Injectable and Conductive Polyurethane Gel with Load-Responsive Antibiosis for Sustained Root Canal Disinfection
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of AT and CPU-Based Prepolymers
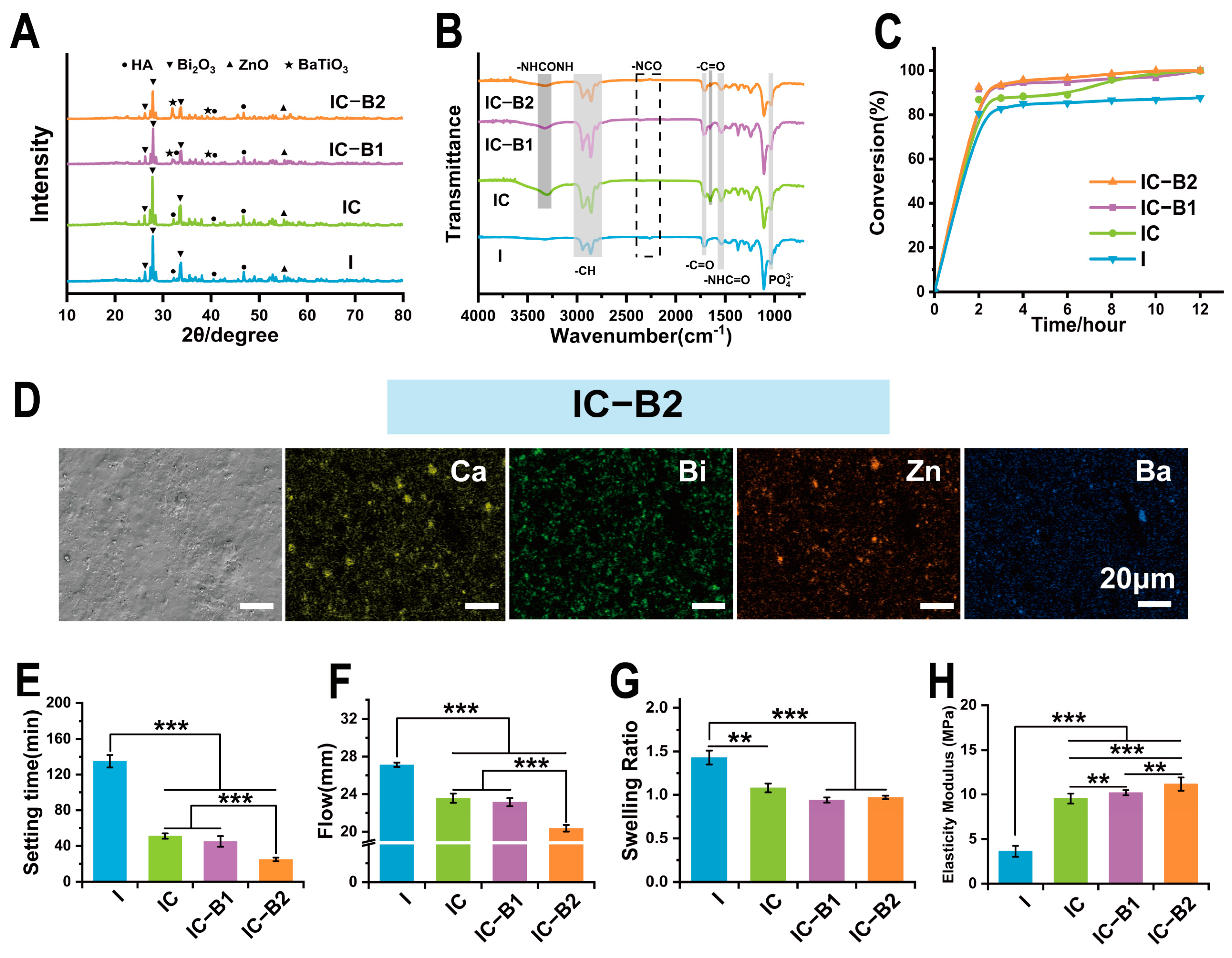
2.2. Physicochemical Properties of Different Composite Gels for Root Canal Therapy
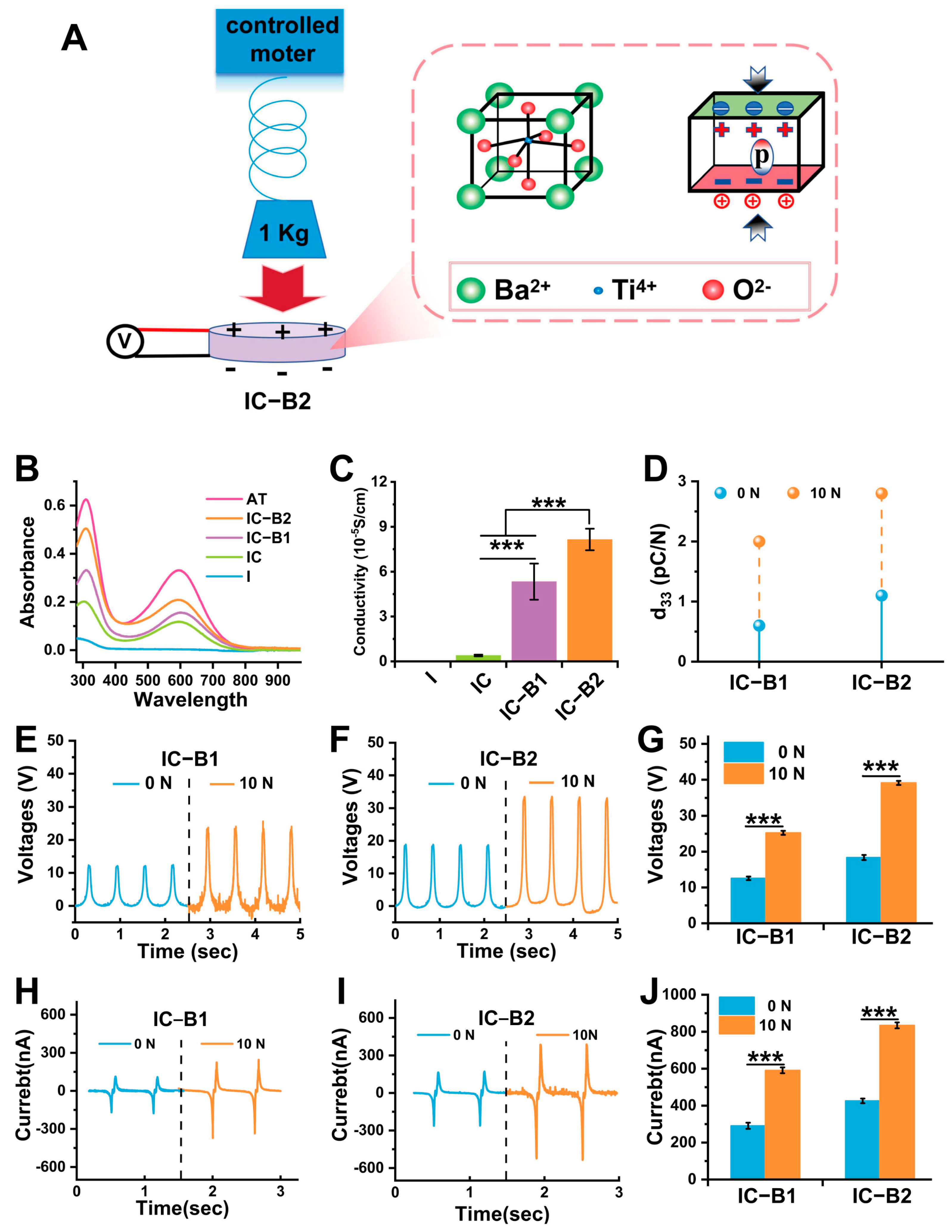
2.3. Piezoelectric Response of Gels Under Cyclic Compressive Load
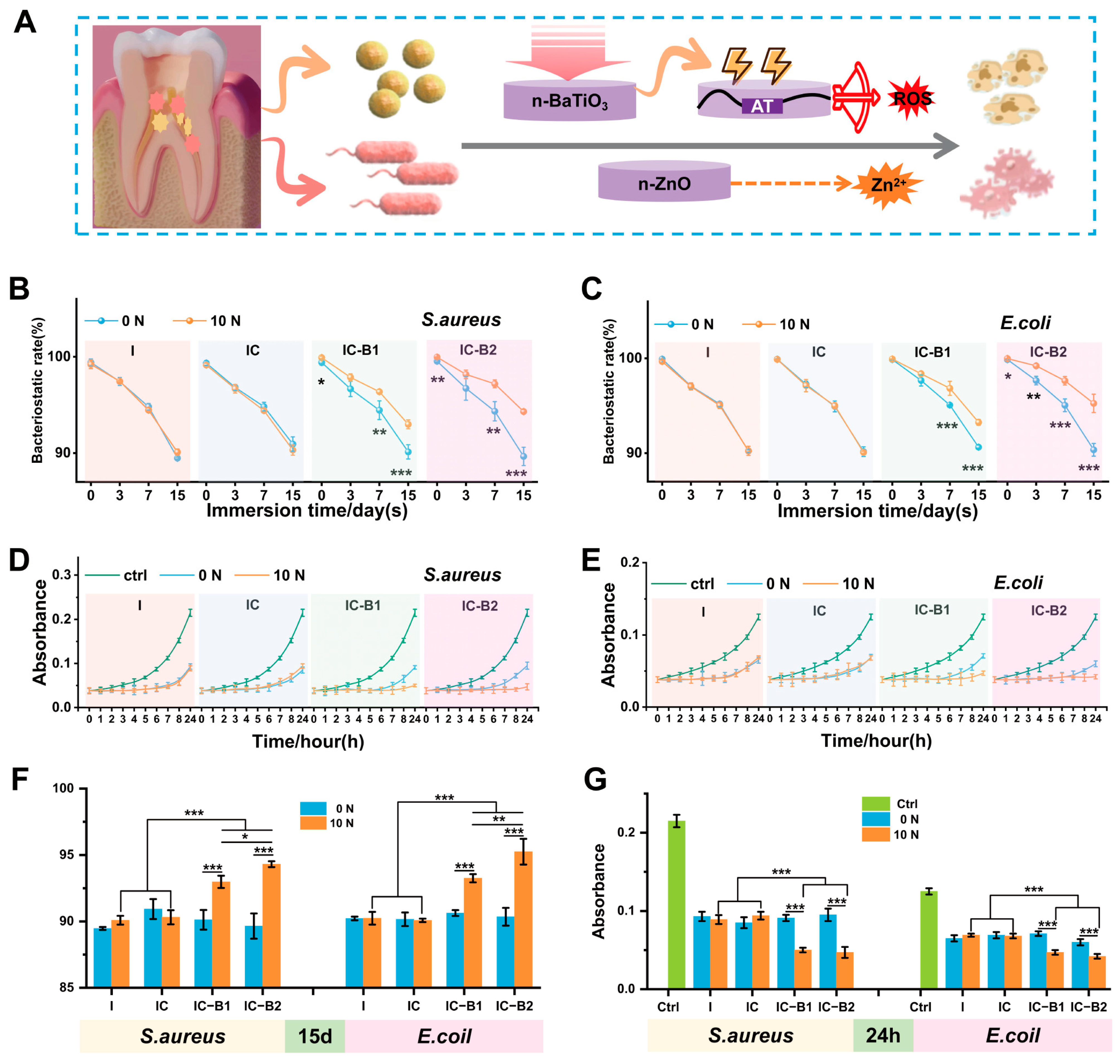
2.4. Antibacterial Properties of Gels Under Cyclic Loading
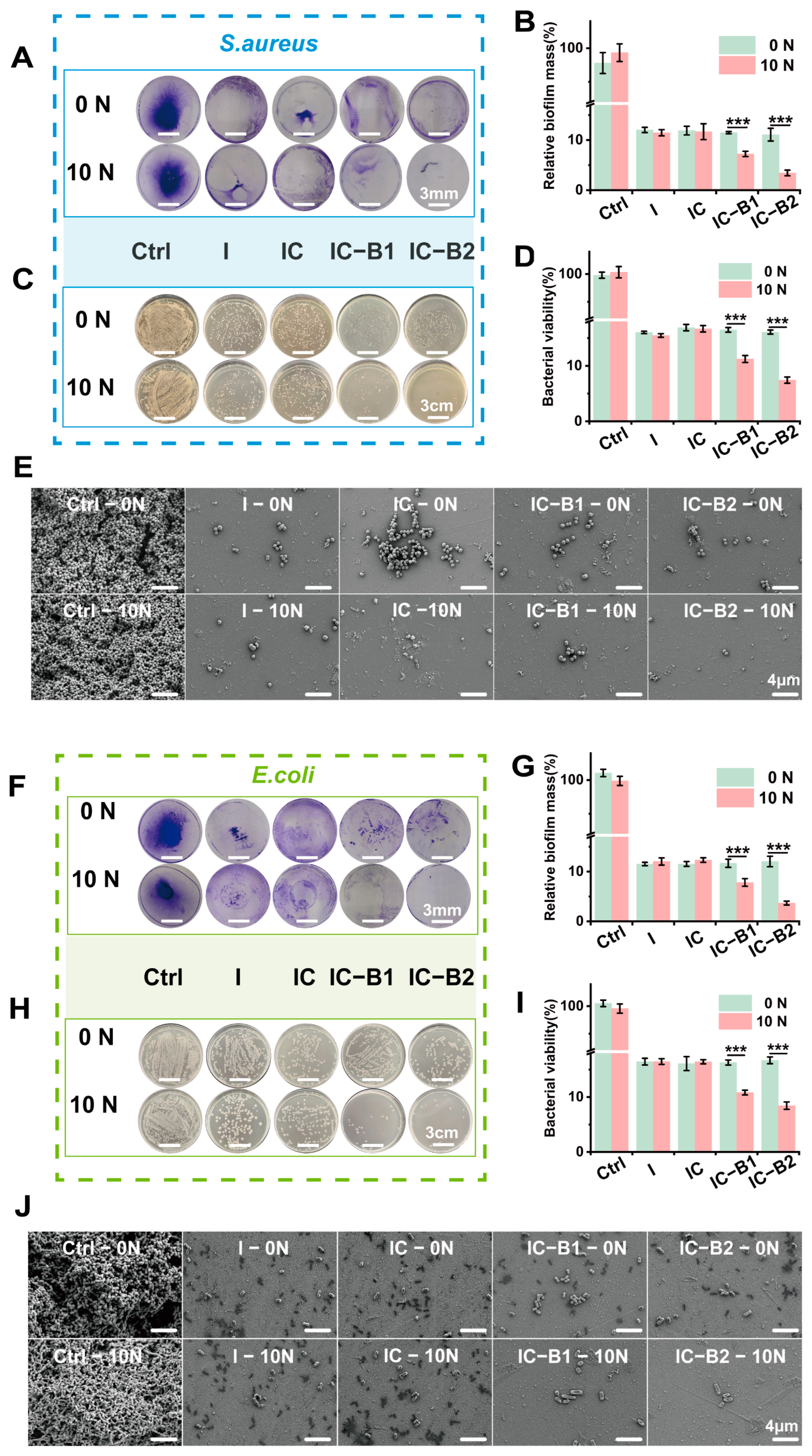
2.5. The Ability of Gels to Eradicate Bacterial Biofilms Under Cyclic Loading
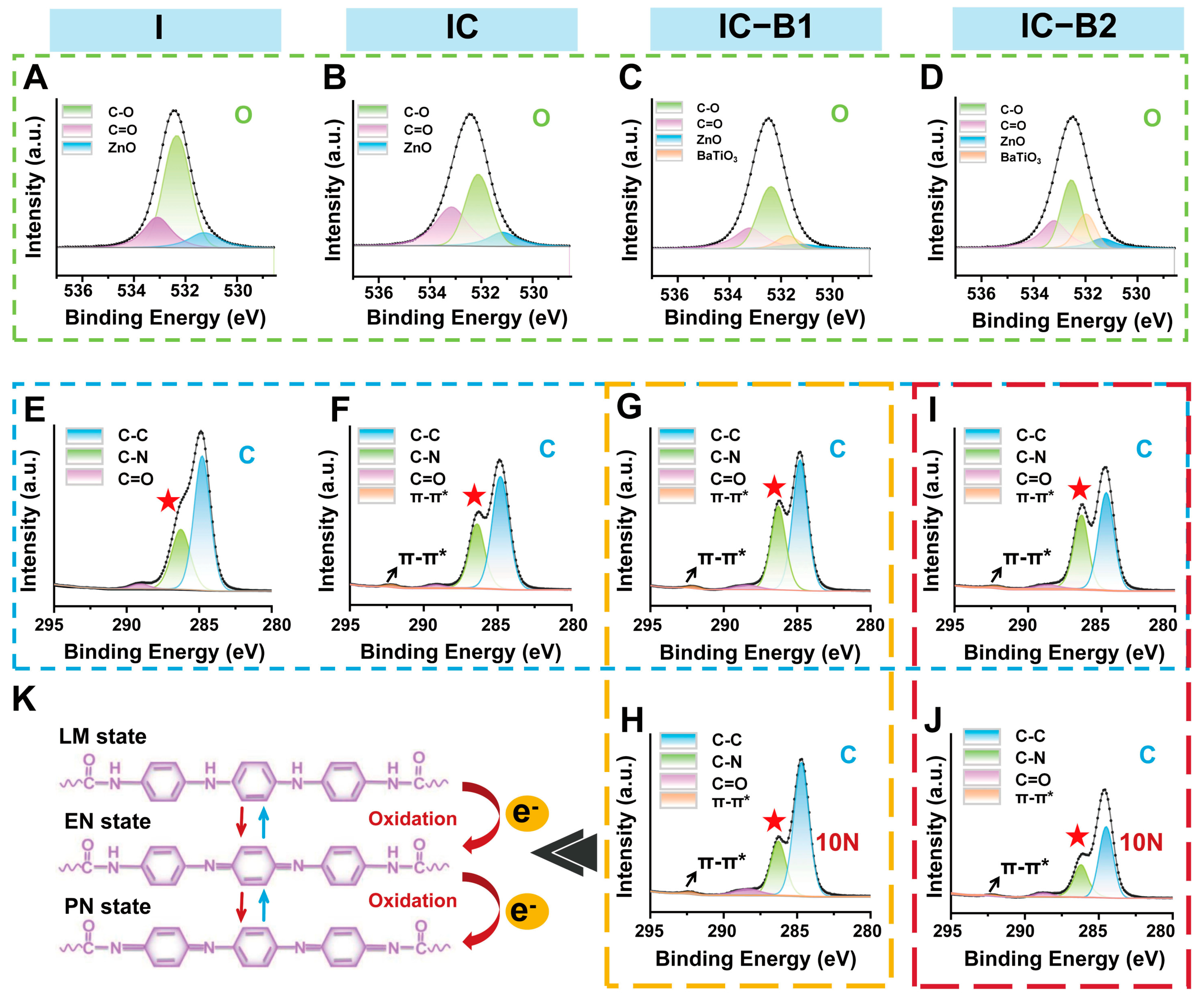
2.6. Chemical Structure Changes and ROS Generation of Gels Under Cyclic Loading
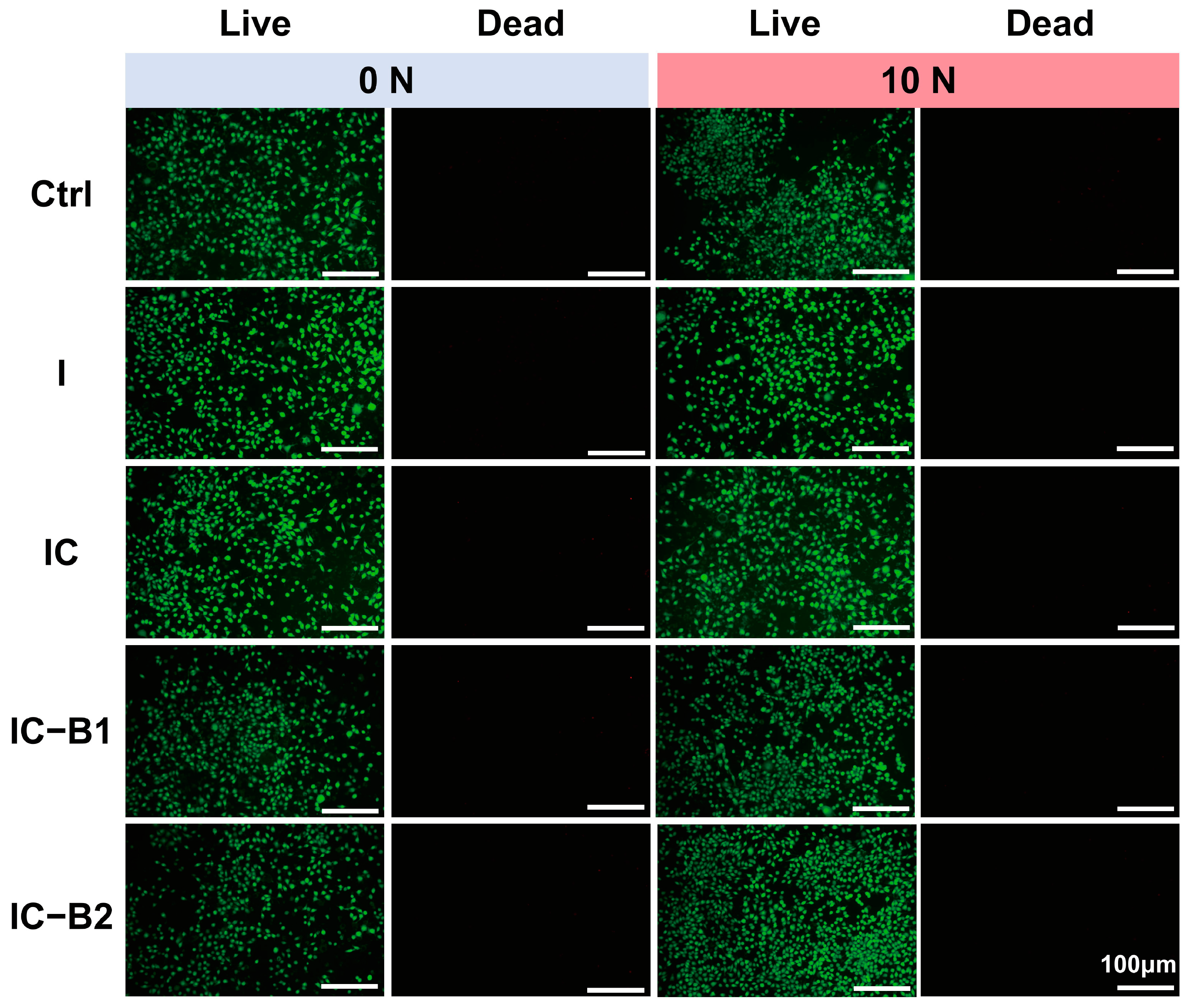
2.7. Biocompatibility of Gels Under Cyclic Loading
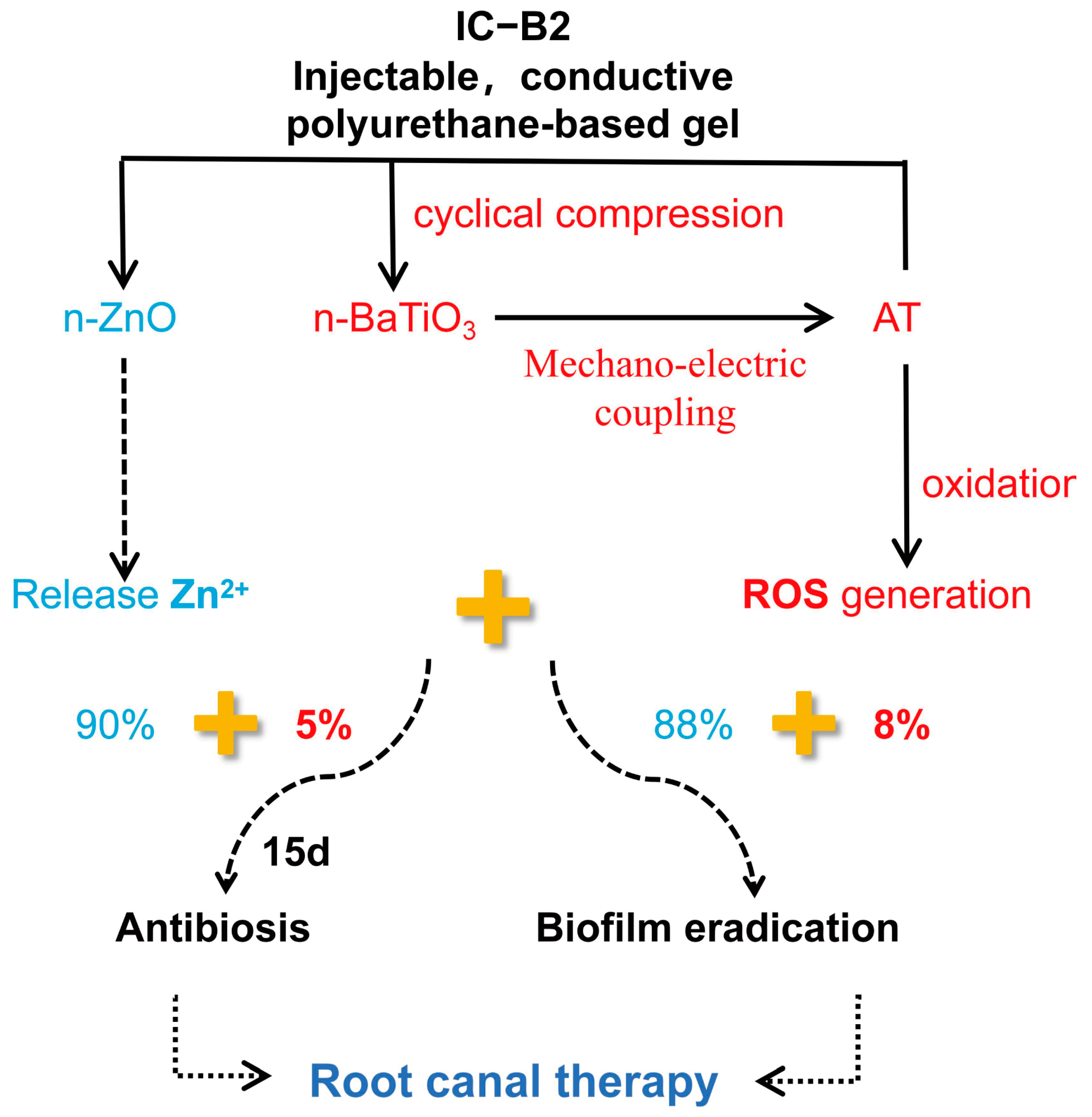
2.8. Discussion
2.8.1. Electro-Responsive Behavior of the Conductive Gels Under Cyclic Loading
2.8.2. Dynamic Antibacterial Activity of the Gels Under Cyclic Loading
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of AT
4.3. Preparation of Injectable Conductive Polyurethane-Based Composite Gels
4.3.1. Component A: Injectable Prepolymer
4.3.2. Component B: Curing Reagent
4.3.3. Preparation of Gels
4.4. Physicochemical Evaluation of Gels for Root Canal Therapy
4.4.1. XRD and FT-IR
4.4.2. Anti-Washout Property
4.4.3. Material Morphology and Element Distribution
4.4.4. Setting Time and Flowing
4.4.5. Degree of Crosslinking
