.; El-Metwaly, N.M. Citric acid-cross linked with magnetic metal-organic framework composite sponge for superior adsorption of indigo carmine blue dye from aqueous solutions: Characterization and adsorption optimization via Box–Behnken design. J. Mol. Struct. 2024, 1299, 137131. [Google Scholar] [CrossRef]
- Al-Hazmi, G.H.; Refat, M.S.; El-Desouky, M.G.; Wali, F.K.; El-Bindary, A.A. Effective removal of industrial dye from aqueous solution using mesoporous nickel oxide: A complete batch system evaluation. Desalin. Water Treat. 2022, 273, 246–260. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elshishini, H.M.; Ghatass, Z.F.; Elsubruiti, G.M. Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. Powder Technol. 2021, 379, 407–416. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Wang, Z.; Chen, L.; Zhu, F.; Ye, Y.; Zheng, Y.; Yu, W.; Zheng, Q. Metal-Coordinated Dynamics and Viscoelastic Properties of Double-Network Hydrogels. Gels 2023, 9, 145. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, N.; Krishnan, S.; Singh, A.K.; Mathur, P.; Rai, D.K. Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid. Sci. Rep. 2020, 10, 111. [Google Scholar] [CrossRef]
- Elsayed, I.; Schueneman, G.T.; El-Giar, E.M.; Hassan, E.B. Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater. Gels 2023, 9, 154. [Google Scholar] [CrossRef]
