Utilization of Cassava Starch–Glycerol Gel as a Sustainable Material to Decrease Metal Ion Surface Contamination
Abstract
1. Introduction
2. Results and Discussion
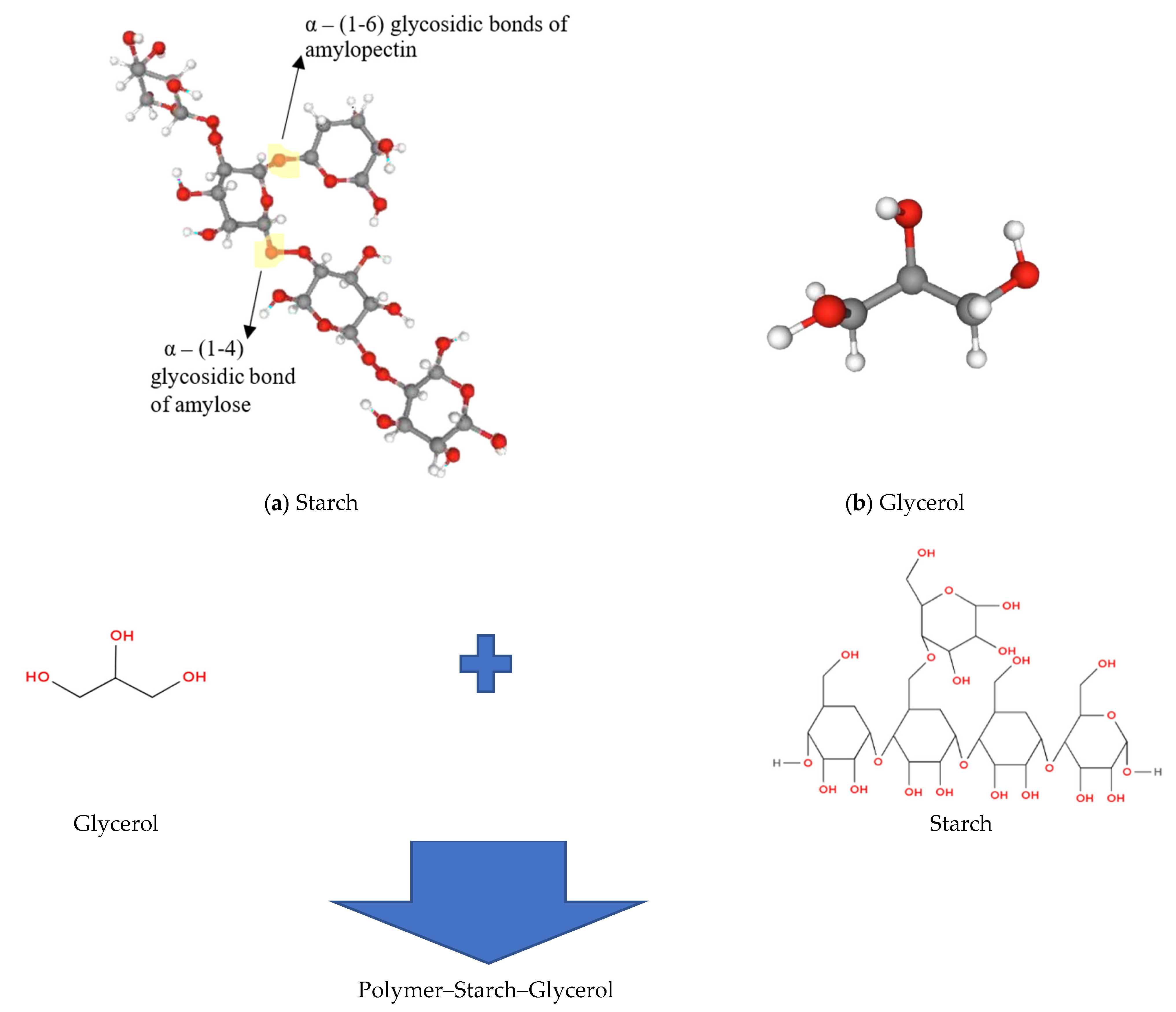
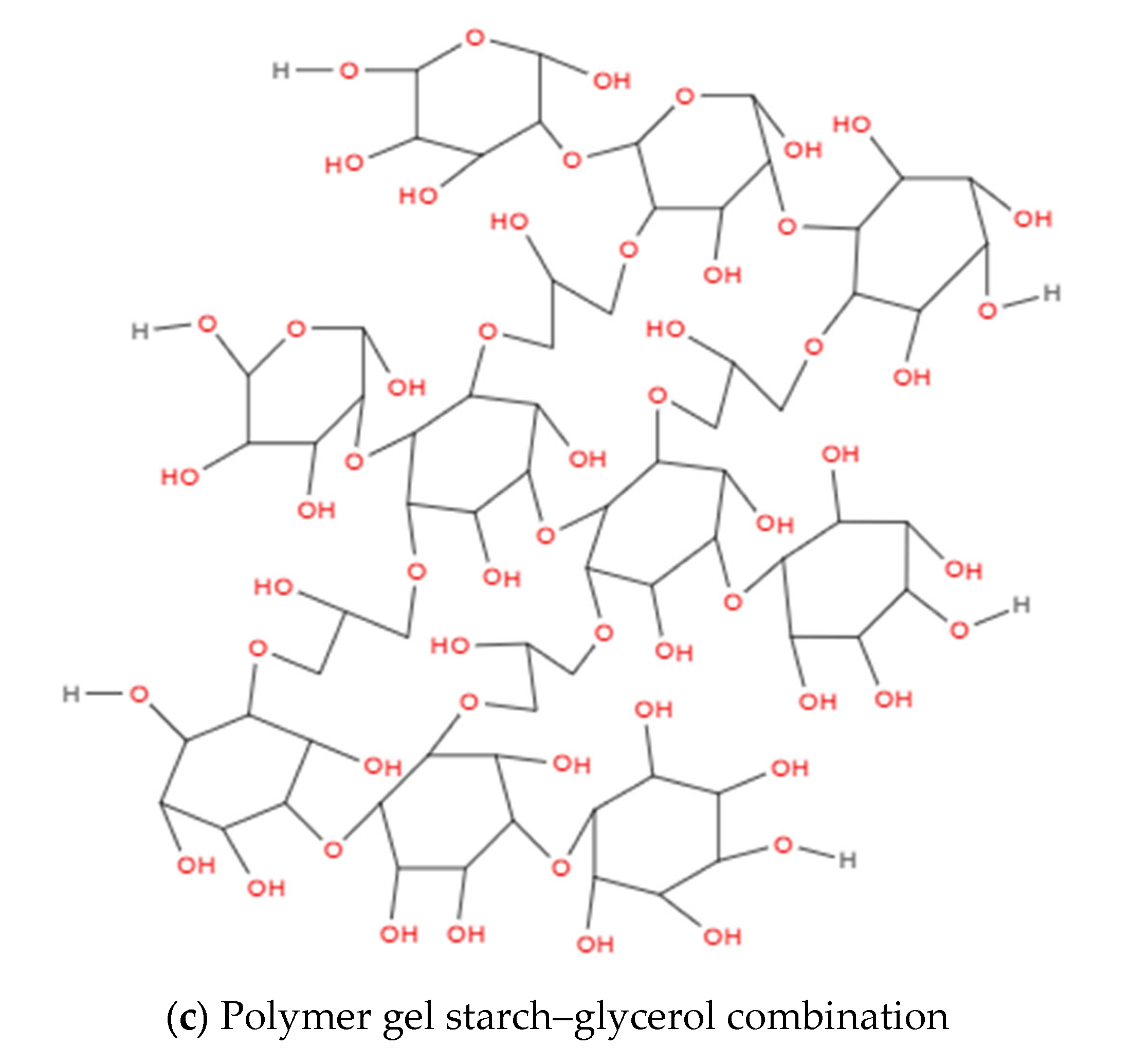
2.1. Starch Cassava–Glycerol Gel
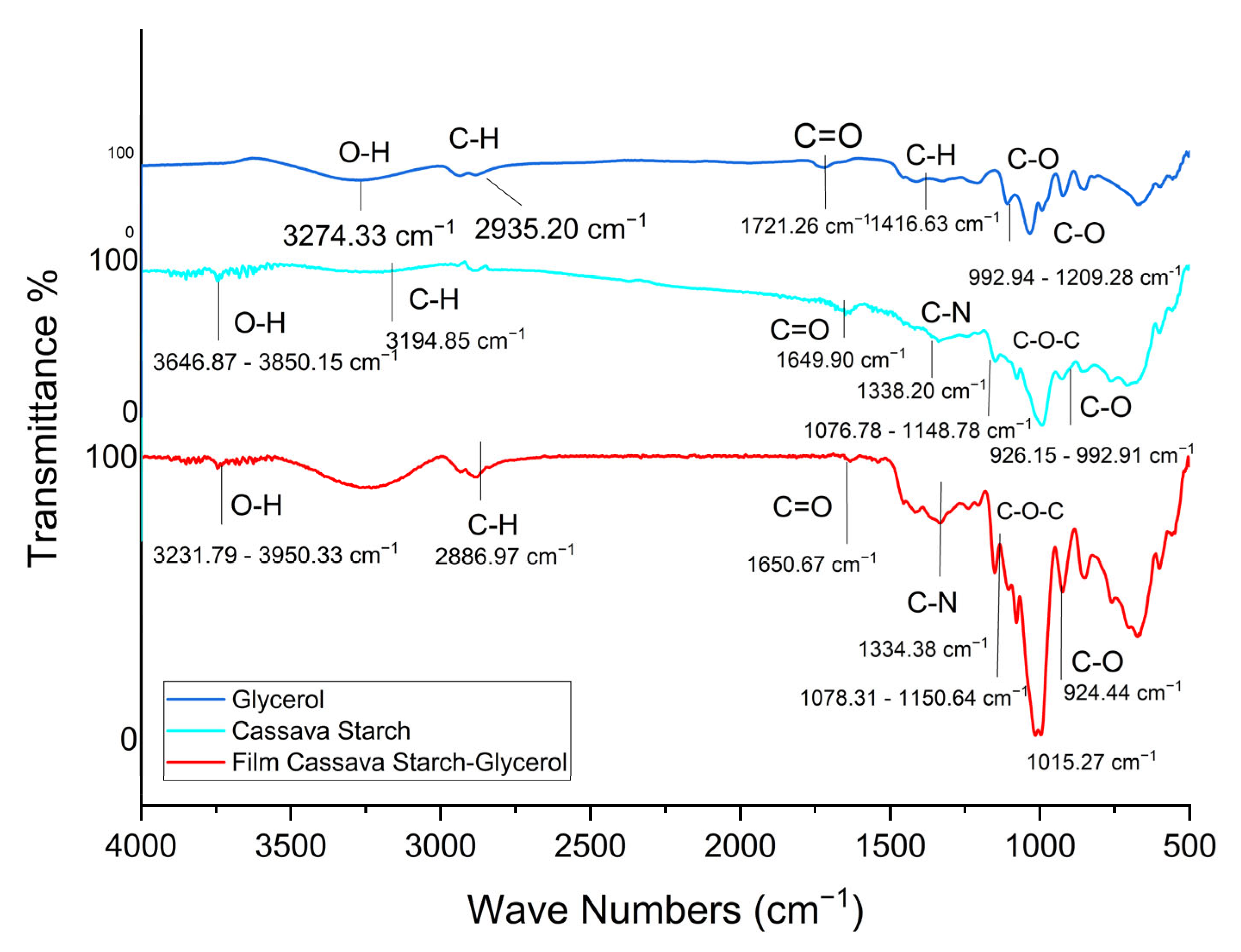
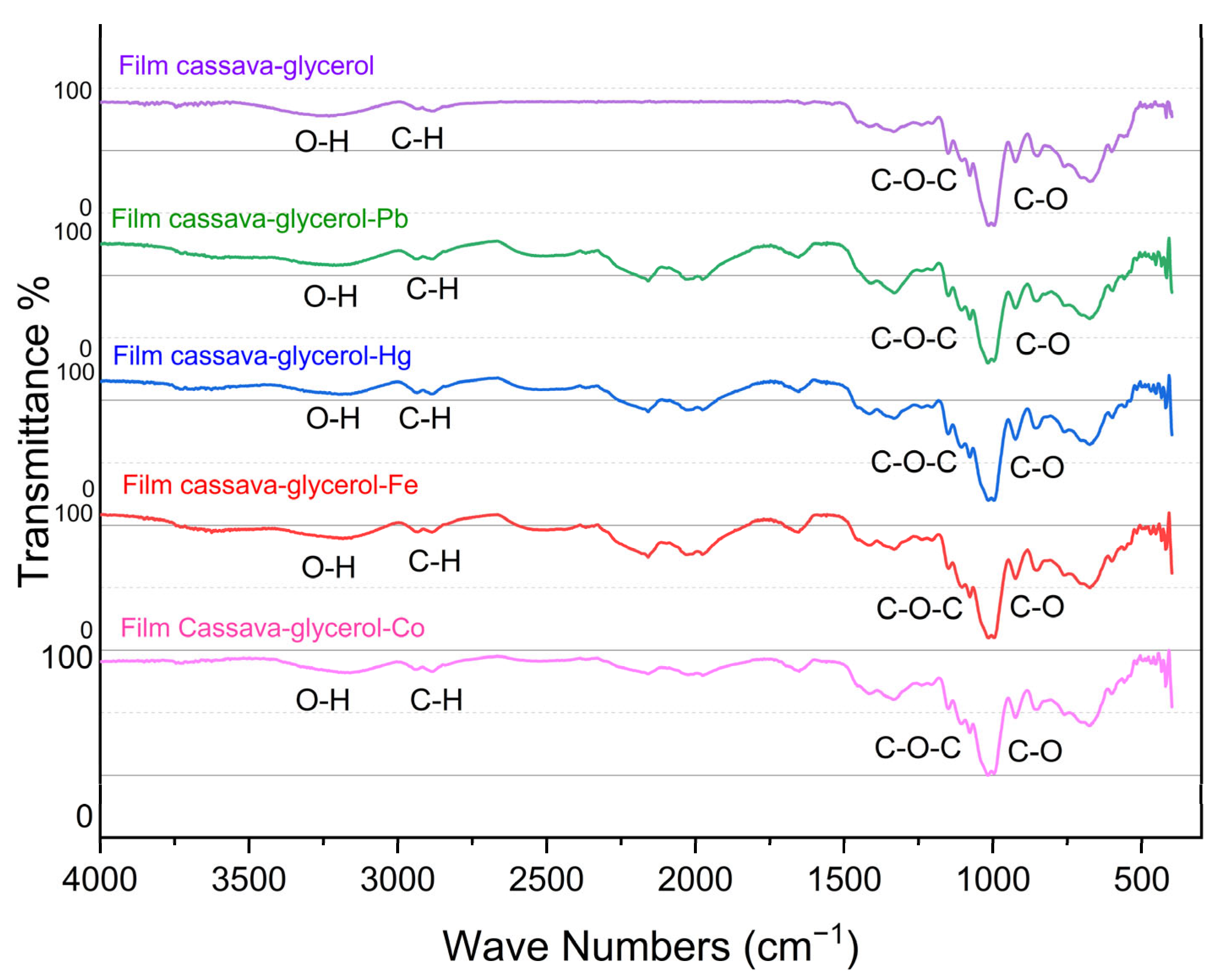
2.2. FTIR Analysis
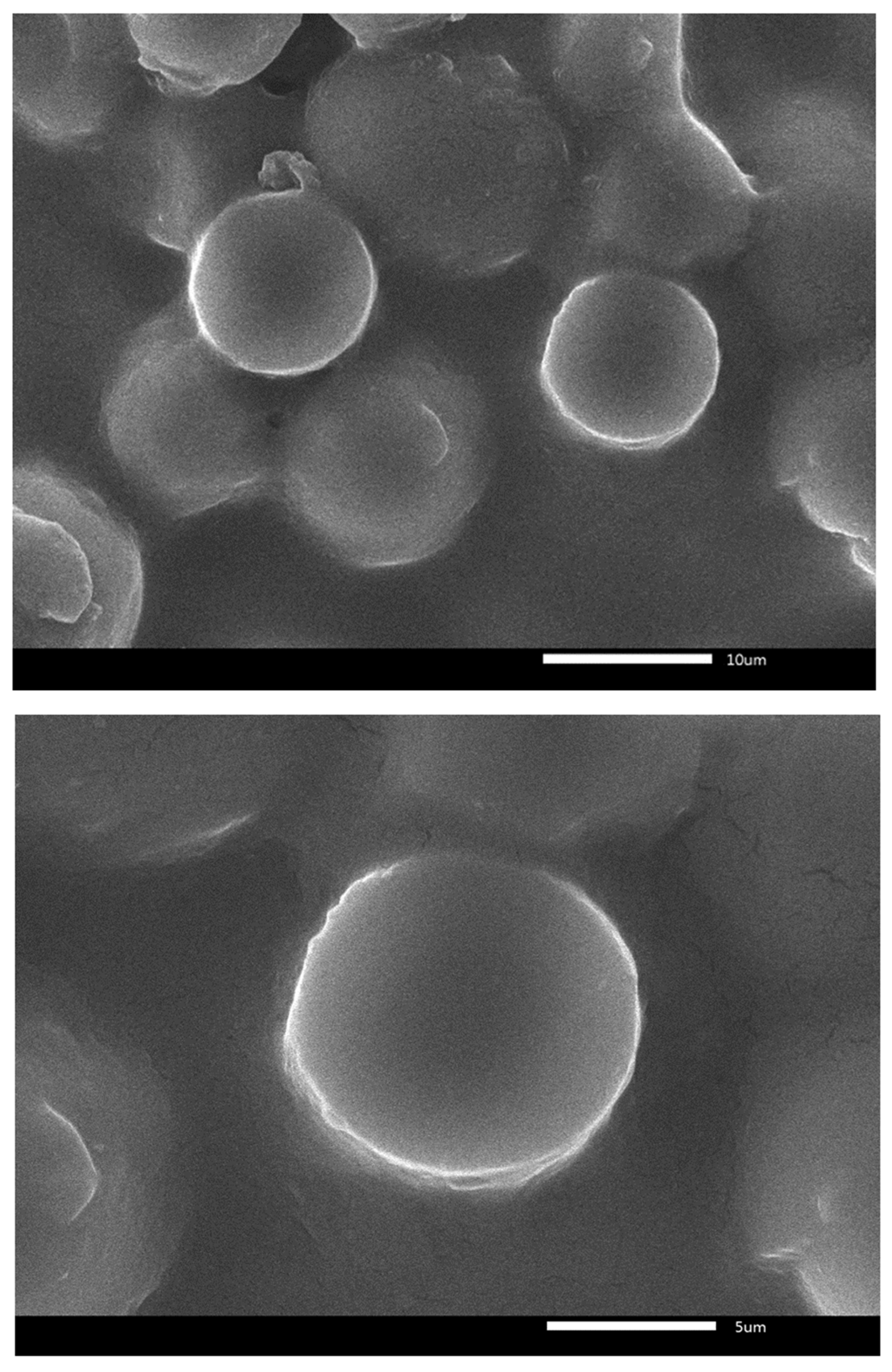
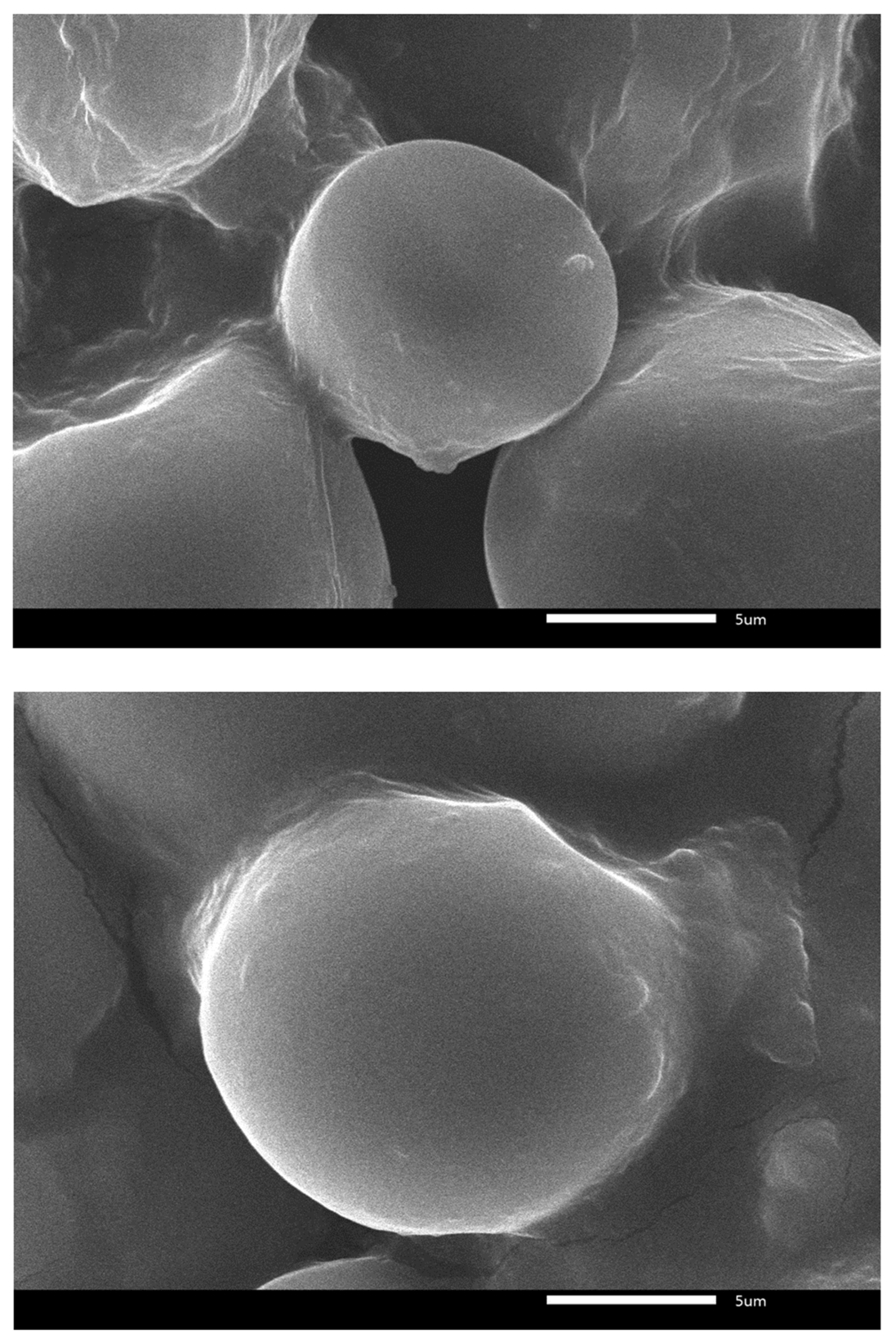
2.3. SEM Analysis
2.4. BET Analysis
2.5. XRF Analysis
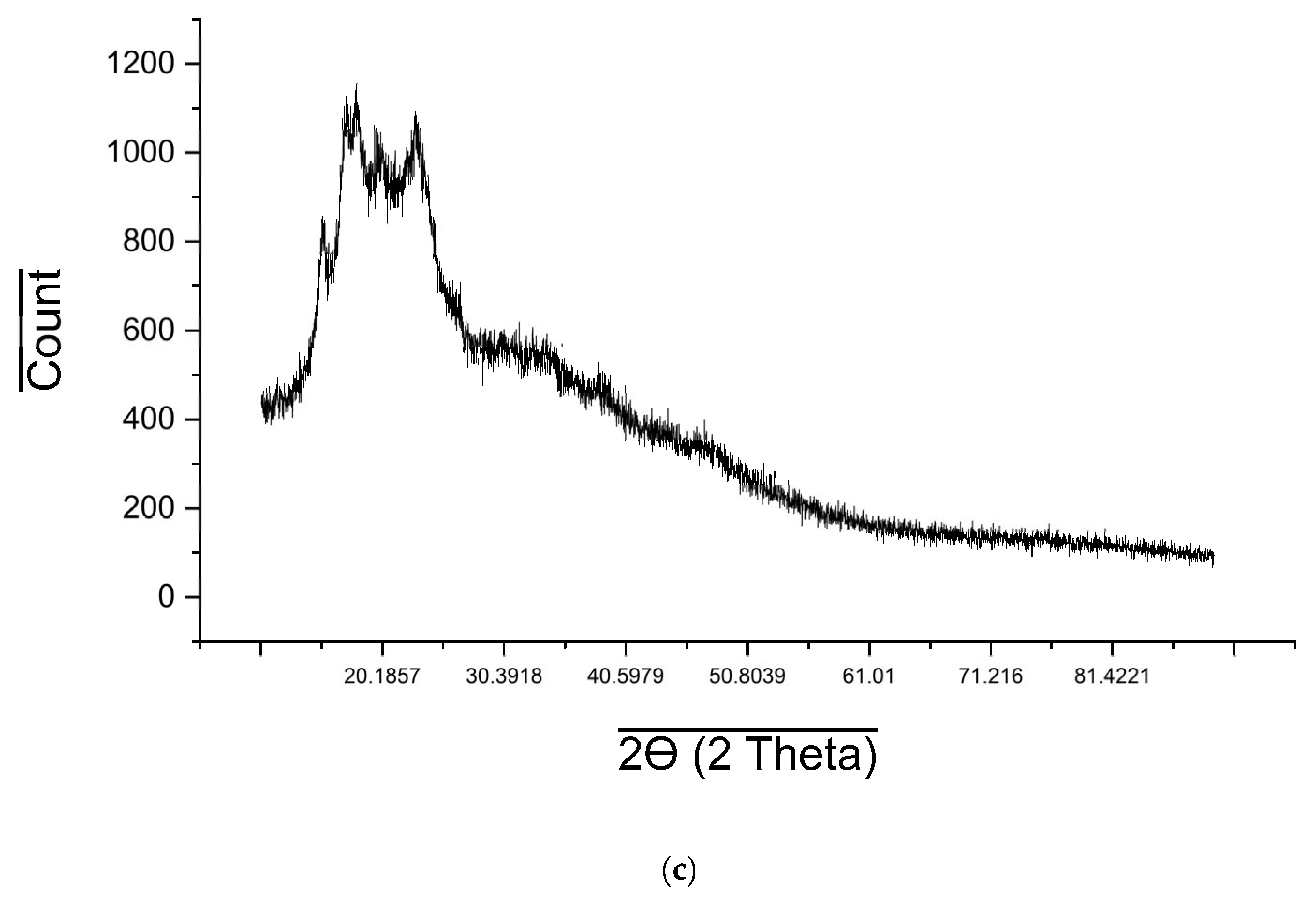
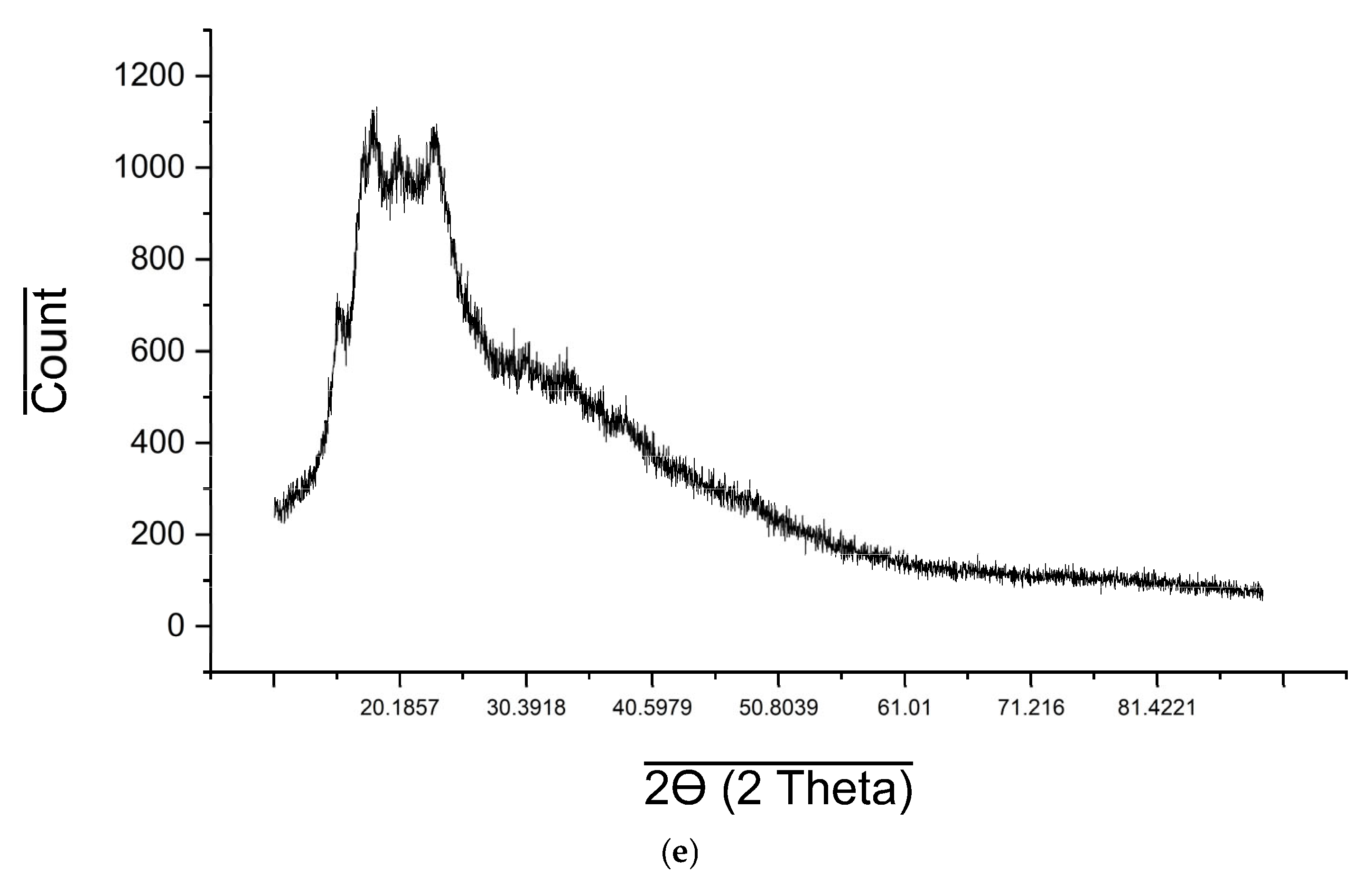
2.6. XRD Analysis
3. Materials and Methods
- Fourier Transform Infrared Spectroscopy (FTIR): ATR Thermo Nicolet iS5. The spectra were read in the 400–4000 cm−1 frequency range in attenuated total reflectance (ATR) mode with a diamond crystal, 64 consecutive scans at a resolution of 4 cm−1.
- Brunauer–Emmett–Teller (BET): Nova2000 Quantaqrom. Sample used without degassing. Outgas time, 3 h; outgas temp, 30 °C; analysis gas, nitrogen; bath temp, 77.3 K; press. tolerance, 0.100/0.100 (ads/des); equil time, 60/60 sec (ads/des); equil timeout, 240/240 sec (ads/des).
- Scanning electron microscopy (SEM): Jeol. signal, secondary electron (SE); voltage, 20 kV; vacuum, high vacuum.