4.4.6. Mechanical Test
4.5. Electrochemical Properties of Composite Gels Under Compressive Loading
4.5.1. Conductivity and Electrochemical Properties
4.5.2. Piezoelectric Properties Under Cyclic Compressive Loading
4.6. Antibacterial Properties Under Cyclic Compressive Loading
4.6.1. Static Contact Test (SCT)
4.6.2. Dynamic Contact Test (DCT)
4.7. In Vitro Bacterial Biofilm Culture and Eradication Under Cyclic Compressive Loading
4.8. Chemical Structural Changes and ROS Generation Under Cyclic Compressive Loading
4.9. In Vitro Cell Evaluation Under Cyclic Compressive Loading
4.9.1. Cell Culture and Extraction Solution Preparation
4.9.2. Cell Proliferation
4.9.3. Live-Dead Cell Staining Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Palmer, R.J., Jr. Composition and development of oral bacterial communities. Periodontology 2000 2013, 64, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.D.; Peirano, G.; Church, D.L. Molecular methods for pathogen and microbial community detection and characterization: Current and potential application in diagnostic microbiology. Infect. Genet. Evol. 2012, 12, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.P.J.; Maciel, S.M.; Cerci Neto, A.; Dezan, C.C.; Fernandes, K.B.P.; de Andrade, F.B. Cariogenic Microorganisms and Oral Conditions in Asthmatic Children. Caries Res. 2011, 45, 386–392. [Google Scholar] [CrossRef]