- Simion, A.-I.; Grigoraș, C.-G.; Favier, L. Batch Adsorption of Orange II Dye on a New Green Hydrogel—Study on Working Parameters and Process Enhancement. Gels 2025, 11, 79. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of Acid Orange 7 and Remazol Black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater. Sci. Eng. C 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Cao, J.; Wu, Y.; Jin, Y.; Yilihan, P.; Yang, S. Dynamic adsorption of anionic dyes by apricot shell activated carbon. Desalination Water Treat. 2015, 53, 2990–2998. [Google Scholar] [CrossRef]
- Tǎmaş, A.; Cozma, I.; Cocheci, L.; Lupa, L.; Rusu, G. Adsorption of orange II onto Zn2Al–layered double hydroxide prepared from zinc ash. Front. Chem. 2020, 8, 573535. [Google Scholar] [CrossRef]
- Yu, J.; He, W.; Liu, B. Adsorption of acid orange Ⅱ with two step modified sepiolite: Optimization, adsorption performance, kinetics, thermodynamics and regeneration. Int. J. Environ. Res. Public Health 2020, 17, 1732. [Google Scholar] [CrossRef]
- Suhag, M.H.; Khatun, A.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. Purification of aqueous orange II solution through adsorption and visible-light-induced photodegradation using ZnO-modified g-C3N4 composites. RSC Adv. 2024, 14, 17888–17900. [Google Scholar] [CrossRef]
- Al-Omari, M.H.; Abu-Rayyan, A.; Ragab, A.H.; Taher, M.A.; El-Sayed, E.-S.M.; Elfiky, A.; Taha, A.; Mubarak, M.F. Optimized Congo Red Dye Adsorption Using ZnCuCr-Based MOF for Sustainable Wastewater Treatment. Langmuir 2025, 41, 5947–5961. [Google Scholar] [CrossRef] [PubMed]
- Zolgharnein, J.; Farahani, S.D.; Bagtash, M.; Amani, S. Application of a new metal-organic framework of [Ni2F2(4,4′-bipy)2(H2O)2](VO3)2·8H2O as an efficient adsorbent for removal of Congo red dye using experimental design optimization. Environ. Res. 2020, 182, 109054. [Google Scholar] [CrossRef]