- X-ray fluorescence (XRF): XRF Thermo Scientific Niton type XL3t 500 Analyzers. X-ray source, X-ray tube with gold anode (Au); maximum voltage, 50 kV; maximum current, 200 μA; maximum power, 4 watts; detector, high-performance semiconductor.
- X-ray diffraction (XRD): Bruker D8. Scanning with Cu Kα radiation (λ = 1.5406 Å), operating at 40 kV and 40 mA. The scanning range of 2θ was from 5 to 90°.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badan Tenaga Nujklir Nasional (BATAN). Buku Putih Pemanfaatan Reaktor Riset BATAN; BATAN: Jakarta, Indonesia, 2016; pp. 1–66. [Google Scholar]
- Alamsyah, R.; Yudhi Pristianto, A.P. Infrastuktur Keselamatan Dekomisioning Fasilitas Nuklir di Indonesia. In Proceedings of the Seminar Nasional Teknologi Pengelolaan Limbah XV-2017, Tanggerang, Indonesia, 26 September 2017; pp. 1–7. [Google Scholar]
- Anggakusuma, R.; Utama, G.L.; Zain, M.K.; Megasari, K. Reducing the Radioactive Surface Contamination Level of Cobalt-60-Contaminated Material with PVA-Glycerol-EDTA Combination Gel. Gels 2025, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Septilarso, A.; Kawasaki, D.; Yanagihara, S. Radioactive waste inventory estimation of a research reactor for decommissioning scenario development. J. Nucl. Sci. Technol. 2020, 57, 253–262. [Google Scholar] [CrossRef]