- Kilian, M. The oral microbiome—Friend or foe? Eur. J. Oral Sci. 2018, 126, 5–12. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Deng, W.-W.; Song, W.-F.; Wu, C.-C.; Liu, J.; Hong, S.; Zhuang, Z.-N.; Cheng, H.; Sun, Z.-J.; Zhang, X.-Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat. Biomed. Eng. 2021, 6, 32–43. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Long, J.; Kreft, J.U.; Camilleri, J. Antimicrobial and ultrastructural properties of root canal filling materials exposed to bacterial challenge. J. Dent. 2020, 93, 103283. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel Bioactive and Therapeutic Root Canal Sealers with Antibacterial and Remineralization Properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Y.; Weir, M.D.; Xu, H.H.K.; Bai, Y.; Melo, M.A.S. Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials 2017, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Verma, N.; Kumar, V.; Apoorva, P.; Agarwal, V. Biofilm inhibition/eradication: Exploring strategies and confronting challenges in combatting biofilm. Arch. Microbiol. 2024, 206, 212. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.A.M.; Pereira, M.D.; Furtado, F.; Prado, G.P.R.; Mestriner, W.; Ferreira, L.M. Masticatory efficiency and bite force in individuals with normal occlusion. Arch. Oral Biol. 2014, 59, 1065–1074. [Google Scholar] [CrossRef]
- Quiudini, P.R.; Pozza, D.H.; Pinto, A.d.S.; de Arruda, M.F.; Guimarães, A.S. Differences in bite force between dolichofacial and brachyfacial individuals: Side of mastication, gender, weight and height. J. Prosthodont. Res. 2017, 61, 283–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Steenberghe, D.; de Vries, J.H. The development of a maximal clenching force between two antagonistic teeth. J. Periodontal Res. 2006, 13, 91–97. [Google Scholar] [CrossRef]