- Singh, K.P.; Wareppam, B.; Raghavendra, K.G.; Singh, N.J.; de Oliveira, A.C.; Garg, V.K.; Ghosh, S.; Singh, L.H. Heterophase Grain Boundary-Rich Superparamagnetic Iron Oxides/Carbon Composite for Cationic Crystal Violet and Anionic Congo Red Dye Removal. Adv. Eng. Mater. 2023, 25, 2300354. [Google Scholar] [CrossRef]
- Muedi, K.L.; Masindi, V.; Maree, J.P.; Haneklaus, N.; Brink, H.G. Effective adsorption of Congo red from aqueous solution using Fe/Al Di-metal nanostructured composite synthesised from Fe (III) and Al (III) recovered from real acid mine drainage. Nanomaterials 2022, 12, 776. [Google Scholar] [CrossRef]
- Hussain, S.; Salman, M.; Youngblood, J.P.; Farooq, U.; Yasmeen, S.; Al-Ahmary, K.M.; Ahmed, M. Enhanced adsorption of Congo red dye by CS/PEG/ZnO composite hydrogel: Synthesis, characterization, and performance evaluation. J. Mol. Liq. 2024, 411, 125704. [Google Scholar] [CrossRef]
- Hamad, K.H.; Yasser, A.M.; Nabil, R.; Tarek, R.; Hesham, E.; El-telbany, A.; Saeed, A.; Selim, S.E.; Abdelhamid, A.E. Nylon fiber waste as a prominent adsorbent for Congo red dye removal. Sci. Rep. 2024, 14, 1088. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, D.M.P.; Abatal, M.; Lima, E.; Franseschi, F.A.; Ucán, C.A.; Tariq, R.; Elías, M.A.R.; Vargas, J. Sorption Behavior of Azo Dye Congo Red onto Activated Biochar from Haematoxylum campechianum Waste: Gradient Boosting Machine Learning-Assisted Bayesian Optimization for Improved Adsorption Process. Int. J. Mol. Sci. 2024, 25, 4771. [Google Scholar] [CrossRef]