- Utami, S.B.; Putero, S.H. Regulatory Framework for the Decommissioning of Indonesian Nuclear Facilities. J. Nucl. Eng. Radiat. Sci. 2017, 3, 044501. [Google Scholar] [CrossRef]
- Lauridsen, K. 11—Recent experience in decommissioning research reactors. In Advances and Innovations in Nuclear Decommissioning; Laraia, M., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2017; pp. 315–343. [Google Scholar]
- Robinson, I.F. A nuclear inspector’s perspective on decommissioning at UK nuclear sites. J. Radiol. Prot. 1999, 19, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, G.; Mancini, M. Competitiveness of small-medium, new generation reactors: A comparative study on decommissioning. J. Eng. Gas Turbines Power 2010, 132, 102906. [Google Scholar] [CrossRef]
- Caldas Neto, A.B.; Silva, A.T. Strategies for decommissioning small nuclear reactors in Brazil. Nucl. Eng. Des. 2023, 414, 112608. [Google Scholar] [CrossRef]
- Bardtenschlager, R.; Bottger, D.; Gasch, A.; Majohr, N. Decommissioning of Light-Water Reactor Nuclear Power Plants. Nucl. Eng. Des. 1978, 45, 1–51. [Google Scholar] [CrossRef]
- Suh, Y.A.; Hornibrook, C.; Yim, M.S. Decisions on nuclear decommissioning strategies: Historical review. Prog. Nucl. Energy 2018, 106, 34–43. [Google Scholar] [CrossRef]