- Fahlgren, A.; Bostrom, M.P.G.; Yang, X.; Johansson, L.; Edlund, U.; Agholme, F.; Aspenberg, P. Fluid pressure and flow as a cause of bone resorption. Acta Orthop. 2010, 81, 508–516. [Google Scholar] [CrossRef]
- Huo, B.; Lu, X.L.; Costa, K.D.; Xu, Q.; Guo, X.E. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 2010, 47, 234–241. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Li, X.; Cui, L.; Fu, M.; Rabie, A.B.; Zhang, D. Functional analysis of core binding factor a1 and its relationship with related genes expressed by human periodontal ligament cells exposed to mechanical stress. Eur. J. Orthod. 2010, 32, 698–705. [Google Scholar] [CrossRef]
- ISO 7405; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. Standardization IOf. International Organization for Standardization: Geneva, Switzerland, 2018.
- Lee, S.J.; Heo, M.; Lee, D.; Han, S.; Moon, J.-H.; Lim, H.-N.; Kwon, I.K. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Popkin, D.L.; Zilka, S.; Dimaano, M.; Fujioka, H.; Rackley, C.; Salata, R.; Griffith, A.; Mukherjee, P.K.; Ghannoum, M.A.; Esper, F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017, 2, 253. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes. Pathophysiology 2008, 15, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Wang, J.; Chen, J.; Gao, J.; Zhang, J.; Zhao, Q.; Tang, J.; Fang, W.; Li, J.; Li, Y.; et al. The in vitro evaluation of