- Harja, M.; Lupu, N.; Chiriac, H.; Herea, D.-D.; Buema, G. Studies on the Removal of Congo Red Dye by an Adsorbent Based on Fly-Ash@Fe3O4 Mixture. Magnetochemistry 2022, 8, 125. [Google Scholar] [CrossRef]
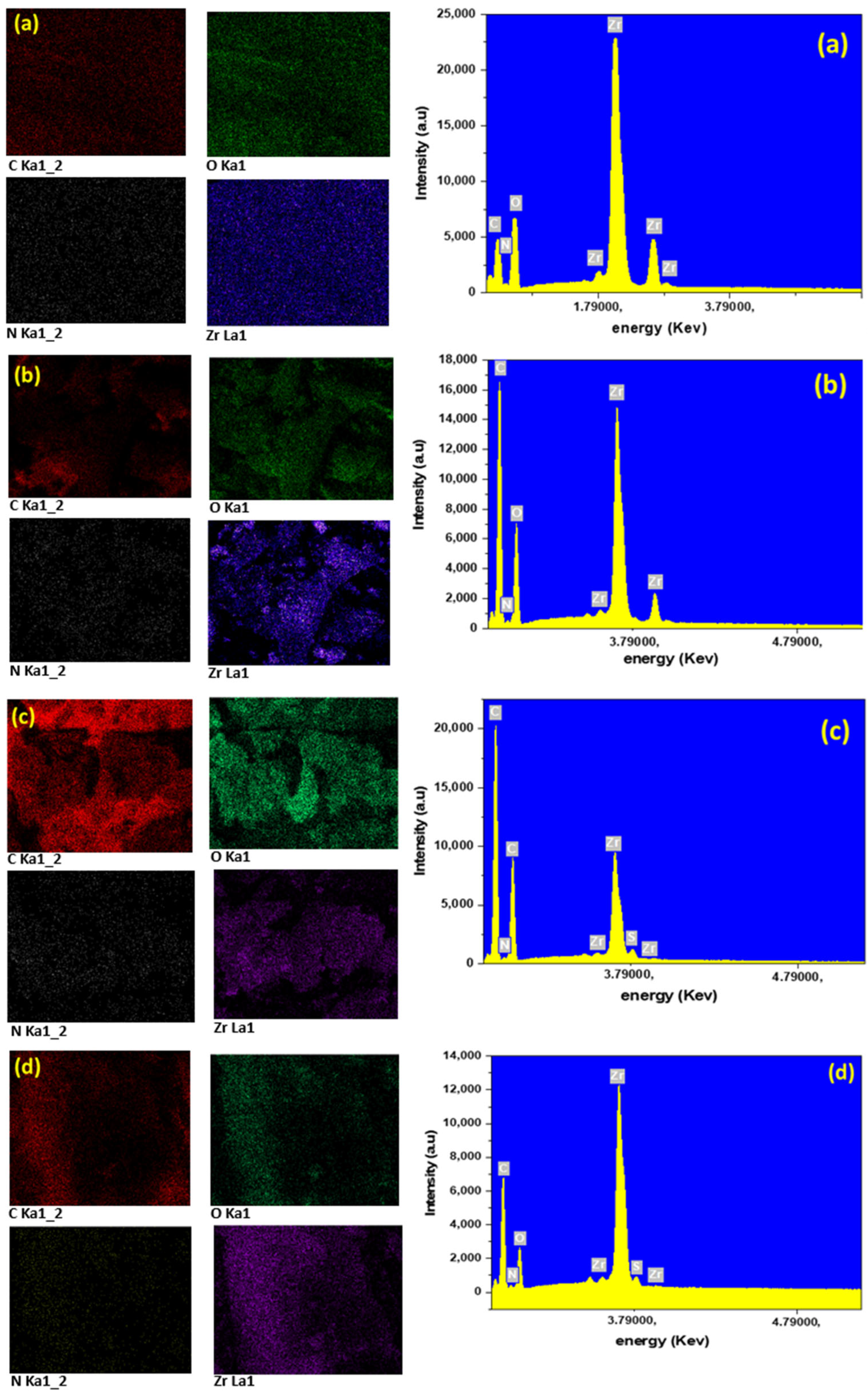
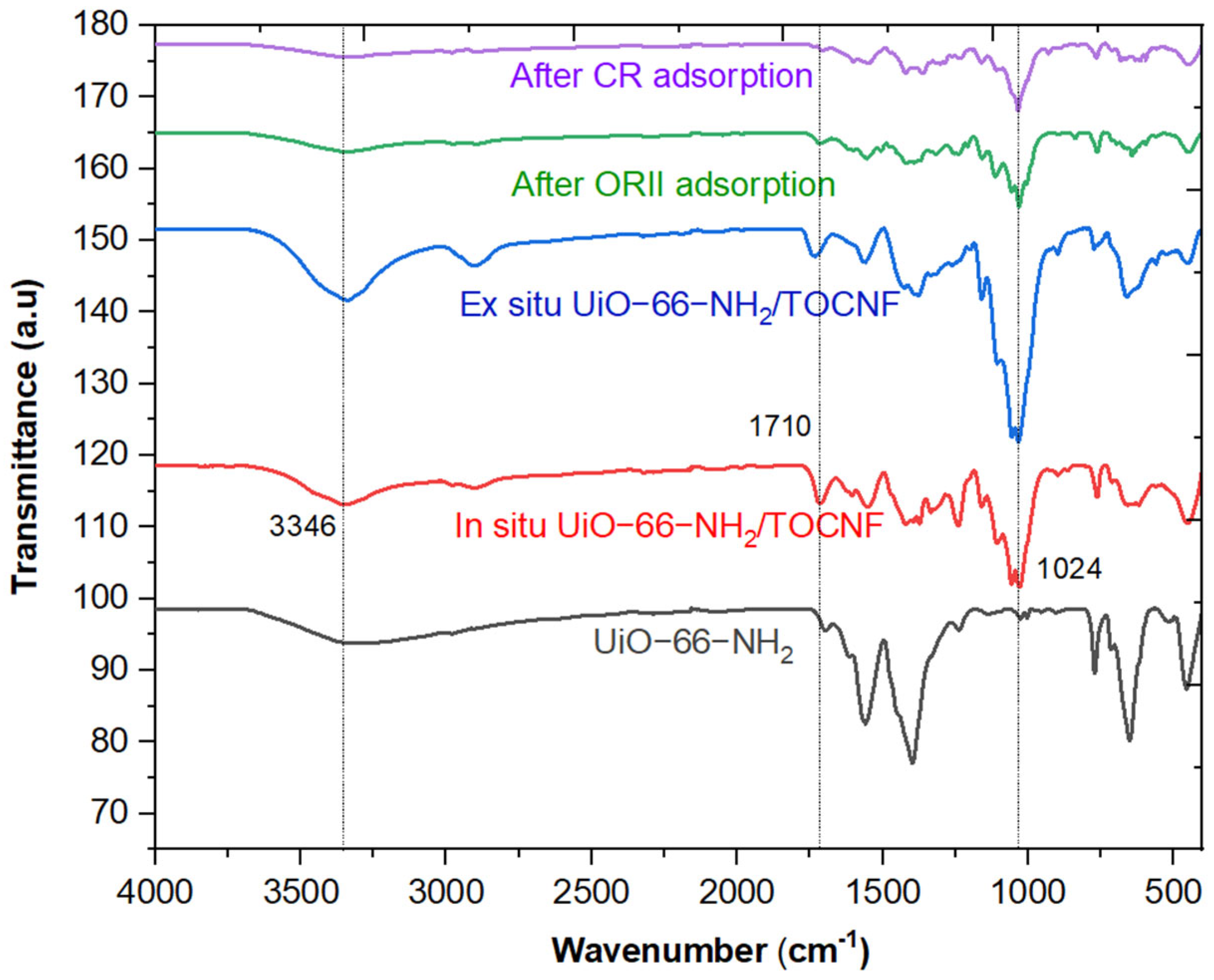
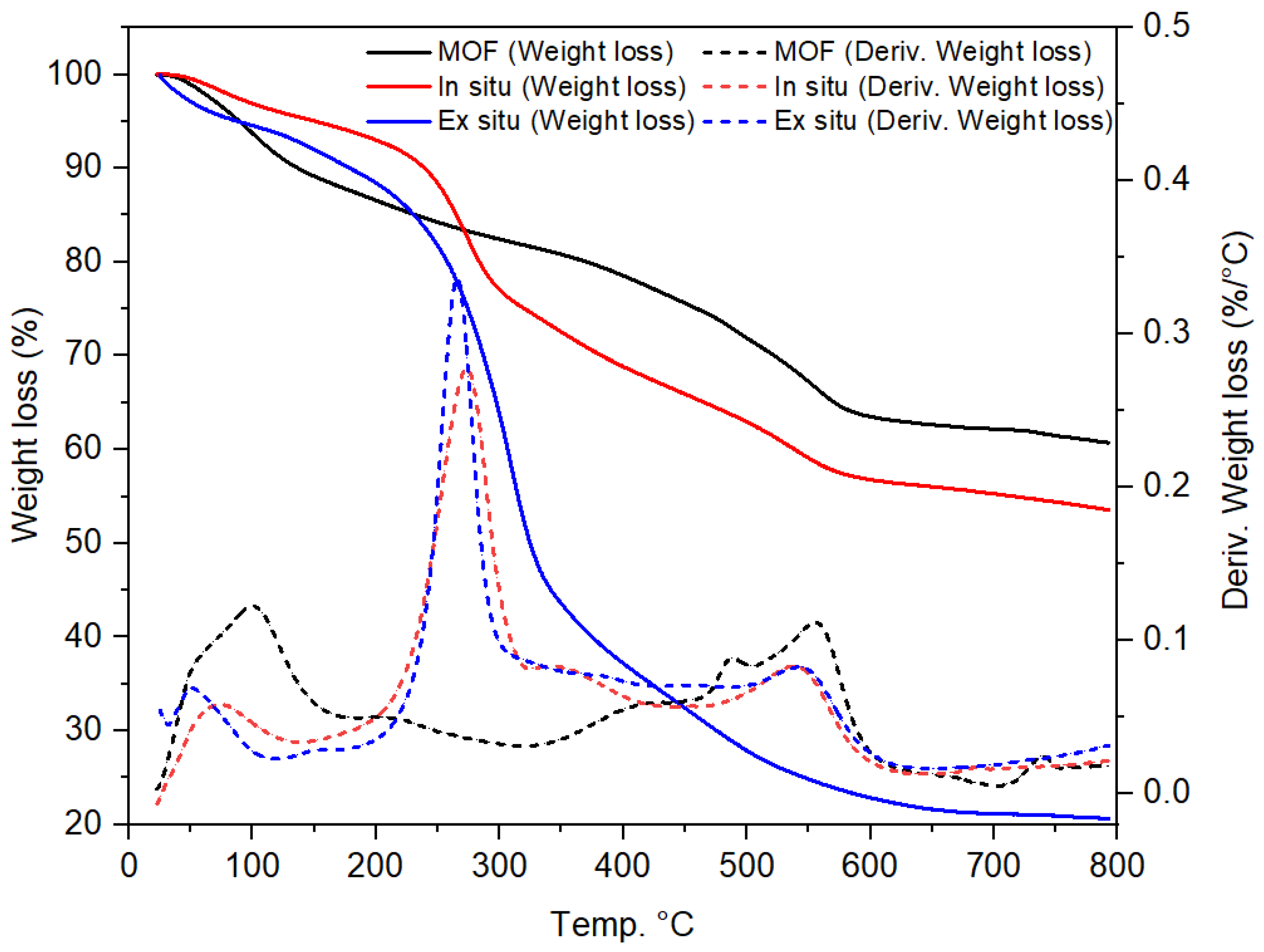

| Materials | BET (m2/g) | Pore Volume cc/g |
|---|---|---|
| UiO-66-NH2 | 60.42 | 0.23 |
| in situ UiO-66-NH2/TOCNF | 145.67 | 0.39 |
| ex situ UiO-66-NH2/TOCNF | 26.70 | 0.15 |
| Models | CR | ORII | |||||
|---|---|---|---|---|---|---|---|
| Co (mg/L) | 100 | 150 | 200 | 100 | 150 | 200 | |
| Pseudo−first order | K1 (1/min) | 0.04708 | 0.03646 | 0.03777 | 0.03771 | 0.03364 | 0.03849 |
| qe (mg/g) | 72.67083 | 122.5097 | 176.7524 | 78.22427 | 113.0274 | 147.831 | |
| R2 | 0.96442 | 0.98535 | 0.96711 | 0.9884 | 0.98676 | 0.99391 | |
| Pseudo−second order | K2 (g/mg/min) | 0.001701 | 0.000493 | 0.000374 | 0.000592 | 0.000251 | 0.000303 |
| qe (mg/g) | 101.626 | 156.9859 | 208.7683 | 100.5025 | 160.5136 | 204.0816 | |
| R2 | 0.99954 | 0.99816 | 0.99788 | 0.9976 | 0.99453 | 0.99804 | |
| Models | CR | ORII | |||||
|---|---|---|---|---|---|---|---|
| Temp (°C) | 15 | 25 | 40 | 15 | 25 | 40 | |
| Langmuir | K1 (L/mg) | 0.384949 | 0.186695 | 0.06587 | 1.915423 | 0.115305 | 0.761408 |
| qmax (mg/g) | 375.9398 | 383.1418 | 549.4505 | 129.8701 | 148.8095 | 171.2329 | |
| R2 | 0.99345 | 0.99554 | 0.99604 | 0.99976 | 0.98957 | 0.99894 | |