- Mariani, C.; Mancini, M. Selection of projects’ primary and secondary mitigation actions through optimization methods in nuclear decommissioning projects. Nucl. Eng. Des. 2023, 407, 112284. [Google Scholar] [CrossRef]
- Invernizzi, D.C.; Locatelli, G.; Brookes, N.J. How benchmarking can support the selection, planning and delivery of nuclear decommissioning projects. Prog. Nucl. Energy 2017, 99, 155–164. [Google Scholar] [CrossRef]
- Invernizzi, D.C.; Locatelli, G.; Brookes, N.J. Managing social challenges in the nuclear decommissioning industry: A responsible approach towards better performance. Int. J. Proj. Manag. 2017, 35, 1350–1364. [Google Scholar] [CrossRef]
- De, P.L. Costs of Decommissioning Nuclear Power Plants: A Report on Recent International Estimates; IAEA Bulletin: Vienna, Austria, 1990; pp. 39–42. [Google Scholar]
- Liu, S.; He, Y.; Xie, H.; Ge, Y.; Lin, Y.; Yao, Z.; Jin, M.; Liu, J.; Chen, X.; Sun, Y.; et al. A State-of-the-Art Review of Radioactive Decontamination Technologies: Facing the Upcoming Wave of Decommissioning and Dismantling of Nuclear Facilities. Sustainability 2022, 14, 4021. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Global Status of Decommissioning of Nuclear Instalation [Internet]; International Atomic Energy Agency: Vienna, Austria, 2023; Available online: www.iaea.org/publications (accessed on 18 January 2025).