antibacterial efficacy optimized with cellular apoptosis on multi-functional polyurethane sealers for the root canal treatment. J. Mater. Chem. B 2021, 9, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, Y.; Dan, N.; Zheng, X.; Feng, R.; Yu, G.; He, X.; Dan, W.; Wang, Y. Flexible nano-piezoelectric membranes with spontaneous electric field generation for bacteria elimination and wound healing. J. Mater. Sci. 2022, 57, 19532–19552. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Buschanga, P.H.; Hayasakib, H.; Throckmorton, G.S. Quantication of human chewing-cycle kinematics. Arch. Oral Biol. 2000, 45, 461–474. [Google Scholar] [CrossRef]
- Jiang, Y.; Parsonnet, E.; Qualls, A.; Zhao, W.; Susarla, S.; Pesquera, D.; Dasgupta, A.; Acharya, M.; Zhang, H.; Gosavi, T.; et al. Enabling ultra-low-voltage switching in BaTiO3. Nat. Mater. 2022, 21, 779–785. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional Dental Composite with Piezoelectric Nanofillers for Combined Antibacterial and Mineralization Effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef]
- Wei, J.; Xia, J.; Liu, X.; Ran, P.; Zhang, G.; Wang, C.; Li, X. Hollow-structured BaTiO3 nanoparticles with cerium-regulated defect engineering to promote piezocatalytic antibacterial treatment. Appl. Catal. B Environ. 2023, 328, 122520. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, Y.; Pang, Y.; Zhang, D.; Wang, Y.; Zhao, H.; Zhang, X.; Leng, H.; Yang, X.; Cai, Q. Improving bone regeneration with composites consisting of piezoelectric poly(l-lactide) and piezoelectric calcium/manganese co-doped barium titanate nanofibers. Compos. Part B Eng. 2022, 234, 109734. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Y.; Fang, W.; Man, Y.; Zhang, J.; Zhao, Q.; Lei, X.; Chen, J.; Li, J.; Li, Y.; et al. Electrophysiological microenvironment and site-specific cell behaviors regulated by fibrous aniline trimer-based polyurethanes in bone progressive regeneration. Chem. Eng. J. 2023, 460, 141630. [Google Scholar] [CrossRef]
- Fang, W.; Sun, F.H.; Tang, J.J.; Zhao, Q.; Chen, J.; Lei, X.Y.; Zhang, J.Z.; Zhang, Y.L.; Zuo, Y.; Li, J.D.; et al. Porous Electroactive and Biodegradable Polyurethane Membrane through Self-Doping Organogel. Macromol. Rapid Commun. 2021, 42, 2100125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, B.; Eyster, T.W.; Ma, P.X. Super Stretchable Electroactive Elastomer Formation Driven by Aniline Trimer Self-Assembly. Chem. Mater. 2015, 27, 5668–5677. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Huang, T.-C.; Lin, J.-C.; Chang, J.-H.; Lee, Y.-T.; Yeh, J.-M. Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane. Mater. Chem. Phys. 2013, 137, 772–780. [Google Scholar] [CrossRef]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Bertó, A.; Primus, C.M.; Watanabe, I. Physical and Chemical Properties of New-generation Endodontic Materials. J. Endod. 2010, 36, 524–528. [Google Scholar] [CrossRef]
- ISO 6876; Dentistry—Root Canal Sealing Materials. Standardization IOf. International Organization for Standardization: Geneva, Switzerland, 2012.