| Freundlich | KF ([mg g−1(Lmg−1)1/n]) | 101.763 | 58.13933 | 33.55135 | 84.30629 | 26.87571 | 74.98424 |
| n | 1.269728 | 1.237517 | 1.103107 | 12.51251 | 3.246964 | 6.436663 | |
| R2 | 0.85886 | 0.87461 | 0.83526 | 0.90941 | 0.99024 | 0.94508 | |
| Adsorbate | T (°C) | Qe (mg/g) | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J·mol−1·K−1) | ||
|---|---|---|---|---|---|---|---|
| CR | 15 | 189.57 | 18.17546 | 2.90007 | −6.94403 | 40.15255 | 164.0981 |
| 25 | 194.87 | 37.98635 | 3.63722 | −9.01149 | |||
| 40 | 197.83 | 91.1659 | 4.51268 | −11.9309 | |||
| ORII | 15 | 185.68 | 12.96648 | 2.56236 | −6.13541 | 19.4291 | 88.83509 |
| 30 | 189.13 | 17.39926 | 2.85642 | −7.07701 | |||
| 40 | 193.1 | 27.98551 | 3.33168 | −8.80849 |
| Adsorbent Material | Dye Type | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| Orange II | |||
| UiO-66-NH2, solvothermal method | ORII | 229.8 | [44] |
| CSSA Hydrogel | ORII | 6.84 | [62] |
| Apricot shell activated carbon | ORII | 13.98 | [63] |
| Canola stalks | ORII | 25.6 | [64] |
| Zn2Al-layered double hydroxide | ORII | 42.5 | [65] |
| cetyltrimethylammonium bromide (CTAB) | ORII | 110.05 | [66] |
| ZnO-modified g-C3N4 composite | ORII | 13.441 | [67] |
| In situ UiO-66-NH2/TOCNF | ORII | 171.2 | This study |
| Congo Red | |||
| UiO-66-NH2@HTC | CR | 277.77 | [23] |
| ZnCuCr-Based MOF | CR | 325 | [68] |
| [Ni2F2(4,4′-bipy)2(H2O)2](VO3)2.8H2O | CR | 242.1 | [69] |
| UiO-66-NH2] | CR | 16.50 | [20] |
| Iron oxide/carbon composite | CR | 40.44 | [70] |
| Polycationic Fe/Al Di-metal nanostructured composite (PDFe/Al) | CR | 411 | [71] |