- Gurau, D.; Deju, R. Radioactive decontamination technique used in decommissioning of nuclear facilities [Internet]. Rom. J. Phys. 2014, 59, 912–919. [Google Scholar]
- Koryakovskiy, Y.S.; Doilnitsyn, V.A.; Akatov, A.A. Improving the efficiency of fixed radionuclides’ removal by chemical decontamination of surfaces in situ. Nucl. Energy Technol. 2019, 5, 155–161. [Google Scholar] [CrossRef]
- Han, S.; Hong, S.; Nam, S.; Kim, W.S.; Um, W. Decontamination of concrete waste from nuclear power plant decommissioning in South Korea. Ann. Nucl. Energy 2020, 149, 107795. [Google Scholar] [CrossRef]
- Hahm, I.; Kim, D.; Ryu, H.J.; Choi, S. A multi-criteria decision-making process for selecting decontamination methods for radioactively contaminated metal components. Nucl. Eng. Technol. 2023, 55, 52–62. [Google Scholar] [CrossRef]
- Hertel, N.; Buttram, C.; Ervin, P.; Lunberg, L.; Marske, S. Research Reactor Decommissioning Planning—It is Never Too Early to Start. In Proceedings of the Safety Analysis, Licensing & Decommissioning Session 1, International Group on Research Reactors—9, Sydney, Australia, 24–28 March 2003. [Google Scholar]
- Cross, M.T.; Green, T.H.; Adsley, I. Characterisation of radioactive materials in redundant nuclear facilities: Key issues for the decommissioning plan. In Nuclear Decommissioning; Elsevier: Amsterdam, The Netherlands, 2012; pp. 87–116. [Google Scholar]
- Moore, J.; Raine, T.; Jenkins, A.; Livens, F.; Law, K.; Morris, K.; Law, G.; Yeates, S. Decontamination of caesium and strontium from stainless steel surfaces using hydrogels. React. Funct. Polym. 2019, 142, 7–14. [Google Scholar] [CrossRef]
- Zhong, L.; Lei, J.; Deng, J.; Lei, Z.; Lei, L.; Xu, X. Existing and potential decontamination methods for radioactively contaminated metals-A Review. Prog. Nucl. Energy 2021, 139, 103854. [Google Scholar] [CrossRef]
- Castellani, R.; Poulesquen, A.; Goettmann, F.; Marchal, P.; Choplin, L. A topping gel for the treatment of nuclear contaminated small items. Nucl. Eng. Des. 2014, 278, 481–490. [Google Scholar] [CrossRef]
- Gossard, A.; Lilin, A.; Faure, S. Gels, coatings and foams for radioactive surface decontamination: State of the art and challenges for the nuclear industry. Prog. Nucl. Energy 2022, 149, 104255. [Google Scholar] [CrossRef]
- Kaminski, M.D.; Lee, S.D.; Magnuson, M. Wide-area decontamination in an urban environment after radiological dispersion: A review and perspectives. J. Hazard. Mater. 2016, 305, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sadegh, H.; Monajjemi, M.; Hamdy, A.S.; Ali, G.A.; Memar, A.O.; Shahryari-Ghoshekandi, R.; Tyagi, I.; Gupta, V.K. Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinyl alcohol. J. Mol. Liq. 2016, 218, 191–197. [Google Scholar] [CrossRef]
- Jung, C.H.; Moon, J.K.; Won, H.J.; Lee, K.W.; Kim, C.K. Chemical Gel for Surface Decontamination. In Proceedings of the Transactions of the Korean Nuclear Society Autumn Meeting, Jeju, Republic of Korea, 21–22 October 2010. [Google Scholar]
- Ma, L.; Wang, J.; Han, L.; Lu, C. Research Progress of Chemical Decontamination Technology in the Decommissioning of Nuclear Facilities. Int. Core J. Eng. 2020, 6, 57–61. [Google Scholar]
- Purna Yudha, E.; Salsabila, A.; Haryati, T. Analisis daya saing ekspor komoditas ubi kayu indonesia, thailand dan vietnam di pasar dunia. J. Maneksi 2023, 12, 417–424. [Google Scholar] [CrossRef]
- Ardyani, N.P.; Gunawan, B.; Harahap, J. Ekologi Politik Budidaya Singkong di Kecamatan Arjasari Kabupaten Bandung Provinsi Jawa Barat. Aceh Anthropol. J. 2022, 6, 137. [Google Scholar] [CrossRef]
- Fathoni, A.; Hartati, N.S.; Wahyuni, H.F.; Rahman, N.; Harmoko, R.; Perwitasari, U. Characterization of cassava starch and its potential for fermentable sugar production. IOP Conf. Ser. Earth Environ. Sci. 2020, 439, 012024. [Google Scholar] [CrossRef]
- Wang, Z.; Mhaske, P.; Farahnaky, A.; Kasapis, S.; Majzoobi, M. Cassava starch: Chemical modification and its impact on functional properties and digestibility, a review. Food Hydrocoll. 2022, 129, 107542. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.I.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Ullah, R.S.; Khan, A.; Nazir, A. Advances in chemical modifications of starches and their applications. In Carbohydrate Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 476, pp. 12–35. [Google Scholar]
- Sakhaei Niroumand, J.; Peighambardoust, S.J.; Mohammadi, R. Tetracycline decontamination from aqueous media using nanocomposite adsorbent based on starch-containing magnetic montmorillonite modified by ZIF-67. Int. J. Biol. Macromol. 2024, 259, 129263. [Google Scholar] [CrossRef]
- de Barros, N.R.; Dos Santos, R.S.; Miranda, M.C.; Bolognesi, L.F.; Borges, F.A.; Schiavon, J.V.; Marques, R.F.; Herculano, R.D.; Norberto, A.M. Natural latex-glycerol dressing to reduce nipple pain and healing the skin in breastfeeding women. Ski. Res. Technol. 2019, 25, 461–468. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Preparation and characterization of cassava bagasse reinforced thermoplastic cassava starch. Fibers Polym. 2017, 18, 162–171. [Google Scholar] [CrossRef]
- Boonsuk, P.; Sukolrat, A.; Kaewtatip, K.; Chantarak, S.; Kelarakis, A.; Chaibundit, C. Modified cassava starch/poly(vinyl alcohol) blend films plasticized by glycerol: Structure and properties. J. Appl. Polym. Sci. 2020, 137, 48848. [Google Scholar] [CrossRef]
- Fan, M.; Hu, T.; Zhao, S.; Xiong, S.; Xie, J.; Huang, Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chem. 2017, 218, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption: Measurement, Classical Theories and Quantum Theory; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Liu, S. Cooperative adsorption on solid surfaces. J. Colloid Interface Sci. 2015, 450, 224–238. [Google Scholar] [CrossRef]
- Jannesarahmadi, S.; Aminzadeh, M.; Helmig, R.; Or, D.; Shokri, N. Quantifying Salt Crystallization Impact on Evaporation Dynamics From Porous Surfaces. Geophys. Res. Lett. 2024, 51, e2024GL111080. [Google Scholar] [CrossRef]
- Kılınç, Ö.; Toplan, N. Amorf Polimerler. ALKU J. Sci. 2023, 5, 131–148. [Google Scholar]
- Toader, G.; Stănescu, P.O.; Zecheru, T.; Rotariu, T.; El-Ghayoury, A.; Teodorescu, M. Water-based strippable coatings containing bentonite clay for heavy metal surface decontamination. Arab. J. Chem. 2019, 12, 4026–4034. [Google Scholar] [CrossRef]
- Liu, F.; Peng, G.; Li, T.; Yu, G.; Deng, S. Au(III) adsorption and reduction to gold particles on cost-effective tannin acid immobilized dialdehyde corn starch. Chem. Eng. J. 2019, 370, 228–236. [Google Scholar] [CrossRef]
- Mahrous, S.S.; Mansy, M.S. Experimental study for the decontamination of various surfaces from 99Mo using PVA/Borax/Al(OH)3 strippable hydrogel. Appl. Radiat. Isot. 2024, 213, 111493. [Google Scholar] [CrossRef]
- Mahrous, S.S.; Borai, E.H.; Mansy, M.S. Polymeric gel for surface decontamination of long-lived gamma and beta-emitting radionuclides. Appl. Radiat. Isot. 2023, 197, 110834. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Chousidis, N. Polyvinyl alcohol (PVA)-based films: Insights from crosslinking and plasticizer incorporation. Eng. Res. Express 2024, 6, 025010. [Google Scholar] [CrossRef]
- Pemerintah Republik Indonesia. Peraturan Presiden Republik Indonesia No. 78 Tahun 2021 tentang Badan Riset dan Inovasi Nasional; Government of the Republic of Indonesia: Jakarta, Indonesia, 2021; pp. 1–38.