- Radev, B.R.; Kase, J.A.; Askew, M.J.; Weiner, S.D. Potential for thermal damage to articular cartilage by PMMA reconstruction of a bone cavity following tumor excision: A finite element study. J. Biomech. 2009, 42, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, Q.; Lin, L.; Sun, F.; Li, J.; Zou, Q.; Zuo, Y.; Li, Y. A comparison of the characteristics of polyurethane-based sealers including various antimicrobial agents. RSC Adv. 2019, 9, 7043–7056. [Google Scholar] [CrossRef]
- Williams, C.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. A Comparison of Cohesive Strength and Stiffness of Resilon and Gutta-Percha. J. Endod. 2006, 32, 553–555. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, R.; Guo, B.; Ma, P.X. Dopamine-Incorporated Dual Bioactive Electroactive Shape Memory Polyurethane Elastomers with Physiological Shape Recovery Temperature, High Stretchability, and Enhanced C2C12 Myogenic Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 29595–29611. [Google Scholar] [CrossRef]
- Moini, N.; Jahandideh, A.; Shahkarami, F.; Kabiri, K.; Piri, F. Linear and star-shaped π-conjugated oligoanilines: A review on molecular design in syntheses and properties. Polym. Chem. 2022, 13, 2714–2756. [Google Scholar] [CrossRef]
- Landis, R.F.; Li, C.-H.; Gupta, A.; Lee, Y.-W.; Yazdani, M.; Ngernyuang, N.; Altinbasak, I.; Mansoor, S.; Khichi, M.A.S.; Sanyal, A.; et al. Biodegradable Nanocomposite Antimicrobials for the Eradication of Multidrug-Resistant Bacterial Biofilms without Accumulated Resistance. J. Am. Chem. Soc. 2018, 140, 6176–6182. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Yang, L.; Tian, B.; Xie, Y.; Dong, S.; Yang, M.; Gai, S.; Lin, J. Oxygen-Vacancy-Rich Piezoelectric BiO2−x Nanosheets for Augmented Piezocatalytic, Sonothermal, and Enzymatic Therapies. Adv. Mater. 2023, 35, 2300648. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, J.; Zang, P.; Feng, L.; Tian, B.; Zhao, R.; Xie, Y.; Wu, L.; Chen, Z.; Yang, P. A Functional “Key” Amplified Piezoelectric Effect Modulates ROS Storm with an Open Source for Multimodal Synergistic Cancer Therapy. Small 2024, 20, 2401931. [Google Scholar] [CrossRef] [PubMed]
- Lay, R.; Deijs, G.S.; Malmström, J. The intrinsic piezoelectric properties of materials—A review with a focus on biological materials. RSC Adv. 2021, 11, 30657–30673. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Z.; Liu, Z.; Zhang, J.; Zhang, Y.; Ding, Y.; Huang, T.; Xiang, D.; Wang, Z.; Dai, Y.; et al. Piezoelectric nanocomposites for sonodynamic bacterial elimination and wound healing. Nano Today 2021, 37, 101104. [Google Scholar] [CrossRef]
- Mokhtari, F.; Azimi, B.; Salehi, M.; Hashemikia, S.; Danti, S. Recent advances of polymer-based piezoelectric composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 122, 104669. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Xia, Y.; Qi, X.; Jiang, C.; Xiao, Y.; Jiang, F.; Jiang, X.; Yuan, G. Strontium-Containing Piezoelectric Biofilm Promotes Dentin Tissue Regeneration. Adv. Mater. 2024, 36, 2313419. [Google Scholar] [CrossRef]
- Kim, J.S.; Nam, I.W.; Lee, H.K. Piezoelectric characteristics of urethane composites incorporating piezoelectric nanomaterials. Compos. Struct. 2020, 241, 112072. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Look, D.C.; Reynolds, D.C.; Sizelove, J.R.; Jones, R.L.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Electrical Properties of Bulk ZnO. Solid State Commun. 1998, 105, 399–401. [Google Scholar] [CrossRef]
- Na, C.W.; Woo, H.-S.; Kim, I.-D.; Lee, J.-H. Selective detection of NO2 and C2H5OH using a Co3O4-decorated ZnO nanowire network sensor. Chem. Commun. 2011, 47, 5148–5150. [Google Scholar] [CrossRef]
- Sadykov, T.T.; Massalimov, I.A.; Mustafin, A.G. Synthesis and physico-chemical properties of composites based on polyaniline and nanosized sulfur. Polym. Bull. 2023, 81, 2757–2776. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Sunthar, T.P.M.; Zanocco, M.; Ohgitani, E.; Zhu, W.; Pezzotti, G. Antibacterial effects of barium titanate reinforced polyvinyl-siloxane scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 425–436. [Google Scholar] [CrossRef]
- Shah, A.A.; Khan, A.; Dwivedi, S.; Musarrat, J.; Azam, A. Antibacterial and Antibiofilm Activity of Barium Titanate Nanoparticles. Mater. Lett. 2018, 229, 130–133. [Google Scholar] [CrossRef]