| CS/PEG/ZnO Composite Hydrogel | CR | 212.76 | [72] |
| Nylon fiber waste | CR | 188 | [73] |
| Activated biochar | CR | 114.8 | [74] |
| Fly-Ash@Fe3O₄ | CR | 154 | [75] |
| In situ UiO-66-NH2/TOCNF | CR | 549.4 | This study |
| Model | Equation |
|---|---|
| Langmuir linear | |
| Langmuir non-linear | |
| Freundlich linear | |
| Freundlich non-linear | |
| Pseudo-first order non-linear | |
| Pseudo-first order linear | |
| Pseudo-second order non-linear | |
| Pseudo-second order linear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amen, R.; Elsayed, I.; Kim, Y.; Schueneman, G.T.; El-Giar, E.M.; Hassan, E.B. A Novel Green In Situ Amine-Functionalized Aerogel UiO-66-NH2/TOCNF for the Removal of Azo Anionic Dyes. Gels 2025, 11, 365. https://doi.org/10.3390/gels11050365
Amen R, Elsayed I, Kim Y, Schueneman GT, El-Giar EM, Hassan EB. A Novel Green In Situ Amine-Functionalized Aerogel UiO-66-NH2/TOCNF for the Removal of Azo Anionic Dyes. Gels. 2025; 11(5):365. https://doi.org/10.3390/gels11050365
Chicago/Turabian StyleAmen, Rabia, Islam Elsayed, Yunsang Kim, Gregory T. Schueneman, Emad M. El-Giar, and El Barbary Hassan. 2025. "A Novel Green In Situ Amine-Functionalized Aerogel UiO-66-NH2/TOCNF for the Removal of Azo Anionic Dyes" Gels 11, no. 5: 365. https://doi.org/10.3390/gels11050365
APA StyleAmen, R., Elsayed, I., Kim, Y., Schueneman, G. T., El-Giar, E. M., & Hassan, E. B. (2025). A Novel Green In Situ Amine-Functionalized Aerogel UiO-66-NH2/TOCNF for the Removal of Azo Anionic Dyes. Gels, 11(5), 365. https://doi.org/10.3390/gels11050365