- Badan Riset dan Inovasi Nasional. Peraturan Badan Riset Dan Inovasi Nasional Republik Indonesia Nomor 6 Tahun 2021 Tentang Tugas, Fungsi, Dan Struktur Organisasi Riset Tenaga Nuklir; BRIN Indonesia: Jakarta, Indonesia, 2021; pp. 1–19. [Google Scholar]
- Badan Riset dan Inovasi Nasional. Peraturan Badan Riset Dan Inovasi Nasional Republik Indonesia Nomor 1 Tahun 2021 Tentang Organisasi Dan Tata Kerja Badan Riset Dan Inovasi Nasional; BRIN Indonesia: Jakarta, Indonesia, 2021; pp. 1–127. [Google Scholar]
- Tyas, R.L.; Deswandri, D.; Intaningrum, D.; Purba, J.H. risk assessment on the decommissioning stage of indonesian triga 2000 research reactor. J. Teknol. Reakt. Nukl. Tri Dasa Mega 2022, 24, 75. [Google Scholar] [CrossRef]
- Badan Pengawas Tenaga Nuklir. Keputusan Kepala Badan Pengawas Tenaga Nuklir No. 500/IO/Ka-BAPETEN/29-V/2017 tentang Perpanjangan Izin Operasi Reaktor Triga 2000 Bandung; BAPETEN: Jakarta, Indonesia, 2017. [Google Scholar]