- Swain, S.; Padhy, R.N.; Rautray, T.R. Polarized piezoelectric bioceramic composites exhibit antibacterial activity. Mater. Chem. Phys. 2020, 239, 122002. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2016, 9, 035004. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Li, Y.; Jin, B.; Jiang, P.; Chen, X.; Wei, C.; Sheng, J.; Liu, Y.-N.; Li, J.; et al. Piezoelectric Nanozyme for Dual-Driven Catalytic Eradication of Bacterial Biofilms. ACS Appl. Mater. Interfaces 2023, 15, 14690–14703. [Google Scholar] [CrossRef]
- Wei, W.; Bing, W.; Ren, J.; Qu, X. Near infrared-caged d-amino acids multifunctional assembly for simultaneously eradicating biofilms and bacteria. Chem. Commun. 2015, 51, 12677–12679. [Google Scholar] [CrossRef] [PubMed]
- Faria-Junior, N.B.; Tanomaru-Filho, M.; Berbert, F.L.; Guerreiro-Tanomaru, J.M. Antibiofilm activity, pH and solubility of endodontic sealers. Int. Endod. J. 2013, 46, 755–762. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Shi, J.; Kang, Y.; Dong, J.; Pei, Z.; Ji, X. Piezoelectricity, Pyroelectricity, and Ferroelectricity in Biomaterials and Biomedical Applications. Adv Mater 2024, 36, e2308726. [Google Scholar] [CrossRef] [PubMed]
- Strzyz, P. Synthetic biology: Designer cells tackle diabetes. Nat. Rev. Mol. Cell Biol. 2017, 18, 69. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Seaver, L.C.; Imlay, J.A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001, 183, 7173–7181. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Y.; Zou, Q.; Yang, B.; Lin, L.; Li, J.; Li, Y. Hierarchical Structure and Mechanical Improvement of an n-HA/GCO–PU Composite Scaffold for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 22618–22629. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Fang, W.; Zhao, Q.; Lei, X.; Zhang, J.; Chen, J.; Li, Y.; Zuo, Y. Synergetic Effect of Electrical and Topographical Cues in Aniline Trimer-Based Polyurethane Fibrous Scaffolds on Tissue Regeneration. J. Funct. Biomater. 2023, 14, 185. [Google Scholar] [CrossRef]
- Ning, X.; Hao, A.; Cao, Y.; Hu, J.; Xie, J.; Jia, D. Effective promoting piezocatalytic property of zinc oxide for degradation of organic pollutants and insight into piezocatalytic mechanism. J. Colloid Interface Sci. 2020, 577, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, Q.; Hosono, E.; Asakura, D.; Miyawaki, J.; Harada, Y. Tetragonal Distortion of a BaTiO3/Bi0.5Na0.5TiO3 Nanocomposite Responsible for Anomalous Piezoelectric and Ferroelectric Behaviors. ACS Omega 2020, 5, 22800–22807. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tao, B.; Wang, T.; Zhang, Y.; Wei, W.; Wang, C. Fast-setting and anti-washout tricalcium silicate/disodium hydrogen phosphate composite cement for dental application. Ceram. Int. 2019, 45, 24182–24192. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices. Standardization IOf. International Organization for Standardization: Geneva, Switzerland, 2019.



| I | IC | IC-B1 | IC-B2 | |
|---|---|---|---|---|
| AT (wt%) | 0 | 2.5 | 2.5 | 2.5 |
| BaTiO3 (wt%) | 0 | 0 | 5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, B.; Lei, X.; Zhang, Y.; Zhang, J.; Du, Q.; Li, Y.; Huang, D.; Wang, L.; Li, J.; Li, Y.; et al. Injectable and Conductive Polyurethane Gel with Load-Responsive Antibiosis for Sustained Root Canal Disinfection. Gels 2025, 11, 346. https://doi.org/10.3390/gels11050346
Mu B, Lei X, Zhang Y, Zhang J, Du Q, Li Y, Huang D, Wang L, Li J, Li Y, et al. Injectable and Conductive Polyurethane Gel with Load-Responsive Antibiosis for Sustained Root Canal Disinfection. Gels. 2025; 11(5):346. https://doi.org/10.3390/gels11050346
Chicago/Turabian StyleMu, Bo, Xiaoyu Lei, Yinglong Zhang, Jingzheng Zhang, Qingda Du, Yuping Li, Dongyu Huang, Li Wang, Jidong Li, Yubao Li, and et al. 2025. "Injectable and Conductive Polyurethane Gel with Load-Responsive Antibiosis for Sustained Root Canal Disinfection" Gels 11, no. 5: 346. https://doi.org/10.3390/gels11050346
APA StyleMu, B., Lei, X., Zhang, Y., Zhang, J., Du, Q., Li, Y., Huang, D., Wang, L., Li, J., Li, Y., & Zuo, Y. (2025). Injectable and Conductive Polyurethane Gel with Load-Responsive Antibiosis for Sustained Root Canal Disinfection. Gels, 11(5), 346. https://doi.org/10.3390/gels11050346