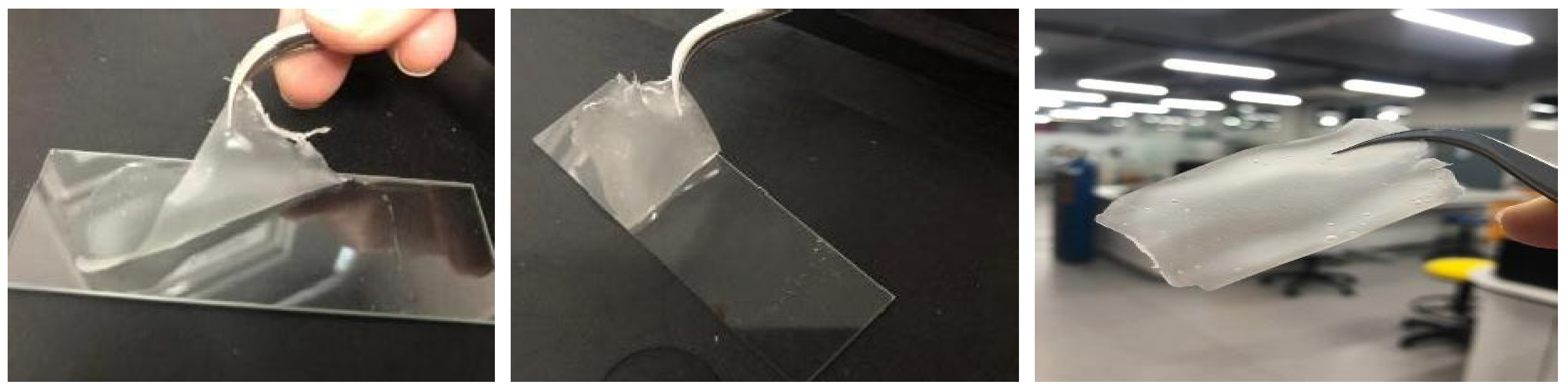

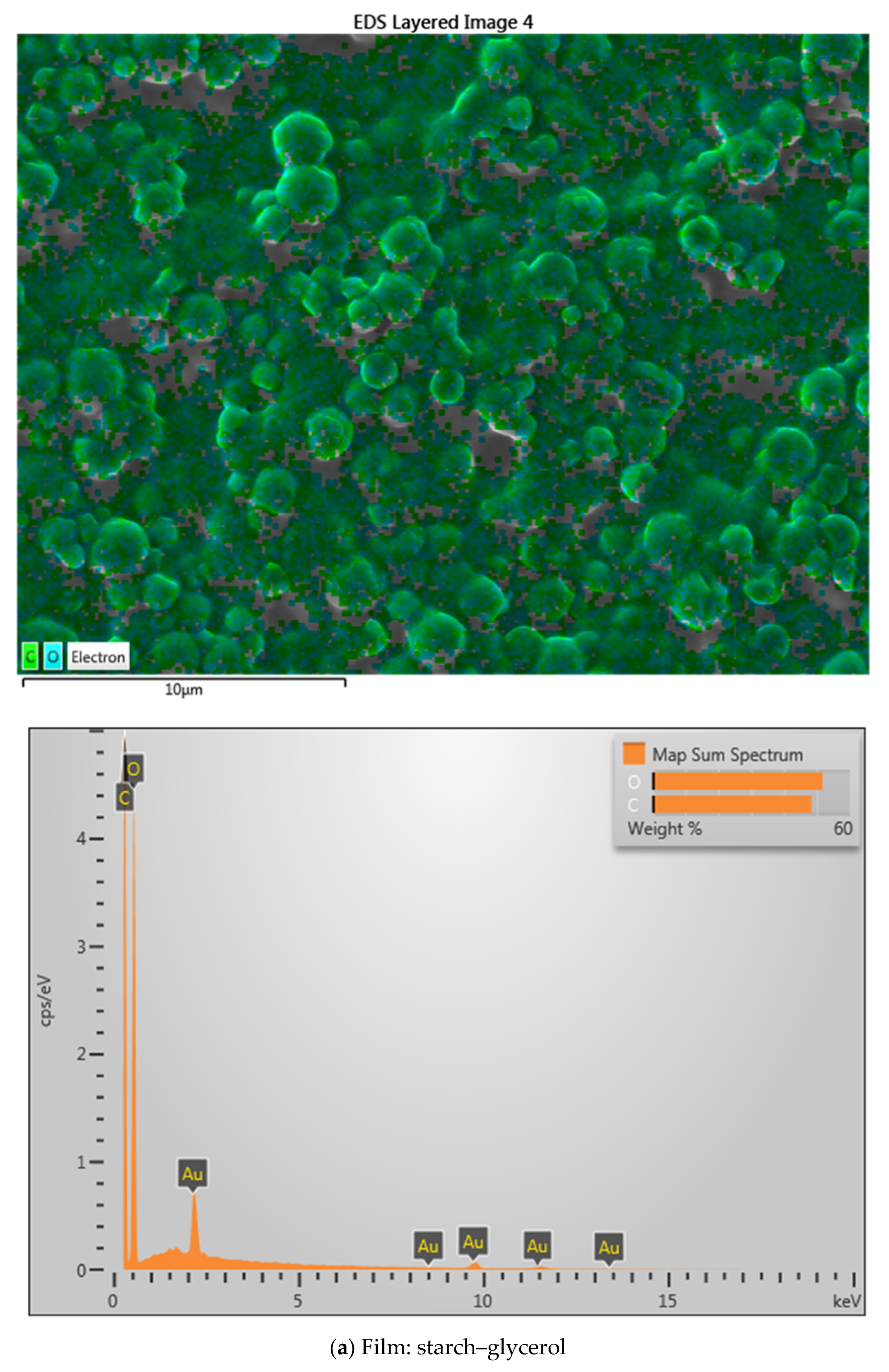



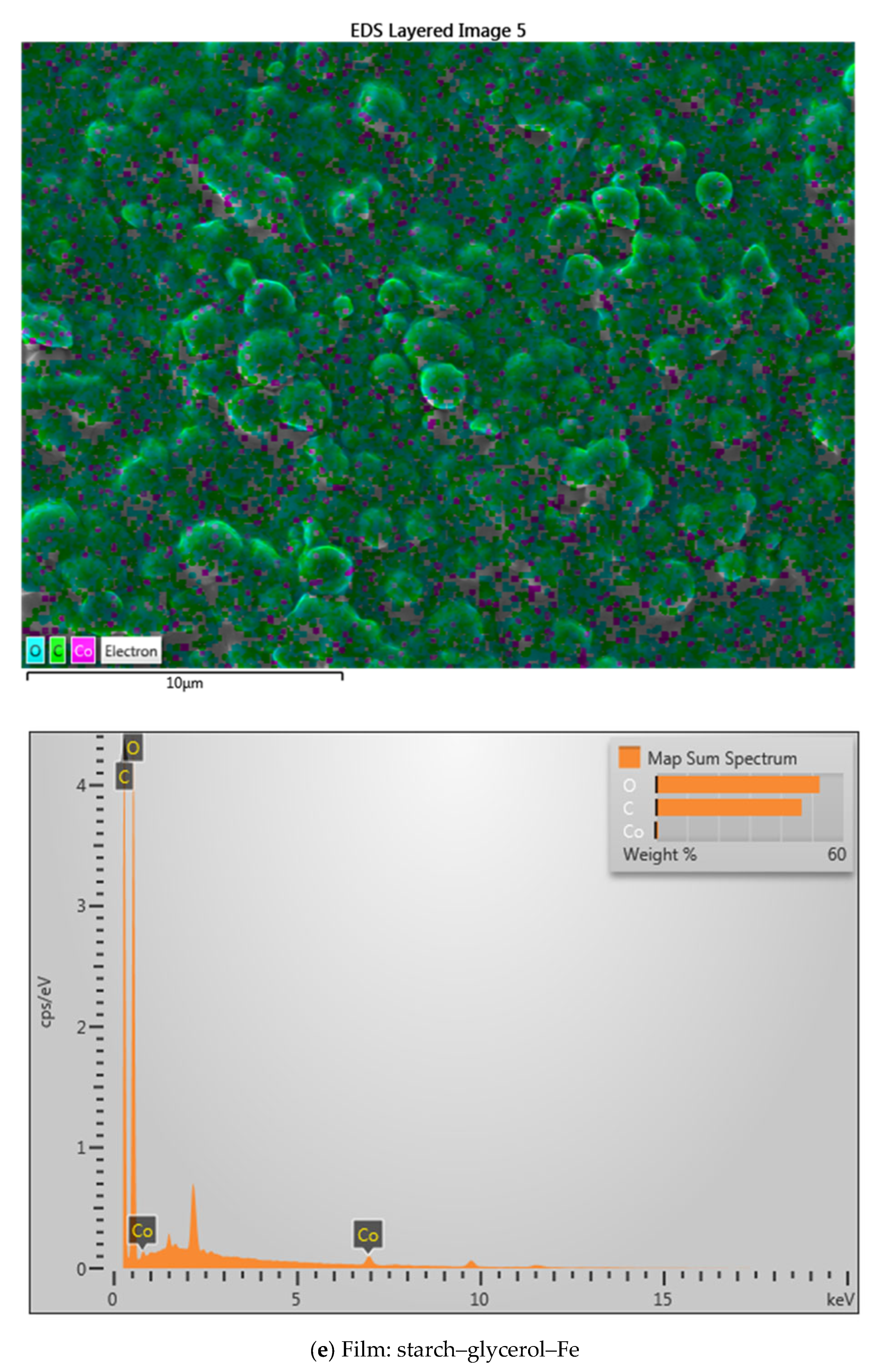
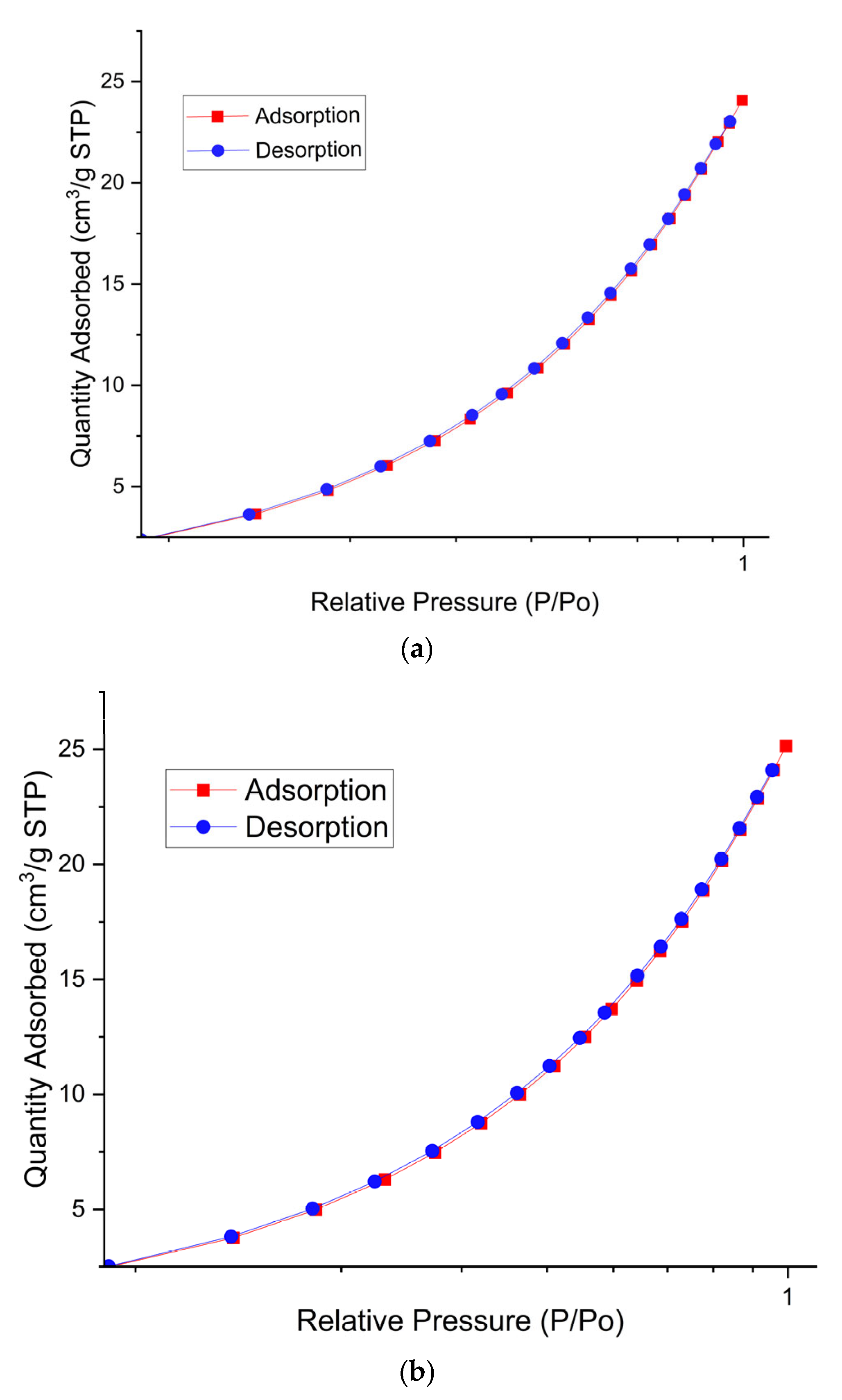
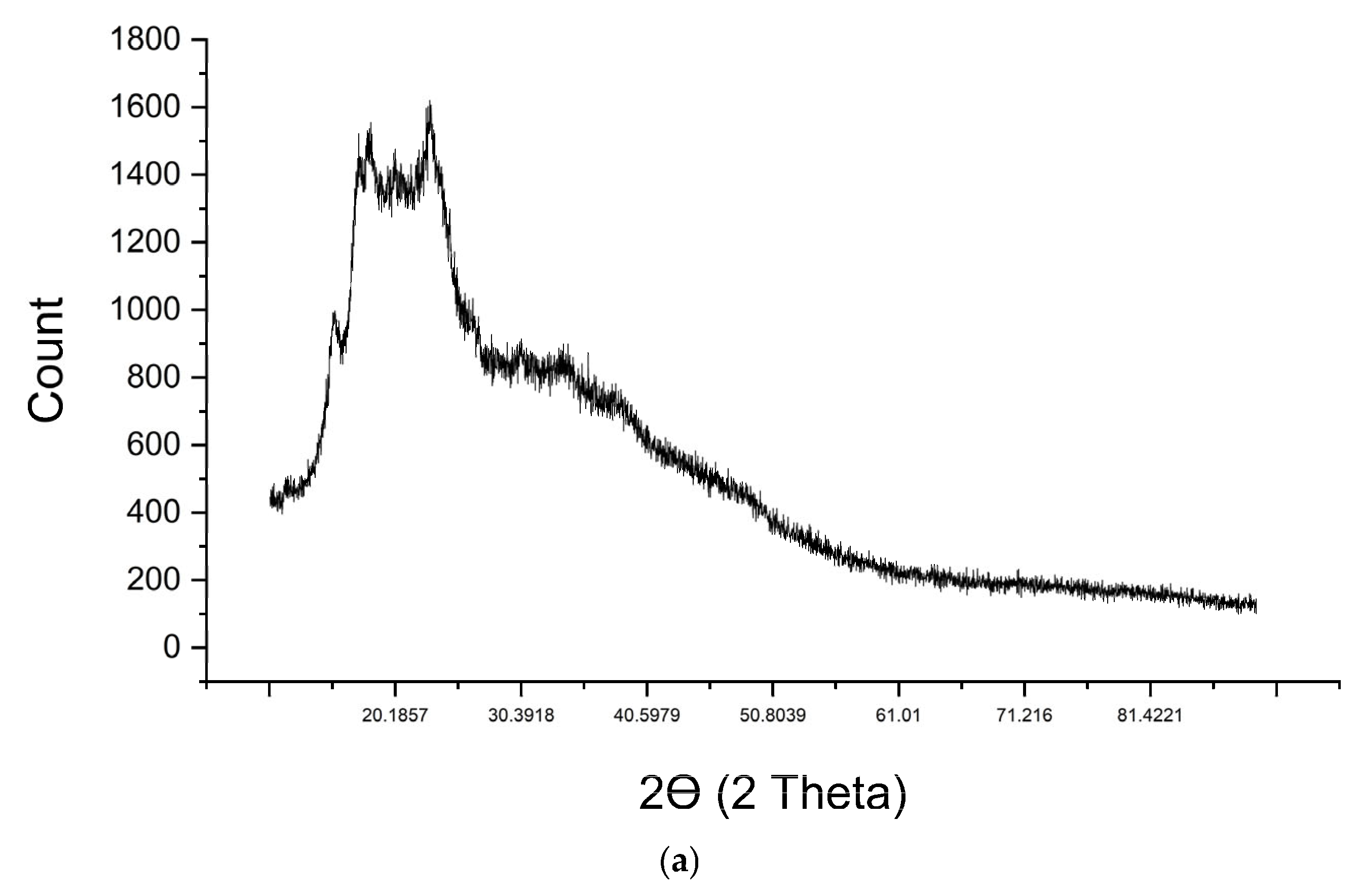
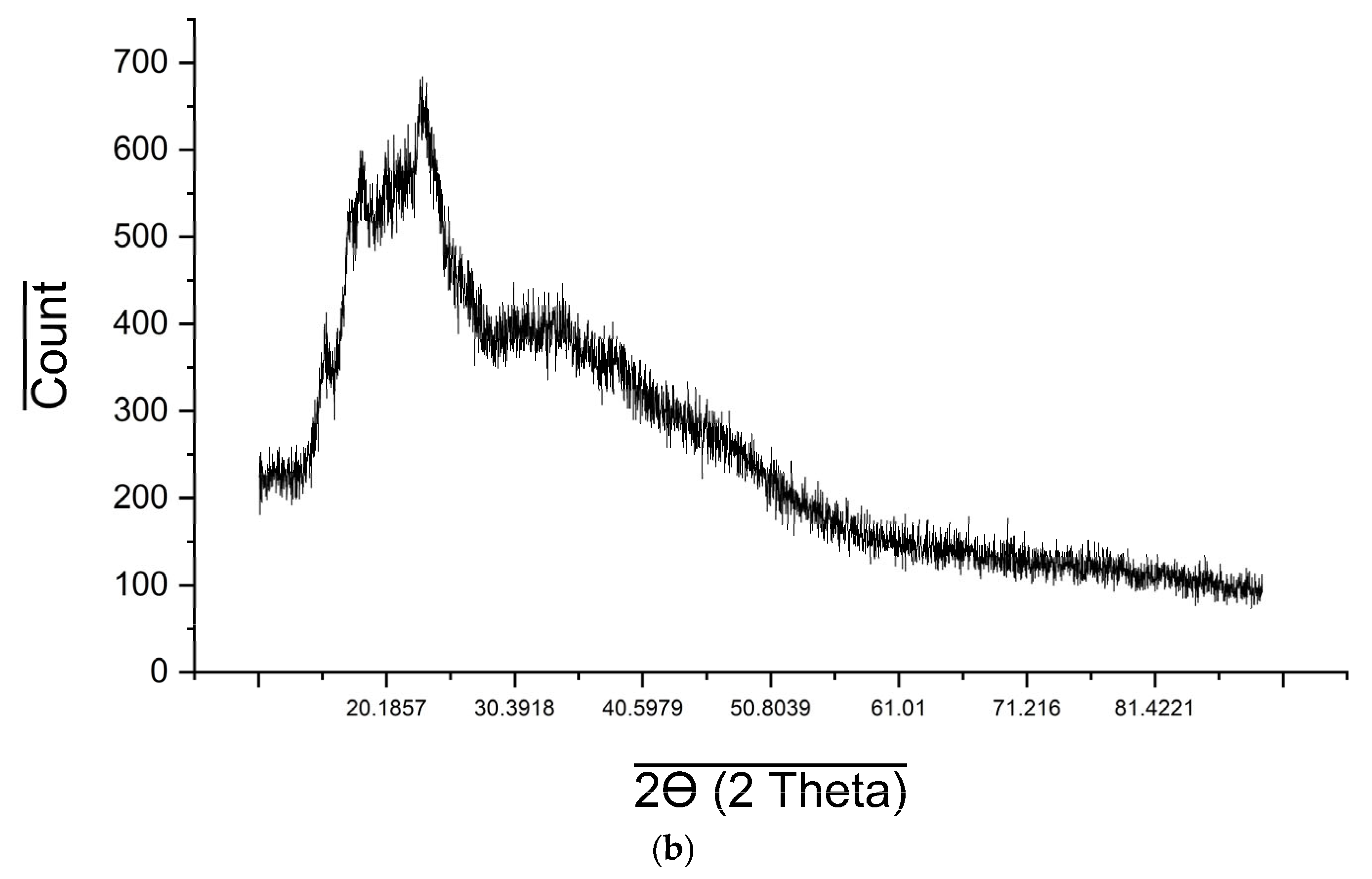

| Material Name | Surface Area (m2/gram) | Mean Pore Diameter (nm) | Total Pore Volume (cm3/gram) |
|---|---|---|---|
| Cassava powder (raw material) | 1202.047 | 0.1774 | 1.064 |
| Starch–glycerol fixed film | 33,703.643 | 0.1776 | 2.993 |
| No. | Metal Ions | Concentration (ppm) | Values Read on Resulting Film—Decontamination of Media | ||
|---|---|---|---|---|---|
| Glass | Ceramics | Aluminum | |||
| 1 | Aquadest | 0 | 0 | 0 | 0 |
| 2 | Hg | 10.000 | 6.600 | 19.957 | 4.309 |
| 3 | Pb | 5.000 | 18.362 | 6.081 | 8.552 |
| 4 | Fe | 10.000 | 68.419 | 123.001 | 111.970 |
| 5 | Co | 20.000 | 28.761 | 16.222 | 64.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anggakusuma, R.; Utama, G.L.; Sumiarsa, D.; Muslimah, P.A.D.; Asgar, A. Utilization of Cassava Starch–Glycerol Gel as a Sustainable Material to Decrease Metal Ion Surface Contamination. Gels 2025, 11, 363. https://doi.org/10.3390/gels11050363
Anggakusuma R, Utama GL, Sumiarsa D, Muslimah PAD, Asgar A. Utilization of Cassava Starch–Glycerol Gel as a Sustainable Material to Decrease Metal Ion Surface Contamination. Gels. 2025; 11(5):363. https://doi.org/10.3390/gels11050363
Chicago/Turabian StyleAnggakusuma, Rezky, Gemilang Lara Utama, Dadan Sumiarsa, Permata Apriliani Dewi Muslimah, and Ali Asgar. 2025. "Utilization of Cassava Starch–Glycerol Gel as a Sustainable Material to Decrease Metal Ion Surface Contamination" Gels 11, no. 5: 363. https://doi.org/10.3390/gels11050363
APA StyleAnggakusuma, R., Utama, G. L., Sumiarsa, D., Muslimah, P. A. D., & Asgar, A. (2025). Utilization of Cassava Starch–Glycerol Gel as a Sustainable Material to Decrease Metal Ion Surface Contamination. Gels, 11(5), 363. https://doi.org/10.3390/gels11050363








